Abstract
Precisely controlling macromolecular stereochemistry and sequences is a powerful strategy for manipulating polymer properties. Controlled synthetic routes to prepare degradable polyester, polycarbonate, and polyether are of recent interest due to the need for sustainable materials as alternatives to petrochemical-based polyolefins. Enantioselective ring-opening polymerization and ring-opening copolymerization of racemic monomers offer access to stereoregular polymers, specifically enantiopure polymers that form stereocomplexes with improved physicochemical and mechanical properties. Here, we highlight the state-of-the-art of this polymerization chemistry that can produce microstructure-defined polymers. In particular, the structures and performances of various homogeneous enantioselective catalysts are presented. Trends and future challenges of such chemistry are discussed.
Subject terms: Polymer synthesis, Polymerization mechanisms, Polymers, Synthetic chemistry methodology
Stereoregular polymers exhibit improved thermal and mechanical properties, making the development of enantioselective polymerization catalysts of significant importance. Here, the authors summarize catalyst design strategies and synthetic routes for enantioselective polymerizations of degradable or recyclable polymers from racemic monomers.
Introduction
Asymmetric catalysts provide invaluable routes to enantiopure pharmaceutical agents and intermediates. On the other side, stereoselective polymerization catalysts are the most commercially viable means to prepare stereoregular polymers, that is, polymers having regularity of the configurations of adjacent stereocenters along the polymer chain. Stereoregular polymers are usually crystalline and have improved thermal and mechanical properties relative to those of atactic polymers1. Ever since the birth of polymer chemistry, polymer stereochemistry, or chirality, has been one of the focal points. In 1929, Staudinger and coworkers predicted a correlation between the polymer’s stereochemistry and its physical properties2. In 1947, Schildknecht synthesized the first stereoregular polymers—isotactic poly(isobutyl vinyl ether)—and attributed its semicrystalline properties to the polymer’s ordered tacticity3. In 1955, Natta and coworkers discovered the first isoselective catalysts to prepare stereoregular polypropylene, leading to the large-scale production of isotactic polypropylene (it-PP)4. Since then, synthesis of stereoregular polymers has been actively pursued in both industrial and academic laboratories1,5–13.
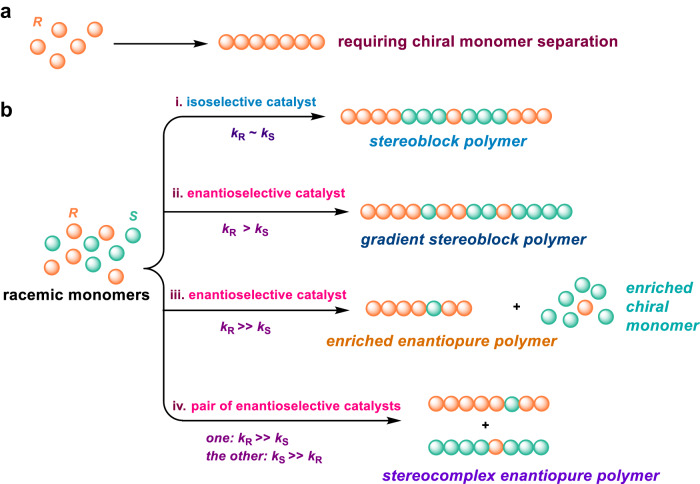
Several methods to prepare isotactic polymers are shown in Fig. 1. In the first route, enantiopure monomers are polymerized to produce isotactic polymers (Fig. 1a). Nevertheless, obtaining enantiopure monomers in industry is challenging, requiring additional purification and separation steps. Alternatively, racemic monomers can be polymerized by isoselective catalysts to produce stereoblock copolymers (Fig. 1b, route i). In lieu of the isoselective polymerization, enantioselective catalysts often lead to the production of gradient stereoblock copolymers when the enantioselectivity is not exclusive (Fig. 1b, route ii). Highly exclusive enantiopure enantioselective catalysts can selectively enchain one enantiopure monomer to produce isotactic polymers, leaving the other enantiomer unreacted (Fig. 1b, route iii). Finally, using a pair of enantioselective catalysts (e.g., the racemic mixture of chiral enantioselective complexes in route iii) may enable the preparation of stereocomplex of two complementary isotactic polymers from racemic monomers (Fig. 1b, route iv). Notably, the interaction between the stereocomplex polymers results in altered physical properties in comparison to the parental polymers14–19. Compared with other routes, the enantioselective polymerization strategy (the last two routes) offers attractive means to produce stereoregular polymers such as stereocomplex enantiopure polymers with improved physical and mechanical properties.
Fig. 1. Stereoselective polymerization of racemic monomers.
a Synthesis of enantiopure polymers from purified enantiopure monomers, which requires additional steps for monomer separation. b Different polymerization strategies to prepare stereoregular copolymers using isoselective or enantioselective polymerization catalysts.
Although there has been long history of producing stereoregular polymers, the development of enantioselective polymerization catalysts for degradable or recyclable polymers has recently undergone a surgency, aware of the excessive non-degradable plastic waste production13,20–25. Ring-opening polymerization (ROP) is a chain-growth polymerization strategy that encompasses many propagation mechanisms such as cationic, anionic, and coordination–insertion26–29. Many degradable polymers, including polyesters, polyethers, and polycarbonates, can be efficiently synthesized via ROPs or ring-opening copolymerizations (ROCOPs)10,30,31. This review focuses on recent advances in developing enantioselective catalysts in ROP and ROCOP to produce degradable polymers from racemic monomers. These monomers or monomer combinations mainly include lactide, lactone, epoxide, epoxide/CO2, epoxide/anhydride, thiirane, and N-carboxyanhydride.
We highlight the novel synthetic routes and catalyst design strategies, and discuss the catalytic mechanisms in these enantioselective polymerizations. We organize this review based on the types of the monomers. We focus on the chemistry for preparing polymers having optical isomerisms in the polymer backbone, but the cis-trans (geometric) isomerism (e.g., polyisoprene) is not discussed. Because the emphasis is on enantioselective polymerization enabled by the catalyst or initiator, the polymerization chemistry of enantiopure monomers is not covered. Notably, stereoselective polymerization without chiral preferences on the monomers will not be focused on, although such systems are mentioned occasionally for comparison.
Basic concepts in enantioselective polymerization
Stereocontrolled polymerization can be mediated by two distinct mechanisms, namely, chain-end control (CEC) or enantiomorphic site control (ESC)1,32. (Fig. 2) In the former case, the chirality of the chain-end monomer determines the chirality of the next monomer to be inserted, even if the catalyst contains a chiral component (Fig. 2a). In the ESC-mediated polymerization, the chirality of the catalyst determines the chirality of the next monomer unit. (Fig. 2b) The monomer enchainment mechanism can be easily identified by observing the stereochemical errors propagating in a polymer chain: stereoerrors can be propagated in the CEC-mediated polymerization; whereas in the ESC-mediated polymerization, correction of stereoerrors can occur because the chiral catalysts direct the stereochemical events, leading to an isolated stereoerror.
Fig. 2. Two distinct mechanisms of stereoselective polymerization.
a Chain-end control mechanism. b Enantiomorphic site control mechanism.
The NMR spectrometry is used to characterize the polymer stereochemistry. The Bovey formalism describes relationships between adjacent stereogenic centers (dyads) in the polymer tacticity, where an ‘m’ for meso (same configuration), and an ‘r’ for racemic (opposite configuration)33. For CEC-mediated enchainment, the parameters Pm and Pr refer to the probability of meso and racemic linkages (Pm + Pr = 1), respectively. For ESC-mediated enchainment, another parameter α—the site control selectivity—is often used to represent the probability of selecting one enantiomer of the monomer for enchainment. When α is 1 or 0, an isotactic polymer forms; an α of 0.5 results in an atactic polymer.
For the enantioselective polymerization, enantiopure catalysts can stereoselectively resolve racemic monomers to produce isotactic polymers enriched in one enantiomer of the monomer, thereby leaving unreacted monomer enriched in the other enantiomer (Fig. 1b, route iii). In most cases, such enantioselectivity is mediated by the ESC mechanism. The selectivity factor, often referred as the s factor (s), is the quantitative measurement of stereocontrol in such a system. The s factor should be calculated using the Eq. (1) based on the monomer conversion (c), which is often determined by NMR, and the enantiomeric excess (ee(m)) of the unreacted racemic monomer mixture, which can be determined by chiral HPLC. Alternatively, NMR spectrometry could be used to measure the enantiomeric purity of repeating units in the polymer (ee(p)) using ESC statistics1,34.
| 1 |
For the ROP of lactide (LA) mediated by ESC, the site control selectivity (α), which can be calculated based on NMR spectra, can also be used to calculate the s factor, as shown in Eq. (1)35. Note that in the literature, the s factor also can be referred as the ratio of the rate constant, krel, for polymerization of the faster-reacting enantiomer to that of the slower-reacting enantiomer of the monomer, assuming that the reaction is first order in monomer. Nevertheless, the krel value should not be calculated using individual enantiomer’s kinetic rates in the absence of the other enantiopure monomer, because the polymerization rate of individual enantiomer in the racemic mixture is different from that of the pure enantiomer. To avoid confusion, we use s factors throughout the review unless the kinetic rates of the enantiomer is determined in the racemic monomer mixture (which is challenging as the isotope-labeled monomer should be used to differentiate the individual enantiomer in the racemic mixture)36. Notably, routine 13C NMR spectroscopic experiments are now often used to reveal both the tacticity, the stereoregularity, and the degree of regioregularity37,38, but such quantification information is often absent in the old literature.
Enantioselective polymerization of lactide
Using different catalysts, the ROP of the racemic lactide (rac-LA, the mixture of d-LA ((R,R)-LA) and l-LA ((S,S)-LA)) can lead to isotactic stereoblock poly (lactic acid) (PLA) or heterotactic PLA, whereas meso-LA ((R,S)-LA) can produce heterotactic or syndiotactic PLA35,39–42. Here we focus on the enantioselective ROP of rac-LA. We discuss the Al catalysts first as they have been extensively applied in the enantioselective ROP of LA, followed by discussions about other metal complexes, and organocatalysts for such polymerization.
Al-based complexes
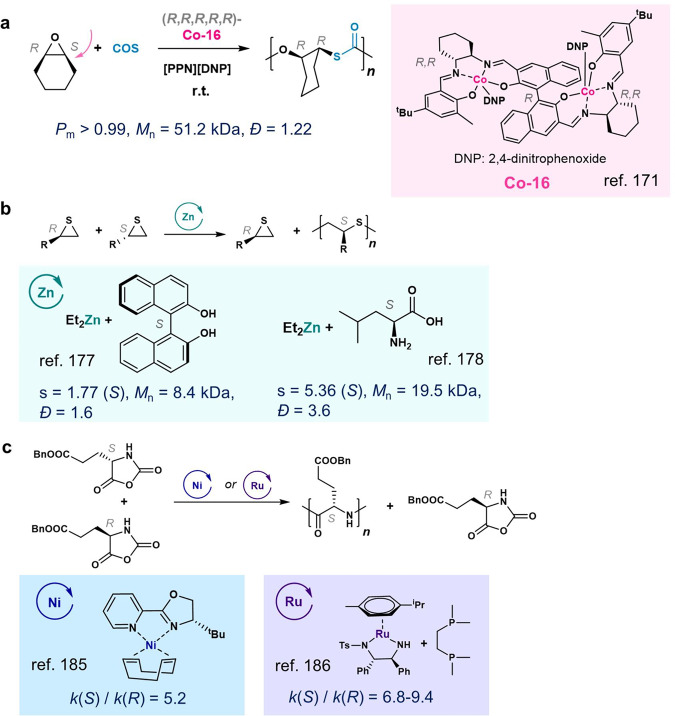
In 1996, Spassky and coworkers first reported using chiral (R)-Al-1 complex for enantioselective ROP of rac-LA (Fig. 3a)43. At 70 °C, the (R)-Al-1 catalyst exhibits a 20:1 preference for the polymerization of d-LA over l-LA. At the LA conversion of less than 50%, the polymer microstructure is predominantly isotactic poly(d-LA). At high monomer conversions, the obtained polymer exhibits a tapered gradient microstructure, where the monomer composition varied from all (R)-units to all (S)-units (Fig. 3a). In 1999, Coates and coworkers showed that the (R)-Al-2 preferentially attacked at the carbonyl group adjacent to the (R)-stereogenic atom in meso-LA, followed by the Al-binding to the (S)-lactic acid unit (Fig. 3b)35,44,45. To this end, the enantiopure (R)-Al-2 polymerizes meso-LA to produce syndiotactic PLA via the ESC mechanism with an s factor of 49 (α = 0.98), while rac-Al-2 polymerizes meso- or rac-LA to heterotactic or isotactic stereoblock PLA, respectively (Fig. 3b). Based on such chiral Al complex, Duda and coworkers developed a two-step polymerization of rac-LA by consecutive addition of homochiral (S)- and (R)-Al-2 complexes into the polymerization mixture, resulting in the stereocomplexation of stereoblock PLAs with a high melting temperature (Tm) of 210 °C (Fig. 3c)46. Later, Coates, Meyer, and coworkers used (R)-Al-2 to regioselectively polymerize (S)-methyl glycolide with the ring-opening exclusively at the glycolide acyl–oxygen bond site, as the chiral Al complex and the steric hindrance all favored the ring-opening on that specific site (Fig. 3d)47. On the other hand, using (S)-Al-2 resulted in a moderate regioselectivity of 78%, presumably due to the mismatch between the chiral Al complex and (S)-LA at the chain end.
Fig. 3. Al complexes for enantioselective ring-opening polymerization (ROP) of lactide (LA).
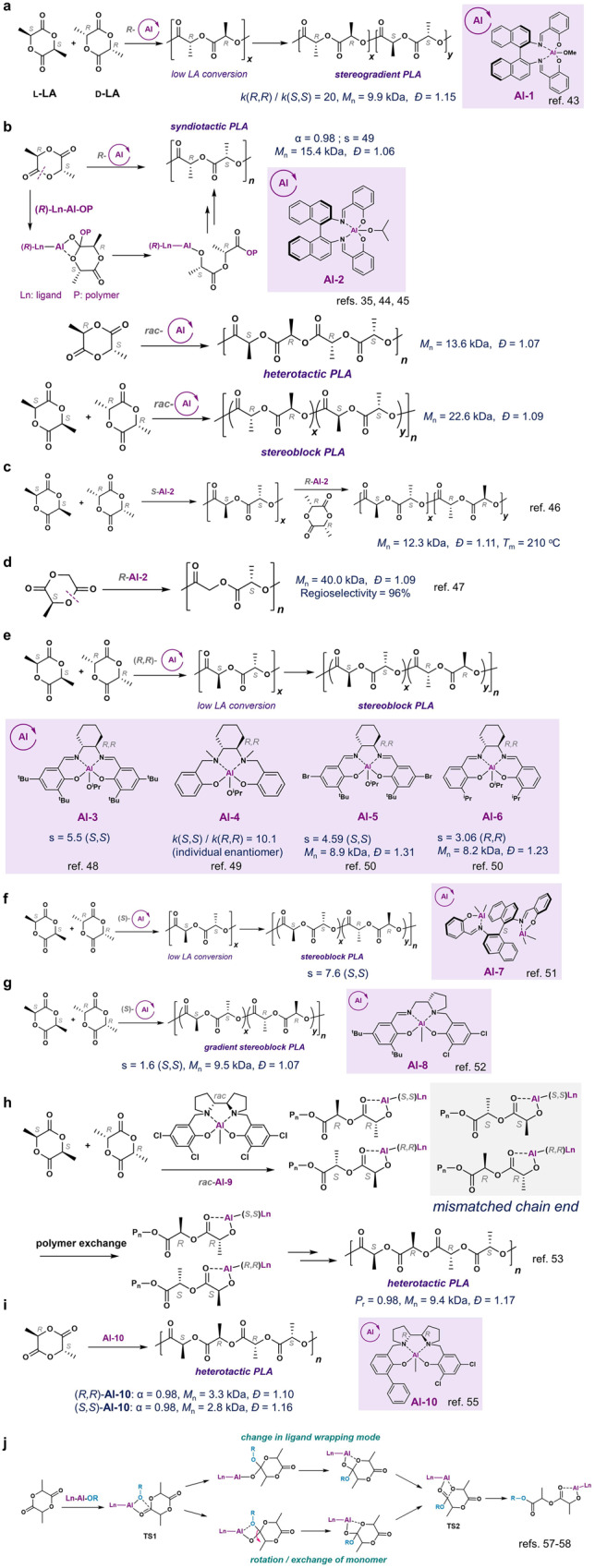
a The enantioselective ROP of racemic-LA (rac-LA) by Al-1 at low LA conversions. b Al-2-mediated regioselective ROP of meso-LA and rac-LA. c Sequential addition of Al-2 enantiomers to prepare stereoblock PLA. d Al-2-mediated regioselective ROP of (S)-methyl glycolide. e The stereoselective polymerization of rac-LA to produce stereoblock PLA by Al-3, Al-4, Al-5 or Al-6. f The enantioselective ROP of rac-LA by Al-7 at low LA conversions. g Al-8-mediated ROP to synthesize gradient stereoblock PLA. h Proposed polymer exchange mechanism in Al-9-mediated ROP of rac-LA. i Al-10-mediated syndioselective ROP of meso-LA. j Proposed possible ring-opening reaction pathways of LA via the enantiomorphic site control mechanism.
Additionally, in 2002, Feijen and coworkers reported that two chiral (R,R)-salcy-Al complexes (salcy, N,N′-bis(salicylidene)cyclohexanediimine), (R,R)-Al-3 and (R,R)-Al-448,49, preferentially polymerized l-LA (Fig. 3e). The reported s factors were lower than the ratio between individual monomer’s kinetic rates, suggesting stereoerrors occurred in the ROP of rac-LA. Very recently, Wang and coworkers studied the phenoxy substituent groups on the (R,R)-salcy ligands, and found that replacing para-tBu with Br in (R,R)-Al-3 (noted as (R,R)-Al-5) could increase the s factor to 4.6 at the conversion of 52%; while replacing the ortho tBu group on the phenolate with iPr (noted as (R,R)-Al-6) switched the monomer chirality preference from l-LA to d-LA with an s factor of 3.1 (Fig. 3e)50.
In addition to monomeric (salen)Al complex (salen, N,N′-bis(salicylidene)ethylene diamine), in 2021, Zhang and coworkers prepared bimetallic Al (bi-Al) complexes based on chiral binaphthalene salen ligands ((S)-Al-7), and showed one of such bi-Al complexes could mediate enantioselective ROP with an s factor around 7 at the conversion ~50% with the preference on l-LA (Fig. 3f)51. The ROP of rac-LA by rac-Al-7 complexes generated a gradient stereoblock PLA with a Mn (Mn, number-average molecular weight) up to 60.7 kDa and a Pm of 0.83.
Apart from salen ligands, other chiral ligands were explored to prepare Al complexes for the enantioselective ROP of rac-LA. In 2014, Lamberti, Kol, and coworkers developed chiral salalen Al complexes (Al-8) to prepare gradient stereoblock copolymers with an s factor of 1.6 (Fig. 3g)52. Mechanistic studies suggested that the combination of ESC and CEC mechanisms might be attributed to the formation of gradient stereoblock PLA52. Kol and coworkers later identified a chloro-substituted racemic salan Al complex (Al-9) mediated heteroselective ROP of rac-LA (Pr = 0.98 at 50 °C), while its enantiopure Al complex had significantly lower hetereoselectivity (Pr = 0.64 at 70 °C, Fig. 3h). They proposed that after each LA insertion, the catalytic site was blocked until an exchange occurred with the opposite polymer chain bound to the opposite salan Al enantiomer53,54. The Kol group also reported the asymmetric chiral salan Al complex (Al-10) followed similar auto-inhibition/exchange mechanism in the ROP of meso-LA with a high syndiotacticity degree (α) of 0.96 (Fig. 3i)54,55. Nevertheless, the stereoregular PLA mediated by these chiral salan catalysts did not have high molecular weight (MW). More highly active chiral Al complexes are needed to prepare high-MW stereoregular PLAs from rac-LA.
Despite intensive catalyst studies, the detailed ESC mechanism for such Al-mediated enantioselective ROP of LA has not been fully understood. On the other hand, the ROP of LA via the CEC mechanism shows that the ROP of LA proceeds via the nucleophilic attack (TS1), followed by the ring-opening process for enchainment (TS2)56. In 2020, Talarico and coworkers re-examined the ESC mechanism of chiral-Al complex-mediated ROP of LA via the DFT computation (DFT, density function theory)57,58. They proposed that between the two transition states (TS1 and TS2), a chain-monomer exchange could exist (equivalent to the backslip in the Ziegler-Natta catalysis59), which may lead to changes in the ligand wrapping mode of the active site (Fig. 3j). The computation results could also agree with Kol and coworker’s proposed chain-exchange mechanism during the ROP54. Such complicated ESC-mediated enantioselective ROP might add difficulties in rationally designing catalyst ligands to prepare exclusively enantioselective catalysts for PLA polymerization.
Other metal complexes
Besides Al complexes, other chiral metal complexes have been tested for enantioselective ROP of rac-LA. Ma et al. showed chiral aminophenolate Zn-1 and Mg-1 complexes exhibited ratios of k(R,R)/k(S,S) around 3, and these complexes usually showed higher polymerization rates compared to Al complexes and produced stereoblock PLA (Fig. 4)60–62. Mehrkhodavandi et al. developed a chiral (R,R)-di-indium complex (R,R)-In-1, which showed a k(S,S)/k(R,R) ratio of 5.1, and the ROP of rac-LA by such In complex led to a gradient stereoblock PLA with a Pm of 0.77 (Fig. 4)63. Recently, Wang and coworkers developed a Zn complex with a chiral bis(oxazolinylphenyl)amido ligand (Zn-2) to mediate the ROP of rac-LA with an s factor around 4 at the LA conversion ~ 50%; the stereoblock PLA was obtained at high monomer conversions with a Pm ~ 0.8 (Fig. 4)64.
Fig. 4. Zn, Mg, and In complexes for stereoselective ring-opening polymerization of racemic lactide.
The complexes lead to the production of gradient stereoblock poly(lactic acid), though the metal complexes show chiral preference on the monomers.
Organocatalyst
The Chen group first investigated the organocatalyst-mediated enantioselective ROP of rac-LA using cinchona alkaloids O-165. The initial identified bifunctional catalyst, β-isocupreidine, consists of a chiral nucleophilic tertiary amine and an acidic phenol moiety (Fig. 5). The β-isocupreidine/benzyl alcohol-mediated ROP of rac-LA in dichloromethane afforded a gradient stereoblock PLA with a Pm of 0.68 at room temperature. Kinetic studies showed a preferential ROP of l-LA using the combination of β-isocupreidine/benzyl alcohol, with an s factor of 4.4 at the rac-LA conversion of 48%. In 2015, the same group furnished the β-isocupreidine core with a thiourea-linked R-binaphthyl-amine, and both moieties were proposed to function as H-bond donors to exert stereo-differentiation (Fig. 5, O-2)66. Indeed, such catalyst with benzyl alcohol in o-difluorobenzene increased the s factor to 53 at the rac-LA conversion of 50%. Meanwhile, Satoh and coworkers also reported chiral BINOL-derived phosphoric acids O-3 (BINOL, 1,1′-bi-2-naphthol) could mediate enantioselective ROP of rac-LA67. For the (R)-phosphoric acid-mediated ROP of rac-LA at 75 °C in toluene using 3-phenyl-1-propanol as an initiator, d-LA was preferred, and the s factor was found as 28.3 at 49.0% conversion of rac-LA.
Fig. 5. Organocatalysts for enantioselective ring-opening polymerization of racemic lactide (rac-LA).
Note that s = 4.4 (S, S) means that the selectivity factor to (S, S)-LA is 4.4.
In 2017, Mecerreyes, Cossío and coworkers showed that diastereomeric N-methylated prolines O-4 could mediate enantioselective ROP of rac-LA in dichloromethane at room temperature (Fig. 5)68. Specifically, for the ROP of rac-LA in the presence of cocatalyst 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) with the monomer conversion ~ 50%, endo-proline promoted the ROP of d-LA, whereas exo-proline preferentially polymerized l-LA. Notably, the ROP of rac-LA in the presence of a mixture of exo- and endo-prolines resulted in atactic PLA (Pm = 0.60). DFT studies suggested the high density of chiral and prochiral centers in the organocatalysts may contribute to the selectivity of chiral LA monomers.
Moreover, other organocatalysts, such as chiral thioureas, were explored for enantioselective ROP of rac-LA. In 2018, Taton, Coulembier, Dove and coworkers found that the chiral Takemoto’s thiourea O-5 (Fig. 5) had a moderate s factor around 3.4 for the ROP of rac-LA in toluene to generate a gradient stereoblock PLA with a Pm of 0.88 at room temperature69. The addition of a phosphazene base into such system could markedly improve the polymerization rate to produce a gradient stereoblock PLA with a Pm of 0.9670. In 2020, Wang and coworkers also showed the iminophosphorane–thiourea catalysts O-6 (Fig. 5) had a k(S,S)/k(R,R) ratio of 1.3 and an s factor of 1.4 for the ROP of rac-LA using benzyl alcohol as an initiator in dichloromethane at room temperature71. Nevertheless, all organocatalyst-mediated enantioselective ROP of LA could not produce stereoregular PLAs with MWs higher than 20 kDa.
Enantioselective polymerization of β-lactone or 8-membered diolide
Polyhydroxyalkanoates (PHAs), including poly-3-hydroxybutyrate (P3HB), is a type of highly crystalline thermoplastic polyesters produced by bacteria and other microorganisms72,73. Isotactic P3HB exhibited PP-like Tm, (160 − 170 °C), and excellent barrier properties superior to PE and PET74. However, P3HB is thermally unstable with a relatively low degradation temperature close to Tm (~ 250 °C)75, and is extremely brittle with a fracture strain (ε) of 4%76. Here we focus on the enantioselective ROP from β-lactone or diolide to produce stereoregular PHAs.
β-lactone
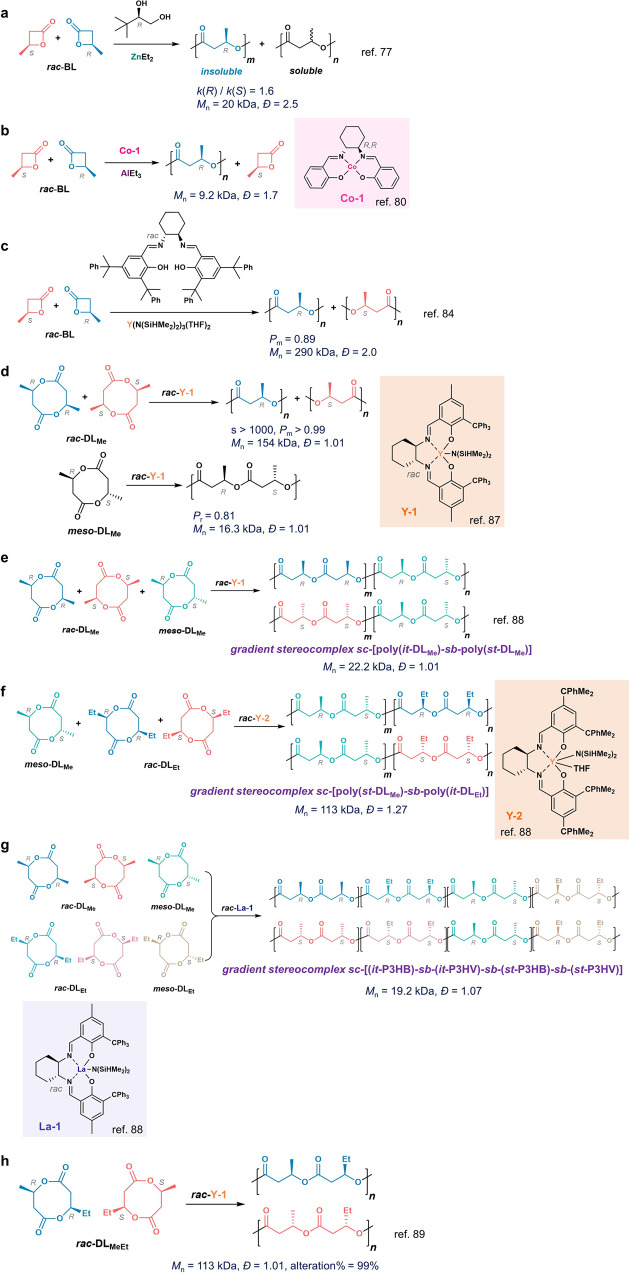
In 1980s, Spassky and coworkers found that the complex of ZnEt2 and (R)-3,3-dimethyl-1,2 butanediol (DMBD) preferentially ring-opened (R)-β-butyrolactone (R-BL) with a k(R)/k(S) ratio of 1.6, resulting in the mixture of isotactic P3HB and amorphous copolymers (Fig. 6a)77. Using CdMe2 and R-DMBD resulted in slightly preferential ROP on R-BL. The complex between ZnEt2 and (R)-DMBD also exhibited moderate stereoselectivities for R-α-methyl-α-ethyl-β-propiolactones (α-Me-β-PL, s = 1.05)78, and R-α-methyl-α-n-propyl-β-propiolactone (s = 1.25)79. Meanwhile, Takayama and coworkers found the (R,R)-(salcy)CoII complex (Co-1) and AlEt3 could preferentially polymerize R-BL, without detailed measurement of selectivity factors (Fig. 6b)80. Later, though various discrete metal complexes were reported for stereoselective ROP of R-BL, many of them exhibited excellent syndioselectivities (Pr > 0.9), instead of high isoselectivities and enantioselectivies81–83. Very recently, Rieger and coworkers’ in-situ-generated catalyst based on Y[N(SiHMe2)2]3(THF)2 and a racemic salan ligand could lead to isoselective ROP of rac-BL to produce stereocomplex P3HB with a Pm of 0.89 (no stereoblock microstructures on NMR), resulting in strong and ductile copolymers whose tensile properties outperform bacterial-synthesized P3HB (Fig. 6c)84.
Fig. 6. Enantioselective ring-opening polymerization (ROP) of racemic β-lactone (rac-BL, a-c) and racemic/meso 8-membered diolides (rac-/meso-DL, d-h).
Enantioselective ROP of rac-BL by a ZnEt2 and (R)-3,3-dimethyl-1,2 butanediol, b Co-1/AlEt3, and c the mixture of the salen ligand /Y complex. d Enantioselective ROP of rac-DLMe or meso-DLMe by rac-Y-1. e Stereosequence controlled polymerization of the mixture of rac-DLMe and meso-DLMe by rac-Y-1. f Stereosequence controlled polymerization of the mixture of meso-DLMe and rac-DLEt by rac-Y-2. g Stereosequence controlled polymerization of the mixture of rac-DLMe, rac-DLEt, meso-DLMe and meso-DLEt by rac-La-1. h Enantioselective and regioselective ROP of rac-DLMeEt by rac-Y-1.
In addition to metal complexes, enzymes have been investigated for the enantioselective ROP of β-lactones. In 1996, the Gross group used lipase PS-30 to prepare S-enriched poly(α-Me-β-PL) in toluene with Mns around 2.9 kDa: at 47% conversion of the racemic monomers, the s factor was 4.7, and the unreacted α-Me-β-PL having 45% ee. Wang et al. identified lipase ESL-001 to preferably polymerize R-BL from racemic BLs85. In 2000, Kobayashi and coworkers found the lipase-mediated copolymerization of racemic β-BL with achiral 12-dodecanolide in diisopropyl ether could give S-enriched copolymers with 76% ee of β-BL in the copolymer at 35% monomer conversion86. Nevertheless, most enzyme-mediated enantioselective ROP of β-lactones could only produce low-MW polymers with MWs less than 20 kDa.
8-membered diolide
To overcome the low reactivities in the enantioselective ROP of β-lactones and produce high-MW stereoregular PHAs, in 2018, Chen and Tang developed another synthetic route to prepare PHAs with high enantioselectivities87. Instead of focusing on the four-membered β-lactone, they prepared a group of eight-membered diolide monomers from bio-sourced dimethyl succinate in four steps. They used enantiopure chiral yttrium complex (Y-1) having bulky salcy ligands to mediate enantioselective ROP of such diolide, and the enantiomerically pure Y-1 complex (either (R, R) or (S, S) enantiomer) preferentially polymerized one enantiomer of diolide to yield optically pure (R) or (S)- P3HB (Pm = 0.99), and mechanistic studies suggested ESC-mediated mechanism attributed to the high enantioselectivity (Fig. 6d). Using rac-Y-1 complex could produce isotactic stereocomplex P3HB with a high isotacticity of up to 99%, a high Mn up to 154 kDa, and a Tm of 175 °C87. Notably, the rac-Y-1 complex could polymerize meso-diolide to generate syndiotactic (st) P3HB with a Pr of 0.81 and a Tm of 141 °C (Fig. 6d)88.
Notably, the synthesis of diolide from dimethyl succinate yields a mixture of rac- and meso- diastereomers (Fig. 6e). The enantioselectivity of Y-1 enables the copolymerization of the mixture of diolide diastereomers to produce stereoregular P3HBs via kinetic resolutions88. Indeed, the Chen group showed the enantioselective ROP of rac-diolide (rac-DLMe) and meso-DLMe could be achieved in a one-pot fashion at room temperature (Fig. 6e): the rac-diolide was consumed first, at which point 24% meso-diolide was also polymerized; this was followed by subsequent ROP of meso-diolide to provide a stereocomplex of tapered (it-P3HB)-sb-(st-P3HB) with improved ductility compared to isotactic it-P3HB88. Replacing the rac-DLMe with rac-DLEt in such one-pot copolymerization using less bulky Y-2 could generate another stereocomplex of (st-P3HB)-sb-(it-P3HV) (P3HV, poly(3-hydroxyvalerate); ROP rates of meso-DLMe more rapid than that of rac-DLEt) with enhanced ductility and strength compared to either isotactic it-P3HB or it-P3HV (Fig. 6f)88. To this end, the Chen group performed one-pot copolymerization of all six DLMe and DLEt diastereomers using racemic La-1 to produce gradient stereocomplex copolymers (it-P3HB)-sb-(it-P3HV)-sb-(st-P3HB)-sb-(st-P3HV), because of the monomer reactivities where rac-DLMe > rac-DLEt and meso-DLMe > meso-DLEt (Fig. 6g). Very recently, the same group applied such enantioselective ROP for asymmetric diolide, as the Y-1 complex preferentially ring-opened the diolide at less hindered site, and thereby generating stereocomplex of isotactic PHAs with alternating pendant side-chain functional groups (Fig. 6h)89.
Enantioselective polymerization of other lactones
ε-Caprolactone
Poly(ε-caprolactone) (PCL) and its derivatives have drawn considerable attentions as PCL can be used as biocompatible materials for medical applications90. Two types of chiral monomers, 6- and 4-alkyl-ε-CL, have been explored for the enantioselective ROP. In 2002, Bisht et al. found the Novozym 435 could enantioselectively polymerize rac-4-Me-ε-CL and rac-4-Et-ε-CL at 60 °C, preferably polymerizing (S)-enantiomers at low monomer conversions91. Meijer and coworkers then determined that the s factors at 45 °C for 4-Me-ε-CL, 4-Et-ε-CL, and 4-nPr-ε-CL were 16.9, 7.1 and 2.0, respectively, in which the enantiomer selectivity changed from (S)- to (R)-enantiomer in the polymerization of 4-nPr-ε-CL (Fig. 7a)92.
Fig. 7. Enantioselective ROP of racemic ε-caprolactone (ε-CL, a-g) and racemic β-thiolactone (h).

a Enantioselective ROP of 4-alkyl-ε-CL by Novozyme 435. b Racemization of (S)-6-Me-ε-CL by the Ru complex to promote the enchainment of (R)-enriched CL. c The switch of chirality preference in Novozyme 435-mediated ROP of medium to large-sized lactones. d (R,R)-Al-3-mediated enantioselective ROP of rac-6-Me-ε-CL. e (R)-O-7-mediated ROP of enantioselective ROP of rac-2-Bn-ε-CL. f (S)-O-8-mediated ROP of enantioselective ROP of rac-6-ε-CL. g (R)-O-7-mediated ROP of enantioselective ROP of rac-6-Me-ε-CL. h Y-3-mediated ROP of stereoselective ROP of rac-β-thiolactone.
In contrast to the ROP of rac-4-Me-ε-CL, Novozym 435 could not mediate the ROP of rac-6-Me-ε-CL. In 2005, Meijer et al. found that the ring-opening reaction of 6-Me-ε-CL using Novozym 435 did occur, and such reaction was (S)-selective; however, after the ring-opening, the (S)-configured terminal secondary alcohol could not efficiently engage with the enzyme enchainment93. To overcome such problem, Meijer et al. identified a Ru complex (the mixture of RuCl2(cymene) and 2-phenyl-2-aminopropionamide, Fig. 7b) to in situ racemize the terminal (S)-alcohol, which resulted in the active (R)-alcohol chain end for propagation. Such iterative tandem catalysis leads to (R)-enriched 6-Me-ε-CL oligomers (degree of polymerization < 5) with 100% monomer conversion and optical purity of up to 95% ee.
In 2007, Meijer and coworkers reported a switch from S- to R-selectivity from small to medium-sized lactone (ring sizes ≤7) to large-sized lactone (ring sizes ≥8) in the ROP of racemic ω-methylated lactones mediated by Novozyme 43594. The ROPs of racemic 7-MeHL, 8-MeOL, and 12-MeDDL all preferred (R)-monomer (Fig. 7c), yielding enantiopure (R)-polyesters with optical purity of up to 99% ee. The conformational change of lactones from cis-oid in small-sized lactones to trans-oid in large lactones might be attributed to such an enantioselectivity switch.
In addition to enzymes for enantioselective ROP of ε-caprolactones, metal complexes were explored for such polymerizations. In 2006, Feijen group found the chiral salen-Al complex could mediate enantioselective ROP of rac-6-Me-ε-CL (Fig. 7d)95. The chiral (R,R)-Al-3 complex exhibited a preference for (R)-6-Me-ε-CL, and at 80% monomer conversion, the ee value was 50%, and the s factor was 1.63. On the other hand, no enantiomeric preference was found for chiral Al complex to polymerize rac-4-Me-ε-CL.
Recently, organocatalyst has been investigated for the enantioselective ROP of substituted ε-CL. In 2020, Wang and coworkers found that the chiral phosphoric acid could catalyze the enantioselective ROP of racemic 2-Bn-ε-CL and 6-Bn-ε-CL at 90 °C, with moderate s factors that prefer (R)-monomers around 2 at 50% monomer conversions, and eventually provided gradient stereoblock polymers (Fig. 7e, f)96. The chiral HPLC analysis showed that (R)-substituted-ε-CL monomers were preferentially polymerized when employing (R)-phosphoric acid (Fig. 7e), whereas (S)-substituted-ε-CL monomers were preferentially consumed when using (S)-phosphoric acid as the catalyst (Fig. 7f). The same group also evaluated chiral phosphoric acid for the enantioselective ROP of racemic 6-aryl-ε-CL97 and 6-Me-ε-CL;98 and the former had an s factor of 4.1 at the monomer conversion of 44% (Fig. 7f), while the latter showed an s factor of 7.3 at conversion of monomer conversion 44% (Fig. 7g). In all cases, gradient stereoblock copolymers were obtained when all monomers were consumed.
Thiolactone
Compared to the ester bond, the thioester bond is more reactive, and recent studies showed that polythioesters could be degraded back to corresponding thiolactones99–102. Most of the ROP of racemic thiolactones reported so far used organocatalysts and produced atactic copolymers. Poly(3-thiobutyrate) (P3TB) with high MW (~175 kDa) was first prepared from a recombinant strain of E.coli cultured with racemic thiobutyric acids and was presumably highly isotactic103. In 2022, Carpentier, Guillaume, and coworkers developed tetradentate aminobis(phenolate) yttrium complexes to mediate either cyclic syndio- or enantioselective cyclic polymer stereocomplex104. The ROP of rac-TBL by the Y-3 complex with less bulky substituents proceeded rapidly to produce isotactic cyclic P3TB with a Pm of 0.90 (Fig. 7h). Notably, the stereocomplex of cyclic polymers were proposed, though kinetic studies and the ee measurement were not performed to eliminate the possibility of forming stereoblock microstructures via the chain-end control mechanism.
Enantioselective polymerization of epoxides
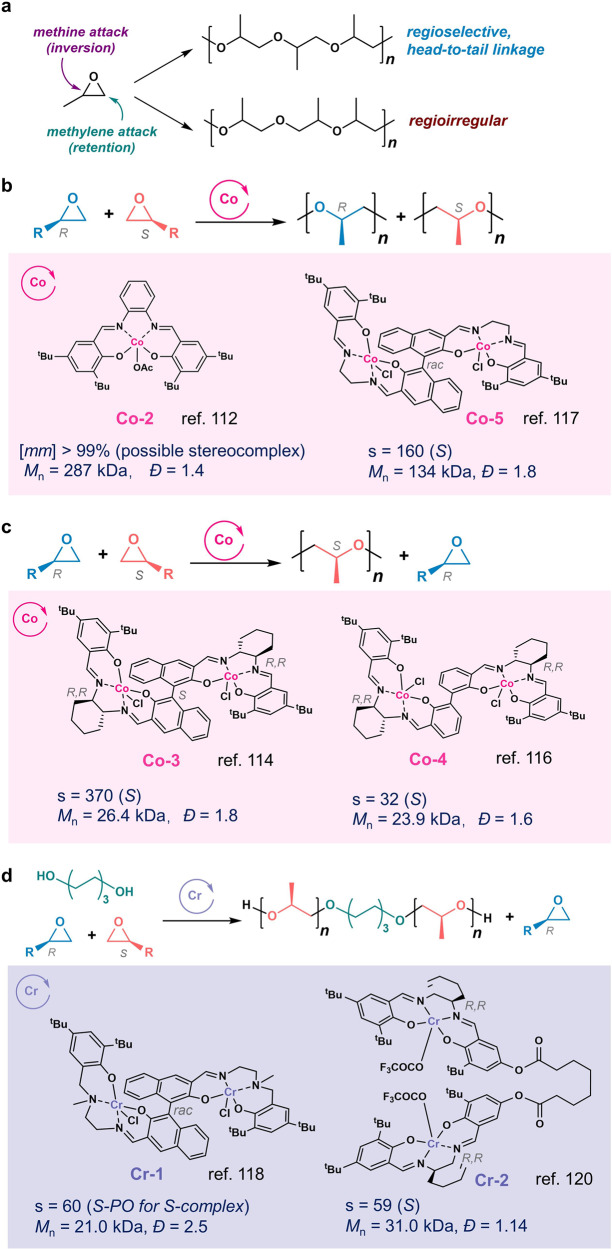
Isotactic polyethers, e.g., isotactic polypropylene oxide (it-PPO), are semi-crystalline thermoplastics. Compared with polyolefins, polyethers have revealed low environmental existence: atactic PPO (a-PPO) can be metabolized by microorganisms through oxidation105, and polyethers are susceptible to photo-oxidative degradation106,107. Commercial production of a-PPO uses double-metal cyanide catalysts in conjunction with alcohol chain transfer agents to produce low-MW polymers without tacticity control108. Though the synthesis of it-PPO was independently explored early in 1950s by Dow chemicals, Natta, and Price et al.109–111, the large production of it-PPO from racemic propylene oxide (PO) has not been realized due to the low reactivities and stereoselectivity ratios10. Notably, in addition to enantioselectivity, regioselectivity plays an important role affecting the polyether microstructures for monosubstituted epoxide, i.e., head-to-tail, head-to-head, tail-to-tail linkages (Fig. 8a). For monosubstituted epoxides such as PO, enchainment can occur either at the methylene to give a secondary metal alkoxide, or at the methine with inversion to give a primary metal alkoxide (Fig. 8a). The resulted polyether is regio-regular when only one process dominates; and the polymer becomes regio-irregular when both processes occur, which give head-to-head and tail-to-tail linkages that can be characterized by NMR.
Fig. 8. Enantioselective ring-opening polymerization (ROP) of racemic monosubstituted epoxide.
a Regio-selective ring-opening polymerization of propylene oxide (PO) to produce regioregular poly(propylene oxide) (PPO). b Co-2 or Co-5 mediated enantioselective ROP of rac-PO to produce stereocomplex PPO. c Co-3 or Co-4 mediated enantioselective ROP of rac-PO to produce (S)-PPO. d Isoselective ROP of rac-PO with the diol as the chain-shuttling agent to produce isotactic PPO.
In 2005, the Coates group first reported enantioselective Co-catalyst Co-2 capable of polymerizing rac-PO to exclusively regioregular stereocomplex it-PPO with over 99% triads and Mns over 287 kDa112. Mechanistic studies suggest the bimetallic mechanism for PO enchainment: Co centers are separated by 7.1 Å, and an empty Co coordination site is allocated for the PO insertion into the polymer that is bound to the other Co site113. The proposed bimetallic mechanism led to the development of a series of chiral bimetallic complexes by the Coates group. In 2008, Coates and coworkers reported the first bimetallic enantioselective Co complex for the ROP of various monosubstituted epoxides, including alkyl, aryl, unsaturated, and fluorinated epoxides as well as glycidyl ethers (Fig. 8b)114. Combined with an cocatalyst salt, e.g., bis(triphenylphosphine)iminium acetate ([PPN][OAc]), the bimetallic Co-3 complex could kinetically resolve various racemic epoxides into valuable enantiopure epoxides and isotactic polyethers with high krel values (>200 for many epoxides), high mm-triad content (>98% for many epoxides), and high TOFs (up to 30,000 h–1)114,115. In addition, the use of the racemic bimetallic complex allows the preparation of isotactic polyethers in quantitative yields (Fig. 8c)115. DFT computation studies reveal that the active form of the catalyst has two active exo anionic ligands (chloride or carboxylate) and an endo polymer alkoxide which can ring-open an adjacent cobalt-coordinated epoxide; additionally, the initiation is favored by an endo chloride ligand, while propagation is favored by the presence of two exo carboxylate ligands116. Notably, the chirality of the diamines has little effect on this class of catalysts when the chirality of the binaphthol linker in the bi-Co complex was fixed117. Switching from a chiral binaphthol linker to an achiral biphenol linker (Co-4) resulted in a bi-Co complex, in which the stereochemistry of the diamines determined the preferred linker conformation116.
Nevertheless, attempts to prepare rac-Co-3 from racemic starting materials produced inseparable diastereomers, which impedes the preparation of stereocomplex polyethers. Coates and coworkers found that replacing the chiral diamines with achiral ethylene diamine in the bi-Co complex (Co-5) could also mediate enantioselective ROP of various monosubstituted epoxides to produce stereocomplex copolymers with mm-triad over 97% when combined with the cocatalyst [PPN][OPiv] (OPiv = pivalate; Fig. 8b)117.
To prepare hydroxy-telechelic isotactic PPO—that is used industrially as a midsegment in polyurethane, in 2017, the Coates group developed one-pot synthesis of bimetallic salenen ligand for Cr complexes (Cr-1), which could tolerate di-alcohol chain-transfer agents in combination with [PNN]Cl; and (S)-Cr-1 preferentially polymerized (S)-PO with an s factor ~ 60 (Fig. 8d)118. Notably, the produced UV light-degradable (S)-it-PPO exhibited dramatic strain hardening with an ultimate fracture strength comparable to that of Nylon-6,6 and an ultimate fracture strain similar to that of it-PP119. To increase the polyether’s MW control and improve MW distributions, the same group introduced flexible inter between the active salen-Cr units to allow the complex to render conformations for cooperative ROP. They found the bi-Cr complex with the linker length of six methylenes (Cr-2) showed an optimal performance in terms of activity and selectivity, with an s factor of 59 in the ROP of rac-PO (Fig. 8d)120.
Enantioselective alternating copolymerization of epoxide/CO2
The ever-increasing emissions of CO2 as waste material from fossil-based industries pose chemists a grand challenge to efficiently transform CO2 to value-added chemicals121,122. In contrast to many reactions requiring expensive stoichiometric reagents and having scale-up problems123, the ring-opening copolymerization (ROCOP) of CO2 and other monomers, e.g., epoxides, is well suited for large-scale deployment because it enables significant CO2 sequestration, and produces valuable polymers with properties to replace existing petrochemicals124.
The ROCOP of epoxide/CO2 can be traced back to 1969125,126, and such polymer chemistry has witnessed a renaissance in the last 20 years due to the development of new catalysts, which has been extensively reviewed by many others10,12,30,127–132. Here we focus on highlighting enantioselective ROCOP of epoxide/CO2. Similar to the homopolymerization of epoxide, in addition to enantioselectivity, the regioselectivity of ROCOP of epoxide/CO2 plays an important role in copolymer’s microstructure.
Monosubstituted epoxide/CO2
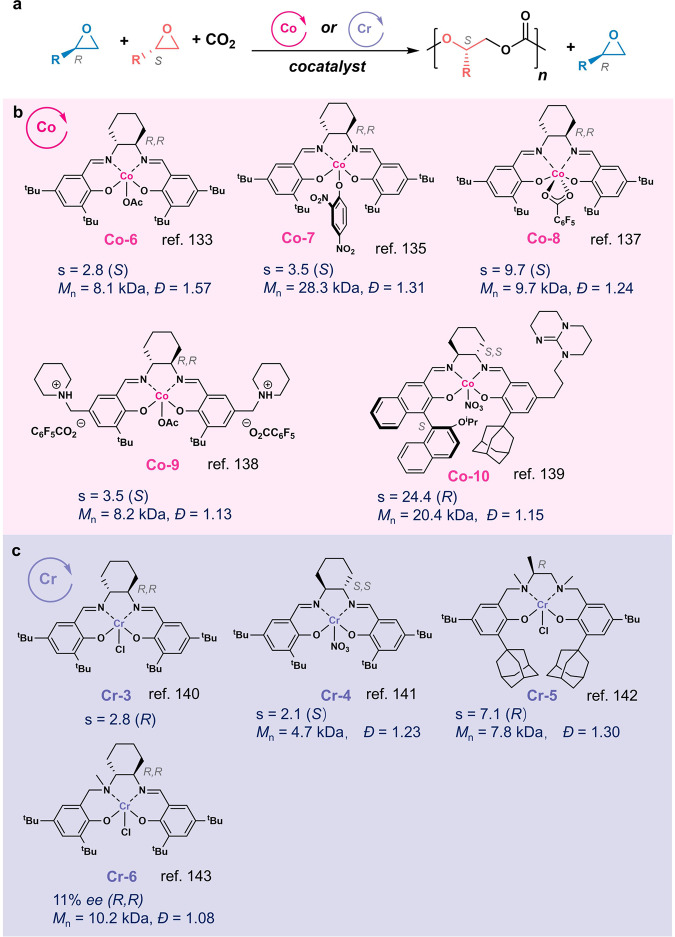
In 2003, Coates and coworkers first developed chiral (salcy)CoIII-based catalysts for the ROCOP of rac-PO and CO2 to produce poly(propylene carbonate) (PPC) with over 90% head-to-tail linkages when combined with PPN salts133,134. The (R,R)-Co-6 preferably polymerized (S)-PO over (R)-PO with an s factor of 2.8 (Fig. 9a, b)133. The Lu group found that replacing the axial group in chiral (salcy)CoIIIX from acetate to binitrophenol (Co-7) and using cocatalyst nBu4NCl or 7-methyl-1,5,7-triazabicyclo[4.4.0]dec-5-ene resulted in the increase of the s factor to 3.5135, and 5.9136, respectively. Coates et al. later found out the (R,R)-Co-8 /[PPN]Cl catalyst system had an s factor of 9.7 for the enchainment of (S)- over (R)-PO at −20 °C, resulting in a near perfectly regioregular PPC with 98% head-to-tail connectivity137. Nozaki et al. incorporated cocatalyst onto the salen ligand (Co-9) to improve the ROCOP reactivities, and produced gradient stereoblock PPC with the head-to-tail ratio of 95% and an s factor value of 3.5138. Such gradient stereoblock PPC showed significantly increased degradation temperature compared with stereocomplex or enantiopure PPCs. Along this line, the Lu group developed an asymmetrical salen ligand containing a chiral BINOL and an appended base 1,5,7-triabicyclo[4.4.0] dec-5-ene for the Co complex (Co-10), which mediated ROCOP of rac-PO/CO2 with a high s factor of 24.4 at −20 °C139.
Fig. 9. Enantioselective ring-opening copolymerization (ROCOP) of racemic monosubstituted epoxide/CO2.
a The enantioselective ROCOP reaction scheme. b Co and c Cr complexes used in the polymerization in a.
Cr-based catalysts have been explored for enantioselective ROCOP (Fig. 9c). In 2000, Jacobsen et al. reported enantioselective copolymerization of CO2/1-hexene oxide catalyzed by chiral (R,R)-salen-Cr catalyst (Cr-3) with an s factor of 2.8140. Later, the Lu group and the Nozaki group individually reported chiral (salen)Cr (Cr-4)141, (salan)Cr (Cr-5)142, and (salalen)Cr (Cr-6)143 for the copolymerization of PO/CO2, with s factors ranging from 2 to 7.
Meso-epoxide/CO2
The opposite configurations in the meso-epoxides allow for the synthesis of isotactic (–RR– or –SS–) and syndiotactic (–RR–SS–) polycarbonates (Fig. 10a). In 1999, the Nozaki group first reported the mixture of ZnEt2 and (S)-α,α-diphenyl(pyrrolidin-2-yl)methanol could mediate ROCOP of CO2 and cyclohexene oxide (CHO) to produce perfectly alternating poly(cyclohexene carbonate) (PCHC, Fig. 10b)144. The subsequent hydrolysis of the obtained PCHC showed the polymer has 73% ee, and no cis-diol was found in the degradation, suggesting the ring-opening reaction proceeds through an SN2-type mechanism. The same group then treated such Zn complex with ethanol to generate a di-Zn complex (Zn-3) that improved ee up to 80% (Fig. 10b)145.
Fig. 10. Enantioselective ring-opening copolymerization (ROCOP) of meso-epoxide/CO2.
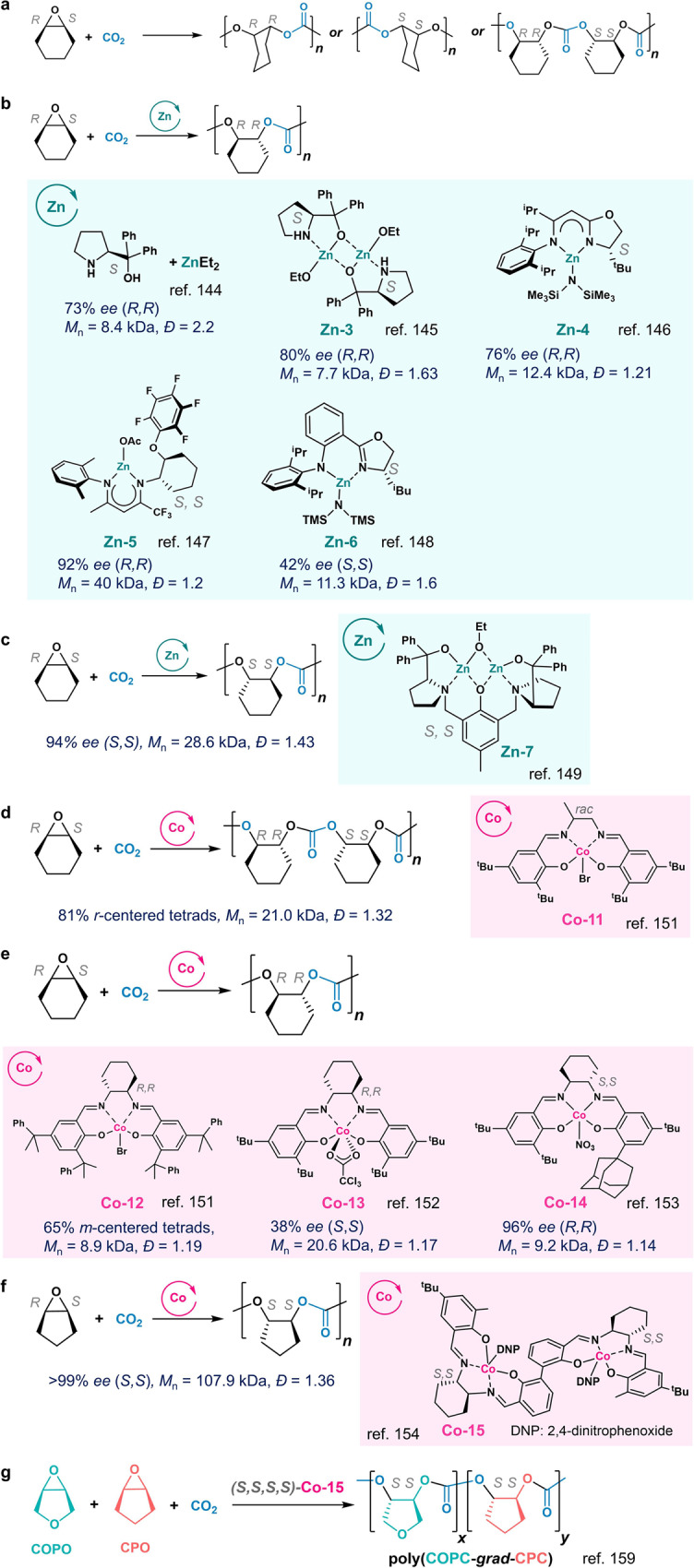
a Stereoselective ROCOP of meso-epoxide (e.g., cyclohexene oxide (CHO))/CO2 to produce various stereoregular polycarbonates. b Zn-based complexes for isoselective ROCOP of CHO/CO2. c bi-Zn complex for stereoselective ROCOP of CHO/CO2 to produce (S,S)-enriched polycarbonates. d Co-11-mediated syndioselective ROCOP of CHO/CO2. e Co-based complexes for isoselective ROCOP of CHO/CO2. f Co-15-mediated isospecific ROCOP of CHO/CO2 to produce (S,S)-enriched polycarbonates. g Co-15-mediated isoselective copolymerization of two meso-epoxides with CO2 to produce (S,S)-enriched gradient polycarbonates.
Meanwhile, Coates and coworkers reported an imine-oxazoline Zn complex (Zn-4) for enantioselective ROCOP of CHO/CO2 to produce PCHC with 76% ee146. Later, the same group identified an asymmetrical β-diiminate Zn complex (Zn-5) that enables the preparation of highly isotactic PCHC with units up to 92%147. Other examples are using chiral Zn catalysts: the amido-oxazoline Zn complexes (Zn-6) producing iso-enriched PCHC with units of 42% ee reported by Du and coworkers (all above in Fig. 10b)148, and bi-Zn complexes (Zn-7)149,150 that have (S,S)-enriched PCHC with 94% ee (Fig. 10c).
In 2006, the Coates group first reported syndioselective ROCOP of CHO/CO2 using racemic (salen)CoIII catalyst Co-11, which provided PCHC with 81% r-centered tetrads (Fig. 10d)151. Notably, adding [PPN]Cl as a cocatalyst decreased syndioselectivity. On the other hand, both Coates and Lu groups found that (R,R)-(salcy)CoIII catalysts with bulky substituents (Co-12 and Co-13) could produce iso-enriched PCHC151,152. In 2012, Lu identified an asymmetrical (salcy)Co III catalyst with the bulky ortho-substituent (Co-14) for enantioselective ROCOP of CHO/CO2 to produce isotactic PCHC with 96% ee at −25 °C (Fig. 10e). The use of (S)-2-methyltetrahydrofuran as a chiral induction agent was found impactful for the enantioselectivity, whereas the inclusion of [PPN]Cl only slightly lower the ee to 90% at 0 °C153.
To improve Co-catalyst’s reactivity for stereoregular high-MW polycarbonate synthesis, in 2013, the Lu group developed bi-Co complexes in which two (salen)CoIII moieties were linked through a biphenol linker. Combined with the cocatalyst [PPN][DNP] (DNP, 2,4-dinitrophenoxide), the enantiopure bi-Co complex Co-15 could improve the reactivity and increase the selectivity over 99% ee in poly(cyclopentene carbonate) at room temperature (Fig. 10f)154. A bimetallic cooperation mechanism was proposed for the extraordinary enantioselectivity: the initiation is triggered by one of the two nucleophilic anions from the inside cleft of the bimetallic catalyst, and enchainment proceeded via the nucleophilic attack of the propagating carboxylate moiety at one Co toward the epoxide bonded at the other Co center. Both the stereochemistry of the biphenol-linker and cyclohexyl diamine skeletons determine the enantiomeric preference of the asymmetric copolymerization155. Such highly reactive and enantioselective chiral bi-Co complex could polymerize various epoxides with CO2, producing the corresponding polycarbonates with more than 99% carbonate units and 99% ee156. The produced polycarbonates were mostly crystalline with Tm values of 179–257 °C. The stereocomplex of these isotactic enantiopure polycarbonates prepared using Co-15 exhibited significant improvement in Tms and thermal stability157,158. Enantioselective terpolymerization of 3,4-epoxytetrahydrofuran, cyclopentene oxide, and CO2 could be achieved by using the (S,S,S,S)-Co-15 complex to produce gradient block copolymers with over 95% (S,S)-configured units, in which crystalline epoxytetrahydrofuran carbonate segment was incorporated first, followed by the amorphous segments consisting of cyclopentene carbonate (Fig. 10g)159.
Enantioselective polymerization of epoxide/anhydride
Aliphatic polyesters are commonly made through ROP of lactones26,28,40,160. The resulting polyesters have a limited range of properties owing to the lack of functional diversity of available lactones161–163. An alternative chain-growth route to both aliphatic and semi-aromatic polyesters is the alternating copolymerization of epoxides and cyclic anhydrides, which has emerged in recent 10 years and has been reviewed elsewhere12,31,129,132. Using two distinct monomer sets in such alternating ROCOP could allow for facile tuning of polyesters properties.
In 2016, the Lu group first reported enantioselective ROP of CHO (a meso-epoxide) with phthalic anhydride (PA) mediated by the enantiopure (R,R,R,R)-bi-Al complex Al-11 with [PPN]Cl at 0 °C to afford (R,R)-enriched poly(cyclopentene phthalate) with 91% ee (Fig. 11a)164. Later, the same group improved the reactivity and enantioselectivity of bi-Al complexes by using a hydrogenated binaphthol linkage; and the iPr group was found to be the optimal phenolate ortho-substituent for the bi-Al complex (Al-12, Fig. 11a)165. The obtained chiral polyesters are usually semicrystalline materials with Tms up to 240 °C, and the obtained stereocomplex polyesters exhibited elevated Tms of ~ 20-45 °C relative to the Tms of the corresponding enantiopure counterparts165,166. The Lu group also identified another chiral bi-Al complex, Al-13, with the co-catalyst [PPN]Cl to mediate enantioselective ROCOP of racemic cis-internal meso-epoxide (e.g., 7-oxabicyclo[4,1,0]hept-2-ene) /PA at 0 °C to produce (R,R)-enriched copolyester with a high s factor of 437 (Fig. 11b)166. Very recently, Lu and coworkers identified a chiral bi-Cr complex Cr-7, along with [PPN]Cl, to mediate enantioselective ROCOP of racemic disubstituted cis-epoxides with cyclic anhydrides to produce (R,R)-enriched polyester with 90% ee and an s factor of 31 (Fig. 11c)167.
Fig. 11. Enantioselective ring-opening copolymerization (ROCOP) of epoxide/anhydride.
a Al complexes for stereoselective ROCOP of meso-epoxide/anhydride. b Al-13 and c Cr-7 mediated enantioselective ROCOP of meso-epoxides/anhydride. d Al-14-mediated enantioselective ROCOP of racemic mono-substituted epoxides/anhydride. e Al-15-mediated stereoselective ROCOP of meso-epoxides and racemic mono-substituted epoxides and anhydride.
In addition to meso-epoxides, the Lu group also applied the bi-Al complex for the enantioselective ROP of racemic monosubstituted epoxide and anhydrides. The chiral bi-Al complex Al-14 having a binaphthol linkage, in conjunction with [PPN]Cl at 0 °C, exhibited exceptional enantioselectivities for the ROCOP of phenyl glycidyl ether (PGE)/PA with an s factor over 300 and over 99% ee (Fig. 11d)168. Such bi-Al complex also can be used for ROCOPs of various racemic epoxide/anhydrides, and all afford highly isotactic copolymers, with over 90% ee for most combinations. Additionally, the Lu group found that the combination of (S,S,S,S,S)-Al-15 and [PPN]Cl could enantioselectively terpolymerize rac-tert-butyl glycidyl ether, meso-cyclopentene oxide, and PA to form a gradient copolymer with a 97% ee for the (S,S)-configuration in cyclopentene oxide units and a 95% ee for the glycidyl ether unit (Fig. 11e)169. During the enchainment, the (R)-tert-butyl glycidyl ether was incorporated first, followed by a gradually increased ratio of (S,S)-cyclopentene oxide, resulting in the copolymers bearing a wide range of Tg/Tm temperatures by adjusting the co-monomer ratios.
Lu and Coates et al. also found that the stereocomplex assembly of discrete (R)- and (S)-aromatic polyesters required a minimal degree of polymerization over 5 and over 74% ee170. In addition, for aliphatic polyesters, the Coates group recently showed that only specific chiral combinations of epoxide and anhydrides would lead to the formation of semicrystalline stereocomplex mixtures, which could significantly increase Tms17.
Other enantioselective ring-opening polymerization and copolymerization
Carbonyl sulfide/meso-epoxide
In 2018, the Lu group first achieved enantioselective synthesis of highly stereoregular poly(monothiocarbonate)s through the asymmetric copolymerization of meso-epoxides and COS (carbonyl sulfide) mediated by enantiopure biaryl-linked bi-CoIII complexes (Co-16)171. A binaphthol-linked, bimetallic complex with an (R,R,R,R,R)-Co-16 exhibited efficient enantioselectivity in mediating this asymmetric transformation, affording a variety of isotactic poly(monothiocarbonate)s with over 99% isotacticity (Fig. 12a). Notably, the resultant chiral, sulfur-containing copolymers exhibit good optical properties with refractive indices of up to 1.56.
Fig. 12. Other enantioselective ring-opening polymerization (ROP) and ring-opening copolymerization (ROCOP) reactions.
a Enantioselective ROCOP of meso-epoxide/COS. b Enantioselective ROP of racemic thiirane. c Enantioselective ROP of racemic N-carboxyanhydride.
Thiirane
Early work on enantioselective ROP of methylthiirane showed low enantioselectivities172–175. In 1981, Spassky and coworkers found the chiral initiator of ZnEt2/(S)-BINOL could selectively polymerize (S)-methylthiirane from the racemic mixture (Fig. 12b)176,177. Kakuchi and coworkers used ZnEt2/l-leucine for the ROP of racemic (phenoxymethyl)thiirane, and found the S-enantiomer was preferably polymerized with an s factor of 5.36 (Fig. 12b)178.
N-carboxyanhydride
Synthetic polypeptides mimicking natural proteins offer potential advantages for biomedical applications with simpler components. Synthetic polypeptides can be prepared from enantiopure α-amino-acid N-carboxyanhydrides (NCAs)179–181. Early efforts included using chiral amines182, AlEt3/(+)-borneol183, or 2-methylbutyrate-tri-n-butylphosphine Ni complex, etc.184, but the overall enantioselectivities were very low. In 1999, Cheng and Deming found that Ni complexes with chiral 2-pyridinyloxazoline ligands could initiate ROP with a modest enantioselectivity (the krel of 5.2, Fig. 12c)185. A higher krel of 9.4 was achieved by the same group using a Ru complex with a dmpe ligand (dmpe, 1,2-bis(dimethylphosphino)ethane; Fig. 12c)186.
Thermal and mechanical properties of stereoregular polymers prepared via ROP
In Table 1, we summarize a few of the common stereoregular degradable polymers’ thermo-mechanical properties. In general, high stereoregularity in the polymer backbone can lead to enhanced properties. In particular, the formation of stereocomplex—the mixture of two opposite enantiomeric polymers in equivalent amounts through an interlocked orderly packing of polymer chains—often results in a higher level of crystallinity and an increased Tm. For example, stereocomplex PLA exhibits a Tm of 230 °C, which is ~ 50 °C higher than that of its enantiopure parent polymers16. Additionally, the stereocomplex PLA significantly increases its hydrolytic and thermal degradation resistance and gas barrier properties187. Coates, Lu, and coworkers showed that the weak hydrogen-bonding interaction between carbonyl and methine of the opposite enantiomeric polyesters that were prepared by ROCOP of epoxides/anhydrides could be the driving force of stereocomplexation that leads to the greater increases in Tms170. Similarly, the stereocomplex of poly(limonene carbonate) was able to easily crystallize whereas its enantiopure parent polymers could not in spite of the high stereoregular configuration188,189. The ROCOP of racemic or meso epoxides with anhydrides using chiral Al complexes (Fig. 11a) could also lead to an increase in Tm of ~ 25–45 °C for stereocomplex polyesters compared to the Tm values of enantiopure constituents165. Coates, Tolman, and coworkers recently found that the specific chiral combinations between the epoxide and anhydride in the ROCOP could induce the change from amorphous to semicrystalline copolymers, and the stereocomplex of such copolymers exhibited increased Tms17.
Table 1.
Thermo-mechanical properties of stereoregular polymers prepared via ROPa.
| Name | Pm | Tg (°C) | Tm (°C) | σ (MPa) | ε (%) | Refs. |
|---|---|---|---|---|---|---|
| poly(l-LA) | 1 | 55–65 | 160–185 | 50–60 | 3–6 | 192 |
| poly(sc-LA) | 1 | 50–60 | 220–230 | 60–70 | 25–35 | 16 |
| poly(sb-LA) | 0.96 | – | 192 | 49 | 5 | 191 |
| poly(ht-LA) | 0.13 | 50 | – | 11 | 533 | 191 |
| poly(sc-3HB) | 0.99 | – | 171 | 35 | 3–5 | 87 |
| poly(sr-3HB) | 0.23 | ~0 | 114 | 34 | 419 | 190 |
| sb-PPO | 0.97 | – | 67 | 65 | 450 | 119 |
| it-PPO | 0.99 | – | 68 | 75 | 450 | 119 |
aPm the probability of meso linkages, Tg glass transition temperature, Tm melting temperature, σ fracture strength, ε fracture strain, sc stereocomplex, sb stereoblock, ht heterotactic, sr syndio-rich, it isotactic, LA lactide, 3HB 3-hydroxybutyric acid, PPO poly(propylene oxide).
Nevertheless, the high levels of stereoregularity and high crystallinity could bring up brittleness for the polymers, which limits the polymer’s applications such as packaging. For instance, purely isotactic P3HB exhibits high crystallinity, high Tm (~ 170–180 °C), high fracture strength (∼35 MPa), and excellent barrier properties but is extremely brittle with low fracture strain (~5%)74,87. The introduction of stereo-defects into P3HB by using a less-stereoselective catalyst to produce syndiorich-P3HB (sr-P3HB) with a lower Tm (114 °C) resulted in a significantly improved fracture strain (over 400%) without sacrificing the fracture strength (34 MPa)190. The change of stereoregularity or stereosequence renders a unique strategy to improve the polymer’s properties without changing its chemical composition163,190,191.
Conclusion and outlook
Enantioselective ROP and ROCOP of cyclic monomers have a long and distinguished history, with early work dating back to the early 20th century. Interest in the field has experienced a resurgence in recent years, due in part to the development of new synthetic methods and the urgency to find alternatives to non-sustainable petroleum chemicals. The application of NMR and chiral chromatography to elucidate polymer microstructures and reaction kinetics becomes critical to provide understanding of the polymerization process. Importantly, the polymerization catalyst dictates the reaction performance. Understanding the reaction mechanism, exemplified by Coates’ work on enantioselective polymerization of epoxides112–114, could aid the design of polymerization catalysts. Nevertheless, identification of qualitative trends in catalyst structures can be often challenging, because the catalyst structural factors influencing selectivity and polymerization efficiency in complex systems are high-dimensional. Notably, the machine learning techniques (e.g., Bayesian optimization) have been recently applied by our group to accelerate the discovery of stereoselective catalyst for the ROP of rac-LA mediated via the chain-end-control mechanism191. The development of such new methods for the quantitative identification of key structural features in catalysts will enable the unbiased optimization of catalyst performance and the discovery of new chemistry in this field. Additionally, as many studies focus on the structurally simple monomers, the expansion of monomers having pendant functional groups will help bestow new materials properties. Most reported polymerization systems have only been demonstrated on a bench scale (and many producing low-MW polymers), and thereby remain at a proof-of-concept stage. The examination of the catalyst and final polymer’s toxicities is of importance if the polymers will be used for packaging or biomedical applications. The demonstration of materials performance and scalability of these new polymers, in addition to their degradability and sustainability, will make them attractive polymers for potential industrial translation in the future.
Supplementary information
Acknowledgements
This work was supported by the National Science Foundation (CHE-1807911).
Author contributions
X.X., Z.H., and R.T. conceived the scope of the article. X.X., Z.H., E.J., and R.T. discussed the content and wrote the manuscript.
Peer review
Peer review information
Communications Chemistry thanks Jian-Bo Zhu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Xiaoyu Xie, Ziyu Huo.
Supplementary information
The online version contains supplementary material available at 10.1038/s42004-023-01007-z.
References
- 1.Coates GW. Precise control of polyolefin stereochemistry using single-site metal catalysts. Chem. Rev. 2000;100:1223–1252. doi: 10.1021/cr990286u. [DOI] [PubMed] [Google Scholar]
- 2.Staudinger H, Ashdown AA, Brunner M, Bruson HA, Wehrli S. Über hochpolymere verbindungen. 22. Mitteilung. Über die konstitution des poly-indens. Helv. Chim. Acta. 1929;12:934–957. [Google Scholar]
- 3.Schildknecht CE, Zoss AO, McKinley C. Vinyl alkyl ethers. Ind. Eng. Chem. 1947;39:180–186. [Google Scholar]
- 4.Natta G, et al. Crystalline high polymers of α-olefins. J. Am. Chem. Soc. 1955;77:1708–1710. [Google Scholar]
- 5.Jayaratne KC, Sita LR. Stereospecific living ziegler−natta polymerization of 1-hexene. J. Am. Chem. Soc. 2000;122:958–959. [Google Scholar]
- 6.Coates, G. W. Polymerization catalysis at the millennium: Frontiers in stereoselective, metal-catalyzed polymerization. J. Chem. Soc. Dalton Trans. 467–475 (2002).
- 7.Corradini P, Guerra G, Cavallo L. Do new century catalysts unravel the mechanism of stereocontrol of old Ziegler-Natta catalysts? Acc. Chem. Res. 2004;37:231–241. doi: 10.1021/ar030165n. [DOI] [PubMed] [Google Scholar]
- 8.Baugh, L. S. & Canich, J. A. M. Stereoselective Polymerization With Single-site Catalysts. (CRC Press, 2007).
- 9.Franssen NMG, Reek JNH, de Bruin B. Synthesis of functional ‘polyolefins’: State of the art and remaining challenges. Chem. Soc. Rev. 2013;42:5809–5832. doi: 10.1039/c3cs60032g. [DOI] [PubMed] [Google Scholar]
- 10.Childers MI, Longo JM, Van Zee NJ, LaPointe AM, Coates GW. Stereoselective epoxide polymerization and copolymerization. Chem. Rev. 2014;114:8129–8152. doi: 10.1021/cr400725x. [DOI] [PubMed] [Google Scholar]
- 11.Worch JC, et al. Stereochemical enhancement of polymer properties. Nat. Rev. Chem. 2019;3:514–535. [Google Scholar]
- 12.Wang X, Huo Z, Xie X, Shanaiah N, Tong R. Recent advances in sequence-controlled ring-opening copolymerizations of monomer mixtures. Chem. Asian J. 2023;18:e202201147. doi: 10.1002/asia.202201147. [DOI] [PubMed] [Google Scholar]
- 13.Xu G, et al. Asymmetric kinetic resolution polymerization. Coord. Chem. Rev. 2020;414:213296. [Google Scholar]
- 14.Mori Y, Tanzawa H. Viscoelastic properties of stereocomplex polymers. J. Appl. Polym. Sci. 1976;20:1775–1785. [Google Scholar]
- 15.Shin EJ, Jones AE, Waymouth RM. Stereocomplexation in cyclic and linear polylactide blends. Macromolecules. 2012;45:595–598. [Google Scholar]
- 16.Tsuji H. Poly(lactic acid) stereocomplexes: a decade of progress. Adv. Drug Deliv. Rev. 2016;107:97–135. doi: 10.1016/j.addr.2016.04.017. [DOI] [PubMed] [Google Scholar]
- 17.Popowski Y, Lu Y, Coates GW, Tolman WB. Stereocomplexation of stereoregular aliphatic polyesters: change from amorphous to semicrystalline polymers with single stereocenter inversion. J. Am. Chem. Soc. 2022;144:8362–8370. doi: 10.1021/jacs.2c02981. [DOI] [PubMed] [Google Scholar]
- 18.Tutoni G, Becker ML. Underexplored stereocomplex polymeric scaffolds with improved thermal and mechanical properties. Macromolecules. 2020;53:10303–10314. [Google Scholar]
- 19.Kawauchi T, Kitaura A, Kumaki J, Kusanagi H, Yashima E. Helix-sense-controlled synthesis of optically active poly(methyl methacrylate) stereocomplexes. J. Am. Chem. Soc. 2008;130:11889–11891. doi: 10.1021/ja8048805. [DOI] [PubMed] [Google Scholar]
- 20.Zhu Y, Romain C, Williams CK. Sustainable polymers from renewable resources. Nature. 2016;540:354–362. doi: 10.1038/nature21001. [DOI] [PubMed] [Google Scholar]
- 21.Deacy AC, Gregory GL, Sulley GS, Chen TTD, Williams CK. Sequence control from mixtures: Switchable polymerization catalysis and future materials applications. J. Am. Chem. Soc. 2021;143:10021–10040. doi: 10.1021/jacs.1c03250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang X, Chen EYX. Toward infinitely recyclable plastics derived from renewable cyclic esters. Chem. 2019;5:284–312. [Google Scholar]
- 23.Häußler M, Eck M, Rothauer D, Mecking S. Closed-loop recycling of polyethylene-like materials. Nature. 2021;590:423–427. doi: 10.1038/s41586-020-03149-9. [DOI] [PubMed] [Google Scholar]
- 24.Abel BA, Snyder RL, Coates GW. Chemically recyclable thermoplastics from reversible-deactivation polymerization of cyclic acetals. Science. 2021;373:783–789. doi: 10.1126/science.abh0626. [DOI] [PubMed] [Google Scholar]
- 25.Coates GW, Getzler YDYL. Chemical recycling to monomer for an ideal, circular polymer economy. Nat. Rev. Mater. 2020;5:501–516. [Google Scholar]
- 26.Dechy-Cabaret O, Martin-Vaca B, Bourissou D. Controlled ring-opening polymerization of lactide and glycolide. Chem. Rev. 2004;104:6147–6176. doi: 10.1021/cr040002s. [DOI] [PubMed] [Google Scholar]
- 27.Kamber NE, et al. Organocatalytic ring-opening polymerization. Chem. Rev. 2007;107:5813–5840. doi: 10.1021/cr068415b. [DOI] [PubMed] [Google Scholar]
- 28.Ajellal N, et al. Metal-catalyzed immortal ring-opening polymerization of lactones, lactides and cyclic carbonates. Dalton Trans. 2010;39:8363–8376. doi: 10.1039/c001226b. [DOI] [PubMed] [Google Scholar]
- 29.Tschan MJL, Gauvin RM, Thomas CM. Controlling polymer stereochemistry in ring-opening polymerization: a decade of advances shaping the future of biodegradable polyesters. Chem. Soc. Rev. 2021;50:13587–13608. doi: 10.1039/d1cs00356a. [DOI] [PubMed] [Google Scholar]
- 30.Paul S, et al. Ring-opening copolymerization (ROCOP): Synthesis and properties of polyesters and polycarbonates. Chem. Commun. 2015;51:6459–6479. doi: 10.1039/c4cc10113h. [DOI] [PubMed] [Google Scholar]
- 31.Longo JM, Sanford MJ, Coates GW. Ring-opening copolymerization of epoxides and cyclic anhydrides with discrete metal complexes: Structure–property relationships. Chem. Rev. 2016;116:15167–15197. doi: 10.1021/acs.chemrev.6b00553. [DOI] [PubMed] [Google Scholar]
- 32.Odian, G. Principles of polymerization Stereochemistry of polymerization. Ch. 8, 619–728 (John Wiley & Sons, Inc., 2004).
- 33.Bovey, F. A. & P. A., M. NMR of Polymers (Academic Press, 1996).
- 34.Miri MJ, Pritchard BP, Cheng HN. A versatile approach for modeling and simulating the tacticity of polymers. J. Mol. Model. 2011;17:1767–1780. doi: 10.1007/s00894-010-0880-8. [DOI] [PubMed] [Google Scholar]
- 35.Ovitt TM, Coates GW. Stereochemistry of lactide polymerization with chiral catalysts: new opportunities for stereocontrol using polymer exchange mechanisms. J. Am. Chem. Soc. 2002;124:1316–1326. doi: 10.1021/ja012052+. [DOI] [PubMed] [Google Scholar]
- 36.Feng Q, et al. Stereoselective photoredox ring-opening polymerization of O-carboxyanhydrides. Nat. Commun. 2018;9:1559. doi: 10.1038/s41467-018-03879-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Busico V, Cipullo R, Monaco G, Vacatello M, Segre AL. Full assignment of the 13C NMR spectra of regioregular polypropylenes: Methyl and methylene region. Macromolecules. 1997;30:6251–6263. [Google Scholar]
- 38.Zell MT, et al. Unambiguous determination of the 13C and 1H NMR stereosequence assignments of polylactide using high-resolution solution NMR spectroscopy. Macromolecules. 2002;35:7700–7707. [Google Scholar]
- 39.Chamberlain BM, et al. Polymerization of lactide with zinc and magnesium β-diiminate complexes: stereocontrol and mechanism. J. Am. Chem. Soc. 2001;123:3229–3238. doi: 10.1021/ja003851f. [DOI] [PubMed] [Google Scholar]
- 40.Stanford MJ, Dove AP. Stereocontrolled ring-opening polymerisation of lactide. Chem. Soc. Rev. 2010;39:486–494. doi: 10.1039/b815104k. [DOI] [PubMed] [Google Scholar]
- 41.Hormnirun P, Marshall EL, Gibson VC, White AJP, Williams DJ. Remarkable stereocontrol in the polymerization of racemic lactide using aluminum initiators supported by tetradentate aminophenoxide ligands. J. Am. Chem. Soc. 2004;126:2688–2689. doi: 10.1021/ja038757o. [DOI] [PubMed] [Google Scholar]
- 42.Marin P, et al. Polymerization of rac-lactide using achiral iron complexes: access to thermally stable stereocomplexes. Angew. Chem. Int. Ed. 2019;58:12585–12589. doi: 10.1002/anie.201903224. [DOI] [PubMed] [Google Scholar]
- 43.Spassky N, Wisniewski M, Pluta C, Le Borgne A. Highly stereoelective polymerization of rac-(d,l)-lactide with a chiral schiff’s base/aluminium alkoxide initiator. Macromol. Chem. Phys. 1996;197:2627–2637. [Google Scholar]
- 44.Ovitt TM, Coates GW. Stereoselective ring-opening polymerization of meso-lactide: Synthesis of syndiotactic poly(lactic acid) J. Am. Chem. Soc. 1999;121:4072–4073. [Google Scholar]
- 45.Ovitt TM, Coates GW. Stereoselective ring-opening polymerization of rac-lactide with a single-site, racemic aluminum alkoxide catalyst: Synthesis of stereoblock poly(lactic acid) J. Polym. Sci. Part A: Polym. Chem. 2000;38:4686–4692. [Google Scholar]
- 46.Majerska K, Duda A. Stereocontrolled polymerization of racemic lactide with chiral initiator: combining stereoelection and chiral ligand-exchange mechanism. J. Am. Chem. Soc. 2004;126:1026–1027. doi: 10.1021/ja0388966. [DOI] [PubMed] [Google Scholar]
- 47.Lu Y, Swisher JH, Meyer TY, Coates GW. Chirality-directed regioselectivity: an approach for the synthesis of alternating poly(lactic-co-glycolic acid) J. Am. Chem. Soc. 2021;143:4119–4124. doi: 10.1021/jacs.1c00248. [DOI] [PubMed] [Google Scholar]
- 48.Zhong Z, Dijkstra PJ, Feijen J. [(salen)Al]-Mediated, controlled and stereoselective ring-opening polymerization of lactide in solution and without solvent: synthesis of highly isotactic polylactide stereocopolymers from racemic d,l-lactide. Angew. Chem. Int. Ed. 2002;41:4510–4513. doi: 10.1002/1521-3773(20021202)41:23<4510::AID-ANIE4510>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 49.Du H, et al. Chiral salan aluminium ethyl complexes and their application in lactide polymerization. Chem. Eur. J. 2009;15:9836–9845. doi: 10.1002/chem.200900799. [DOI] [PubMed] [Google Scholar]
- 50.Peng Z, et al. Exploring ligand substituent effects on stereoselective polymerization of racemic lactide using aluminium salen-type complexes. Polym. Chem. 2023;14:2174–2180. [Google Scholar]
- 51.Wan Y, Bai Y, Xu H, He J, Zhang Y. Highly isoselective ring-opening polymerization of rac-lactide using chiral binuclear aluminum catalyst. Macromol. Rapid. Commun. 2021;42:2000491. doi: 10.1002/marc.202000491. [DOI] [PubMed] [Google Scholar]
- 52.Pilone A, et al. Gradient isotactic multiblock polylactides from aluminum complexes of chiral salalen ligands. J. Am. Chem. Soc. 2014;136:2940–2943. doi: 10.1021/ja412798x. [DOI] [PubMed] [Google Scholar]
- 53.Press K, Goldberg I, Kol M. Mechanistic insight into the stereochemical control of lactide polymerization by salan–aluminum catalysts. Angew. Chem. Int. Ed. 2015;54:14858–14861. doi: 10.1002/anie.201503111. [DOI] [PubMed] [Google Scholar]
- 54.Peterson A, et al. Defining stereochemistry in the polymerization of lactide by aluminum catalysts: Insights into the dual-stereocontrol mechanism. J. Am. Chem. Soc. 2022;144:20047–20055. doi: 10.1021/jacs.2c09073. [DOI] [PubMed] [Google Scholar]
- 55.Hador R, et al. The dual-stereocontrol mechanism: heteroselective polymerization of rac-lactide and syndioselective polymerization of meso-lactide by chiral aluminum salan catalysts. Angew. Chem. Int. Ed. 2019;58:14679–14685. doi: 10.1002/anie.201906848. [DOI] [PubMed] [Google Scholar]
- 56.Marshall EL, Gibson VC, Rzepa HS. A computational analysis of the ring-opening polymerization of rac-lactide initiated by single-site β-diketiminate metal complexes: defining the mechanistic pathway and the origin of stereocontrol. J. Am. Chem. Soc. 2005;127:6048–6051. doi: 10.1021/ja043819b. [DOI] [PubMed] [Google Scholar]
- 57.D’Alterio MC, De Rosa C, Talarico G. Stereoselective lactide polymerization: the challenge of chiral catalyst recognition. ACS Catal. 2020;10:2221–2225. [Google Scholar]
- 58.D’Alterio MC, De Rosa C, Talarico G. Syndiotactic pla from meso-LA polymerization at the Al-chiral complex: a probe of DFT mechanistic insights. Chem. Commun. 2021;57:1611–1614. doi: 10.1039/d0cc07787a. [DOI] [PubMed] [Google Scholar]
- 59.Resconi L, Cavallo L, Fait A. Selectivity in propene polymerization with metallocene catalysts. Chem. Rev. 2000;100:1253–1346. doi: 10.1021/cr9804691. [DOI] [PubMed] [Google Scholar]
- 60.Wang H, Chem. Commun Ma H. Highly diastereoselective synthesis of chiral aminophenolate zinc complexes and isoselective polymerization of rac-lactide. Chem. Commun. 2013;49:8686–8688. doi: 10.1039/c3cc44980g. [DOI] [PubMed] [Google Scholar]
- 61.Wang H, Yang Y, Ma H. Stereoselectivity switch between zinc and magnesium initiators in the polymerization of rac-lactide: Different coordination chemistry, different stereocontrol mechanisms. Macromolecules. 2014;47:7750–7764. [Google Scholar]
- 62.Hu J, Kan C, Wang H, Ma H. Highly active chiral oxazolinyl aminophenolate magnesium initiators for isoselective ring-opening polymerization of rac-lactide: Dinuclearity induced enantiomorphic site control. Macromolecules. 2018;51:5304–5312. [Google Scholar]
- 63.Aluthge DC, Patrick BO, Mehrkhodavandi P. A highly active and site selective indium catalyst for lactide polymerization. Chem. Commun. 2013;49:4295–4297. doi: 10.1039/c2cc33519k. [DOI] [PubMed] [Google Scholar]
- 64.Peng Z, et al. Isoselective mechanism for asymmetric kinetic resolution polymerization of rac-lactide catalyzed by chiral tridentate bis(oxazolinylphenyl)amido ligand supported zinc complexes. Eur. Polym. J. 2022;180:111571. [Google Scholar]
- 65.Miyake GM, Chen EYX. Cinchona alkaloids as stereoselective organocatalysts for the partial kinetic resolution polymerization of rac-lactide. Macromolecules. 2011;44:4116–4124. [Google Scholar]
- 66.Zhu J-B, Chen EYX. From meso-lactide to isotactic polylactide: epimerization by B/N lewis pairs and kinetic resolution by organic catalysts. J. Am. Chem. Soc. 2015;137:12506–12509. doi: 10.1021/jacs.5b08658. [DOI] [PubMed] [Google Scholar]
- 67.Makiguchi K, Yamanaka T, Kakuchi T, Terada M, Satoh T. Binaphthol-derived phosphoric acids as efficient chiral organocatalysts for the enantiomer-selective polymerization of rac-lactide. Chem. Commun. 2014;50:2883–2885. doi: 10.1039/c4cc00215f. [DOI] [PubMed] [Google Scholar]
- 68.Sanchez-Sanchez A, et al. Enantioselective ring-opening polymerization of rac-lactide dictated by densely substituted amino acids. J. Am. Chem. Soc. 2017;139:4805–4814. doi: 10.1021/jacs.6b13080. [DOI] [PubMed] [Google Scholar]
- 69.Orhan B, et al. Isoselective ring-opening polymerization of rac-lactide from chiral takemoto’s organocatalysts: Elucidation of stereocontrol. ACS Macro Lett. 2018;7:1413–1419. doi: 10.1021/acsmacrolett.8b00852. [DOI] [PubMed] [Google Scholar]
- 70.Zaky MS, Wirotius A-L, Coulembier O, Guichard G, Taton D. A chiral thiourea and a phosphazene for fast and stereoselective organocatalytic ring-opening-polymerization of racemic lactide. Chem. Commun. 2021;57:3777–3780. doi: 10.1039/d0cc08022e. [DOI] [PubMed] [Google Scholar]
- 71.Lv C, et al. Isoselective ring-opening polymerization and asymmetric kinetic resolution polymerization of rac-lactide catalyzed by bifunctional iminophosphorane–thiourea/urea catalysts. N. J. Chem. 2020;44:1648–1655. [Google Scholar]
- 72.Laycock B, Halley P, Pratt S, Werker A, Lant P. The chemomechanical properties of microbial polyhydroxyalkanoates. Prog. Polym. Sci. 2013;38:536–583. [Google Scholar]
- 73.Wang Y, Yin J, Chen G-Q. Polyhydroxyalkanoates, challenges and opportunities. Curr. Opin. Biotechnol. 2014;30:59–65. doi: 10.1016/j.copbio.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 74.Sangroniz A, et al. Packaging materials with desired mechanical and barrier properties and full chemical recyclability. Nat. Commun. 2019;10:3559. doi: 10.1038/s41467-019-11525-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Aoyagi Y, Yamashita K, Doi Y. Thermal degradation of poly[(r)-3-hydroxybutyrate], poly[ε-caprolactone], and poly[(s)-lactide] Polym. Degrad. Stab. 2002;76:53–59. [Google Scholar]
- 76.Zhou L, et al. Chemically circular, mechanically tough, and melt-processable polyhydroxyalkanoates. Science. 2023;380:64–69. doi: 10.1126/science.adg4520. [DOI] [PubMed] [Google Scholar]
- 77.Le Borgne A, Spassky N. Stereoelective polymerization of β-butyrolactone. Polymer. 1989;30:2312–2319. [Google Scholar]
- 78.Leborgne A, Grenier D, Prud’homme RE, Spassky N. Preparation of optically active poly-β-propiolactones—II. Preparation of optically active poly-α-methyl-α-ethyl-β-propiolactones. Eur. Polym. J. 1981;17:1103–1106. [Google Scholar]
- 79.Leborgne A, Spassky N, Sigwalt P. Preparation of optically active poly β-propiolactones. Polym. Bull. 1979;1:825–832. [Google Scholar]
- 80.Takeichi T, Hieda Y, Takayama Y. Asymmetric selective polymerization of β-butyrolactone catalyzed by optically active cobalt complex/triethylaluminum system. Polym. J. 1988;20:159–162. [Google Scholar]
- 81.Ligny R, Hänninen MM, Guillaume SM, Carpentier J-F. Highly syndiotactic or isotactic polyhydroxyalkanoates by ligand-controlled yttrium-catalyzed stereoselective ring-opening polymerization of functional racemic β-lactones. Angew. Chem. Int. Ed. 2017;56:10388–10393. doi: 10.1002/anie.201704283. [DOI] [PubMed] [Google Scholar]
- 82.Westlie AH, Quinn EC, Parker CR, Chen EYX. Synthetic biodegradable polyhydroxyalkanoates (PHAs): recent advances and future challenges. Prog. Polym. Sci. 2022;134:101608. [Google Scholar]
- 83.Ligny R, Guillaume SM, Carpentier J-F. Yttrium-mediated ring-opening copolymerization of oppositely configurated 4-alkoxymethylene-β-propiolactones: effective access to highly alternated isotactic functional phas. Chem. Eur. J. 2019;25:6412–6424. doi: 10.1002/chem.201900413. [DOI] [PubMed] [Google Scholar]
- 84.Bruckmoser J, Pongratz S, Stieglitz L, Rieger B. Highly isoselective ring-opening polymerization of rac-β-butyrolactone: access to synthetic poly(3-hydroxybutyrate) with polyolefin-like material properties. J. Am. Chem. Soc. 2023;145:11494–11498. doi: 10.1021/jacs.3c02348. [DOI] [PubMed] [Google Scholar]
- 85.Xie W, Li J, Chen D, Wang PG. Ring-opening polymerization of β-butyrolactone by thermophilic lipases. Macromolecules. 1997;30:6997–6998. [Google Scholar]
- 86.Kikuchi H, Uyama H, Kobayashi S. Lipase-catalyzed enantioselective copolymerization of substituted lactones to optically active polyesters. Macromolecules. 2000;33:8971–8975. [Google Scholar]
- 87.Tang X, Chen EYX. Chemical synthesis of perfectly isotactic and high melting bacterial poly(3-hydroxybutyrate) from bio-sourced racemic cyclic diolide. Nat. Commun. 2018;9:2345. doi: 10.1038/s41467-018-04734-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tang X, Westlie AH, Watson EM, Chen EY-X. Stereosequenced crystalline polyhydroxyalkanoates from diastereomeric monomer mixtures. Science. 2019;366:754–758. doi: 10.1126/science.aax8466. [DOI] [PubMed] [Google Scholar]
- 89.Zhang Z, Shi C, Scoti M, Tang X, Chen EYX. Alternating isotactic polyhydroxyalkanoates via site- and stereoselective polymerization of unsymmetrical diolides. J. Am. Chem. Soc. 2022;144:20016–20024. doi: 10.1021/jacs.2c08791. [DOI] [PubMed] [Google Scholar]
- 90.Pohlmann AR, et al. Poly(ϵ-caprolactone) microcapsules and nanocapsules in drug delivery. Expert Opin. Drug Deliv. 2013;10:623–638. doi: 10.1517/17425247.2013.769956. [DOI] [PubMed] [Google Scholar]
- 91.Al-Azemi TF, Kondaveti L, Bisht KS. Solventless enantioelective ring-opening polymerization of substituted ε-caprolactones by enzymatic catalysis. Macromolecules. 2002;35:3380–3386. [Google Scholar]
- 92.Peeters JW, van Leeuwen O, Palmans ARA, Meijer EW. Lipase-catalyzed ring-opening polymerizations of 4-substituted ε-caprolactones: mechanistic considerations. Macromolecules. 2005;38:5587–5592. [Google Scholar]
- 93.van As BAC, et al. Chiral oligomers by iterative tandem catalysis. J. Am. Chem. Soc. 2005;127:9964–9965. doi: 10.1021/ja052347d. [DOI] [PubMed] [Google Scholar]
- 94.van Buijtenen J, et al. Switching from s- to r-selectivity in the candida antarctica lipase b-catalyzed ring-opening of ω-methylated lactones: tuning polymerizations by ring size. J. Am. Chem. Soc. 2007;129:7393–7398. doi: 10.1021/ja071241a. [DOI] [PubMed] [Google Scholar]
- 95.Breteler MRT, et al. Ring-opening polymerization of substituted ε-caprolactones with a chiral (salen) aloipr complex. J. Polym. Sci. A Polym. Chem. 2007;45:429–436. [Google Scholar]
- 96.Lv C, Yang R, Xu G, Zhou L, Wang Q. Phosphoric acids catalyzed asymmetric kinetic resolution polymerization of benzyl substituted ε-caprolactones: efficient protocol for stereogradient polycaprolactones. Eur. Polym. J. 2020;133:109792. [Google Scholar]
- 97.Lv C, Xu G, Yang R, Zhou L, Wang Q. Chiral phosphoric acid catalyzed asymmetric kinetic resolution polymerization of 6-aryl-ε-caprolactones. Polym. Chem. 2020;11:4203–4207. [Google Scholar]
- 98.Lv C, Xu G, Yang R, Zhou L, Wang Q. Stereogradient polycaprolactones formed by asymmetric kinetic resolution polymerization of 6-methyl-ε-caprolactone. Polym. Chem. 2021;12:4856–4863. [Google Scholar]
- 99.Yuan J, et al. 4-hydroxyproline-derived sustainable polythioesters: controlled ring-opening polymerization, complete recyclability, and facile functionalization. J. Am. Chem. Soc. 2019;141:4928–4935. doi: 10.1021/jacs.9b00031. [DOI] [PubMed] [Google Scholar]
- 100.Xiong W, et al. Geminal dimethyl substitution enables controlled polymerization of penicillamine-derived β-thiolactones and reversed depolymerization. Chem. 2020;6:1831–1843. [Google Scholar]
- 101.Shi C, et al. High-performance pan-tactic polythioesters with intrinsic crystallinity and chemical recyclability. Sci. Adv. 2020;6:eabc0495. doi: 10.1126/sciadv.abc0495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wang Y, Zhu Y, Lv W, Wang X, Tao Y. Tough while recyclable plastics enabled by monothiodilactone monomers. J. Am. Chem. Soc. 2023;145:1877–1885. doi: 10.1021/jacs.2c11502. [DOI] [PubMed] [Google Scholar]
- 103.Lütke-Eversloh T, et al. Biosynthesis of novel thermoplastic polythioesters by engineered escherichia coli. Nat. Mater. 2002;1:236–240. doi: 10.1038/nmat773. [DOI] [PubMed] [Google Scholar]
- 104.Li H, Ollivier J, Guillaume SM, Carpentier J-F. Tacticity control of cyclic poly(3-thiobutyrate) prepared by ring-opening polymerization of racemic β-thiobutyrolactone. Angew. Chem. Int. Ed. 2022;61:e202202386. doi: 10.1002/anie.202202386. [DOI] [PubMed] [Google Scholar]
- 105.Kawai F. Microbial degradation of polyethers. Appl. Microbiol. Biotechnol. 2002;58:30–38. doi: 10.1007/s00253-001-0850-2. [DOI] [PubMed] [Google Scholar]
- 106.Gardette J-L, Mailhot B, Posada F, Rivaton A, Wilhelm C. Photooxidative degradation of polyether-based polymers. Macromol. Symp. 1999;143:95–109. [Google Scholar]
- 107.Morlat S, Gardette J-L. Phototransformation of water-soluble polymers. I: photo- and thermooxidation of poly(ethylene oxide) in solid state. Polymer. 2001;42:6071–6079. [Google Scholar]
- 108.Herzberger J, et al. Polymerization of ethylene oxide, propylene oxide, and other alkylene oxides: Synthesis, novel polymer architectures, and bioconjugation. Chem. Rev. 2016;116:2170–2243. doi: 10.1021/acs.chemrev.5b00441. [DOI] [PubMed] [Google Scholar]
- 109.Çolak N, Alyürük K. Polymerization of propylene oxide by the pruitt-baggett catalyst. Polymer. 1989;30:1709–1713. [Google Scholar]
- 110.Price CC, Osgan M, Hughes RE, Shambelan C. The polymerization of l-propylene oxide. J. Am. Chem. Soc. 1956;78:690–691. [Google Scholar]
- 111.Natta G, Corradini P, Dall’Asta G. Structure of the crystalline poly(propylene oxide)chain. Atti Accad. Nazl. Lincei Rend. Cl. Mat. e Nat. 1956;20:408–413. [Google Scholar]
- 112.Peretti KL, Ajiro H, Cohen CT, Lobkovsky EB, Coates GW. A highly active, isospecific cobalt catalyst for propylene oxide polymerization. J. Am. Chem. Soc. 2005;127:11566–11567. doi: 10.1021/ja053451y. [DOI] [PubMed] [Google Scholar]
- 113.Ajiro, H., Peretti, K. L., Lobkovsky, E. B. & Coates, G. W. On the mechanism of isospecific epoxide polymerization by salen cobalt(III) complexes: evidence for solid-state catalysis. Dalton Trans. 8828–8830 (2009). [DOI] [PubMed]
- 114.Hirahata W, Thomas RM, Lobkovsky EB, Coates GW. Enantioselective polymerization of epoxides: a highly active and selective catalyst for the preparation of stereoregular polyethers and enantiopure epoxides. J. Am. Chem. Soc. 2008;130:17658–17659. doi: 10.1021/ja807709j. [DOI] [PubMed] [Google Scholar]
- 115.Thomas RM, et al. Enantioselective epoxide polymerization using a bimetallic cobalt catalyst. J. Am. Chem. Soc. 2010;132:16520–16525. doi: 10.1021/ja1058422. [DOI] [PubMed] [Google Scholar]
- 116.Ahmed SM, et al. Enantioselective polymerization of epoxides using biaryl-linked bimetallic cobalt catalysts: a mechanistic study. J. Am. Chem. Soc. 2013;135:18901–18911. doi: 10.1021/ja409521z. [DOI] [PubMed] [Google Scholar]
- 117.Widger PCB, et al. Isospecific polymerization of racemic epoxides: a catalyst system for the synthesis of highly isotactic polyethers. Chem. Commun. 2010;46:2935–2937. doi: 10.1039/b926888j. [DOI] [PubMed] [Google Scholar]
- 118.Childers MI, et al. Isospecific, chain shuttling polymerization of propylene oxide using a bimetallic chromium catalyst: a new route to semicrystalline polyols. J. Am. Chem. Soc. 2017;139:11048–11054. doi: 10.1021/jacs.7b00194. [DOI] [PubMed] [Google Scholar]
- 119.Lipinski BM, Morris LS, Silberstein MN, Coates GW. Isotactic poly(propylene oxide): aphotodegradable polymer with strain hardening properties. J. Am. Chem. Soc. 2020;142:6800–6806. doi: 10.1021/jacs.0c01768. [DOI] [PubMed] [Google Scholar]
- 120.Morris LS, Childers MI, Coates GW. Bimetallic chromium catalysts with chain transfer agents: a route to isotactic poly(propylene oxide)s with narrow dispersities. Angew. Chem. Int. Ed. 2018;57:5731–5734. doi: 10.1002/anie.201801380. [DOI] [PubMed] [Google Scholar]
- 121.Burkart MD, Hazari N, Tway CL, Zeitler EL. Opportunities and challenges for catalysis in carbon dioxide utilization. ACS Catal. 2019;9:7937–7956. [Google Scholar]
- 122.Artz J, et al. Sustainable conversion of carbon dioxide: an integrated review of catalysis and life cycle assessment. Chem. Rev. 2018;118:434–504. doi: 10.1021/acs.chemrev.7b00435. [DOI] [PubMed] [Google Scholar]
- 123.Hepburn C, et al. The technological and economic prospects for CO2 utilization and removal. Nature. 2019;575:87–97. doi: 10.1038/s41586-019-1681-6. [DOI] [PubMed] [Google Scholar]
- 124.Sulley GS, et al. Switchable catalysis improves the properties of CO2-derived polymers: Poly(cyclohexene carbonate-b-ε-decalactone-b-cyclohexene carbonate) adhesives, elastomers, and toughened plastics. J. Am. Chem. Soc. 2020;142:4367–4378. doi: 10.1021/jacs.9b13106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Inoue S, Koinuma H, Tsuruta T. Copolymerization of carbon dioxide and epoxide. J. Polym. Sci., Part B: Polym. Lett. 1969;7:287–292. [Google Scholar]
- 126.Inoue S, Koinuma H, Tsuruta T. Copolymerization of carbon dioxide and epoxide with organometallic compounds. Makromol. Chem. 1969;130:210–220. [Google Scholar]
- 127.Lu X-B, Darensbourg DJ. Cobalt catalysts for the coupling of CO2 and epoxides to provide polycarbonates and cyclic carbonates. Chem. Soc. Rev. 2012;41:1462–1484. doi: 10.1039/c1cs15142h. [DOI] [PubMed] [Google Scholar]
- 128.Plajer AJ, Williams CK. Heterocycle/heteroallene ring-opening copolymerization: selective catalysis delivering alternating copolymers. Angew. Chem. Int. Ed. 2022;61:e202104495. doi: 10.1002/anie.202104495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lidston CAL, Severson SM, Abel BA, Coates GW. Multifunctional catalysts for ring-opening copolymerizations. ACS Catal. 2022;12:11037–11070. [Google Scholar]
- 130.Diment WT, Lindeboom W, Fiorentini F, Deacy AC, Williams CK. Synergic heterodinuclear catalysts for the ring-opening copolymerization (ROCOP) of epoxides, carbon dioxide, and anhydrides. Acc. Chem. Res. 2022;55:1997–2010. doi: 10.1021/acs.accounts.2c00197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Li, J. & Lu, X.-B. Catalytic asymmetric synthesis Asymmetric polymerization (eds Takahiko, A. & Iwao, O) Ch. 21, p. 803-829 (John Wiley & Sons, Inc., 2022).
- 132.Liu Y, Lu X-B. Current challenges and perspectives in CO2-based polymers. Macromolecules. 2023;56:1759–1777. [Google Scholar]
- 133.Qin Z, Thomas CM, Lee S, Coates GW. Cobalt-based complexes for the copolymerization of propylene oxide and CO2: active and selective catalysts for polycarbonate synthesis. Angew. Chem. Int. Ed. 2003;42:5484–5487. doi: 10.1002/anie.200352605. [DOI] [PubMed] [Google Scholar]
- 134.Cohen CT, Chu T, Coates GW. Cobalt catalysts for the alternating copolymerization of propylene oxide and carbon dioxide: Combining high activity and selectivity. J. Am. Chem. Soc. 2005;127:10869–10878. doi: 10.1021/ja051744l. [DOI] [PubMed] [Google Scholar]
- 135.Lu X-B, Wang Y. Highly active, binary catalyst systems for the alternating copolymerization of CO2 and epoxides under mild conditions. Angew. Chem. Int. Ed. 2004;43:3574–3577. doi: 10.1002/anie.200453998. [DOI] [PubMed] [Google Scholar]
- 136.Lu X-B, et al. Design of highly active binary catalyst systems for CO2/epoxide copolymerization: polymer selectivity, enantioselectivity, and stereochemistry control. J. Am. Chem. Soc. 2006;128:1664–1674. doi: 10.1021/ja056383o. [DOI] [PubMed] [Google Scholar]
- 137.Cohen CT, Coates GW. Alternating copolymerization of propylene oxide and carbon dioxide with highly efficient and selective (salen)Co(III) catalysts: effect of ligand and cocatalyst variation. J. Polym. Sci. A Polym. Chem. 2006;44:5182–5191. [Google Scholar]
- 138.Nakano K, Hashimoto S, Nakamura M, Kamada T, Nozaki K. Stereocomplex of poly(propylene carbonate): Synthesis of stereogradient poly(propylene carbonate) by regio- and enantioselective copolymerization of propylene oxide with carbon dioxide. Angew. Chem. Int. Ed. 2011;50:4868–4871. doi: 10.1002/anie.201007958. [DOI] [PubMed] [Google Scholar]
- 139.Ren W-M, Liu Y, Wu G-P, Liu J, Lu X-B. Stereoregular polycarbonate synthesis: alternating copolymerization of CO2 with aliphatic terminal epoxides catalyzed by multichiral cobalt(III) complexes. J. Polym. Sci. A Polym. Chem. 2011;49:4894–4901. [Google Scholar]
- 140.Jacobsen, E. N., Tokunaga, M. & Larrow, J. F. Stereoselective Ring Opening Reactions. PCT Int. Appl. WO 00/09463 (2000).
- 141.Li B, Zhang R, Lu X-B. Stereochemistry control of the alternating copolymerization of CO2 and propylene oxide catalyzed by salencrx complexes. Macromolecules. 2007;40:2303–2307. [Google Scholar]
- 142.Li B, et al. Asymmetric, regio- and stereo-selective alternating copolymerization of CO2 and propylene oxide catalyzed by chiral chromium salan complexes. J. Polym. Sci. A Polym. Chem. 2008;46:6102–6113. [Google Scholar]
- 143.Nakano K, Nakamura M, Nozaki K. Alternating copolymerization of cyclohexene oxide with carbon dioxide catalyzed by (salalen)CrCl complexes. Macromolecules. 2009;42:6972–6980. [Google Scholar]
- 144.Nozaki K, Nakano K, Hiyama T. Optically active polycarbonates: asymmetric alternating copolymerization of cyclohexene oxide and carbon dioxide. J. Am. Chem. Soc. 1999;121:11008–11009. [Google Scholar]
- 145.Nakano K, Nozaki K, Hiyama T. Asymmetric alternating copolymerization of cyclohexene oxide and CO2 with dimeric zinc complexes. J. Am. Chem. Soc. 2003;125:5501–5510. doi: 10.1021/ja028666b. [DOI] [PubMed] [Google Scholar]
- 146.Cheng, M., Darling, N. A., Lobkovsky, E. B. & Coates, G. W. Enantiomerically-enriched organic reagents polymer synthesis: Enantioselective copolymerization of cycloalkene oxides and CO2 using homogeneous, zinc-based catalysts. Chem. Commun. 2007–2008 (2000).
- 147.Ellis WC, et al. Copolymerization of CO2 and meso epoxides using enantioselective β-diiminate catalysts: a route to highly isotactic polycarbonates. Chem. Sci. 2014;5:4004–4011. [Google Scholar]
- 148.Abbina S, Du G. Chiral amido-oxazolinate zinc complexes for asymmetric alternating copolymerization of CO2 and cyclohexene oxide. Organometallics. 2012;31:7394–7403. [Google Scholar]
- 149.Xiao Y, Wang Z, Ding K. Copolymerization of cyclohexene oxide with CO2 by using intramolecular dinuclear zinc catalysts. Chem. Eur. J. 2005;11:3668–3678. doi: 10.1002/chem.200401159. [DOI] [PubMed] [Google Scholar]
- 150.Hua Y-Z, et al. Highly enantioselective catalytic system for asymmetric copolymerization of carbon dioxide and cyclohexene oxide. Chem. Eur. J. 2014;20:12394–12398. doi: 10.1002/chem.201403088. [DOI] [PubMed] [Google Scholar]
- 151.Cohen, C. T., Thomas, C. M., Peretti, K. L., Lobkovsky, E. B. & Coates, G. W. Copolymerization of cyclohexene oxide and carbon dioxide using (salen)Co(III) complexes: synthesis and characterization of syndiotactic poly(cyclohexene carbonate). Dalton Trans. 237–249 (2006). [DOI] [PubMed]
- 152.Shi L, et al. Asymmetric alternating copolymerization and terpolymerization of epoxides with carbon dioxide at mild conditions. Macromolecules. 2006;39:5679–5685. [Google Scholar]
- 153.Wu G-P, et al. Enhanced asymmetric induction for the copolymerization of CO2 and cyclohexene oxide with unsymmetric enantiopure salenCo(III) complexes: synthesis of crystalline CO2-based polycarbonate. J. Am. Chem. Soc. 2012;134:5682–5688. doi: 10.1021/ja300667y. [DOI] [PubMed] [Google Scholar]
- 154.Liu Y, Ren W-M, Liu J, Lu X-B. Asymmetric copolymerization of CO2 with meso-epoxides mediated by dinuclear cobalt(III) complexes: unprecedented enantioselectivity and activity. Angew. Chem. Int. Ed. 2013;52:11594–11598. doi: 10.1002/anie.201305154. [DOI] [PubMed] [Google Scholar]
- 155.Liu Y, et al. Mechanistic understanding of dinuclear cobalt(III) complex mediated highly enantioselective copolymerization of meso-epoxides with CO2. Macromolecules. 2014;47:7775–7788. [Google Scholar]
- 156.Liu Y, et al. Stereospecific CO2 copolymers from 3,5-dioxaepoxides: Crystallization and functionallization. Macromolecules. 2014;47:1269–1276. [Google Scholar]
- 157.Liu Y, Ren W-M, Wang M, Liu C, Lu X-B. Crystalline stereocomplexed polycarbonates: Hydrogen-bond-driven interlocked orderly assembly of the opposite enantiomers. Angew. Chem. Int. Ed. 2015;54:2241–2244. doi: 10.1002/anie.201410692. [DOI] [PubMed] [Google Scholar]
- 158.Liu Y, Fang L-M, Ren B-H, Lu X-B. Asymmetric alternating copolymerization of CO2 with meso-epoxides: Ring size effects of epoxides on reactivity, enantioselectivity, crystallization, and degradation. Macromolecules. 2020;53:2912–2918. [Google Scholar]
- 159.Liu Y, Ren W-M, He K-K, Lu X-B. Crystalline-gradient polycarbonates prepared from enantioselective terpolymerization of meso-epoxides with CO2. Nat. Commun. 2014;5:5687. doi: 10.1038/ncomms6687. [DOI] [PubMed] [Google Scholar]
- 160.Tong R. New chemistry in functional aliphatic polyesters. Ind. Eng. Chem. Res. 2017;56:4207–4219. [Google Scholar]
- 161.Martin Vaca B, Bourissou D. O-carboxyanhydrides: Useful tools for the preparation of well-defined functionalized polyesters. ACS Macro Lett. 2015;4:792–798. doi: 10.1021/acsmacrolett.5b00376. [DOI] [PubMed] [Google Scholar]
- 162.Wang X, Chin AL, Tong R. Controlled ring-opening polymerization of O-carboxyanhydrides to synthesize functionalized poly(α-hydroxy acids) Org. Mater. 2021;03:041–050. [Google Scholar]
- 163.Wang X, Chin AL, Zhou J, Wang H, Tong R. Resilient poly(α-hydroxy acids) with improved strength and ductility via scalable stereosequence-controlled polymerization. J. Am. Chem. Soc. 2021;143:16813–16823. doi: 10.1021/jacs.1c08802. [DOI] [PubMed] [Google Scholar]
- 164.Li J, Liu Y, Ren W-M, Lu X-B. Asymmetric alternating copolymerization of meso-epoxides and cyclic anhydrides: efficient access to enantiopure polyesters. J. Am. Chem. Soc. 2016;138:11493–11496. doi: 10.1021/jacs.6b07520. [DOI] [PubMed] [Google Scholar]
- 165.Li J, et al. Development of highly enantioselective catalysts for asymmetric copolymerization of meso-epoxides and cyclic anhydrides: Subtle modification resulting in superior enantioselectivity. ACS Catal. 2019;9:1915–1922. [Google Scholar]
- 166.He G-H, Ren B-H, Chen S-Y, Liu Y, Lu X-B. Enantioselective, stereoconvergent resolution copolymerization of racemic cis-internal epoxides and anhydrides. Angew. Chem. Int. Ed. 2021;60:5994–6002. doi: 10.1002/anie.202011259. [DOI] [PubMed] [Google Scholar]
- 167.He G-H, Liu Y-L, Liu Y, Lu X-B. Enantioselective resolution copolymerization of racemic cis-epoxides and cyclic anhydrides mediated by multichiral bimetallic chromium complexes. Macromolecules. 2022;55:3869–3876. [Google Scholar]
- 168.Li J, et al. Enantioselective resolution copolymerization of racemic epoxides and anhydrides: efficient approach for stereoregular polyesters and chiral epoxides. J. Am. Chem. Soc. 2019;141:8937–8942. doi: 10.1021/jacs.9b02722. [DOI] [PubMed] [Google Scholar]
- 169.Li J, Liu Y, Ren W-M, Lu X-B. Enantioselective terpolymerization of racemic and meso-epoxides with anhydrides for preparation of chiral polyesters. Proc. Natl Acad. Sci. USA. 2020;117:15429–15436. doi: 10.1073/pnas.2005519117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Wan Z-Q, et al. Comprehensive understanding of polyester stereocomplexation. J. Am. Chem. Soc. 2019;141:14780–14787. doi: 10.1021/jacs.9b07058. [DOI] [PubMed] [Google Scholar]
- 171.Yue T-J, et al. Synthesis of chiral sulfur-containing polymers: asymmetric copolymerization of meso-epoxides and carbonyl sulfide. Angew. Chem. Int. Ed. 2018;57:12670–12674. doi: 10.1002/anie.201805200. [DOI] [PubMed] [Google Scholar]
- 172.Furukawa, J., Kawabata, N. & Kato, A. Asymmetric-selection polymerization of propylene sulfide. J. Polym. Sci. Part B: Polym. Lett. 5, 1073–1076 (1967).
- 173.Aliev AD, Solomatina IP, Krentsel BA. Asymmetric reactions during the polymerization of (RS)-propylene sulfide by n-butyllithium-lithium (–)-menthoxide. Macromolecules. 1973;6:797–801. [Google Scholar]
- 174.Dumas P, Sigwalt P, Guérin P. Temperature effect on the stereoelective polymerization of methylthiirane in homogeneous phase. Makromol. Chem. 1981;182:2225–2231. [Google Scholar]
- 175.Dumas PH, Sigwalt P. Living enantiosymmetric and enantioasymmetric polymerization of methylthiirane in homogeneous phase. Chirality. 1991;3:484–491. [Google Scholar]
- 176.Sépulchre M, Spassky N, Mark C, Schurig V. The use of atropoisomeric chiral initiators in the polymerization of heterocyclic monomers: An example of almost ideal stereoelection in the case of methylthiirane. Makromol. Chem. Rapid Commun. 1981;2:261–266. [Google Scholar]
- 177.Sepulchre M. Differently substituted thiiranes using the chiral system ZnEt2/(S)-2,2’-dihydroxy-1,1’-binaphthyl .1. Stereoelectivity of the polymerization. Makromol. Chem. 1987;188:1583–1596. [Google Scholar]
- 178.Imai T, Hayakawa K, Satoh T, Kaga H, Kakuchi T. Enantiomer-selective polymerization of (RS)-(phenoxymethyl)thiirane with diethylzinc/L-amino acid. J. Polym. Sci. A Polym. Chem. 2002;40:3443–3448. [Google Scholar]
- 179.Deming TJ. Facile synthesis of block copolypeptides of defined architecture. Nature. 1997;390:386–389. doi: 10.1038/37084. [DOI] [PubMed] [Google Scholar]
- 180.Kricheldorf HR. Polypeptides and 100 years of chemistry of α-amino acid N-carboxyanhydrides. Angew. Chem. Int. Ed. 2006;45:5752–5784. doi: 10.1002/anie.200600693. [DOI] [PubMed] [Google Scholar]
- 181.Cheng, J. & Deming, T. J. Peptide-based materials (ed Deming, T) Ch.1, p. 1–26 (Springer Berlin Heidelberg, 2012).
- 182.Bührer HG, Elias H-G. Asymmetric-selective polymerization of dl-leucine N-carboxy anhydride with optically active amines. Makromol. Chem. 1973;169:145–162. [Google Scholar]
- 183.Matsuura K, Inoue S, Tsuruta T. Stereospecific polymerization of N-carboxy-dl-alanine anhydride. Makromol. Chem. 1964;80:149–157. [Google Scholar]
- 184.Yamashita S, Yamawaki N, Tani H. Stereoselective polymerization of dl-amino acid N-carboxyanhydrides by nickel d-2-methylbutyrate-tri-n-butylphosphine catalyst system. Macromolecules. 1974;7:724–727. [Google Scholar]
- 185.Cheng J, Deming TJ. Screening of optically active nickel initiators for enantioasymmetric polymerization of γ-benzyl glutamate-N-carboxyanhydride. Macromolecules. 1999;32:4745–4747. [Google Scholar]
- 186.Seidel SW, Deming TJ. Use of chiral ruthenium and iridium amido−sulfonamidate complexes for controlled, enantioselective polypeptide synthesis. Macromolecules. 2003;36:969–972. [Google Scholar]
- 187.Bertin A. Emergence of polymer stereocomplexes for biomedical applications. Macromol. Chem. Phys. 2012;213:2329–2352. [Google Scholar]
- 188.Auriemma F, et al. Stereocomplexed poly(limonene carbonate): a unique example of the cocrystallization of amorphous enantiomeric polymers. Angew. Chem. Int. Ed. 2015;54:1215–1218. doi: 10.1002/anie.201410211. [DOI] [PubMed] [Google Scholar]
- 189.Auriemma F, De Rosa C, Di Caprio MR, Di Girolamo R, Coates GW. Crystallization of alternating limonene oxide/carbon dioxide copolymers: Determination of the crystal structure of stereocomplex poly(limonene carbonate) Macromolecules. 2015;48:2534–2550. [Google Scholar]
- 190.Quinn EC, et al. Installing controlled stereo-defects yields semicrystalline and biodegradable poly(3-hydroxybutyrate) with high toughness and optical clarity. J. Am. Chem. Soc. 2023;145:5795–5802. doi: 10.1021/jacs.2c12897. [DOI] [PubMed] [Google Scholar]
- 191.Wang X, et al. Bayesian-optimization-assisted discovery of stereoselective aluminum complexes for ring-opening polymerization of racemic lactide. Nat. Commun. 2023;14:3647. doi: 10.1038/s41467-023-39405-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Cywar RM, Rorrer NA, Hoyt CB, Beckham GT, Chen EYX. Bio-based polymers with performance-advantaged properties. Nat. Rev. Mater. 2022;7:83–103. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.