A recent study published in Nature Immunology by Peter Savage and colleagues sought to characterize regulatory T-cell (Treg)-suppressed self-reactive CD4+ T cells using an elegant system combining the depletion of Tregs with T-cell receptor (TCR) sequencing of expanded conventional CD4+ T-cell clones. By tracking the fate of T cells engineered to express the identified TCRs, Lee et al. discovered a ‘new’ subpopulation of CD4+ T cells bearing antigen receptors with high levels of self-reactivity that take on a T follicular helper (TFH)-like phenotype in the periphery in the absence of immune challenge [1].
Antigen-specific αβTCRs are generated through the somatic recombination of V(D)J gene segments during thymic development, giving rise to T cells that can recognize an enormously diverse set of peptides presented by major histocompatibility complex (pMHC) molecules. A risk of this process is that it can also lead to the generation of potentially highly self-reactive TCRs. T cells with TCRs with high affinity for self-pMHC molecules are purged in the thymus by clonal deletion, a key tolerance mechanism that prevents autoimmunity. However, this process is incomplete, and highly self-reactive T cells can escape negative selection and join the peripheral naive T-cell pool. Indeed, while the naive CD4+ T-cell pool is heterogeneous with regard to an individual T cell’s reactivity for self-pMHC molecules, the distribution of T cells with differing strengths of self-reactivity leaving the thymus is skewed toward those with relatively higher self-pMHC binding strengths [2]. A subset of these highly self-reactive CD4+ T cells in the periphery are deleted, some become functionally anergic, and others differentiate into Tregs, which go on to play an important role in peripheral tolerance, holding other CD4+ T cells with potentially dangerous levels of self-reactivity in check [3]. What is the fate of these Treg-suppressed self-reactive cells?
Treg ablation leads to the expansion and tissue infiltration of CD4+ T cells [4, 5]. Lee et al. took advantage of this to perform deep sequencing of the (less diverse) TCRα chains of CD4+ T cells expanded in the prostate upon Treg depletion (but not in other contexts such as prostate tumors or negative selection deficiencies) in transgenic mice with a fixed TCRβ chain. The advantage of this approach was that the researchers were then able to generate TCR retrogenic mice expressing the TCRs of Treg-suppressed cells; the sequenced TCRα chains were cloned and introduced into bone marrow cells, leading to the development and selection of the self-reactive T cells of interest with the same fixed TCRβ transgene. Among the recurrent prostate-localized clones, Lee et al. identified three groups of TCRs that varied in self-reactivity strength as assessed by determining the extent of proliferation in coculture with syngeneic splenic dendritic cells and by measuring the levels of CD5 surface expression (a marker of self-reactivity strength). Careful controls indicated that the group containing overtly self-reactive TCR clones was peptide specific and not pan-MHCII reactive. Next, Lee et al. generated retrogenic mice expressing a TCR representative of each group, showing that those with the highest self-reactivity resembled TFH cells in the periphery at both the transcriptome and protein levels [6]; the cells expressed markers such as CXCR5, ICOS, Bcl6, and CD200 and were PD-1hi (but did not express other helper T-cell lineage-defining transcription factors or cytokines) and were found to localize in B-cell follicles more than their nonautoreactive T-cell counterparts. Following Treg depletion, the T cells bearing a more highly self-reactive TCR clone accumulated in the prostate and produced IFNγ. Importantly, this unique PD-1+Bcl6+Eomes+ CD4+ T-cell population was also detectable in Treg-replete polyclonal wild-type mice and thus represents a ‘new’ population of conventional CD4+ cells with relatively high reactivity to self-peptides (Fig. 1).
Fig. 1.
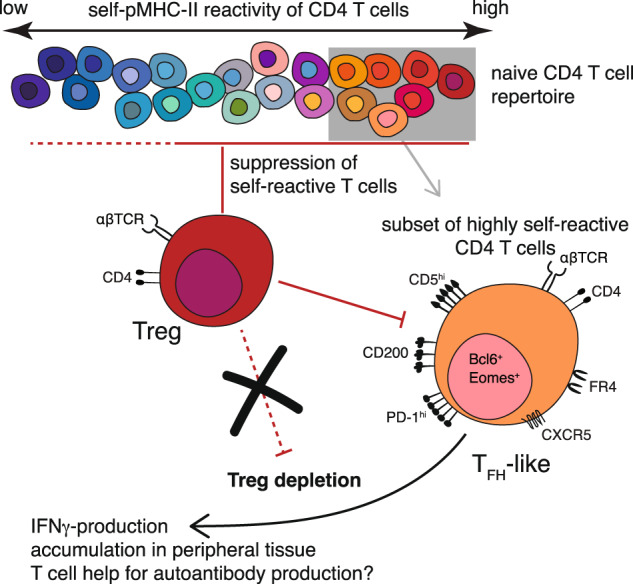
Self-reactive CD4+ T cells in the mature T-cell population are controlled by diverse peripheral tolerance mechanisms that include Treg-mediated suppression. A subset of CD4+ T cells with relatively high self-reactivity takes on a TFH-like phenotype in the peripheral lymphoid organs at a steady state; depletion of Tregs leads to their accumulation in tissues and the production of IFNγ
An increasing number of studies have highlighted the phenotypic and functional biases among T cells arising from differences in self-reactivity strength [7]. Consistent with findings from Lee et al., CD4+ T cells at the higher end of the self-reactivity spectrum were shown to be enriched for a TFH cell gene signature and to preferentially differentiate into TFH cells after activation [8]. However, the factors that regulate the distinct cell fates of highly self-reactive T cells are not yet clear. While the TFH-like phenotype only becomes apparent in the periphery, whether a precursor to this population is thymically derived has not been ruled out. In addition, although single-cell sequencing of CD4+ T cells has thus far failed to uncover any distinct subsets among the naive population [8, 9], improvements in single-cell technologies that enable deeper sequencing in combination with the incorporation of surface markers that can identify naturally occurring TFH-like cells will shed light on this and potentially other not-yet-described CD4+ T-cell subpopulations. One limitation of the current study is the restriction of the TCRβ chain, which may itself bias T cells to a cell fate, given that the β chain on its own can already determine the strength of self-reactivity [7, 10]. Future studies employing single-cell TCR sequencing of clones expanded in the absence of Tregs in a fully polyclonal environment will address this.
The identification of natural TFH-like cells leads to many exciting new questions, and we look forward to future studies on the biology of these cells. In particular, the work from Lee et al. raises the intriguing possibility that natural TFH-like cells play a role in autoantibody-driven immunopathology. If we better understand what signals, other than Treg depletion, might push natural TFH-like cells to become activated, provide help to autoreactive B cells, and cause disease, this may be a point of intervention in future therapies to prevent autoimmunity before it begins.
Competing interests
The authors declare no competing interests.
Contributor Information
Judith N. Mandl, Email: judith.mandl@mcgill.ca
Heather J. Melichar, Email: heather.melichar@umontreal.ca
References
- 1.Lee V, Rodriguez DM, Ganci NK, Zeng S, Ai J, Chao JL, et al. The endogenous repertoire harbors self-reactive CD4+ T cell clones that adopt a follicular helper T cell-like phenotype at steady state. Nat Immunol. 2023;24:487–500. doi: 10.1038/s41590-023-01425-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mandl JN, Monteiro JP, Vrisekoop N, Germain RN. T cell-positive selection uses self-ligand binding strength to optimize repertoire recognition of foreign antigens. Immunity. 2013;38:263–74. doi: 10.1016/j.immuni.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.This S, Valbon SF, Lebel MÈ, Melichar HJ. Strength and numbers: the role of affinity and avidity in the ‘quality’ of T cell tolerance. Cells. 2021;10:1530. doi: 10.3390/cells10061530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Richards DM, Ruggiero E, Hofer AC, Sefrin JP, Schmidt M, von Kalle C, et al. The contained self-reactive peripheral T cell repertoire: size, diversity, and cellular composition. J Immunol. 2015;195:2067–79. doi: 10.4049/jimmunol.1500880. [DOI] [PubMed] [Google Scholar]
- 5.Yi J, Jung J, Hong SW, Lee JY, Han D, Kim KS, et al. Unregulated antigen-presenting cell activation by T cells breaks self tolerance. Proc Natl Acad Sci USA. 2019;116:1007–16. doi: 10.1073/pnas.1818624116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crotty S. T follicular helper cell biology: a decade of discovery and diseases. Immunity. 2019;50:1132–48. doi: 10.1016/j.immuni.2019.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.This S, Rogers D, Mallet Gauthier È, Mandl JN, Melichar HJ. What’s self got to do with it: sources of heterogeneity among naive T cells. Semin Immunol. 2023;65:101702. doi: 10.1016/j.smim.2022.101702. [DOI] [PubMed] [Google Scholar]
- 8.Rogers D, Sood A, Wang H, van Beek JJP, Rademaker TJ, Artusa P, et al. Pre-existing chromatin accessibility and gene expression differences among naive CD4+ T cells influence effector potential. Cell Rep. 2021;37:110064. doi: 10.1016/j.celrep.2021.110064. [DOI] [PubMed] [Google Scholar]
- 9.ElTanbouly MA, Zhao Y, Nowak E, Li J, Schaafsma E, Le Mercier I, et al. VISTA is a checkpoint regulator for naive T cell quiescence and peripheral tolerance. Science. 2020;367:eaay0524. doi: 10.1126/science.aay0524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Textor J, Buytenhuijs F, Rogers D, Gauthier EM, Sultan S, Wortel IMN, et al. Machine learning analysis of the T cell receptor repertoire identifies sequence features that predict self-reactivity. bioRxiv [Preprint] 2022: p. 2022.11.23.517563. [DOI] [PubMed]


