Abstract
Lysolecithin is widely used as emulsifier to improve the digestibility and retention of fat. The current study aimed to investigate the effects of dietary lysolecithin supplementation on growth performance, nutrients absorption, lipid metabolism, and redox status of weaned pigs. A total of 60 weaned piglets were assigned into 2 dietary treatments in a randomized complete block design, receiving basal diet with 0 or 1,000 mg/kg lysolecithin for a period of 28 d. Each dietary treatment had 10 replicates with 3 piglets per replicate. Growth performance and fecal score were monitored during trial. Samples of blood, ileum, and liver tissues were collected and analyzed for serology, intestinal histomorphology, and lipid metabolism-related gene and protein expressions. Dietary lysolecithin supplementation increased average daily gain (+15%, P < 0.05) and tended to increase average daily feed intake (+14%, P = 0.08) in overall experimental period. At final, the average body weight of piglets in lysolecithin group was 10% greater than that of control group (P = 0.09). In addition, dietary lysolecithin supplementation improved the ability of nutrients absorption as indicated by the higher d-xylose level in plasma (P < 0.05). Moreover, piglets from lysolecithin group had higher concentration of high-density lipoprotein (P < 0.05), but lower triglyceride (P < 0.05) in plasma. The inclusion of lysolecithin in diet increased the level of reduced glutathione (GSH) and GSH to oxidized glutathione (GSSG) ratio in plasma and liver (P < 0.05), but attenuated the levels of malondialdehyde and GSSG in ileum (P < 0.05). The upregulation of lipogenesis-related genes (FAS and ACC), downregulation of lipolysis (PNPLA2 and PABP1), and lipid mobilization (PGC-1α and SRIT1) genes were observed in lysolecithin relative to control piglets. Compared with control group, dietary lysolecithin supplementation upregulated protein expressions of GPX4, SREBP1, and LPL in liver and LPL in ileum (P < 0.05). Collectively, our study indicates that dietary lysolecithin supplementation improved growth performance of weaned piglets, which may be associated with the improved nutrients absorption, redox status, and lipid metabolism.
Keywords: lysolecithin, growth performance, lipid metabolism, redox status
Dietary lysolecithin supplementation improves growth performance, nutrients availability, lipid metabolism, and redox status of weaned piglets.
Introduction
To improve reproductive efficiency of sows, piglets are generally weaned early in the current pig production, which is one of the most stressful events for piglets that causes a series of problems in intestinal and immune systems that result in adverse impacts such as diarrhea, poor growth performance, and even death, particularly during the first week after weaning (Campbell et al., 2013; Zhang et al., 2022a). After weaning, piglets are forced to cope with the feed transition from highly digestible and palatable liquid milk to a solid dry diet with the immature digestive system. As a consequence, the newly weaned piglets commonly become mal-nourished and have reduced feed intake. As reported before, the metabolizable energy intake of piglets during the first week post-weaning is about 60% to 70% of preweaning energy intake from milk (Campbell et al., 2013). The deficient energy intake compromises growth rate of piglets and importantly, the body weight gain in the first week after weaning is associated with the total days to market (Wolter and Ellis, 2001). Therefore, it is crucial to increase energy utilization and nutrients retention for weaned piglets to maintain optimal growth performance.
Lysolecithin, generated from phospholipase A2 hydrolysis reaction of soy lecithin to remove one molecule of fatty acid, is widely used as emulsifying agent to improve digestion, absorption, and retention of fats and oils (Zhang et al., 2022b). Relative to natural lecithin, lysolecithin is more hydrophilic and has better oil-in-water emulsification due to the removal of fatty acid (Weng et al., 2022). Lysolecithin contains diverse mixtures of lysophosphatidic acid (LPA), lysophosphatidylcholine (LPC), lysophosphatidylethanolamine (LPE), and lysophosphatidylinositol (LPI). Current research suggested that LPA promotes proliferation of intestinal epithelial cells and induces nutrients transportation and absorption (Jang et al., 2020). Besides, dietary LPC supplementation improved the utilization of fat and amino acids in hens (Juntanapum et al., 2020). LPI has been reported to stimulate the mammalian target rapamycin (mTOR) signaling pathway by activation of protein kinase B (Akt) (Menon et al., 2017; Zhang et al., 2022b), modulating protein synthesis. Recent study demonstrated that lysolecithin supplementation in sows improved energy digestibility and retention with greater litter weight at weaning (Papadopoulos et al., 2022).
Overall, it could be hypothesized that dietary lysolecithin supplementation could improve the retention of energy as well as other nutrients. In this context, the objective of this study was to investigate the effects of lysolecithin supplementation on growth performance and selected parameters associated with metabolism, intestinal morphology, redox status, and related gene and protein expressions in weaned piglets.
Materials and Methods
All institutional and national guidelines for the care and use of laboratory animals were followed. The animal experiment was approved by the Animal Care and Use Committee of the Sichuan Agricultural University (DKY-B20121602) and was performed in accordance with the National Research Council’s Guidelines for Care and Use of Laboratory Animals.
Animals and dietary treatments
A total of 60 piglets (Duroc × Landrace × Yorkshire), weaned at 21 ± 2 d of age with an average body weight of 6.14 ± 0.11 kg, were assigned into 2 dietary treatments in a randomized complete block design with body weight as block. Each dietary treatment was replicated 10 times, with 3 piglets per replicate (i.e., pen). The piglets received basal diet with 0 or 1,000 mg/kg lysolecithin for a period of 28 d. The lysolecithin complex used in this study was provided by Kemin (China) Technologies Co., Ltd., contained LPC ≥ 2.5%, LPI ≥ 1.2%, LPE ≥ 1.2%, and LPA ≥ 0.5%, with soy oil and silica as the carrier. The 2-phase basal diets were formulated referring to the nutrient requirements of pigs with 5 to 7 and 7 to 11 kg, respectively, estimated by National Research Council (NRC, 2012) (Table 1). Zinc oxide (ZnO) was supplied at 2,000 mg/kg in the first 2 wk only. To reduce the stress caused by feed change, the feed transition was completed gradually over first 3 d in the third week. Piglets were housed in an environmentally controlled room and room temperature was kept at 30 °C in the first week with an ~1 °C reduction each week thereafter. All piglets had free access to feed and water throughout the experimental period. The feed supply and feed refusals were recorded twice a week and piglets were weighed every 2 weeks, thus average daily gain (ADG), average daily feed intake (ADFI), and ratio of ADFI to ADG (F:G) could then be calculated. Fecal scores were visually assessed 3 times a day throughout the experiment as previous described (Li et al., 2021). Briefly, firm and well-formed feces were scored as 0; soft and formed feces were scored as 1; fluid and usually yellowish feces were scored as 2; and watery and projectile feces were scored as 3.
Table 1.
Composition of ingredients and nutrient level of the diet (as-fed basis)
| Ingredient, % | 1-14 d | 15-28 d |
|---|---|---|
| Corn (7.8% CP) | 53.22 | 61.06 |
| Dehulled soybean meal (46% CP) | 7.50 | 15.00 |
| Fermented soybean meal (50% CP) | 10.00 | 5.00 |
| Extruded soybean (35.5% CP) | 3.00 | 3.00 |
| Whey powder )2% CP) | 10.00 | 5.00 |
| Fish meal (62.5 % CP) | 4.00 | 2.00 |
| Milk powder (16% CP) | 2.00 | — |
| Soybean oil | 2.00 | 1.00 |
| Sucrose | 2.00 | 2.00 |
| Glucose | 2.00 | 2.00 |
| l-Lysine·HCl (98%) | 0.64 | 0.51 |
| dl-Methionine (98.5%) | 0.25 | 0.20 |
| l-Threonine (98%) | 0.25 | 0.18 |
| l-Tryptophan (98%) | 0.05 | 0.04 |
| Choline chloride (50%) | 0.16 | 0.16 |
| Calcium carbonate | 1.00 | 1.00 |
| Dicalcium phosphate monohydrate | 0.58 | 0.70 |
| Sodium chloride | 0.40 | 0.40 |
| Zinc oxide (75%) | 0.20 | - |
| Acidifier a | 0.50 | 0.50 |
| Mineral premix b | 0.20 | 0.20 |
| Vitamin premix c | 0.05 | 0.05 |
| Total | 100.00 | 100.00 |
| Calculated nutrient levels | ||
| Digestible energy, MCal/kg | 3.48 | 3.39 |
| Crude protein, % | 18.08 | 17.47 |
| Ca, % | 0.80 | 0.70 |
| Total P, % | 0.53 | 0.47 |
| Available P, % | 0.40 | 0.33 |
| SID Lys, % | 1.47 | 1.34 |
| SID Met, % | 0.55 | 0.48 |
| SID Met + Cys, % | 0.84 | 0.75 |
| SID Thr, % | 0.91 | 0.84 |
| SID Trp, % | 0.24 | 0.22 |
aThe acidifier (Rusuanbao) was provided by Kemin (China) Technologies Co., Ltd.
bMineral premix provided per kilogram of diet: Fe as ferrous sulfate,100 mg; Cu as copper sulphate, 150 mg; Mn as manganese sulphate, 20 mg; Zn as zinc sulphate, 100 mg; I as potassium iodide, 0.3 mg; Se as sodium selenite, 0.3 mg.
cVitamin premix provided per kilogram of diet: VA as vitamin A acetate, 9,000IU; VD3 as 25-hydroxyvitamin D3, 3,000IU; VE as α tocopheryl acetate, 20IU; VK3 as menadione nicotinamide bisulfite, 3.0 mg; VB1 as thiamine nitrate, 1.5 mg; VB2 4.0 mg; VB6, 3.0 mg; VB12, 0.2 mg; nicotinic acid, 30.0 mg; pantothenic acid, 15.0 mg; folic acid, 0.75 mg; biotin, 0.1 mg.
Samples collection
After the 28-d lysolecithin supplementation, piglets were weighed individually on the morning (0600 hours) of day 29 after an overnight fast. The 2 pens with largest and smallest average body weight each treatment were excluded from sampling. For the remaining pens, the piglet with body weight closest to the average body weight of this pen were selected for d-xylose administration and sampling (n = 8). The d-xylose powder (Sigma-Aldrich, St. Louis, MO, USA) was dissolved into deionized water at the dose of 50 mg/mL and d-xylose solution was orally administrated to piglets at 10 mL/kg body weight. Blood samples of 10 mL were collected from jugular vein into sodium heparinised tubes 1 hour after d-xylose administration. The blood samples were then centrifuged at 1000 × g for 15 min and plasma was harvested and stored at −20 °C for later analysis.
After blood sampling, all blood-sampled piglets received intramuscular injection of anaesthetic (Shu Mianling II Injection, 0.1 mL/kg body weight; Animal disease laboratory, Chengdu, China) and then euthanized. The ileal tissue samples of ~6 cm in length were opened longitudinally, of which 2 cm samples were preserved in 4% paraformaldehyde solution for histological measurements and another 4 cm samples were washed with physiological saline to remove chyme and then snap frozen and stored immediately in liquid nitrogen. Samples of liver tissue were also collected and stored in liquid nitrogen. All the samples stored in liquid nitrogen were then transferred to −80 °C refrigerator for long-term storage.
Determination of plasma metabolites and d-xylose
Frozen plasma samples were thawed on the ice and centrifuged at 3,000 × g for 5 min. Then 300 µL of supernatant were used for measuring the concentrations of glucose (GLU), triglyceride (TG), nonesterified fatty acid (NEFA), total cholesterol (TC), high-density lipoprotein (HDL), low-density lipoprotein (LDL), and urea using an automatic biochemical analyzer (Model 7020, Hitachi, Tokyo, Japan) with corresponding kits.
The plasma concentration of d-xylose was measured using spectrophotometry with specific assay kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). Standard working solutions were prepared with standard stock solution by 10-fold dilution with standard dilution buffer. Then both plasma samples and standard working solutions (30 µL) were mixed with 3 mL of phloroglucinol color reagent solution and cooling in the water after heating at 100 °C for 4 min. Finally, 200 µL of supernatant was taken into 96-well plates and optical density values were determined by microplate reader (Model 680, BIO-RAD, Japan) at 554 nm.
Ileal morphology and goblet cell counting
Ileal samples preserved in 4% paraformaldehyde solution were washed, dehydrated, and then embedded in paraffin. The paraffin was cut into 5 µm thick slices (5 slices per sample) using microtome (Leica RM2235), which were stained with hematoxylin and eosin for intestinal morphology analysis. All the intact well-oriented crypt–villus units were measured (Olympus BX43) and thus the ratio of villus to crypt depth (VCR) could be calculated. The slices were stained with Periodic Acid Schiff and Alcian Blue (PAS-AB) to measure the number of goblet cells, and the values obtained from 10 villi by each ileal segment were averaged.
Oxidative stress parameters
Frozen plasma samples were thawed on the ice and centrifuged at 3,000 × g for 5 min. The liver and ileum samples were thawed and homogenized on ice with cold buffer, then homogenate was centrifuged at 4,000 × g at 4 °C for 15 min to obtain the supernatant. Protein concentrations of liver and ileum samples were determined by bicinchoninic acid (BCA) protein kit (Beyotime, Shanghai, China). Activities of catalase (CAT), total superoxide dismutase (T-SOD), total antioxidant capacity (T-AOC), malondialdehyde (MDA), glutathione peroxidase (GSH-Px), reduced glutathione (GSH), and oxidized glutathione (GSSG) in plasma, liver ,and ileum samples (supernatant) were measured using enzyme-linked immunosorbent assay (ELISA), according to the kit instructions (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). The ratio of GSH:GSSG was also calculated.
RNA extraction and real-time quantitative PCR
Total RNA of liver and ileum tissues was extracted using Trizol reagent (TaKaRa Biotechnology, Dalian, China). The concentration and purity of the extracted RNA were determined by nucleic acid analyzer (Beckman DU-800; Beckman Coulter, Inc., Brea, CA). Reverse transcription of RNA and real-time quantitative polymerase chain reaction (RT-qPCR) were performed by PrimeScript RT reagent Kit with gDNA Eraser and TB Green Premix Ex Taq II (Takara), respectively. The total PCR reaction system was 10 μL, consisting of 5.0 μL TB Green Premix Ex TaqII, 1.0 μL forward primer, 1.0 μL reverse primer, 0.2 μL ROX reference dye II, and 2.8 μL cDNA. β-Actin was treated as housekeeping gene to normalize the expression of target genes according to the 2−ΔΔCt method. The PCR primers used in this study are listed in Table 2.
Table 2.
Primer sequences of target and reference genes
| Gene1 | Primer sequence (5ʹ→3ʹ) | Accession no. |
|---|---|---|
| FAS | F:CGTTGGGTCGACTCACTGAA R:GAGACAGTTCACCATGCCCA |
NM_001099930.1 |
| ACC | F:GGATGAACCGTCTCCCTTGG R:GTGTAAGGCCAAGCCATCCT |
NM_001114269.1 |
| PNPLA2 | F:TGTTCCCCAAAGAGACGACG R:CGTTGGCCACTAGGGAGGA |
NM_001098605.1 |
| FABP1 | F:TCAAGGGGACATCGGAAATCG R:ACTCCTCTCCCAAGGTGAACT |
NM_001004046.2 |
| PGC-1α | F:TGTGCAACCAGGACTCTGTA R:TGGTCACTGCACCACTTGAG |
NM_213963.2 |
| SIRT1 | F:GAGCCGCTCCGCAAGAAA R:TAAGCCCGGCCCATTGTTT |
NM_001145750.2 |
FAS, fatty acid synthase; ACC, acetyl-CoA carboxylase; PNPLA2, patatin-like phospholipase domain containing 2; FABP1, fatty acid binding protein 1; PGC-1α, peroxisome proliferator activated receptor γ coactivator-1α; SIRT1, silent information regulator 1.
Western blot analysis
Six liver and ileum samples each treatment were randomly selected for western blot analysis. Protein extraction and western blotting were performed as described previously (Wu et al., 2021a). Briefly, about 50 mg samples of liver and ileum were homogenated mixed with RIPA lysate buffer (Servicebio, Wuhan, China) to separate protein, then BCA protein kit was used to measured protein concentrations. Prepared protein samples were separated by gel electrophoresis and transferred to solid phase carrier (PVDF) membranes, which were then incubated with diluent primary antibodies (1:1000) and second antibodies (1:3,000) and washed with TBST (Tris buffer solution + Tween) before developing.
Statistical analysis
The Shapiro–Wilk test and Levene’s test procedures of SAS 9.4 (SAS Institute, Inc., Cary, NC, USA) were used to evaluate the variance homogeneity and normality, respectively. For growth performance and fecal score, pens served as the experimental unit, and piglet data were reported as a mean for the pen. Data were analyzed using mixed model with the dietary treatment as fixed effect and initial body weight as random effect, using the following statistical model:
where Y is the parameter to be tested, µ is the mean, αi is the effect of the diet (i = 1, 2), υj the random effect of the body weight, and εij the error term. Pearson correlation analysis was applied to further explore potential associations between growth performance and redox related parameters. For all statistical analyses, significance was declared at P < 0.05 and trends at 0.05 ≤ P < 0.10.
Results
Growth performance
Piglets fed lysolecithin diet had increased ADG during days 15 to 28 and overall experimental period (P < 0.05), compared with pigs fed control diet. In parallel, dietary lysolecithin supplementation increased ADFI of piglets during days 15 to 28 (P < 0.05) and tended to increase the ADFI in overall experimental period (P = 0.08). Accordingly, piglets fed lysolecithin diet tended to have greater body weights at final (P = 0.09) than control ones. No significant difference was observed among dietary treatments in F:G and fecal score at all phases (Table 3, P > 0.05).
Table 3.
Growth performance and faecal score in weaned piglets
| Item | Control | Lysolecithin | P—value |
|---|---|---|---|
| Body weights, kg | |||
| Day 1 | 6.13 ± 0.66 | 6.15 ± 0.69 | 0.95 |
| Day 14 | 9.64 ± 0.52 | 10.23 ± 1.40 | 0.30 |
| Day 28 | 15.37 ± 0.82 | 16.86 ± 2.56 | 0.09 |
| ADG, g/d | |||
| Days 1 to 14 | 255 ± 24 | 291 ± 63 | 0.13 |
| Days 15 to 28 | 410 ± 39 | 473 ± 93 | 0.03 |
| Overall period | 333 ± 23 | 382 ± 76 | 0.04 |
| ADFI, g/d | |||
| Days 1 to 14 | 420 ± 28 | 462 ± 83 | 0.18 |
| Days 15 to 28 | 630 ± 57 | 744 ± 130 | 0.03 |
| Overall period | 525 ± 35 | 598 ± 107 | 0.08 |
| F:G | |||
| Days 1 to 14 | 1.65 ± 0.12 | 1.60 ± 0.14 | 0.49 |
| Days 15 to 28 | 1.54 ± 0.13 | 1.59 ± 0.19 | 0.58 |
| Overall period | 1.58 ± 0.05 | 1.57 ± 0.13 | 0.94 |
| Faecal score | |||
| Days 1 to 14 | 0.60 ± 0.19 | 0.62 ± 0.19 | 0.86 |
| Days 15 to 28 | 0.60 ± 0.29 | 0.53 ± 0.13 | 0.55 |
| Overall period | 0.61 ± 0.21 | 0.59 ± 0.14 | 0.81 |
ADG, average daily gain; ADFI, average daily feed intake; F:G, the ratio of ADFI to ADG. Firm and well-formed feces were scored as 0; soft and formed feces were scored as 1; fluid and usually yellowish feces were scored as 2; and watery and projectile feces were scored as 3. n = 10.
Plasma metabolic parameters and D-xylose concentration
As shown in Table 4, dietary lysolecithin supplementation increased the plasma concentration of HDL (P < 0.05), but decreased the plasma concentration of TG (P < 0.05) and tended to decrease the plasma concentration of urea (P = 0.07). In addition, dietary lysolecithin supplementation increased the plasma concentration of d-xylose in piglets (P < 0.01).
Table 4.
Plasma metabolites and D-xylose concentrations in weaned piglets
| Item | Control | Lysolecithin | P—value |
|---|---|---|---|
| GLU, mmol/L | 5.19 ± 0.82 | 5.45 ± 0.86 | 0.57 |
| TG, mmol/L | 0.95 ± 0.23 | 0.67 ± 0.16 | 0.04 |
| NEFA, mmol/L | 0.96 ± 0.30 | 0.87 ± 0.36 | 0.60 |
| TC, mmol/L | 2.00 ± 0.35 | 2.06 ± 0.35 | 0.76 |
| HDL, mmol/L | 0.74 ± 0.07 | 0.87 ± 0.10 | 0.03 |
| LDL, mmol/L | 1.12 ± 0.27 | 1.15 ± 0.23 | 0.81 |
| Urea, mmol/L | 4.77 ± 1.06 | 3.79 ± 0.78 | 0.07 |
| d-xylose, mmol/L | 1.51 ± 0.58 | 2.40 ± 0.44 | <0.01 |
GLU, glucose; TG, triglyceride; NEFA, nonesterified fatty acid; TC, total cholesterol; HDL, high-density lipoprotein; LDL, low-density lipoprotein. n = 8.
Intestinal morphology and goblet cell density
Compared with piglets fed control diet, piglets fed lysolecithin diet tended to have shorter crypt depth of ileum (P = 0.06). There was no significant difference among dietary treatments in villus height, VCR, and number of villous goblet cells (Figure 1, P > 0.05).
Figure 1.
Intestinal morphology and number of goblet cells in the ileum of weaned piglets. (A) Villous height and crypt depth; (B) the ratio of villous height to crypt depth (VCR) and goblet cell numbers per villous; (C, D) representative micrographs of villous goblet cell stained with periodic acid Schiff method (C, control group; D, lysolecithin group). n = 8.
Redox status
The results pertaining to the redox parameters are shown in Table 5. Dietary lysolecithin supplementation increased activity of CAT in plasma (P < 0.05) and tended to increase activity of CAT in liver (P = 0.09). Besides, dietary lysolecithin supplementation increased the concentration of GSH in both plasma and liver (P < 0.05). Piglets fed lysolecithin diet had lower levels of MDA and GSSG in ileum (P < 0.05) and tended to have lower levels of MDA (P = 0.06) and GSSG (P = 0.05) in liver, relative to control ones. In addition, increased ration of GSH to GSSG was observed in plasma (P < 0.05), liver (P < 0.05) and ileum (P = 0.06). Diet treatment had no significant influence on T-SOD, T-AOC, and GSH-Px (P > 0.05).
Table 5.
Redox status in plasma, liver and ileum of weaned piglets
| Item | Control | Lysolecithin | P—value |
|---|---|---|---|
| Plasma | |||
| CAT, U/mL | 7.21 ± 2.86 | 53.01 ± 17.87 | <0.01 |
| T-SOD, U/mL | 29.25 ± 2.38 | 28.70 ± 1.95 | 0.64 |
| T-AOC, U/mL | 1.20 ± 0.61 | 1.84 ± 0.63 | 0.18 |
| MDA, nmol/mL | 13.05 ± 2.78 | 12.54 ± 1.52 | 0.68 |
| GSH-Px, U/mL | 33.27 ± 0.08 | 33.29 ± 0.20 | 0.79 |
| GSH, µmol/L | 11.57 ± 3.33 | 21.15 ± 7.29 | 0.02 |
| GSSG, µmol/L | 23.86 ± 5.61 | 20.52 ± 7.47 | 0.36 |
| GSH:GSSG | 0.46 ± 0.19 | 1.04 ± 0.38 | 0.01 |
| Liver | |||
| CAT, U/mg prot | 3.56 ± 0.64 | 4.54 ± 0.90 | 0.09 |
| T-SOD, U/mg prot | 7.53 ± 1.14 | 7.24 ± 0.68 | 0.58 |
| T-AOC, U/mg prot | 0.62 ± 0.12 | 0.58 ± 0.18 | 0.45 |
| MDA, nmol/mg prot | 2.19 ± 0.30 | 1.90 ± 0.17 | 0.06 |
| GSH-Px, U/mg prot | 4.60 ± 0.42 | 4.50 ± 0.47 | 0.66 |
| GSH, µmol/mg prot | 9.07 ± 1.33 | 15.15 ± 3.24 | <0.01 |
| GSSG, µmol/mg prot | 2.34 ± 1.08 | 1.27 ± 0.22 | 0.05 |
| GSH:GSSG | 4.21 ± 1.51 | 12.23 ± 3.30 | <0.01 |
| Ileum | |||
| CAT, U/mg prot | 2.55 ± 0.55 | 2.60 ± 1.52 | 0.94 |
| T-SOD, U/mg prot | 9.27 ± 1.95 | 9.42 ± 1.92 | 0.90 |
| T-AOC, U/mg prot | 0.60 ± 0.25 | 0.60 ± 0.08 | 0.98 |
| MDA, nmol/mg prot | 1.89 ± 0.16 | 1.52 ± 0.22 | <0.01 |
| GSH-Px, U/mg prot | 7.04 ± 1.27 | 7.04 ± 0.99 | 0.99 |
| GSH, µmol/mg prot | 9.84 ± 1.13 | 9.93 ± 0.87 | 0.87 |
| GSSG, µmol/mg prot | 1.82 ± 0.76 | 0.87 ± 0.41 | 0.03 |
| GSH:GSSG | 6.29 ± 2.26 | 12.56 ± 7.12 | 0.06 |
CAT, catalase; T-SOD, total superoxide dismutase; T-AOC, total antioxidant capacity; MDA, malondialdehyde; GSH-Px, glutathione peroxidase; GSH, reduced glutathione; GSSG, oxidized glutathione. n = 8.
Lipid metabolism-related gene expression
As shown in Table 6, relative to control ones, piglets fed lysolecithin diet had higher mRNA expressions of ACC (P < 0.05), but lower expressions of PNPLA2, FABP1, PGC-1α, and SIRI1 in liver (P < 0.05). Meanwhile, dietary lysolecithin supplementation elevated mRNA expressions of FAS and ACC (P < 0.05), but decreased expression of PNPLA2 in ileum (P < 0.05).
Table 6.
Gene expressions in liver and ileum of weaned piglets
| Item | Control | Lysolecithin | P—value |
|---|---|---|---|
| Liver | |||
| FAS | 1.00 ± 0.19 | 0.86 ± 0.20 | 0.21 |
| ACC | 1.00 ± 0.55 | 2.15 ± 0.97 | 0.02 |
| PNPLA2 | 1.00 ± 0.33 | 0.66 ± 0.13 | 0.03 |
| FABP1 | 1.00 ± 0.40 | 0.52 ± 0.11 | 0.02 |
| PGC-1α | 1.00 ± 0.21 | 0.73 ± 0.15 | 0.03 |
| SIRT1 | 1.00 ± 0.20 | 0.75 ± 0.20 | 0.04 |
| Ileum | |||
| FAS | 1.00 ± 0.11 | 1.24 ± 0.15 | 0.01 |
| ACC | 1.00 ± 0.28 | 1.37 ± 0.16 | 0.02 |
| PNPLA2 | 1.00 ± 0.25 | 0.73 ± 0.18 | 0.03 |
| FABP1 | 1.00 ± 0.22 | 0.98 ± 0.21 | 0.88 |
| PGC-1α | 1.00 ± 0.37 | 1.08 ± 0.41 | 0.74 |
| SIRT1 | 1.00 ± 0.32 | 0.93 ± 0.22 | 0.62 |
FAS, fatty acid synthase; ACC, acetyl-CoA carboxylase; PNPLA2, patatin-like phospholipase domain containing 2; FABP1, fatty acid binding protein 1; PGC-1α, peroxisome proliferator activated receptor γ coactivator-1α; SIRT1, silent information regulator 1. n = 8.
Relative expression of proteins
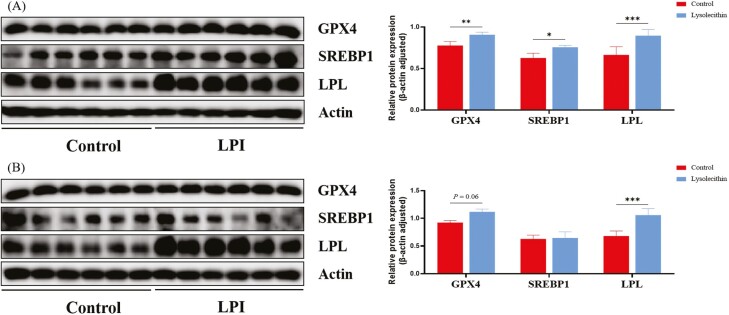
Compared with the control group, dietary lysolecithin supplementation upregulated protein expressions of GPX4, SREBP1, and LPL in liver (Figure 2A; P < 0.05). In addition, piglets fed lysolecithin diet had elevated protein expression of LPL in ileum (Figure 2B; P < 0.05), and tended to have higher protein expression of GPX4 (Figure 2B; P = 0.06).
Figure 2.
Relative protein expression in liver (A) and ileum (B) of weaned piglets. GPX4, glutathione peroxidase 4; SREBP1, sterol regulatory element binding protein 1; LPL, lipoprotein lipase. *, P < 0.05; **, P < 0.01, ***, P < 0.001. n = 6.
Correlations
Based on Pearson correlation analyses, associations between growth performance and redox-related parameters in plasma, liver, and ileum are shown as heatmaps. The growth performance involves in 7 indexes, BW at final, ADG in the first and last 2 wk and in whole period, ADFI in the first and last 2 wk and in whole period. As presented in Figure 3, CAT in ileum was positive correlated with all the 7 indexes (all Ps < 0.05), while CAT in plasma was positive correlated with 5 indexes (all Ps < 0.05) except for ADG (P = 0.06, r = 0.61) and ADFI (P = 0.09, r = 0.57) in the first 2 wk. Negative correlations between MDA in ileum and final BW, ADG in the first 2 wk and ADFI (all Ps < 0.05) were observed. Meanwhile, MDA in ileum tended to have negative correlation with ADG in whole period (P = 0.05, r = −0.53). Moreover, GSH in plasma was positive correlated with 6 indexes (all Ps < 0.05) except for ADFI (P = 0.07, r = 0.55) in the last 2 wk.
Figure 3.
Heatmap of correlation of growth performance and redox related parameters of weaned piglets. BW, body weight; ADG, average daily gain; ADFI, average daily feed intake; SOD, superoxide dismutase; CAT, catalase; MDA, malondialdehyde; GSH, reduced glutathione; GSSG, oxidized glutathione. *, P < 0.05; **, P < 0.01.
Discussion
Due to early weaning, piglets have to face physiological, environmental, and social challenges in advance that predispose the pig to subsequent diseases and other production losses, such as lower digestive ability and growth rate (Campbell et al., 2013; Heo et al., 2013). The weaned piglets are highly sensitive to factors that cause diarrhea due to their immature gastrointestinal tract and undeveloped immune system, which then decrease the utilization of nutrients. In this study, we found that inclusion of dietary lysolecithin improved growth performance, nutrients absorption, redox status, and lipid metabolism in weaned piglets.
The intrinsic nature of growth process is essentially cell proliferation (mitosis as well as endoreduplication cycles) and protein deposition (Moyano-Rodriguez and Queralt, 2020). Intriguingly, previous studies pointed that LPI is a potent mitogenic factor, stimulating cell growth in an autocrine loop mechanism (Falasca and Corda, 1994; Falasca et al., 1998). In this study, consistently, distinct features were observed in the ADG and ADFI between groups, especially in the last 2 wk of experimental period. Subsequently, piglets fed lysolecithin diet had 16% greater total weight gain and 10% greater final body weights. Urea, the product of protein degradation, reflects amino acid metabolism and protein turnover (Malcolm and Wu, 2018). Dietary lysolecithin supplementation tended to decrease plasma concentration of urea, implying positive nitrogen balance by lysolecithin supplementation, which aligned with the better growth performance. The d-xylose absorption test is clinically used to evaluate absorptive function of intestine (Che et al., 2016; Cicalese et al., 2016). Dietary lysolecithin supplementation increased the plasma concentration of d-xylose, suggesting that the availability of nutrients may be improved by lysolecithin administration. Villous height and crypt depth were also determined to reflect the digestive and absorptive functions of the small intestine (Peng et al., 2019). It should be stressed that weaning would lead to both acute and long-lasting structural and functional changes in gut, including villous atrophy and crypt elongatione (Boudry et al., 2004). Likewise, our previous study pointed that the crypt depth of piglets increased dramatically under pathological status, such as the challenge of enterotoxigenic Escherichia coli K88 (Peng et al., 2019), indicating a faster turnover of new villous cells. In this study, we found that crypt depth tended to be shorter by dietary lysolecithin supplementation. Taken together, these results indicated that lysolecithin improved nutrients availability of weaned piglets.
G-protein coupled receptor 55 (GPR55) has been identified as the receptor of LPI (Oka et al., 2007). In mammalian, GPR55 is expressed in several tissues, including jejunum and ileum (Petitet et al., 2006; Ryberg et al., 2007). Noteworthy, the profound link between LPI-GPR55 axis and obesity has been demonstrated that LPI activates GPR55 and signaling cascades downstream of the receptor stimulating cell proliferation (Andradas et al., 2011; Hu et al., 2011). In humans, elevated circulating LPI concentration was observed in obese patients and positively correlated with fat mass and BMI (José et al., 2012). Based on the current knowledge, we investigated the mRNA expression of genes related to lipid metabolism. FAS catalyzes de novo fatty acids synthesis and ACC regulates the metabolism of fatty acids (Kim, 1997; Mashima et al., 2009). Our results showed that dietary lysolecithin supplementation increased the mRNA expressions of FAS and ACC, which were in agreement with the perspective that LPI increased the expression of lipogenic genes in visceral adipose tissue explants (José et al., 2012).
Apart from genes, we also conducted western bolt to analyze the expression of specific proteins involved in fatty acid utilization, including SREBP1 and LPL. SREBP1 is reported to enhance the transcription of genes that encode enzymes of cholesterol and fatty acid biosynthesis and uptake (Jiao et al., 2020). LPL is highly expressed in adipocytes (Kersten, 2014) and is one of the most essential factors in systemic lipid partitioning and metabolism (Wu et al., 2021b). The findings that protein expressions of SREBP1 and LPL were increased by lysolecithin supplementation supported our results of gene expressions. Importantly, LPL also functions in catalyzing the hydrolysis of intravascular triglycerides (Young and Zechner, 2013). In addition, lipids and apolipoproteins derived from hydrolysis of triglyceride-rich lipoproteins are essential for the generation of HDL (Stadler and Marsche, 2020). Therefore, a strong inverse relationship of HDL and triglyceride levels are often observed. In support, we found that dietary lysolecithin supplementation decreased plasma concentration of triglyceride but increased HDL concentration. In contrast to the upregulation of lipogenesis, the genes related to lipolysis (PNPLA2 and FABP1) were downregulated by lysolecithin supplementation. PGC-1α is related to energy metabolism and enhances adaptive thermogenesis in fat (Lin et al., 2002), and SIRT1 targets PGC-1α to induce fatty oxidation. The decreased mRNA expression of PGC-1α and SIRT1 further indicated the elevated energy retention by lysolecithin supplementation, which was in line with previous report that LPE is involved in suppressing lipolysis and fatty acid biosynthesis in a cultured human liver-derived cell line (Yamamoto et al., 2022). Taken together, these results indicated that dietary lysolecithin supplementation promoted anabolism but inhibited catabolism of fat in weaned piglets.
Oxidative stress, resulting from the imbalance of pro-oxidant (generally refers to reactive oxygen species, ROS) and antioxidant systems (Zeng et al., 2021), has been implicated to negatively affect growth performance of piglets. In this study, we also provided evidence for the strong associations between redox status and growth performance of weaned piglets by Pearson correlation analysis. Interestingly, only CAT, MDA, and GSH in plasma and ileum, but not in liver, were associated with growth performance. As mentioned above, piglets have to cope with the sudden transition from liquid sow milk to less digestible solid feed with immature digestive system at weaning, which may lead to severe oxidative stress. MDA is the major product of lipid peroxidation caused by ROS and is regarded as a marker for oxidative damage (Liu et al., 2021). In contrast, CAT, T-SOD, and GPX4 are important enzymes that functions as enzymatic antioxidants to scavenge ROS (Peng et al., 2021). We found that dietary lysolecithin supplementation increased activity of CAT, but decreased concentration of MDA. Likewise, the protein expression of GPX4 was increased by lysolecithin administration. GSH is the most abundant non-protein thiol in cells and plays role in anti-oxidation (Zhu et al., 2021). GSH and GSSG (oxidized form of GSH) constitute a main intracellular reduction–oxidation system. The ratio of GSH to GSSG is generally accepted as an important indicator of oxidative stress and lower GSH/GSSG ratio represents an imbalance of redox status (Schafer and Buettner, 2001). In the current study, we found that lysolecithin supplementation increased level of GSH, but decreased level of GSSG, thereby a higher GSH/GSSG ratio. Taken together, these results indicated that dietary lysolecithin supplementation improved anti-oxidative capacity and alleviated oxidative stress of weaned piglets.
In summary, our study revealed that dietary lysolecithin supplementation improved growth performance of weaned piglet, which may be attributed to the improved deposition of nutrients, as well as the alleviation of oxidative stress.
Acknowledgments
This work was supported by Kemin (China) Technologies Co., Ltd., the China Agriculture Research System of MOF and MARA (CARS-35) and Precise and Intelligent Feeding with Environmental Control System of Pig Production in Sichuan (2021ZDZX0011).
Glossary
Abbreviations:
- LPA
lysophosphatidic acid
- LPC
lysophosphatidylcholine
- LPE
lysophosphatidylethanolamine
- LPI
lysophosphatidylinositol
- CP
crude protein
- ADG
average daily gain
- ADFI
average daily feed intake
- GLU
glucose
- TG
triglyceride
- NEFA
non-esterified fatty acid
- TC
total cholesterol
- HDL
high-density lipoprotein
- LDL
low-density lipoprotein
- FAS
fatty acid synthase
- ACC
acetyl-CoA carboxylase
- PNPLA2
Patatin-like phospholipase domain containing 2
- FABP1
fatty acid binding protein 1
- PGC-1α
peroxisome proliferator activated receptor γ coactivator-1α
- SIRT1
silent information regulator 1
- CAT
catalase
- T-SOD
total superoxide dismutase
- T-AOC
total antioxidant capacity
- MDA
malondialdehyde
- GSH-Px
glutathione peroxidase
- GSH
reduced glutathione
- GSSG
oxidized glutathione
Contributor Information
Yang Liu, Key Laboratory for Animal Disease-Resistance Nutrition of China Ministry of Education, Institute of Animal Nutrition, Sichuan Agricultural University, Chengdu, Sichuan 611130, China.
Aimin Wu, Key Laboratory for Animal Disease-Resistance Nutrition of China Ministry of Education, Institute of Animal Nutrition, Sichuan Agricultural University, Chengdu, Sichuan 611130, China.
Ruixia Mo, Key Laboratory for Animal Disease-Resistance Nutrition of China Ministry of Education, Institute of Animal Nutrition, Sichuan Agricultural University, Chengdu, Sichuan 611130, China.
Qiang Zhou, Key Laboratory for Animal Disease-Resistance Nutrition of China Ministry of Education, Institute of Animal Nutrition, Sichuan Agricultural University, Chengdu, Sichuan 611130, China.
Lianghui Song, Key Laboratory for Animal Disease-Resistance Nutrition of China Ministry of Education, Institute of Animal Nutrition, Sichuan Agricultural University, Chengdu, Sichuan 611130, China.
Zheng Li, Kemin (China) Technologies Co., Ltd., Sanzao, Zhuhai 519040, China.
Hua Zhao, Key Laboratory for Animal Disease-Resistance Nutrition of China Ministry of Education, Institute of Animal Nutrition, Sichuan Agricultural University, Chengdu, Sichuan 611130, China.
Zhengfeng Fang, Key Laboratory for Animal Disease-Resistance Nutrition of China Ministry of Education, Institute of Animal Nutrition, Sichuan Agricultural University, Chengdu, Sichuan 611130, China.
Yan Lin, Key Laboratory for Animal Disease-Resistance Nutrition of China Ministry of Education, Institute of Animal Nutrition, Sichuan Agricultural University, Chengdu, Sichuan 611130, China.
Shengyu Xu, Key Laboratory for Animal Disease-Resistance Nutrition of China Ministry of Education, Institute of Animal Nutrition, Sichuan Agricultural University, Chengdu, Sichuan 611130, China.
Bin Feng, Key Laboratory for Animal Disease-Resistance Nutrition of China Ministry of Education, Institute of Animal Nutrition, Sichuan Agricultural University, Chengdu, Sichuan 611130, China.
Yong Zhuo, Key Laboratory for Animal Disease-Resistance Nutrition of China Ministry of Education, Institute of Animal Nutrition, Sichuan Agricultural University, Chengdu, Sichuan 611130, China.
De Wu, Key Laboratory for Animal Disease-Resistance Nutrition of China Ministry of Education, Institute of Animal Nutrition, Sichuan Agricultural University, Chengdu, Sichuan 611130, China.
Lianqiang Che, Key Laboratory for Animal Disease-Resistance Nutrition of China Ministry of Education, Institute of Animal Nutrition, Sichuan Agricultural University, Chengdu, Sichuan 611130, China.
Authors’ Contributions
In this work, Lianqiang Che and Yang Liu designed the study. Yang Liu, Ruixia Mo and Lianghui Song carried out the animal experiment. Yang Liu and Aimin Wu carried out the laboratory experiments. Yang Liu, Aimin Wu, Qiang Zhou, Zheng Li, Zhengfeng Fang, Yan Lin, Shengyu Xu, Bin Feng, Yong Zhuo, De Wu, and Lianqiang Che analyzed the data. Yang Liu wrote the manuscript, then Aimin Wu and Lianqiang Che helped to revise the manuscript.
Conflict of Interest Statement
The authors declare that there is no conflict of interest.
References
- Andradas, C., Caffarel M. M., Perez-Gomez E., Salazar M., Lorente M., Velasco G., Guzman M., and Sanchez C... 2011. The orphan G protein-coupled receptor GPR55 promotes cancer cell proliferation via ERK. Oncogene 30:245–252. doi: 10.1038/onc.2010.402. [DOI] [PubMed] [Google Scholar]
- Boudry, G., Peron V., Huerou-Luron I. L., Lalles J. P., and Seve B... 2004. Weaning induces both transient and long-lasting modifications of absorptive, secretory, and barrier properties of piglet intestine. J. Nutr. 134:2256–2262. doi: 10.1093/jn/134.9.2256. [DOI] [PubMed] [Google Scholar]
- Campbell, J. M., Crenshaw J. D., and Polo J... 2013. The biological stress of early weaned piglets. J. Anim. Sci. Biotechnol. 4:124–127. doi: 10.1186/2049-1891-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Che, L., Hu L., Liu Y., Yan C., Peng X., Xu Q., Wang R., Cheng Y., Chen H., Fang Z.,. et al. 2016. Dietary nucleotides supplementation improves the intestinal development and immune function of neonates with intra-uterine growth restriction in a pig model. PLoS One 11:e0157314. doi: 10.1371/journal.pone.0157314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicalese, L., Corsello T., Stevenson H. L., Damiano G., Tuveri M., Zorzi D., Montalbano M., Shirafkan A., and Rastellini C... 2016. Evidence of absorptive function in vivo in a neo-formed bio-artificial intestinal segment using a rodent model. J. Gastrointest. Surg. 20:34–42; discussion 42. doi: 10.1007/s11605-015-2974-1. [DOI] [PubMed] [Google Scholar]
- Falasca, M., and Corda D... 1994. Elevated levels and mitogenic activity of lysophosphatidylinositol in k-ras-transformed epithelial cells. Eur. J. Biochem. 221:383–389. doi: 10.1111/j.1432-1033.1994.tb18750.x. [DOI] [PubMed] [Google Scholar]
- Falasca, M., Iurisci C., Carvelli A., Sacchetti A., and Corda D... 1998. Release of the mitogen lysophosphatidylinositol from H-Ras-transformed fibroblasts; a possible mechanism of autocrine control of cell proliferation. Oncogene 16:2357–2365. doi: 10.1038/sj.onc.1201758. [DOI] [PubMed] [Google Scholar]
- Heo, J. M., Opapeju F. O., Pluske J. R., Kim J. C., Hampson D. J., and Nyachoti C. M... 2013. Gastrointestinal health and function in weaned pigs: a review of feeding strategies to control post-weaning diarrhoea without using in-feed antimicrobial compounds. J. Anim. Physiol. Anim. Nutr 97:207–237. doi: 10.1111/j.1439-0396.2012.01284.x. [DOI] [PubMed] [Google Scholar]
- Hu, G., Ren G., and Shi Y... 2011. The putative cannabinoid receptor GPR55 promotes cancer cell proliferation. Oncogene 30:139–141. doi: 10.1038/onc.2010.502. [DOI] [PubMed] [Google Scholar]
- Jang, K. B., Purvis J. M., and Kim S. W... 2020. Supplemental effects of dietary lysophospholipids in lactation diets on sow performance, milk composition, gut health, and gut-associated microbiome of offspring. J. Anim. Sci. 98:1–8. doi: 10.1093/jas/skaa227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao, A., Yu B., He J., Yu J., Zheng P., Luo Y., Luo J., Mao X., and Chen D... 2020. Short chain fatty acids could prevent fat deposition in pigs via regulating related hormones and genes. Food Funct. 11:1845–1855. doi: 10.1039/c9fo02585e. [DOI] [PubMed] [Google Scholar]
- José, M. M., Victoria C. L. W., Rafael V., Fernando R., Rocío G., Javier G., Marina R. P., Wendy R. R., Mónica I., and Ruth A. R... 2012. The L-α-lysophosphatidylinositol/GPR55 system and its potential role in human obesity. Diabetes 2:281–291. doi: 10.2337/db11-0649. [DOI] [Google Scholar]
- Juntanapum, W., Bunchasak C., Poeikhampha T., Rakangthong C., and Poungpong K... 2020. The effects of supplementing lysophosphatidylcholine (LPC) in the diet on production performance, fat digestibility, blood lipid profile, and gene expression related to nutrients transport in small intestine of laying hens. J. Anim. Feed Sci. 29:258–265. doi: 10.22358/jafs/127689/2020. [DOI] [Google Scholar]
- Kersten, S. 2014. Physiological regulation of lipoprotein lipase. Biochim. Biophys. Acta 1841:919–933. doi: 10.1016/j.bbalip.2014.03.013. [DOI] [PubMed] [Google Scholar]
- Kim, K. H. 1997. Regulation of mammalian acetyl-coenzyme A carboxylase. Annu. Rev. Nutr. 17:77–99. doi: 10.1146/annurev.nutr.17.1.77. [DOI] [PubMed] [Google Scholar]
- Li, Y. J., Liu Y., Wu J. N., Chen Q. H., Zhou Q., Wu F. L., and Che L. Q... 2021. Comparative effects of enzymatic soybean, fish meal and milk powder in diets on growth performance, immunological parameters, SCFAs production and gut microbiome of weaned piglets. J. Anim. Sci. Biotechnol. 12:106. doi: 10.1186/s40104-021-00625-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, J., Wu H., Tarr P. T., Zhang C. Y., Wu Z., Boss O., Michael L. F., Puigserver P., Isotani E., Olson E. N.,. et al. 2002. Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature 418:797–801. doi: 10.1038/nature00904. [DOI] [PubMed] [Google Scholar]
- Liu, Y., Zhou Q., Theil P. K., Fang Z. F., Lin Y., Xu S. Y., and Che L. Q... 2021. The differences in energy metabolism and redox status between sows with short and long farrowing duration. Animal 15:100355. doi: 10.1016/j.animal.2021.100355. [DOI] [PubMed] [Google Scholar]
- Malcolm, W., and Wu G. Y... 2018. Protein. Adv. Nutr. 9:651–653. doi: 10.1093/advances/nmy027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashima, T., Seimiya H., and Tsuruo T... 2009. De novo fatty-acid synthesis and related pathways as molecular targets for cancer therapy. Br. J. Cancer 100:1369–1372. doi: 10.1038/sj.bjc.6605007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon, D., Salloum D., Bernfeld E., Gorodetsky E., Akselrod A., Frias M. A., and Foster D. A... 2017. Lipid sensing by mTOR complexes via de novo synthesis of phosphatidic acid. J. Biol. Chem. 292:6303–6311. doi: 10.1074/jbc.M116.772988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyano-Rodriguez, Y., and Queralt E... 2020. PP2A functions during mitosis and cytokinesis in yeasts. Int. J. Mol. Sci. 21:264. doi: 10.3390/ijms21010264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NRC. 2012. Nutrient requirements of swine. 11th ed. Washington (DC): National Academy Press. [Google Scholar]
- Oka, S., Nakajima K., Yamashita A., Kishimoto S., and Sugiura T... 2007. Identification of GPR55 as a lysophosphatidylinositol receptor. Biochem. Biophys. Res. Commun. 362:928–934. doi: 10.1016/j.bbrc.2007.08.078. [DOI] [PubMed] [Google Scholar]
- Papadopoulos, G. A., Wealleans A. L., Delis G. A., Janssens G. P. J., Benedetto M., and Fortomaris P... 2022. Effects of dietary lysolecithin supplementation during late gestation and lactation on sow reproductive performance, sow blood metabolic parameters and piglet performance. Animals 12:623. doi: 10.3390/ani12050623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng, X., Wang R., Hu L., Zhou Q., Liu Y., Yang M., and Che L. Q... 2019. Enterococcus faecium NCIMB 10415 administration improves the intestinal health and immunity in neonatal piglets infected by enterotoxigenic Escherichia coli K88. J. Anim. Sci. Biotechno 10:72. doi: 10.1186/s40104-019-0376-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng, X., Cai X., Li J., Huang Y., Liu H., He J., and Che L. Q... 2021. Effects of melatonin supplementation during pregnancy on reproductive performance, maternal–placental–fetal redox status, and placental mitochondrial function in a sow model. Antioxidants 10:1867. doi: 10.3390/antiox10121867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petitet, F., Donlan M., and Michel A... 2006. GPR55 as a new cannabinoid receptor: still a long way to prove it. Chem. Biol. Drug Des. 67:252–253. doi: 10.1111/j.1747-0285.2006.00370.x. [DOI] [PubMed] [Google Scholar]
- Ryberg, E., Larsson N., Sjogren S., Hjort S., Hermansson N. O., Leonova J., and Greasley P. J... 2007. The orphan receptor GPR55 is a novel cannabinoid receptor. Br. J. Pharmacol. 152:1092–1101. doi: 10.1038/sj.bjp.0707460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer, F. Q., and Buettner G. R... 2001. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic. Biol. Med. 30:1191–1212. doi: 10.1016/s0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- Stadler, J. T., and Marsch G... 2020. Obesity-related changes in high-density lipoprotein metabolism and function. Int. J. Mol. Sci. 21:8985. doi: 10.3390/ijms21238985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng, M., Zhang W., Zhang Z., Tang Y., La W., Dan Z., and Mai K... 2022. Effects of dietary lysolecithin on growth performance, serum biochemical indexes, antioxidant capacity, lipid metabolism and inflammation-related genes expression of juvenile large yellow croaker (Larimichthys crocea). Fish Shellfish Immunol. 128:50–59. doi: 10.1016/j.fsi.2022.07.020. [DOI] [PubMed] [Google Scholar]
- Wolter, B., and Ellis M... 2001. The effects of weaning weight and rate of growth immediately after weaning on subsequent pig growth performance and carcass characteristics. Can. J. Anim. Sci. 81:363–369. doi: 10.4141/A00-100. [DOI] [Google Scholar]
- Wu, A., Feng B., Yu J., Yan L., Che L., Wu D., and Chen D... 2021a. Fibroblast growth factor 21 attenuates iron overload-induced liver injury and fibrosis by inhibiting ferroptosis. Redox Biol. 46:102131. doi: 10.1016/j.redox.2021.102131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, S. A., Kersten S., and Qi L... 2021b. Lipoprotein lipase and its regulators: An unfolding story. Trends Endocrinol. Metab. 32:48–61. doi: 10.1016/j.tem.2020.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto, Y., Sakurai T., Chen Z., Inoue N., Chiba H., and Hui S... 2022. Lysophosphatidylethanolamine affects lipid accumulation and metabolism in a human liver-derived cell line. Nutrients 14:579. doi: 10.3390/nu14030579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young, S. G., and Zechner R... 2013. Biochemistry and pathophysiology of intravascular and intracellular lipolysis. Genes Dev. 27:459–484. doi: 10.1101/gad.209296.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng, Y., Shinada K., Hano K., Sui L., Yang T., Li X., and Himaki T... 2021. Effects of tris (2-carboxyethyl) phosphine hydrochloride treatment on porcine oocyte in vitro maturation and subsequent in vitro fertilized embryo developmental capacity. Theriogenology 162:32–41. doi: 10.1016/j.theriogenology.2020.12.027. [DOI] [PubMed] [Google Scholar]
- Zhang, R., Huang G., Ren Y., Wang H., Ye Y., Guo J., and Yu K... 2022a. Effects of dietary indole-3-carboxaldehyde supplementation on growth performance, intestinal epithelial function, and intestinal microbial composition in weaned piglets. Front. Nutr 9:896815. doi: 10.3389/fnut.2022.896815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Z., Zhang S., Nie K., Zheng H., Luo Z., and Kim I... 2022b. Lysolecithin improves broiler growth performance through upregulating growth-related genes and nutrient transporter genes expression independent of experimental diet nutrition level. Animals 12:3365. doi: 10.3390/ani12233365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, Y., Wu J., Wang K., Xu H., Qu M., Gao Z., Guo L., and Xie J... 2021. Facile and sensitive measurement of GSH/GSSG in cells by surface-enhanced Raman spectroscopy. Talanta 224:121852. doi: 10.1016/j.talanta.2020.121852. [DOI] [PubMed] [Google Scholar]