Abstract
Although DNA mutation drives stem cell aging, how mutation-accumulated stem cells obtain clonal advantage during aging remains poorly understood. Here, using a mouse model of irradiation-induced premature aging and middle-aged mice, we show that DNA mutation accumulation in hematopoietic stem cells (HSCs) during aging upregulates their surface expression of major histocompatibility complex class II (MHCII). MHCII upregulation increases the chance for recognition by bone marrow (BM)-resident regulatory T cells (Tregs), resulting in their clonal expansion and accumulation in the HSC niche. On the basis of the establishment of connexin 43 (Cx43)-mediated gap junctions, BM Tregs transfer cyclic adenosine monophosphate (cAMP) to aged HSCs to diminish apoptotic priming and promote their survival via activation of protein kinase A (PKA) signaling. Importantly, targeting the HSC–Treg interaction or depleting Tregs effectively prevents the premature/physiological aging of HSCs. These findings show that aged HSCs use an active self-protective mechanism by entrapping local Tregs to construct a prosurvival niche and obtain a clonal advantage.
Keywords: Hematopoietic stem cell, regulatory T cell, Aging
Subject terms: Mechanisms of disease, Myelopoiesis
Introduction
Deregulation of tissue-resident somatic stem cell homeostasis is considered to be the pivotal factor underlying the aging process. The aging of hematopoietic stem cells (HSCs) underpins the limited health span of systemic organs [1–3] and HSCs are a well-described paradigm for the investigation of stem cell aging. HSC aging is characterized by reduced reconstitution capacity, expansion of myeloid-biased HSCs, increased replication stress, accumulated DNA mutations, increased resistance to apoptosis, loss of autophagy capacity, and epigenetic, transcriptional, and translational alterations [4]. However, the mechanistic interrelationships and hierarchical orders of these characteristics of HSC aging have not yet been clarified. In particular, the nature and timing of initiating events that cause HSC aging remain largely unknown, thus precluding the prevention of HSC aging from an early age.
DNA damage, especially DNA mutation, has been proposed as a principle and central mechanism underlying aging and aging-related diseases [5–7]. Nevertheless, cells have evolved multiple strategies to safeguard their fitness in the face of DNA mutation. For instance, cells are equipped with an elaborate network of highly sophisticated DNA repair and damage response systems to counteract DNA damage, and when the network fails, mutated cells undergo cell cycle arrest or apoptosis through oncogene-induced replication stress or immune eradication by effector immune cells through the presentation of immunogenic neoantigens [5]. Unexpectedly, stem cells are apt to accumulate DNA mutations due to their quiescent nature [8]. In addition, recent studies have unveiled that cells with high mutation loads, such as those in the skin, bronchus, endometrium, and esophagus, as well as HSCs, actually expand and even gain clonal advantages during aging [9, 10]. In particular, it was recently reported that the clonal expansion of HSCs harboring mutations begins by middle age [11, 12].
The specialized bone marrow (BM) microenvironment, termed the HSC niche, maintains HSC pool size and function through intercellular signals in the form of cell-bound or secreted factors or physical interactions [9]. Although accumulating studies have reported that a deregulated niche substantially contributes to the functional deficits of aged HSCs [10], its roles in the clonal advantage of mutation-accumulated, aged HSCs are scarcely appreciated. Immune cells constitute the majority of cells surrounding BM HSCs, and it appears likely that HSCs more readily take their cues from immune cells under physiopathological conditions, including aging [13]. Although the promotive roles of innate and adaptive effector cells in HSC aging have been well demonstrated [14, 15], there is almost no evidence regarding the role of immunosuppressive components of immune cells in stem cell aging. BM is normally maintained in an immune-privileged and anti-inflammatory state, kept in check principally by BM-resident regulatory T cells (Tregs) [16–19]. Understanding how BM Tregs adapt to the aged HSC niche and whether they are endowed with distinct functions to modulate HSC aging can identify novel targets for the prevention of HSC aging.
In this study, using a mouse model of premature aging and middle-aged mice, we identified and characterized a distinct function of BM Tregs in HSC aging. We discovered that DNA mutation accumulation in aged HSCs induces major histocompatibility complex class II (MHCII) upregulation and BM Treg clonal expansion. Moreover, bidirectional interaction is established between aged HSCs and BM Tregs via MHCII–T-cell receptor (TCR) engagement, by which BM Tregs are expanded through TCR recognition of MHCII on aged HSCs, whereas the apoptotic priming of aged HSCs is diminished through gap junction (GJ)-mediated transfer of cyclic adenosine monophosphate (cAMP) from BM Tregs. Importantly, targeting the HSC–Treg interaction prevents HSC aging from an early age.
Materials and methods
Animals
Young (2 months), middle-aged (12 months), and aged (24 months) C57BL/6 mice were purchased from Beijing HFK Bioscience Co., Ltd. (Beijing, China). B6.Cg-Foxp3tm2Tch/J (Foxp3EGFP), B6.129(Cg)-Foxp3tm3(DTR/GFP)Ayr/J (Foxp3GFP-DTR) and B6.129S2-H2dlAb1-Ea/J (MHCII–/–) mice were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). B6-Tg(TcraTcrb)1Cya (OTII) mice and B6-Foxp3tm1(YFP/icre)/Cya (Foxp3YFP-DTR) mice were purchased from Cyagen Biosciences (Guangzhou, China). B6/JGpt-Ptprcem1Cin(p. K302E)/Gpt (CD45.1) mice were purchased from GemPharmatech (Nanjing, China). Male transgenic mice and their wild-type littermates were used in the experiments. All mice used were randomized, background-matched, and age-matched. All mice used were housed in specific pathogen-free conditions and fed autoclaved water and food. Animal experiments were approved by the Animal Care Committee of the Army Medical University (NO. AMUWEC2019092) and conducted according to institutional guidelines.
Irradiation
To create a mouse model of premature aging, mice were exposed to a single dose of 5 Gy total body irradiation with a 60Co γ-ray source at the Irradiation Center (Army Medical University, Chongqing, China). The dose rate of IR was 92.8 to 95.5 cGy/min. IR mice and their age-matched control mice were sacrificed for analysis at 3 mpi.
Flow cytometry and cell sorting
For HSC (Lineage–Sca-1+c-Kit+CD150+Flt3–CD48–) phenotypic analysis, BM cells were stained with Sca-1 (D7), c-Kit (2B8), CD150 (TC15-12F12.2), CD48 (HM48-1), Flt3 (A2F10), MHCII (M5/114.15.2), and mature lineage marker mix [CD3e (145-2C11), B220 (RA3-6B2), Gr-1 (RB6.8C5), Mac-1 (M1/70), and Ter-119 (Ter119)] (all eBioscience, San Diego, CA, USA). For Treg (CD3+CD4+NK1.1–Foxp3+) phenotypic analysis, cells were stained with CD45 (30-F11), CD3e (145-2C11), NK1.1 (PK136), CD4 (GK1.5), CD8a (53.6.7) and CD25 (PC61) antibodies (all Biolegend, San Diego, CA, USA). Subsequently, cells were fixed, permeabilized, and stained using a Foxp3/Transcription Factor Staining Buffer Set (eBioscience) following the manufacturer’s instructions. Then, the cells were stained with Foxp3 antibody (FJK-16s, Biolegend) at room temperature for another 30 minutes and analyzed by flow cytometry. Cells were sorted using a FACSAria II or analyzed using an LSRFortessa (all BD Biosciences, San Jose, CA, USA) flow cytometer. The antibodies used are listed in Supplementary Table 1. All flow cytometry data were analyzed using FlowJo V10 software (Treestar Inc., San Carlos, CA, USA).
CRISPR–Cas9
CRISPR RNA (crRNA) and trans-activating crRNA (tracrRNA) were purchased from Synbio-tech (Suzhou, China), and the crRNA–tracrRNA duplex was obtained according to the manufacturer’s instructions. Transfection was performed by using a P4 Primary Cell 4D-Nucleofector X kit S (Lonza, Basel, Switzerland) according to the manufacturer’s instructions as previously described [20]. The guide sequences are listed in Supplementary Table 2.
Adoptive Treg transfer
Diphtheria toxin (DT) administration was conducted according to a regimen previously reported, which effectively eliminates BM Tregs without provoking autoimmune manifestations [21]. Foxp3GFP-DTR mice at 11 months or 2 mpi were weekly administered two intraperitoneal (i.p.) injections of DT (10 ng/g body weight; Sigma‒Aldrich, St. Louis, MO, USA) 1 day apart for 4 weeks. For adoptive transfer of BM Tregs, 1 × 105 BM Tregs with or without Cx43 knockout from Foxp3YFP-DTR mice at 2 months were intravenously transfused into Foxp3GFP-DTR mice at 2 mpi following the first week of DT treatment, and recipient mice were sacrificed for analysis at 3 mpi.
Pharmacological treatment
αCD25 was administered according to a previously reported regimen, which efficiently depletes BM Tregs [22]. A total of 0.5 mg/mouse of InVivoPlus anti-mouse CD25 (PC-61.5.3; BioXCell, Lebanon, NH, USA) was administered i.p. weekly for 4 weeks to mice at 11 months or 2 mpi. For Gap27 treatment, mice at 11 months or 2 mpi were administered i.p. with 0.5 µg/kg GAP27 (MedChemExpress, Monmouth Junction, NJ, USA) every other day for 4 weeks. For CD8+ T-cell depletion, 75 μg/mouse of InVivoMAb anti-mouse CD8α (53-6.7; BioXCell) was administered i.p. together with DT.
RNA-seq
HSCs and Tregs were sorted from mouse BM, and RNA was extracted using an RNeasy Micro Kit (QIAGEN, Hilden, Germany) following the manufacturer’s instructions. The amount and purity of RNA were quantified using a NanoDrop ND-1000 (Thermo Fisher Scientific). Library construction and RNA-seq were performed on an Illumina NovaSeq™ 6000. Fastp software (https://github.com/OpenGene/fastp) was used to remove adaptor contamination, undetermined bases, and low-quality bases. After sequence alignment, gene expression was quantified by RSEM (version: v1.2.12, http://deweylab.biostat.wisc.edu/RSEM), and differentially expressed genes (DEGs) were analyzed by DEseq2 using a fold change >2.00 and adjusted P-value < 0.05. Gene set enrichment analysis (GSEA, Broad Institute) was performed based on Molecular Signatures Database v6.0 (http://software.broadinstitute.org/gsea/msigdb). Ingenuity Pathway Analysis (QIAGEN) was used to analyze signaling pathways.
TCRβ amplification and sequencing
For TCRβ sequencing, BM Tregs were sorted, and RNA was extracted using an RNeasy Micro Kit (QIAGEN). Subsequently, the samples were analyzed by high-throughput sequencing of full-length TCR using the ImmuHub TCR profiling system (ImmuQuad Biotech, Hangzhou, China) with a 5’ RACE unbiased amplification protocol. Sequencing was performed using an Illumina NovaSeq system (ImmuQuad Biotech) in PE150 mode. The UMI attached to each raw sequence read was applied to correct PCR and sequencing errors. The resulting nucleotide and amino acid sequences of CDR3 of TCRβ were determined, and those with out-of-frame and stop codon sequences were removed from the identified TCRβ repertoire. Then, the amounts of each TCRβ clonotype were defined by adding the numbers of TCRβ clones that share the same nucleotide sequence as CDR3. A donor chart of BM Treg TCRβ-seq is presented to profile the clonal signatures. The fan-shaped area exhibits the corresponding clonal frequency. The top 5 clones’ amino acid sequences of complementary determination region 3 (CDR3) of TCR are displayed in the donut chart, and the radian of the region marked with the amino acid sequence demonstrates the frequency of the relevant Treg clones. The area of “1” and “2” is the summed frequencies of T-cell clones that only had one sequence read. The area of “3+” is the summed frequencies of T-cell clones that had more than two sequence reads. The clonal phenotype of the TCR repertoire was measured by the TCR productive frequency and inverse Simpson index.
Deep WES
BM HSCs were sorted and pooled from 5 mice, and genomic DNA was extracted for deep WES. A total amount of 0.2 μg DNA/sample was used as input material for the library preparations. A sequencing library was generated using a NEBNext® Ultra™ DNA Library Prep Kit for Illumina (New England Biolabs, Ipswich, MA, USA) following the manufacturer’s recommendations. Then, the clustering of the index-coded samples was processed on a cBot Cluster Generation System using an Illumina® TruSeq® PE Cluster Kit (Illumina Inc., San Diego, California, USA). After cluster generation, the DNA libraries were sequenced on the Illumina platform, and 150 bp paired-end reads were generated. WES data were mapped to the mouse genome GRCm38/mm10 reference to achieve an average depth coverage >250× for the targeted exon regions. Next, high-quality WES data were analyzed for variant information (SNV and INDEL) across samples, and analytical interpretation of detected variants was performed. Mutations shared in all the samples were considered to be germline variants. After removing these germline variants, variant information was determined using a normalized (SNV and INDEL) count with the following equation:
HSC–Treg interaction assay
HSC culture was performed according to a previously reported method, which efficiently maintains functional HSCs ex vivo [23]. Briefly, sorted HSCs were plated in 24-well fibronectin-coated plates and cultured in medium composed of F12 medium, 1% insulin–transferrin–selenium–ethanolamine, 1% penicillin/streptomycin/glutamine, 10 mM HEPES (all Gibco), SCF (10 ng/ml), TPO (100 ng/ml; all PeproTech, Rocky Hill, NJ, USA), and polyvinyl alcohol (PVA; 1 mg/ml, Sigma‒Aldrich) at 37 °C and 5% CO2. To mimic the HSC–Treg interaction ex vivo, HSCs (1 × 103) were cocultured with autologous BM Tregs (1 × 104) at a ratio of 1:10. For intervention of HSC–Treg interactions, a Transwell system and coculture system with the addition of αMHCII (10 mg/mL, M5/114.15.2; BioXCell), GAP27 (300 μM, MedChemExpress) or H89 (10 μM, MedChemExpress) were used as indicated. Treg proliferation was determined using a CFSE Cell Proliferation Tracker Kit (Thermo Fisher Scientific) on Day 3. HSC expansion and differentiation were assessed on Day 10. For the colony assay, 1000 sorted Lineage– cells from HSC cultures were plated into methylcellulose medium (STEMCELL Technologies) according to the manufacturer’s protocols. Colonies were assessed after 12 days using inverted light microscopy (Thermo Fisher Scientific).
Apoptotic priming assay
Apoptotic priming of HSCs was performed as previously reported [24]. Briefly, Sca-1+ BM cells were enriched using an EasySep™ Mouse SCA1 Positive Selection Kit (StemCell Technologies) and stained with antibodies to identify HSCs as described above. Subsequently, cells were resuspended at 5 × 105 cells/ml and incubated with 8 μM BIM or 3 μM BID peptide (all EIAab, Wuhan, China) in DTEB buffer (0.1% BSA, 5 mM succinate 10 mM HEPES-KOH, 135 mM trehalose, 20 μM EDTA, 20 μM EGTA and 50 mM KCl, final pH 7.4) for 30 min. Fifteen minutes before the completion of the peptide incubation, tetramethylrhodamine (TMRE, Thermo Fisher Scientific) was added at a final concentration of 20 nM. Cells were analyzed by flow cytometry to determine mitochondrial depolarization (compared with the vehicle, dimethyl sulfoxide (DMSO, Sigma‒Aldrich)) and positive control p-trifluoromethoxy carbonyl cyanide phenyl hydrazone (FCCP, Sigma‒Aldrich). To assess the impact of the HSC–Treg interaction on apoptotic priming, 5 × 105 Sca-1+ cells were cocultured with 1 × 105 autologous BM Tregs. The percentage of depolarization (priming) was calculated by the MFI of the TMRE peak as follows:
Statistical analysis
Statistical analysis was performed using Prism 9.0 (GraphPad Software, USA). All results are expressed as the mean ± standard deviation (SD). n represents the number of independent experiments, as detailed in the figure legends. Comparisons between the two groups were determined by a paired or unpaired two-tailed Student’s t-test. Three or more groups were compared by one-way analysis of variance (ANOVA) followed by Tukey‒Kramer post hoc analysis. Tconv–Treg phenotypic conversion signature analysis was examined by the chi-square test. The Pearson correlation coefficient was examined in correlation analysis. Kaplan‒Meier curves and log-rank tests were used for survival analysis. P < 0.05 was considered statistically significant. The vast majority of ex vivo experiments were performed multiple times. Most in vivo experiments were performed at least twice.
Results
Premature HSC aging accompanies the clonal expansion of BM Tregs
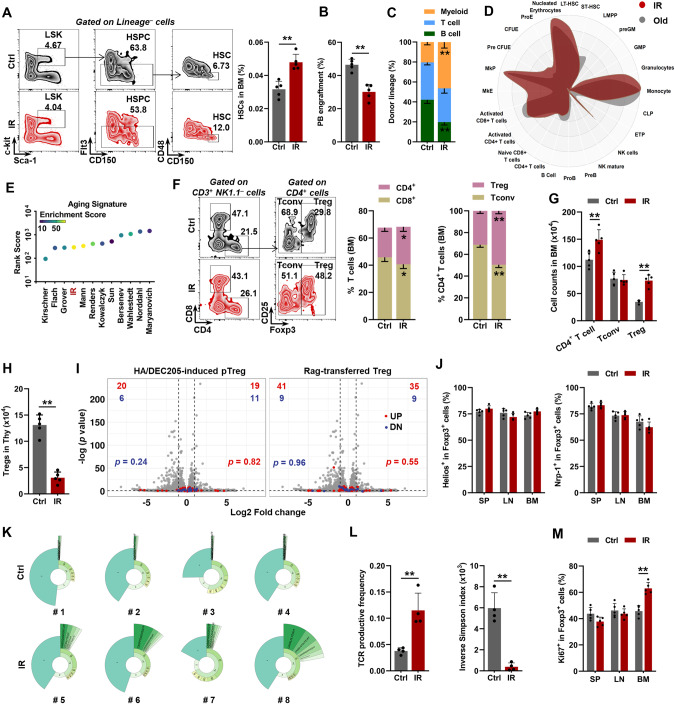
Initially, we employed a well-recognized mouse model of premature aging by exposing mice to ionizing radiation (IR), which is a well-known mutagenic agent [25, 26]. Analogous to physiological hematopoietic aging [27], features of HSC aging, including expansion (Fig. 1A; Supplementary Fig. 1a), impaired reconstitution capacity (Fig. 1B), and myeloid-biased differentiation (Fig. 1C), were apparent in mice at 3 months post IR (mpi), along with modestly increased BM cellularity (Supplementary Fig. 1b). At the transcriptomic level, analogous to those of aged mice (18–24 months old), the BM HSCs of IR mice exhibited myeloid bias (Fig. 1D) and an aging signature as cross-validated using Aging Signature (Fig. 1E), which provides a comprehensive transcriptome signature of murine HSC aging [28]. Surprisingly, with respect to BM T cells, we observed that CD4+ T cells were expanded in IR mice (Fig. 1F, G). Among CD4+ T cells, the fraction and number of Foxp3+ Tregs other than conventional CD4+ T cells (Tconv) were dramatically increased in IR mice (Fig. 1F, G). The pool of mesenchymal stromal cells (MSCs), which play crucial roles in regulating the maintenance of both HSCs and Tregs [16, 29], was negligibly influenced in the BM of IR mice (Supplementary Fig. 1c).
Fig. 1.
Premature HSC aging accompanies clonal expansion of BM Tregs. A Flow cytometric gating strategy and frequency of HSCs in the BM of mice with or without IR (n = 5). B Frequency of PB chimerism in BM HSCs at 16 weeks postcompetitive transplantation (n = 5). C Frequency of donor-derived myeloid, B, and T cells at 16 weeks postcompetitive transplantation (n = 5). D CellRadar plot indicating the lineage potential of the BM HSCs in IR mice relative to the BM HSCs in old mice (18–24 mo). E Aging signature evaluation of the BM HSCs in IR mice using the webtool site Aging Signature. F Flow cytometric gating strategy and frequency of T-cell subpopulations in the BM of mice with or without IR (n = 5). G Total number of T-cell subpopulations in the BM of mice with or without IR (n = 5). H Frequency and total number of T-cell subpopulations in the thymus (Thy) of mice with or without IR (n = 5). I Volcano plots comparing the transcriptomes of Tregs in the BM of mice with or without IR overlaid with the indicated pTreg transcriptomic signatures. j Flow cytometric analysis of Helios or Nrp-1 expression in spleen (SP), lymph nodes (LN), and BM Tregs of mice with or without IR (n = 5). K Donut chart summarizing the clonal signatures of the BM Tregs in mice with or without IR (n = 4). l TCR productive frequency and inverse Simpson index of the BM Tregs in mice with or without IR (n = 4). M Flow cytometric analysis of Ki67+ cells in the SP, LN and BM Tregs in mice with or without IR (n = 5). Data represent the mean ± SD. *P < 0.05, **P < 0.01. Two-tailed unpaired Student’s t test unless stated otherwise. The chi-square test was used to calculate P values in i
Most Tregs located in peripheral tissues are exported from the thymus (and are termed tTregs). However, analogous to physiological aging [30], thymic cellularity, including Treg numbers, remarkably declined in IR mice due to IR-induced thymic atrophy [31] (Fig. 1H; Supplementary Fig. 1d). The accumulation of Tregs can also originate from Treg phenotypic conversion from Tconv (and is termed pTreg) or the local expansion of tissue-resident Tregs [21]. As reported, pTregs can be generated from dendritic cells (Dc) by administering a low-dose of antigen (influenza hemagglutinin [HA] peptide) fused with the monoclonal antibody DEC205 or by transferring naïve Tconv cells into lymphopenic Rag-deficient mice [21, 32]. Based on the transcriptomic data generated using these pTregs, we found no apparent signs of Tconv–Treg phenotypic conversion after superimposing the pTreg signatures onto volcano plots and comparing the transcriptome of BM Tregs from IR mice with that from control (Ctrl) mice (Fig. 1I). Similar to splenic (SP) and lymphoid node (LN) Tregs, BM Tregs had a HelioshiNrp-1hi phenotype (Fig. 1J), which is purported to distinguish between tTregs and pTregs [33]. These data indicate a small contribution of pTregs to BM Treg expansion post IR. Interestingly, the TCR repertoire revealed considerable clonal expansion of BM Tregs in IR mice (Fig. 1K, L). In addition, we also identified a significantly increased fraction of Ki67+ Tregs in the BM instead of the SP or LN of IR mice (Fig. 1M), reflecting the exclusive clonal proliferation of BM Tregs.
MHCII–TCR engagement triggers a bidirectional interaction between aged HSCs and BM Tregs
The exclusive clonal expansion of BM Tregs indicates that BM Tregs may be activated by TCR recognition of cognate antigens presented by MHCII displayed on the surface of BM-resident antigen presentation cells (APCs). Indeed, significant activation of TCR signaling in BM Tregs of IR mice was observed by RNA-seq (Fig. 2A). We then analyzed MHCII expression on professional APCs, including Dc, B cells, and macrophages (Macs), in the BM, as well as on HSCs, which were recently identified as proficient APCs [34]. Intriguingly, dramatically upregulated expression of MHCII but not MHCI was exclusively detected on HSCs from IR mice (Fig. 2B; Supplementary Fig. 1e). Correspondingly, RNA-seq also revealed evident activation of the MHCII-mediated antigen presentation pathway in the HSCs of IR mice (Fig. 2A). Given that BM Tregs localize adjacent to HSCs in both humans and mice during homeostasis [17, 18, 35], the presentation of cognate antigens by MHCII on the HSCs of IR mice might be responsible for the clonal expansion of BM Tregs. In support of this finding, histological analysis showed that compared to those of control mice, the HSCs, particularly the MHCII+ HSCs, of IR mice were much closer to Tregs than Tconv or CD8+ T cells in the BM (Fig. 2C–E; Supplementary Fig. 1f, g), with 47.2% of HSCs making direct contact with Tregs (within 10 μm of Tregs). Based on MHCII expression, we partitioned HSCs into MHCIIhi and MHCIIlo subpopulations (Fig. 2F). We found that the fraction and number of MHCIIhi HSCs gradually and dramatically increased from 1 mpi, while the fraction of MHCIIlo HSCs was dramatically reduced, although their number only slightly declined (Fig. 2G; Supplementary Fig. 1h). In cocultures with OTII naïve CD4+ T cells, we confirmed that MHCIIhi HSCs from IR mice had a stronger antigen presentation capacity than their MHCIIlo counterparts (Supplementary Fig. 1i). Ex vivo, remarkable Treg proliferation was observed in a coculture system of MHCIIhi HSCs with autologous BM Tregs from IR mice, while their separation by Transwell chambers or MHCII blocking with a monoclonal antibody (αMHCII) dramatically abrogated the proliferative effect (Fig. 2H). These data indicate that TCR recognition of MHCII on the HSCs of IR mice stimulates the clonal expansion of BM Tregs.
Fig. 2.
MHCII–TCR engagement triggers a bidirectional interaction between aged HSCs and BM Tregs. A GSEA of the T-cell receptor signaling gene set or MHCII-mediated antigen presentation gene set in BM Tregs or BM HSCs, respectively (IR vs. control). B Flow cytometric analysis of the BM HSC and APC MHCII surface expression in mice with or without IR (n = 6). C Immunostaining analysis of the positional relationship between the BM HSCs and Tregs in mice with or without IR. Scale bar, 5 μm. The white arrow indicates HSCs. The yellow arrow indicates Tregs. D Distances from HSCs with respect to the nearest observable Tregs in the BM of mice with or without IR (n = 90). E Distances from MHCII+ or MHCII– HSCs with respect to the nearest observable Tregs in the BM of mice with or without IR (n = 60–77). F Flow cytometric gating strategy for partitioning BM HSCs from mice with or without IR into MHCIIhi and MHCIIlo subpopulations based on surface MHCII expression. G Fractions of MHCIIhi and MHCIIlo HSCs in the BM of mice with or without IR at the indicated time (n = 6). H CFSE staining analysis of BM Treg proliferation in a coculture or Transwell system with autologous HSCs, MHCIIlo or MHCIIhi HSCs from IR mice. αMHCII was added to the coculture system in the indicated group. The gate indicates the percentage of proliferating Tregs. I Principal component analysis (PCA) using the MHCIIhi and MHCIIlo BM HSC transcriptomes from mice with or without IR (n = 3). J Aging signature evaluation of the MHCIIhi and MHCIIlo HSCs in IR mice. K Frequency of PB chimerism as well as donor-derived myeloid, B, and T cells at 16 weeks postcompetitive transplantation (n = 5). L Flow cytometric analysis of BM HSC expansion and differentiation in the Transwell system or coculture system with the addition of αMHCII or autologous BM Tregs from IR mice (n = 6). M Frequencies of HSCs in the BM as well as MHCIIhi and MHCIIlo HSCs in IR-exposed Foxp3GFP-DTR mice with or without DT treatment (n = 6). Tregs are adoptively transferred post-DT treatment in the indicated group. N Frequency of PB chimerism as well as donor-derived myeloid, B, and T cells at 16 weeks postcompetitive transplantation (n = 5). O Flow cytometric analysis of Ki67+ cells within Foxp3-YFP+ BM Tregs in donor and recipient mice (n = 6). Data represent the mean ± SD. *P < 0.05, **P < 0.01, NS no significance. Two-tailed unpaired Student’s t test unless stated otherwise. One-way ANOVA was used to calculate P values in G, L–N
Interestingly, principal component analysis (PCA) revealed that the MHCIIhi HSCs of IR mice were much different from the MHCIIhi and MHCIIlo HSCs of control mice, while the MHCIIlo HSCs of IR mice were only moderately different from them (Fig. 2I). Simultaneously, the MHCIIhi HSCs of IR mice exhibited a higher aging signature rank score (Fig. 2J) and were more myeloid biased (Supplementary Fig. 2a) relative to their MHCIIlo counterparts, implying that MHCII upregulation might mark premature HSC aging. In favor of this finding, compared with the MHCIIlo HSCs from IR mice, their MHCIIhi counterparts showed a significantly lower reconstitution capacity in both peripheral blood (PB) and BM, accompanied by more apparent myeloid bias (Fig. 2K; Supplementary Fig. 2b). Notably, both MHCIIhi and MHCIIlo HSCs were capable of interconverting during repopulation after transplantation (Supplementary Fig. 2c). Furthermore, although the MHCIIhi and MHCIIlo HSCs from IR mice showed similar expansion and differentiation dynamics ex vivo, coculture of MHCIIhi HSCs, rather than MHCIIlo HSCs, with autologous BM Tregs resulted in remarkable expansion of phenotypic and functional HSCs (Supplementary Fig. 2d–f) as well as myeloid bias (Supplementary Fig. 2g). However, blocking MHCII or Transwell culture nearly abrogated these effects (Fig. 2L; Supplementary Fig. 2f). In vivo, the overrepresentation of MHCIIhi HSCs and the aging phenotype of HSCs were also significantly rescued by Treg depletion using Foxp3GFP-DTR mice (Fig. 2M, N; Supplementary Fig. 2h, i) without inducing significant activation of Tconv and CD8+ T cells (Supplementary Fig. 2j). Treg number rebound after short-term Treg depletion via DT administration [36, 37] only partially held true in IR mice (Supplementary Fig. 2k), probably due to thymic atrophy and the lack of conversion of Tregs from activated Tconvs [37]. In particular, a comparable proportion of BM Tregs localizes close to HSCs and CD8+ T cells during homeostasis, and CD8+ T cells directly and/or indirectly modulate the maintenance of normal and aberrant HSPCs [35, 38–41]. However, CD8+ T-cell depletion had nearly no impact on the HSC aging phenotype or the therapeutic effect of Treg depletion in IR mice (Supplementary Fig. 2l). Nevertheless, the rescue effects were abrogated by the adoptive transfer of BM Tregs (Fig. 2M, N; Supplementary Fig. 2h, i). Moreover, transferred Tregs constituted the majority of BM Tregs (Supplementary Fig. 2m) and showed significant proliferation (Fig. 2O) and accumulation in the HSC niche (Supplementary Fig. 2n) in recipient IR mice. Altogether, these results demonstrate that MHCII–TCR engagement triggers a bidirectional interaction between aged HSCs and BM Tregs that is implicated in driving HSC aging.
Accumulated DNA mutations contribute to MHCII upregulation in aged HSCs
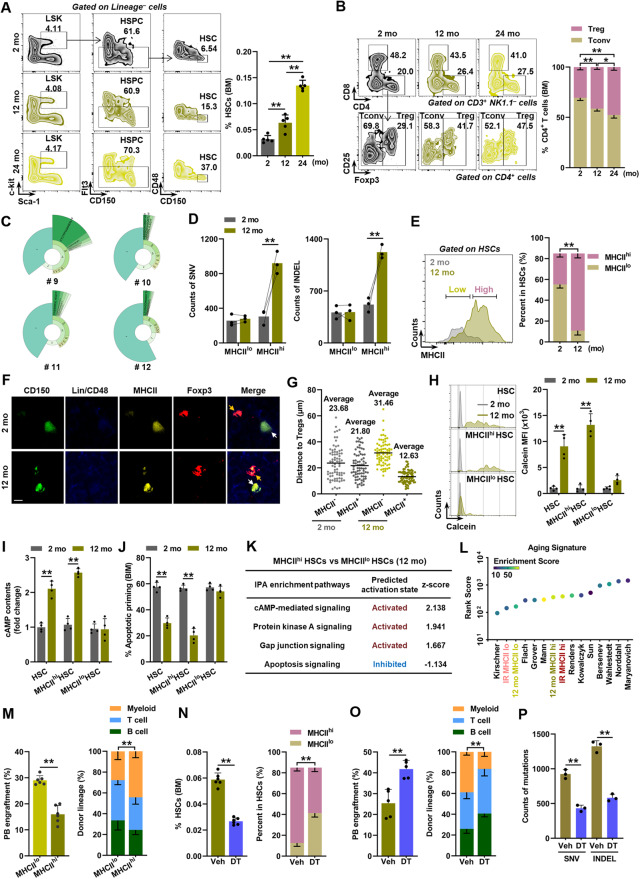
The exclusive MHCII upregulation in the HSCs of IR mice argues against the contributions of accumulating microbially-derived compounds in circulation and heightened systemic inflammation, which are characteristics of aged mice and can induce MHCII upregulation [4, 42]. DNA damage, including mutation, which is the general effect of IR, may stimulate MHCII expression by stimulating innate immune signaling, such as the cGAS-STING pathway [42, 43], and/or by generating potentially immunogenic neoantigens [44, 45]. Intriguingly, we observed that the MHCIIhi HSCs of IR mice displayed a dramatically higher mutation load than the MHCIIlo HSCs, as revealed by whole exome sequencing (WES; Fig. 3A, B). Nevertheless, the DNA mutation load was markedly reduced in the HSCs of IR mice when Tregs were depleted, while the adoptive transfer of BM Tregs nearly abrogated this effect (Fig. 3C, D). In addition, based on published data [46, 47], we also confirmed that human MHCII [also known as leukocyte antigen class II (HLAII)] expression also tended to positively correlate with loads of potentially pathogenic mutations in patients with aging-related hematopoietic diseases, including myeloid leukemia and aplastic anemia (Supplementary Fig. 3a, b). These data indicate that mutation accumulation contributes to MHCII upregulation in the HSCs of IR mice probably by generating MHCII-restricted neoantigens, and antigen-specific Tregs may facilitate the survival of these mutation-accumulated MHCIIhi HSCs.
Fig. 3.
Accumulated DNA mutation contributes to MHCII upregulation in aged HSCs. A Representative mutation circos plots of the MHCIIhi and MHCIIlo HSCs from mice with or without IR based on WES analysis. B Normalized SNV and INDEL counts in the MHCIIhi and MHCIIlo HSCs from mice with or without IR based on WES analysis (n = 3 independent experiments). C Representative mutation circos plots of the BM HSCs from IR-exposed Foxp3GFP-DTR mice with or without DT treatment and Treg adoptive transfer. D Normalized BM HSC SNV and INDEL counts of IR-exposed Foxp3GFP-DTR mice with or without DT treatment and Treg adoptive (n = 3 independent experiments). Data represent the mean ± SD. *P < 0.05, **P < 0.01, NS no significance. Two-tailed paired Student’s t test was used to calculate P values in B. One-way ANOVA was used to calculate P values in D
Aged HSCs establish functional GJs with BM Tregs via Cx43
We next sought to identify the molecular mechanism underlying MHCIIhi HSC expansion. We first analyzed the top enrichment of signaling pathways by IPA and noticed that GJ signaling was robustly upregulated in both the BM HSCs and Tregs of IR mice (Fig. 4A). As reported, MHCII–TCR engagement facilitates GJ formation between T cells and APCs [48]. Indeed, we observed that GJ signaling was much more activated in the MHCIIhi HSCs than in the MHCIIlo HSCs of IR mice (Fig. 4B). GJs are built from connexins (Cx). Among them, we found that Cx43/GJA1 was prominently and exclusively upregulated in both the HSCs (especially MHCIIhi HSCs) and Tregs of IR mice (Fig. 4C, D; Supplementary Fig. 4a). Using immunostaining, we also frequently observed the potential formation of Cx43-based GJs between the HSCs and Tregs in the BM of IR mice (Fig. 4E). Ex vivo, by employing calcein, which can only be transferred between cells via GJs, we verified that functional GJs were established between the HSCs, especially the MHCIIhi HSCs, and Tregs, from IR mice (Fig. 4F, G) and that this effect was cell contact- and MHCII-dependent (Fig. 4H). Moreover, calcein transfer almost vanished when the BM HSCs from IR mice were cocultured with autogenous Tregs with Cx43 knockout (Cx43–/–; Fig. 4H; Supplementary Fig. 4b). Simultaneously, the adoptive transfer of Cx43–/– BM Tregs, which had comparable immunosuppression and activation phenotypes compared with Cx43-proficient BM Tregs (Supplementary Fig. 4c–e), failed to reverse the rescue effect of Treg depletion on HSC aging (Fig. 4I, J; Supplementary Fig. 4f, g). These results indicate that HSC aging involves the establishment of Cx43-based GJs between aged HSCs and BM Tregs via MHCII–TCR engagement.
Fig. 4.
Aged HSCs establish functional GJs with BM Tregs via Cx43. A Top IPA enrichment items in BM HSCs and Tregs (IR vs. control). B IPA enrichment analysis of the BM HSCs in IR mice (MHCIIhi vs. MHCIIlo). C, D Flow cytometric analysis of Cx43 expression in BM cells (IR vs. control; n = 6). Mono, monocyte; Megakaryo, megakaryocyte; Endo, endothelium. E Immunostaining analysis of the potential formation of Cx43-based GJs between the BM HSCs and Tregs in IR mice. Scale bar, 5 μm. F Schematic for the calcein transfer experiment. G Flow cytometric analysis of the calcein transfer from the autologous BM Tregs to HSCs in mice with or without IR within the coculture system (n = 5). H Calcein transfer from the autologous BM Tregs to HSCs in IR mice within a Transwell system or a coculture system with the addition of αMHCII- or Cx43-knockout BM Tregs (n = 5). I Frequencies of the HSCs in the BM as well as the MHCIIhi and MHCIIlo HSCs of IR-exposed Foxp3GFP-DTR mice with DT treatment (n = 6). Tregs are adoptively transferred post-DT treatment in the indicated group. J Frequency of PB chimerism as well as donor-derived myeloid, B, and T cells at 16 weeks postcompetitive transplantation (n = 5). Data represent the mean ± SD. *P < 0.05, **P < 0.01, NS no significance. One-way ANOVA unless stated otherwise. Two-tailed unpaired Student’s t test was used to calculate P values in D, G
BM Tregs transfer cAMP via GJs to diminish the apoptotic priming of aged HSCs
We then investigated the outcome of aged HSCs when establishing GJs with Tregs. Among the top enriched signaling pathways, we noticed that repressed apoptosis signaling was robustly present in the HSCs of IR mice (Fig. 4A). Moreover, apoptosis signaling was more repressed in the MHCIIhi HSCs than in the MHCIIlo HSCs of IR mice (Fig. 4B), inferring that BM Treg interactions play a role in HSC apoptosis signaling. In support of this finding, although the fractions of apoptotic cells within the MHCIIhi and MHCIIlo HSCs of IR mice were comparable at steady state (Supplementary Fig. 5a), BH3 profiling revealed that the apoptotic priming of the MHCIIhi HSCs in IR mice was much lower than their MHCIIlo counterparts in response to BIM (Fig. 5A) and BID (Supplementary Fig. 5b). In addition, the apoptosis resistance of HSCs, particularly that of MHCIIhi HSCs, in IR mice was BM Treg-dependent (Fig. 5B; Supplementary Fig. 5c). Ex vivo, the apoptotic priming of the MHCIIhi HSCs from IR mice, rather than the MHCIIlo HSCs, was diminished in the presence of autologous BM Tregs (Fig. 5C; Supplementary Fig. 5d), and this effect was cell contact- and MHCII-dependent (Fig. 5D; Supplementary Fig. 5e).
Fig. 5.
BM Tregs transfer cAMP via GJs to diminish the apoptotic priming of aged HSCs. A Flow cytometric analysis of BM HSC apoptotic priming in mice with or without IR in response to BIM (n = 4). B Apoptotic priming of the BM HSCs from IR-exposed Foxp3GFP-DTR mice with or without DT treatment and Treg transfer (n = 4). C Apoptotic priming of the MHCIIhi HSCs from IR mice in the presence of autologous BM Tregs (n = 5). D Apoptotic priming of the MHCIIhi HSCs from IR mice in the Transwell system or with the addition of αMHCII- or Cx43-knockout BM Tregs (n = 5). E Bubble plots showing the quantification of intracellular cAMP levels in the BM cells of mice with or without IR (n = 6). F, G cAMP levels and PKA kinase activity in the BM HSCs of mice with or without IR (n = 4). H Flow cytometric analysis of p-CREB expression in the BM HSCs of mice with or without IR (n = 4). I cAMP levels, PKA kinase activity, and p-CREB expression in the BM HSCs of IR-exposed Foxp3GFP-DTR mice with or without DT treatment and Treg transfer (n = 4). J Apoptotic priming of the MHCIIhi HSCs in IR mice with autologous Treg coculture in the presence of H89 (n = 4). K Flow cytometric analysis of the 488-cAMP transfer from the autologous BM Tregs to HSCs in mice with or without IR within the coculture system (n = 4). L Flow cytometric analysis of the 488-cAMP transfer from the autologous BM Tregs to HSCs in IR mice within a Transwell system or a coculture system with the addition of αMHCII- or Cx43-knockout BM Tregs (n = 4). Data represent the mean ± SD. *P < 0.05, **P < 0.01, NS: no significance. Two-tailed unpaired Student’s t test unless stated otherwise. One-way ANOVA was used to calculate P values in B, D, I, J and L
Notably, we also observed that Cx43 was indispensable for the apoptosis resistance of MHCIIhi HSCs in IR mice both in vivo (Fig. 5B; Supplementary Fig. 5c) and ex vivo (Fig. 5D; Supplementary Fig. 5e), indicating the involvement of GJs. GJs regulate intracellular signaling in adjacent cells through the transfer of soluble factors smaller than 1200 Da [49]. Interestingly, RNA-seq revealed that PKA signaling, which is activated by the secondary messenger cAMP, was robustly activated in both the BM HSCs and Tregs of IR mice (Fig. 4A), and the activation was more pronounced in the MHCIIhi HSCs of IR mice than in their MHCIIlo counterparts (Fig. 4B). Unexpectedly, the expression of cAMP synthetases was even downregulated, whereas the expression of cAMP-degrading enzymes was upregulated in the MHCIIhi HSCs of IR mice (Supplementary Fig. 5f). As reported, Tregs are particularly enriched for cAMP. Upon Treg activation, cAMP production is further augmented, and cAMP can be very efficiently transferred from Tregs to recipient cells via GJs [50]. Consistently, we found exclusive enrichment and remarkable elevation of cAMP levels in the BM Tregs of IR mice (Fig. 5E). cAMP–protein kinase A (PKA) signaling positively regulates HSC survival [51], suggesting that Tregs may transfer cAMP via GJs to aged HSCs to promote their survival. Indeed, we measured dramatically increased cAMP levels, PKA kinase activity, and its downstream CREB activity in the MHCIIhi HSCs of IR mice (Fig. 5F–H), and the hyperactivation of cAMP–PKA signaling depended on Cx43 expression in BM Tregs (Fig. 5I). However, inhibition of PKA by the selective inhibitor H89 nearly abrogated the prosurvival effect of analogous BM Tregs on the MHCIIhi HSCs from IR mice (Fig. 5J). To further test our hypothesis, we tracked cAMP transfer using Alexa Fluor 488-labeled cAMP (488-cAMP). As shown, BM Tregs rapidly and considerably transferred 488-cAMP to the MHCIIhi HSCs rather than the MHCIIlo HSCs of IR mice (Fig. 5K), and this effect was cell contact-, MHCII-, and Cx43-dependent (Fig. 5L). These data demonstrate that BM Tregs transfer cAMP via GJs to diminish the apoptotic priming of aged HSCs.
HSC–Treg bidirectional interaction is implicated in physiological HSC aging
Physiological HSC aging is also characterized by DNA mutation accumulation that is apparent in middle age [11, 12]. Interestingly, the HSC aging phenotype (Fig. 6A; Supplementary Fig. 6a) and clonal expansion of BM Tregs (Fig. 6B, C; Supplementary Fig. 6b–d) were already apparent by middle age to the level of old age, accompanied by a dramatic accumulation of DNA mutations, especially in MHCIIhi HSCs (Fig. 6D; Supplementary Fig. 6e). Similar to IR mice, we also identified an overrepresentation of MHCIIhi HSCs in the HSC compartment of middle-aged mice (Fig. 6E). Accordingly, distinctive adjacency to Tregs (Fig. 6F, G; Supplementary Fig. 6f), GJ formation with Tregs (Fig. 6H) and cAMP–PKA signaling hyperactivation (Fig. 6I; Supplementary Fig. 6g–i), as well as diminished apoptotic priming (Fig. 6J; Supplementary Fig. 6j), were observed in the HSCs, particularly in the MHCIIhi HSCs, of middle-aged mice. These alterations were also confirmed at the transcriptomic level (Fig. 6K). Both at the transcriptomic and functional levels, the MHCIIhi HSCs of middle-aged mice exhibited an aging signature and lineage bias resembling those of aged mice (Fig. 6L, M; Supplementary Fig. 6k). However, Treg depletion remarkably mitigated the HSC aging phenotype in middle-aged mice (Fig. 6N–P; Supplementary Fig. 6l–o), demonstrating the involvement of the HSC–Treg bidirectional interaction in physiological HSC aging.
Fig. 6.
HSC–Treg bidirectional interaction is implicated in physiological HSC aging. A Flow cytometric analysis of the HSC frequencies in the BM of 2-, 12-, and 24-month-old (mo) mice (n = 5). B Flow cytometric analysis of the T-cell subpopulation frequencies in the BM of mice at 2, 12, and 24 mo (n = 5). C Donut chart summarizing the BM Treg clonal signatures in mice at 12 mo (n = 4). D Normalized BM HSC SNV and INDEL counts in mice at 2 and 12 mo (n = 3 independent experiments). E Flow cytometric gating strategy for partitioning BM HSCs into MHCIIhi and MHCIIlo subpopulations and fractions of MHCIIhi and MHCIIlo HSCs in the BM of mice at 2 and 12 mo (n = 5). F Immunostaining analysis of the positional relationship between the HSCs and Tregs in the BM of mice at 2 and 12 mo. Scale bar, 5 μm. The white arrow indicates HSCs. The yellow arrow indicates Tregs. G Distances from MHCII+ or MHCII– HSCs with respect to the nearest observable Tregs in the BM of mice at 2 and 12 mo (n = 75). H Flow cytometric analysis of the calcein transfer from autologous BM Tregs to HSCs from mice at 2 and 12 mo in the coculture system (n = 4). I cAMP levels of the HSCs in the BM of mice at 2 and 12 mo (n = 4). J Flow cytometric analysis of HSC apoptotic priming in the BM of mice at 2 and 12 mo in response to BIM (n = 4). K IPA enrichment analysis of the HSCs in the BM of mice at 12 mo (MHCIIhi vs. MHCIIlo). L Aging signature evaluation of the MHCIIhi and MHCIIlo HSCs in mice at 12 mo and IR mice. M Frequency of PB chimerism as well as donor-derived myeloid, B, and T cells at 16 weeks postcompetitive transplantation (n = 6). N Frequencies of HSCs in the BM as well as MHCIIhi and MHCIIlo HSCs of Foxp3GFP-DTR mice at 12 mo with or without DT treatment (n = 6). O Frequency of PB chimerism as well as donor-derived myeloid, B, and T cells at 16 weeks postcompetitive transplantation (n = 5). P Normalized BM HSC SNV and INDEL counts in Foxp3GFP-DTR mice at 12 mo with or without DT treatment (n = 3 independent experiments). Data represent the mean ± SD. *P < 0.05, **P < 0.01. Two-tailed unpaired Student’s t test unless stated otherwise. One-way ANOVA was used to calculate P values in B. Two-tailed paired Student’s t test was used to calculate P values in D
To further explore the human relevance of our findings, we first reanalyzed published bulk RNA-seq data of aged human HSCs (hHSCs) [52]. In line with our mouse data, enhanced MHCII-mediated antigen presentation (Supplementary Fig. 7a) and GJ formation, cAMP–PKA signaling hyperactivation, and diminished apoptotic priming (Supplementary Fig. 7b) were also found in aged hHSCs. Correspondingly, we also found significant activation of TCR signaling, cAMP signaling, and GJ signaling (Supplementary Fig. 7c, d) when reanalyzing the published single-cell RNA-seq data of aged BM hTregs [53]. Then, based on published single-cell RNA-seq data of aged hHSCs [54], we also observed remarkable upregulation of several MHCII genes, including HLA-DRB5 (Supplementary Fig. 7e). Subsequently, the aged hHSCs were classified into MHCIIhi and MHCIIlo subsets by HLA-DRB5 expression (Supplementary Fig. 7f). Consistent with these findings, the aging phenotypes were more evident in MHCIIhi hHSCs than in MHCIIlo hHSCs (Supplementary Fig. 7g, h). From these data, we can infer that the aged HSC–Treg interaction may also be implicated in hHSC aging.
Prevention of HSC aging by intervening in the aged HSC–Treg interaction
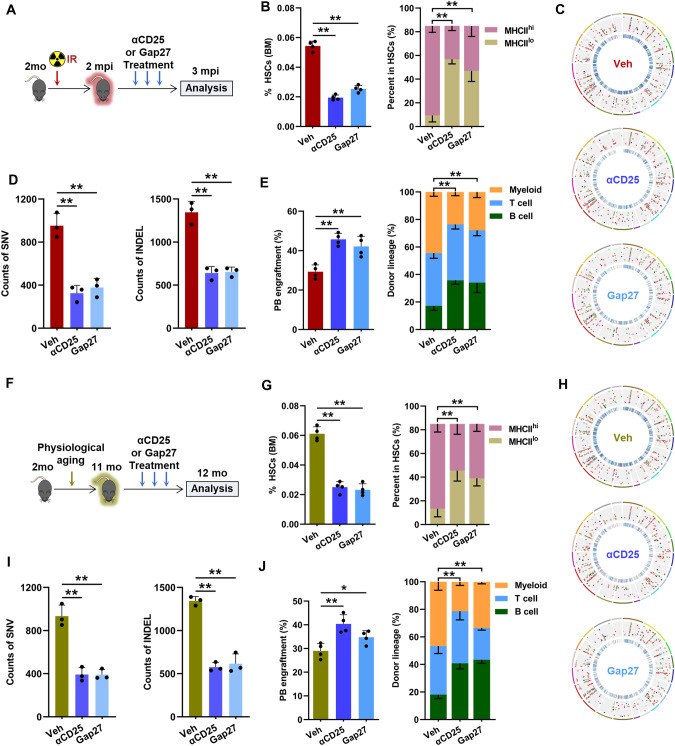
Finally, we evaluated the translational potential of our findings. Since humanized anti-hCD25 antibody (daclizumab) is available and widely used in clinical settings, we first tested the therapeutic potential of depleting Tregs by anti-mouse CD25 (αCD25) in HSC aging (Fig. 7A). αCD25 administration dramatically reduced the number of BM Tregs in IR mice (Supplementary Fig. 8a) without eliciting significant activation of CD8+ T cells (Supplementary Fig. 8b). In parallel, MHCIIhi HSC expansion was remarkably attenuated in IR mice after αCD25 administration (Fig. 7B), accompanied by significantly relieved DNA mutation load (Fig. 7C, D), repressed cAMP–PKA signaling (Supplementary Fig. 8c) and enhanced apoptotic priming (Supplementary Fig. 8d) of HSCs. Finally, the aging phenotype of HSCs in IR mice was markedly rescued by αCD25 administration (Fig. 7E). Gap27 is a peptide that has been proven in vivo to be suitable for interfering with both the formation and stability of Cx43-based GJs [55]. Ex vivo, we confirmed that Gap27 effectively blocked GJ-mediated cAMP transfer between the BM HSCs and Tregs of IR mice (Supplementary Fig. 8e). Correspondingly, GAP27 administration significantly relieved cAMP–PKA signaling activation and apoptotic resistance in HSCs in vivo (Supplementary Fig. 8c, d), thereby mitigating the aging phenotype of HSCs in IR mice (Fig. 7B–E). Likewise, the premature aging phenotype of HSCs in middle-aged mice was significantly rescued by αCD25 or GAP27 administration (Fig. 7F–J; Supplementary Fig. 8f–i). Therefore, systemic administration of αCD25 or GAP27 holds promise in the prevention of HSC aging, at least partially by intervening in the aged HSC–Treg interaction.
Fig. 7.
Prevention of HSC aging by intervening in the aged HSC–Treg interaction. A Experimental design. B HSC frequencies in the BM as well as MHCIIhi and MHCIIlo HSCs in IR mice after αCD25 or Gap27 treatment (n = 4). C Representative mutation circos plots of BM HSCs from IR mice after αCD25 or Gap27 treatment. D Normalized BM HSC SNV and INDEL counts in IR mice after αCD25 or Gap27 treatment (n = 3 independent experiments). E Frequency of PB chimerism as well as donor-derived myeloid, B, and T cells at 16 weeks postcompetitive transplantation (n = 4). F Experimental design. G Frequencies of HSCs in the BM as well as MHCIIhi and MHCIIlo HSCs in mice at 12 mo after αCD25 or Gap27 treatment (n = 4). H Representative mutation circos plots of BM HSCs from mice at 12 mo after αCD25 or Gap27 treatment (n = 5). I Normalized BM HSC SNV and INDEL counts in mice at 12 mo after αCD25 or Gap27 treatment (n = 3 independent experiments). J Frequency of PB chimerism as well as donor-derived myeloid, B, and T cells at 16 weeks postcompetitive transplantation (n = 4). Data represent the mean ± SD. *P < 0.05, **P < 0.01. One-way ANOVA
Discussion
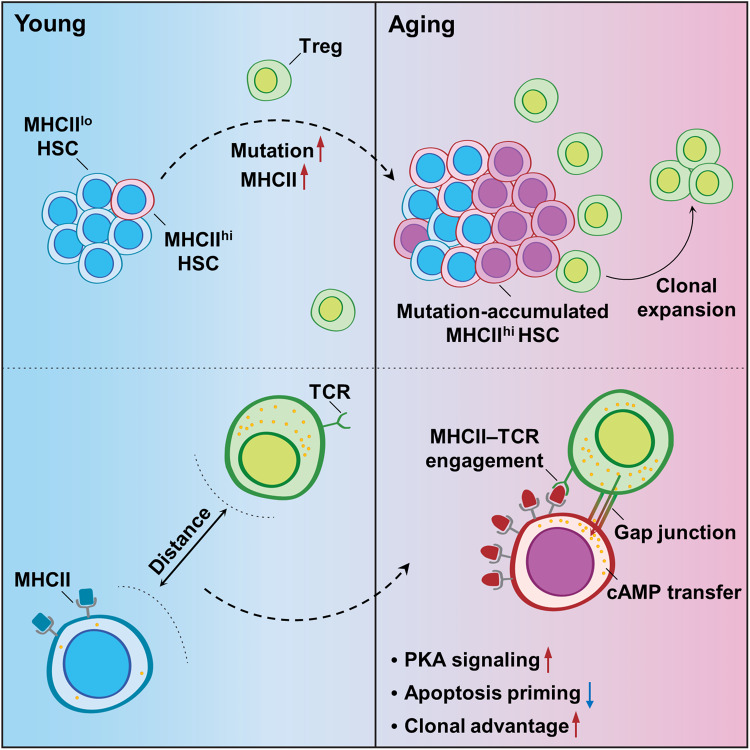
Tissue Tregs have been recently identified as an essential constituent of stem cell niches positioned centrally in stem cell immunobiology [33]. Here, we further advance our understanding of the noncanonical regulatory role of Tregs in stem cell aging that is exemplified by HSC aging (Fig. 8).
Fig. 8.
Schematic illustration of the regulatory role of BM Tregs in HSC aging
Tregs: beyond immunosuppressive and anti-inflammatory functions in the stem cell niche
Tregs are substantially enriched and widely distributed in the BM [16, 35]. Interestingly, apart from closely localizing with effector T cells (Teff) cells, especially CD8+ T cells, a subset of BM Tregs (~30%) has also been reported to localize closely with HSCs in both humans and mice [17, 35]. Reasonably, the conserved link between inflammation and stem cell aging across species infers an antiaging role of tissue Tregs. Unexpectedly, using mouse models of IR-induced premature aging and physiological aging, we revealed that BM Tregs predominantly favor the survival of mutation-accumulated HSCs during aging and drive HSC aging via a noncanonical mechanism, that is, MHCII-based and GJ-mediated cAMP transfer. In addition, BM Tregs in young mice also maintain HSC quiescence and number via extracellular adenosine generation. Constitutive depletion of BM Tregs in young mice causes HSC proliferation [17], in contrast to the effects of BM Treg depletion in aged mice, as we revealed here. This discrepancy reflects a remodeling of the HSC–Treg interaction during aging. In addition, Tconv and CD8+ T-cell activation was not detected in the BM of aged mice with or without Treg depletion, reinforcing the direct prosurvival function of BM Tregs during HSC aging. Thus, Tregs seem to be versatile in the stem cell niche, beyond their well-known immunosuppressive and anti-inflammatory functions. Notably, other immune cells, especially Teff cells, are extremely sensitive to IR [56], although physiological Treg homeostasis and function seem to be minorly influenced by IR [57]. This notion should be considered when investigating the interactions of aged HSCs with other immune cells using a mouse model of IR-induced premature aging.
Antigen presentation by MHCII: a distress signal of mutation-accumulated, aged stem cells
Mutated cells are susceptible to immune attack by upregulating the expression of MHC machinery and subsequent neoantigen presentation to Teff cells [42]. This immune surveillance mechanism is also applicable to the stem cell compartment. For instance, HSCs [34], and stem cells of the intestine [42, 58], as well as of the skin, ovary, and mammary gland [59], display MHC machinery on their surface. However, DNA mutations actually accumulate in a wide variety of somatic stem cells, and mutation-accumulated stem cells even expand with age [5]. Accordingly, in this study, we show that MHCIIhi HSCs, which have stronger immunogenicity and more accumulation of DNA mutations, are overrepresented in the aged HSC pool. It seems plausible that the preferential recognition of HSC antigens by niche Tregs precludes the immune eradication of mutation-accumulated HSCs by Teff cells. In favor of this notion, the clonal expansion of Tregs instead of Tconv informs that BM Tregs are responding to one or more HSC antigens, albeit the identity of the culprit antigen(s) is unknown. MHCII expression on stem cells and Treg expansion upon aging have also been verified in other tissues [21, 33, 42, 58]. Together with our findings, it is reasonable to expect that MHCII upregulation as a distress signal of mutation-accumulated, aged stem cells plays an analogous role in crosstalk with Tregs in other tissues as a general mechanism by which Tregs maintain the survival of mutation-accumulated, aged tissue stem cells. Notably, to fully delineate the MHCII-mediated HSC–Treg interaction, it would be better to utilize currently commercially available MHCII–/– mice. However, the high radiosensitivity (Supplementary Fig. 9a) and constitutive impacts on immune homeostasis [60] preclude the usage of MHCII–/– mice in this study. The development of tools such as punctually deleting MHCII in aged murine HSCs in the future will be helpful to characterize the aged HSC–Treg interaction in greater detail.
Aged stem cells actively shape their niche
Cell-intrinsic and cell-extrinsic mechanisms are independent yet synergistic drivers of stem cell aging. It is generally thought that stem cells passively receive pro-aging signals from their niche with age [10]. Amazingly, although young HSCs transplanted into old recipients acquired aging-related phenotypes, transplantation into young recipients without niche-damaging myeloablation failed to rejuvenate the aging phenotype of old HSCs, particularly the expansion of myeloid-biased HSCs [61]. It seems paradoxical that aging-associated cell-intrinsic defects play a pivotal role in HSC aging. As reported, transplanted HSCs rapidly recruit Tregs to their vicinity in the recipient regardless of irradiation [17, 19]. Based on our findings, it is plausible that aged, myeloid-biased HSCs may utilize the BM Tregs of recipients to maintain their survival, thus reconciling this contradiction. In contrast, acute IR exposure alters the niche [62] and thus possibly changes Treg localization given the active interactions of Tregs with niche cells, especially MSCs [16]. In this study, we investigated the Treg–HSC interaction in the BM at 3 mpi, when the niche architecture, including the MSC pool, was restored to normal, seemingly excluding this possibility. Intrinsically, MHCIIhi HSCs also represent a deeper quiescent state that is more resistant to stress-induced proliferation than MHCIIlo HSCs [43]. However, homeostatic restoration of the hematopoietic compartment can be achieved within 3 weeks post IR [63, 64]. Consistent with these results, the cell cycle state of the HSCs in IR mice was comparable to that at steady state (Supplementary Fig. 9b) and negligibly affected by Treg manipulations (Supplementary Fig. 9c), indicating that the survival advantage of MHCIIhi HSCs during aging is quiescent state-independent. Overall, this study emphasizes an active self-protection mechanism by which aged stem cells hijack otherwise health-beneficial Tregs via MHCII upregulation to shape their microenvironment into a prosurvival niche.
Membrane-related processes drive stem cell aging
Recently, a comprehensive transcriptomic analysis of aged murine HSCs identified a robust and stable transcriptional activation signature of HSC aging that is highly enriched for membrane-related processes, including cell junction organization, although their physiopathological importance is poorly understood [28]. In the present study, we further show that the activation of membrane-related processes, including MHCII-mediated antigen presentation and GJs, is already robustly presented during premature aging and by middle age. In addition, the activation of these membrane-related processes is not simply a consequence of HSC aging but also marks HSC aging and drives HSC aging by promoting the survival of aged HSCs. Deciphering the alterations in membrane-related processes during HSC aging can substantially aid in the development of reliable markers and feasible targeted therapies for stem cell aging. Notably, the expression of membrane markers of HSC aging, including MHCII, IL27Ra [65], and CD41 [66], seems to be a reversible process during repopulation after transplantation. Given the great intrinsic differences such as those in functionality and mutation load between the two opposing subpopulations, interconversion seems unlikely to contribute to the alteration of HSC composition in situ in aged mice with or without therapeutic treatment.
Implications for age-related diseases
It is becoming increasingly evident that the crosstalk between tissue stem cells and immune cells influences not only differentiation and homeostasis but also cancer risk, which is unusually high in elderly individuals. Cancer stem cells (CSCs), which are thought to arise from the malignant transformation of one or several stem cells or progenitors with pathogenic mutations, now serve as a pivotal framework to understand aggressive and therapeutically resistant cancers [67]. For example, leukemia is initiated and propagated by a subpopulation of leukemia cells called leukemia stem cells (LSCs), the malignant counterpart of HSCs. There are many similarities shared by aged HSCs and LSCs, including the acquisition of genomic aberrations (although the mutation load in LSCs is relatively lower than that in other cancers [46, 68]), active neoantigen presentation via MHCII, Treg accumulation in BM, clonal advantage, GJ tendency, and apoptosis resistance [16, 35, 49, 69]. Correspondingly, it has been recently reported that antigen presentation via MHCII enhances the CD4+ Teff cell-mediated eradication of potentially malignant HSCs during the onset of HSC-derived leukemias in young mice, while this surveillance mechanism is not operational in mice with established myeloid leukemia [34]. This finding could be attributed to the activation and accumulation of BM Tregs potentially invoked by mutated antigen stimulation after leukemia establishment [35]. Thus, the interaction of HSCs with Tregs, as we reported here, may also be involved in LSC maintenance and provide a rationale for the pathogenesis and treatment of leukemia. Moreover, this framework may also be compatible with other age-related hematopoietic disorders, such as clonal hematopoiesis [12], aplastic anemia [70], and myelodysplastic syndrome [71], in which mutated HSCs may acquire a survival advantage and dominate BM hematopoiesis through a similar mechanism.
Additionally, although MHCII expression always positively instructs immunogenicity, sometimes tumor-specific MHCII expression is unexpectedly negatively associated with immunogenicity and positively associated with therapy resistance and relapse [44]. For example, LSCs usually have strong MHCII expression but are generally considered immunologically ignored [35]. This paradox may also be explained by the framework we propose here, as CSCs may be capable of hijacking Tregs to create an immunosuppressive and prosurvival microenvironment and evade immune clearance. Targeting the CSC–Treg axis, which may synergize with the effect of traditional cancer therapies, could be of particular clinical interest in cancer treatment, especially in dealing with therapy resistance and relapse. Notably, currently available therapeutic agents that may target the stem cell-Treg axis, such as αCD25 and GAP27 used in this study, may have potential off-target effects. Identifying targets specific to stem cell–Treg interactions will provide superior therapeutic options.
Supplementary information
Acknowledgements
We thank Liting Wang for technical assistance with immunofluorescence. This study was supported by the Key Program of the National Natural Science Foundation of China (No. 81930090), the National Science Foundation for Distinguished Young Scholars of China (No. 81725019), and the National Natural Science Foundation of China (Nos. 82273571, 32171104, U22A20279, 81874256, and 81872556), Chongqing Natural Science Foundation (2023NSCQ-JQX0076).
Author contributions
WL, CL, and KY designed and performed experiments, analyzed data, and wrote the manuscript. JC, YW, SZ, and KY contributed to flow cytometric analysis and animal experiments. LW and LR contributed to mouse model creation and animal experiments. MC and FC contributed to the ex vivo experiments and data analysis. YX and SW contributed to bioinformatics analysis and assisted with writing the manuscript. FW, QZ, and JZ contributed to the experimental design and data interpretation. LY, CD, and JW conceived and supervised the study, interpreted the data, and revised the manuscript.
Data availability
Transcriptome, TCR-seq, and WES datasets generated in this study are available in the GEO database (GSE211007, GSE211136, GSE211203, GSE211205, GSE211206) and in the Sequence Read Archive (PRJNA866671, PRJNA866602, PRJNA866684). Published datasets (GSE156807, GSE20366, GSE69408, GSE104379, GSE175604) were reanalyzed in this paper with the authors’ permission.
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: Weinian Liao, Chaonan Liu, Ke Yang
Contributor Information
Lilin Ye, Email: yelilinlcmv@163.com.
Changhong Du, Email: changhongdu@tmmu.edu.cn.
Junping Wang, Email: wangjunping@tmmu.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41423-023-01072-3.
References
- 1.Yousefzadeh MJ, Flores RR, Zhu Y, Schmiechen ZC, Brooks RW, Trussoni CE, et al. An aged immune system drives senescence and ageing of solid organs. Nature. 2021;594:100–5. doi: 10.1038/s41586-021-03547-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bogeska R, Mikecin A-M, Kaschutnig P, Fawaz M, Büchler-Schäff M, Le D, et al. Inflammatory exposure drives long-lived impairment of hematopoietic stem cell self-renewal activity and accelerated aging. Cell Stem Cell. 2022;29:1273–84.e8. doi: 10.1016/j.stem.2022.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jeon OH, Mehdipour M, Gil T-H, Kang M, Aguirre NW, Robinson ZR, et al. Systemic induction of senescence in young mice after single heterochronic blood exchange. Nat Metab. 2022;4:995–1006. doi: 10.1038/s42255-022-00609-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kovtonyuk LV, Caiado F, Garcia-Martin S, Manz E-M, Helbling P, Takizawa H, et al. IL-1 mediates microbiome-induced inflammaging of hematopoietic stem cells in mice. Blood. 2022;139:44–58. doi: 10.1182/blood.2021011570. [DOI] [PubMed] [Google Scholar]
- 5.Schumacher B, Pothof J, Vijg J, Hoeijmakers JHJ. The central role of DNA damage in the ageing process. Nature. 2021;592:695–703. doi: 10.1038/s41586-021-03307-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cagan A, Baez-Ortega A, Brzozowska N, Abascal F, Coorens THH, Sanders MA, et al. Somatic mutation rates scale with lifespan across mammals. Nature. 2022;604:517–24. doi: 10.1038/s41586-022-04618-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brengdahl MI, Kimber CM, Elias P, Thompson J, Friberg U. Deleterious mutations show increasing negative effects with age in Drosophila melanogaster. BMC Biol. 2020;18:128. doi: 10.1186/s12915-020-00858-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garaycoechea JI, Crossan GP, Langevin F, Mulderrig L, Louzada S, Yang F, et al. Alcohol and endogenous aldehydes damage chromosomes and mutate stem cells. Nature. 2018;553:171–7. doi: 10.1038/nature25154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Batsivari A, Haltalli MLR, Passaro D, Pospori C, Lo Celso C, Bonnet D. Dynamic responses of the haematopoietic stem cell niche to diverse stresses. Nat Cell Biol. 2020;22:7–17. doi: 10.1038/s41556-019-0444-9. [DOI] [PubMed] [Google Scholar]
- 10.Ya-Hsuan H, Simón M-F. Microenvironmental contributions to hematopoietic stem cell aging. Haematologica. 2020;105:38–46. doi: 10.3324/haematol.2018.211334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mitchell E, Spencer Chapman M, Williams N, Dawson KJ, Mende N, Calderbank EF, et al. Clonal dynamics of haematopoiesis across the human lifespan. Nature. 2022;606:343–50. doi: 10.1038/s41586-022-04786-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fabre MA, de Almeida JG, Fiorillo E, Mitchell E, Damaskou A, Rak J, et al. The longitudinal dynamics and natural history of clonal haematopoiesis. Nature. 2022;606:335–42. doi: 10.1038/s41586-022-04785-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Naik S, Larsen SB, Cowley CJ, Fuchs E. Two to Tango: dialog between immunity and stem cells in health and disease. Cell. 2018;175:908–20. doi: 10.1016/j.cell.2018.08.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frisch BJ, Hoffman CM, Latchney SE, LaMere MW, Myers J, Ashton J, et al. Aged marrow macrophages expand platelet-biased hematopoietic stem cells via Interleukin1B. JCI Insight. 2019;5:e124213. doi: 10.1172/jci.insight.124213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pioli PD, Casero D, Montecino-Rodriguez E, Morrison SL, Dorshkind K. Plasma cells are obligate effectors of enhanced myelopoiesis in aging bone marrow. Immunity. 2019;51:351–66.e6. doi: 10.1016/j.immuni.2019.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Riether C. Regulation of hematopoietic and leukemia stem cells by regulatory T cells. Front Immunol. 2022;13:1049301. doi: 10.3389/fimmu.2022.1049301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirata Y, Furuhashi K, Ishii H, Li HW, Pinho S, Ding L, et al. CD150high bone marrow tregs maintain hematopoietic stem cell quiescence and immune privilege via adenosine. Cell Stem Cell. 2018;22:445–53.e5. doi: 10.1016/j.stem.2018.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yuichi H, Miwako K, Simon CR, Joji F. CD150high CD4 T cells and CD150high regulatory T cells regulate hematopoietic stem cell quiescence via CD73. Haematologica. 2019;104:1136–42. doi: 10.3324/haematol.2018.198283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fujisaki J, Wu J, Carlson AL, Silberstein L, Putheti P, Larocca R, et al. In vivo imaging of Treg cells providing immune privilege to the haematopoietic stem-cell niche. Nature. 2011;474:216–9. doi: 10.1038/nature10160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ito M, Komai K, Mise-Omata S, Iizuka-Koga M, Noguchi Y, Kondo T, et al. Brain regulatory T cells suppress astrogliosis and potentiate neurological recovery. Nature. 2019;565:246–50. doi: 10.1038/s41586-018-0824-5. [DOI] [PubMed] [Google Scholar]
- 21.Kolodin D, van Panhuys N, Li C, Magnuson Angela M, Cipolletta D, Miller Christine M, et al. Antigen- and cytokine-driven accumulation of regulatory T cells in visceral adipose tissue of lean mice. Cell Metab. 2015;21:543–57. doi: 10.1016/j.cmet.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Camacho V, Matkins VR, Patel SB, Lever JM, Yang Z, Ying L, et al. Bone marrow Tregs mediate stromal cell function and support hematopoiesis via IL-10. JCI Insight. 2020;5:e135681. doi: 10.1172/jci.insight.135681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilkinson AC, Ishida R, Nakauchi H, Yamazaki S. Long-term ex vivo expansion of mouse hematopoietic stem cells. Nat Protoc. 2020;15:628–48. doi: 10.1038/s41596-019-0263-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gutierrez-Martinez P, Hogdal L, Nagai M, Kruta M, Singh R, Sarosiek K, et al. Diminished apoptotic priming and ATM signalling confer a survival advantage onto aged haematopoietic stem cells in response to DNA damage. Nat Cell Biol. 2018;20:413–21. doi: 10.1038/s41556-018-0054-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang J, Wang Y, Shao L, Laberge R-M, Demaria M, Campisi J, et al. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat Med. 2016;22:78–83. doi: 10.1038/nm.4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dou Z, Ghosh K, Vizioli MG, Zhu J, Sen P, Wangensteen KJ, et al. Cytoplasmic chromatin triggers inflammation in senescence and cancer. Nature. 2017;550:402–6. doi: 10.1038/nature24050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun X, Cao B, Naval-Sanchez M, Pham T, Sun YBY, Williams B, et al. Nicotinamide riboside attenuates age-associated metabolic and functional changes in hematopoietic stem cells. Nat Commun. 2021;12:2665. doi: 10.1038/s41467-021-22863-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Flohr Svendsen A, Yang D, Kim K, Lazare S, Skinder N, Zwart E, et al. A comprehensive transcriptome signature of murine hematopoietic stem cell aging. Blood. 2021;138:439–51. doi: 10.1182/blood.2020009729. [DOI] [PubMed] [Google Scholar]
- 29.Crippa S, Bernardo ME. Mesenchymal stromal cells: role in the BM niche and in the support of hematopoietic stem cell transplantation. HemaSphere. 2018;2:e151. doi: 10.1097/HS9.0000000000000151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chougnet CA, Tripathi P, Lages CS, Raynor J, Sholl A, Fink P, et al. A major role for Bim in regulatory T Cell homeostasis. J Immunol. 2011;186:156–63. doi: 10.4049/jimmunol.1001505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reiko I, Laura PH, Susan MG, Jie L, Andrew S, Junko K, et al. Late effects of exposure to ionizing radiation and age on human thymus morphology and function. Radiat Res. 2017;187:589–98. doi: 10.1667/RR4554.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feuerer M, Hill JA, Kretschmer K, von Boehmer H, Mathis D, Benoist C. Genomic definition of multiple ex vivo regulatory T cell subphenotypes. Proc Natl Acad Sci USA. 2010;107:5919–24. doi: 10.1073/pnas.1002006107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muñoz-Rojas AR, Mathis D. Tissue regulatory T cells: regulatory chameleons. Nat Rev Immunol. 2021;21:597–611. doi: 10.1038/s41577-021-00519-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hernández-Malmierca P, Vonficht D, Schnell A, Uckelmann HJ, Bollhagen A, Mahmoud MAA, et al. Antigen presentation safeguards the integrity of the hematopoietic stem cell pool. Cell Stem Cell. 2022;29:760–75.e10. doi: 10.1016/j.stem.2022.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hinterbrandner M, Rubino V, Stoll C, Forster S, Schnüriger N, Radpour R, et al. Tnfrsf4-expressing regulatory T cells promote immune escape of chronic myeloid leukemia stem cells. JCI Insight. 2021;6:e151797. doi: 10.1172/jci.insight.151797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol. 2007;8:191–7. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 37.Nyström SN, Bourges D, Garry S, Ross EM, van Driel IR, Gleeson PA. Transient Treg-cell depletion in adult mice results in persistent self-reactive CD4 + T-cell responses. Eur J Immunol. 2014;44:3621–31. doi: 10.1002/eji.201344432. [DOI] [PubMed] [Google Scholar]
- 38.Radpour R, Riether C, Simillion C, Höpner S, Bruggmann R, Ochsenbein AF. CD8 + T cells expand stem and progenitor cells in favorable but not adverse risk acute myeloid leukemia. Leukemia. 2019;33:2379–92. doi: 10.1038/s41375-019-0441-9. [DOI] [PubMed] [Google Scholar]
- 39.Schürch Christian M, Riether C, Ochsenbein, Adrian F. Cytotoxic CD8 + T cells stimulate hematopoietic progenitors by promoting cytokine release from bone marrow mesenchymal stromal cells. Cell Stem Cell. 2014;14:460–72. doi: 10.1016/j.stem.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 40.Riether C, Gschwend T, Huguenin AL, Schürch CM, Ochsenbein AF. Blocking programmed cell death 1 in combination with adoptive cytotoxic T-cell transfer eradicates chronic myelogenous leukemia stem cells. Leukemia. 2015;29:1781–5. doi: 10.1038/leu.2015.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schürch C, Riether C, Amrein MA, Ochsenbein AF. Cytotoxic T cells induce proliferation of chronic myeloid leukemia stem cells by secreting interferon-γ. J Exp Med. 2013;210:605–21. doi: 10.1084/jem.20121229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beyaz S, Chung C, Mou H, Bauer-Rowe KE, Xifaras ME, Ergin I, et al. Dietary suppression of MHC class II expression in intestinal epithelial cells enhances intestinal tumorigenesis. Cell Stem Cell. 2021;28:1922–35.e5. doi: 10.1016/j.stem.2021.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li J, Williams MJ, Park HJ, Bastos HP, Wang X, Prins D, et al. STAT1 is essential for HSC function and maintains MHCIIhi stem cells that resist myeloablation and neoplastic expansion. Blood. 2022;140:1592–606. doi: 10.1182/blood.2021014009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jardim DL, Goodman A, de Melo Gagliato D, Kurzrock R. The challenges of tumor mutational burden as an immunotherapy biomarker. Cancer Cell. 2021;39:154–73. doi: 10.1016/j.ccell.2020.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.He K, Wan T, Wang D, Hu J, Zhou T, Tao W, et al. Gasdermin D licenses MHCII induction to maintain food tolerance in small intestine. Cell. 2023;186:3033–48.e20. [DOI] [PubMed]
- 46.Ley TJ, Miller C, Ding L, Raphael BJ, Mungall AJ, Robertson A, et al. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Engl J Med. 2013;368:2059–74. doi: 10.1056/NEJMoa1301689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nowak J, Wozniak J, Mendek-Czajkowska E, Dlugokecka A, Mika-Witkowska R, Rogatko-Koros M, et al. Potential link between MHC-self-peptide presentation and hematopoiesis; the analysis of HLA-DR expression in CD34-positive cells and self-peptide presentation repertoires of MHC molecules associated with paroxysmal nocturnal hemoglobinuria. Cell Biochem Biophys. 2013;65:321–33. doi: 10.1007/s12013-012-9435-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mendoza-Naranjo A, Bouma G, Pereda C, Ramírez M, Webb KF, Tittarelli A, et al. Functional gap junctions accumulate at the immunological synapse and contribute to T cell activation. J Immunol. 2011;187:3121. doi: 10.4049/jimmunol.1100378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Singh AK, Cancelas JA. Gap junctions in the bone marrow lympho-hematopoietic stem cell niche, leukemia progression, and chemoresistance. Int J Mol Sci. 2020;21:796. doi: 10.3390/ijms21030796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Klein M, Bopp T. Cyclic AMP represents a crucial component of Treg cell-mediated immune regulation. Front Immunol. 2016;7:315. doi: 10.3389/fimmu.2016.00315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Negrotto S, Pacienza N, D’Atri LP, Pozner RG, Malaver E, Torres O, et al. Activation of cyclic AMP pathway prevents CD34+ cell apoptosis. Exp Hematol. 2006;34:1420–8. doi: 10.1016/j.exphem.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 52.Rundberg Nilsson A, Soneji S, Adolfsson S, Bryder D, Pronk CJ. Human and murine hematopoietic stem cell aging is associated with functional impairments and intrinsic megakaryocytic/erythroid bias. PLoS ONE. 2016;11:e0158369. doi: 10.1371/journal.pone.0158369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Luo Y, Xu C, Wang B, Niu Q, Su X, Bai Y, et al. Single-cell transcriptomic analysis reveals disparate effector differentiation pathways in human Treg compartment. Nat. Commun. 2021;12:3913. doi: 10.1038/s41467-021-24213-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Adelman ER, Huang H-T, Roisman A, Olsson A, Colaprico A, Qin T, et al. Aging human hematopoietic stem cells manifest profound epigenetic reprogramming of enhancers that may predispose to leukemia. Cancer Discov. 2019;9:1080–101. doi: 10.1158/2159-8290.CD-18-1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tirosh A, Tuncman G, Calay ES, Rathaus M, Ron I, Tirosh A, et al. Intercellular transmission of hepatic ER stress in obesity disrupts systemic metabolism. Cell Metab. 2021;33:319–33.e6. doi: 10.1016/j.cmet.2020.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lumniczky K, Candéias SM, Gaipl US, Frey B. Editorial: Radiation and thE Immune System: Current Knowledge and Future Perspectives. Front Immunol. 2018;8:1933. doi: 10.3389/fimmu.2017.01933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Persa E, Balogh A, Sáfrány G, Lumniczky K. The effect of ionizing radiation on regulatory T cells in health and disease. Cancer Lett. 2015;368:252–61. doi: 10.1016/j.canlet.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 58.Biton M, Haber AL, Rogel N, Burgin G, Beyaz S, Schnell A, et al. T helper cell cytokines modulate intestinal stem cell renewal and differentiation. Cell. 2018;175:1307–20.e22. doi: 10.1016/j.cell.2018.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Agudo J, Park ES, Rose SA, Alibo E, Sweeney R, Dhainaut M, et al. Quiescent tissue stem cells evade immune surveillance. Immunity. 2018;48:271–85.e5. doi: 10.1016/j.immuni.2018.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Madsen L, Labrecque N, Engberg J, Dierich A, Svejgaard A, Benoist C, et al. Mice lacking all conventional MHC class II genes. Proc Natl Acad Sci USA. 1999;96:10338–43. doi: 10.1073/pnas.96.18.10338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kuribayashi W, Oshima M, Itokawa N, Koide S, Nakajima-Takagi Y, Yamashita M, et al. Limited rejuvenation of aged hematopoietic stem cells in young bone marrow niche. J Exp Med. 2020;218:e20192283. doi: 10.1084/jem.20192283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cao X, Wu X, Frassica D, Yu B, Pang L, Xian L, et al. Irradiation induces bone injury by damaging bone marrow microenvironment for stem cells. Proc Natl Acad Sci USA. 2011;108:1609–14. doi: 10.1073/pnas.1015350108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu C, Liao W, Chen J, Yu K, Wu Y, Zhang S, et al. Cholesterol confers ferroptosis resistance onto myeloid-biased hematopoietic stem cells and prevents irradiation-induced myelosuppression. Redox Biol. 2023;62:102661. doi: 10.1016/j.redox.2023.102661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pietras Eric M, Reynaud D, Kang Y-A, Carlin D, Calero-Nieto Fernando J, Leavitt, et al. Functionally distinct subsets of lineage-biased multipotent progenitors control blood production in normal and regenerative conditions. Cell Stem Cell. 2015;17:35–46. doi: 10.1016/j.stem.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.He H, Xu P, Zhang X, Liao M, Dong Q, Cong T, et al. Aging-induced IL27Ra signaling impairs hematopoietic stem cells. Blood. 2020;136:183–98. doi: 10.1182/blood.2019003910. [DOI] [PubMed] [Google Scholar]
- 66.Yamamoto R, Wilkinson AC, Ooehara J, Lan X, Lai C-Y, Nakauchi Y, et al. Large-scale clonal analysis resolves aging of the mouse hematopoietic stem cell compartment. Cell Stem Cell. 2018;22:600–7.e4. doi: 10.1016/j.stem.2018.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bayik D, Lathia JD. Cancer stem cell–immune cell crosstalk in tumour progression. Nat Rev Cancer. 2021;21:526–36. doi: 10.1038/s41568-021-00366-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science. 2015;348:69–74. doi: 10.1126/science.aaa4971. [DOI] [PubMed] [Google Scholar]
- 69.Riether C, Schürch CM, Ochsenbein AF. Regulation of hematopoietic and leukemic stem cells by the immune system. Cell Death Differ. 2015;22:187–98. doi: 10.1038/cdd.2014.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Visconte V, Maciejewski JP. Clonal dynamics of hematopoietic stem cell compartment in aplastic anemia. Semin Hematol. 2022;59:47–53. doi: 10.1053/j.seminhematol.2021.12.003. [DOI] [PubMed] [Google Scholar]
- 71.Chen J, Kao Y-R, Sun D, Todorova TI, Reynolds D, Narayanagari S-R, et al. Myelodysplastic syndrome progression to acute myeloid leukemia at the stem cell level. Nat Med. 2019;25:103–10. doi: 10.1038/s41591-018-0267-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Transcriptome, TCR-seq, and WES datasets generated in this study are available in the GEO database (GSE211007, GSE211136, GSE211203, GSE211205, GSE211206) and in the Sequence Read Archive (PRJNA866671, PRJNA866602, PRJNA866684). Published datasets (GSE156807, GSE20366, GSE69408, GSE104379, GSE175604) were reanalyzed in this paper with the authors’ permission.