Abstract
Objective:
To analyze the influence of exergaming (EXE) quality of life, cancer-related fatigue (CRF), electromyography, and strength and endurance muscle in a randomized crossover trial.
Methods:
We conducted a single-blinded, randomized, and crossover trial, which included 38 cancer volunteers undergoing chemotherapy (Age = 60.07 ± 12.10 years; body mass index = 26.79 ± 5.33 kg/m2). All volunteers were randomized into two intervention moments: EXE and without intervention (WI) and after 1-month washout period of crossing of the evaluated moments. The intervention was performed on an EXE protocol using Xbox 360 Kinect®, with the game “Your Shape Fitness Evolved 2012” two to three times per week for 20 sessions. All volunteers were assessed the CRF and quality-of-life levels through the Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F) questionnaire, the median frequency (MDF) by surface electromyography, maximal voluntary isometric contraction (MVIC), and the muscle endurance time at 80% MVIC of the dorsiflexors and plantar flexors using dynamometer.
Results:
In the comparison between EXE and WI moments, were observed increase in the scores for quality of life (P < 0.001), subscale fatigue (P < 0.001), in the MDF values of right lateral gastrocnemius muscles: P = 0.017, muscle endurance time (left dorsiflexion [LDF]: P < 0.001; right dorsiflexion [RDF]: P < 0.001; left plantar flexion [LPF]: P < 0.001; RPF: P = 0.039), and muscle strength (LDF: P < 0.001; RDF: P < 0.001; LPF: P = 0.002).
Conclusion:
The crossover study, the EXE protocol promoted improvement in cancer-related fatigue (CRF) and quality of life, increased MVIC, endurance time, and MDF values of the dorsiflexor and plantar flexor muscles of cancer volunteers undergoing chemotherapy.
Keywords: Cancer, Muscle fatigue, Electromyography, Rehabilitation
Introduction
In the last decade, there has been an increase in the number of intervention studies for individuals with cancer using physical exercise as a safe and well-tolerated method that promotes health benefits both during and after cancer treatment.1 Several studies have demonstrated the benefits of physical exercise in individuals with cancer, such as improving depression,1 sleep,1,2 vitality, emotional wellbeing,1 aerobic capacity,1,3 and muscle strength,2,4 as well as reducing fatigue levels.1–4 Increased physical activity, especially recreational activity, is associated with a lower risk of mortality for these individuals.5 One of the ways to engage in recreational exercise is through videogames, an exercise method that can be applied to various health disorders, including cancer.6,7
Exergaming (EXE) is a therapeutic, innovative modality that provides an interactive environment for the player, which includes gestures and movements of the upper and lower limbs to simulate a particular practice of physical exercises on a game screen without needing controls.8 Some studies used EXE only as an intervention protocol for cancer survivors regardless of each individual's stage of treatment to reduce cancer-related fatigue (CRF)6,7 and shoulder disability,9 improve quality of life,7,10 achieve psychological well-being,11 restore the patterns of myoelectric activity in the leg muscles6 and middle deltoid muscle,7 and increase muscle strength of the dorsiflexor and plantar flexor muscles.6 In addition, the visual and auditory feedback provided by EXE can constantly stimulate learning and improve the player's performance during the activity.12
In the literature, there are few clinical trials involving only the use of EXE to treat CRF.6,7,13,14 Most studies include EXE as a part of the intervention protocol, suggesting that the interpretation of the results obtained from the use of this resource should be made with caution.13 Moreover, the benefits of EXE in individuals with cancer still need to be better explored, especially in relation to the study designs used, described as quasiexperimental and controlled trials with protocols of 20 sessions of exclusive use of EXE for cancer survivors.6,7,10 These study designs are characterized by parallel-group assays, in which individuals are exposed only to an intervention model.4,15,16 Thus, there is a need for new study models that are able to measure the potential effect of the practice of EXE on cancer patients, such as crossover study designs.
The crossover study design is characterized by volunteers receiving two or more interventions, in random order, at moments of intervention separated by a washout period to avoid possible residual effects of interventions, in addition to exposing individuals to each experimental intervention.15,16 With this model, each volunteer participates in the study as their own control, which makes crossover studies potentially more efficient than parallel-group trials, in which participants are allocated a single intervention for comparison with other interventions.16 We hypothesize that EXE can promote physical and functional changes in cancer patients, and such effects may be better evidenced with crossover studies, since the same individuals participate in different moments of intervention.
To date, no crossover trial has been found regarding the use of EXE in cancer patients. Therefore, the objective of this study was to analyze through a randomized and crossover clinical trial the effects of EXE on CRF, muscle activation, and muscle strength and endurance in the ankle dorsiflexor and plantar flexor muscles of cancer volunteers.
Materials and Methods
Study design
The single-blinded, randomized, and crossover trial was conducted between January 2018 and March 2019. The study was approved by the Research Ethics Committee of the Federal University of Alfenas (Protocol n. 1.980.365) and registered in the Registration Platform of Clinical Trials (RBR-9tmm4d; www.ensaiosclinicos.gov.br/rg/?q=RBR-9tmm4d). All volunteers were informed of the procedures involved in the study; upon their agreement to participate, they signed an informed consent form.
Volunteers
A total of 75 volunteers undergoing chemotherapy for cancer in stages 0, I, II, and III were eligible for the study, including males and females between the ages of 18 and 80 years who attended the oncology unit of Santa Casa de Alfenas and presented medical approval to perform physical exercises.
This study excluded volunteers with stage IV cancer, subjects who had difficulties in carrying out the evaluation methods, practitioners of physical exercises regularly for 3 months after medical release, those who underwent any surgical procedure in recent months, individuals who had an infectious disease, those with hematological problems, people with diseases that compromised the movements of the upper and lower limbs, and those who could not participate in the study for personal reasons.
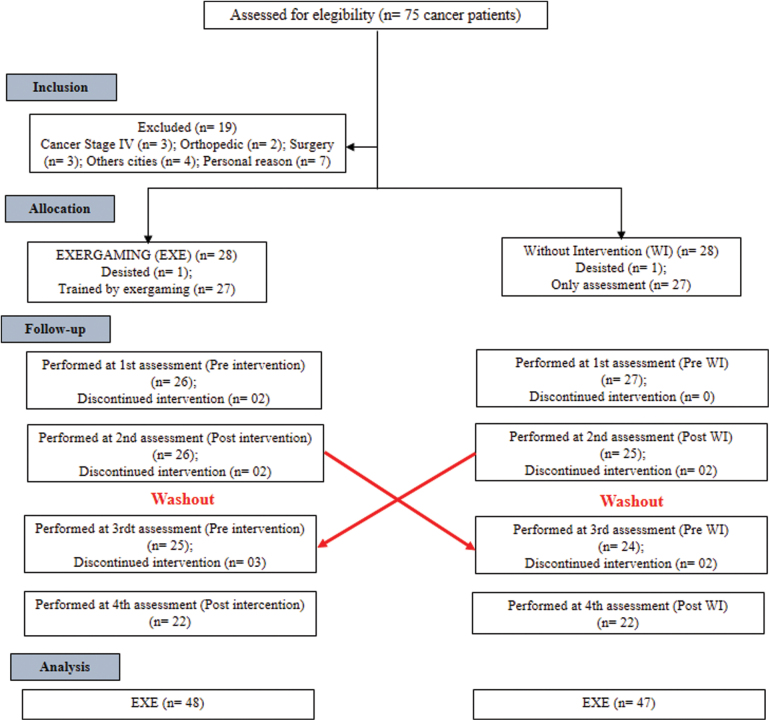
Before starting the study protocol, participants were randomized using an electronic device to the beginning of the intervention. At the time of EXE, the volunteers first received the intervention protocol for 20 sessions. At the moment without intervention (WI), the volunteers performed only the evaluations. After completing the first phase, all volunteers performed a month of washout, preceded by the crossing as shown in Figure 1.
FIG. 1.
Study design model. CONSORT flowchart. Color images are available online.
Measures
All assessment and intervention procedures by EXE were performed at the Human Movement Analysis Laboratory of the Federal University of Alfenas-Minas Gerais (LAM-Unifal-MG). All volunteers were assessed at the beginning and after 20 sessions. The evaluations were carried out using the procedures described below.
CRF and quality of life
For all cancer volunteers, related fatigue was measured using a Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F) questionnaire through interviews. Validated and translated into Portuguese, this instrument is available for cancer patients.17 The FACIT-F addresses five domains: Physical Well-Being (PWB = 7 items), Social/Familiar Well-Being (SWB = 7 items), Emotional Well-Being (EWB = 6 items), Functional Well-Being (FWB = 7 items), and Fatigue Subscale (FS = 13 items). Each item in the domains allows for measuring the intensity of the symptom reported on a scale of 0 to 4 (0, no symptoms; 4, severe symptoms). Each item of the Functional Assessment of Cancer Therapy-General (FACT-G) score is obtained by summing up individual domains (PWB+SWB+EWB+FWB) that are important in evaluating the quality of life. Total FACIT-F scores consist of the sum of the FACT-G domains and the fatigue subscale. All scales of this instrument determine that high scores indicate good conditions.17
Assessment for sEMG and dynamometry
The electromyographic assessment of the medial, lateral, and anterior tibial gastrocnemius muscles of both lower limbs was performed using the electromyography Trigno 8® Channel Wireless (EMGworks; Delsys, Inc., Boston) to obtain the median of frequency parameters. The electrodes were positioned on the muscles evaluated according to the European Union Surface EMG for Noninvasive Assessment of Muscles (SENIAM) standards.
The positioning of the electrodes and each volunteer followed the description performed by Alves et al.6 The electromyography parameters used had a rejection ratio equal to 80 dB and were set at an effective EMG signal gain of 909 (V/V) and a sampling frequency of 1000 Hz. Bandpass filtering at cutoffs of 20 and 500 Hz and 60-Hz filters were used to prevent power grid interference. The signal was processed with EMGworks Analysis® 4.0.13, evaluating the median frequency (MDF) of the signal at 80% of the maximal voluntary isometric contraction (MVIC).
Associated with electromyography, MVIC was obtained using the EMG System® model 830c (EMG System, São José dos Campos, São Paulo, Brazil) equipped with a load cell with a capacity of 200 kgf. Records of dorsiflexion and plantar flexion dynamometry were obtained using three MVICs, each lasting 5 seconds with a 5-minute rest period.6 The same verbal command stimulus was issued to all participants: “tiptoes up, force, force, force” for dorsiflexion and “toe down, force, force, force” for plantar flexion. The average obtained in the three MVIC values was considered the dynamometry analysis, identifying the maximum strength obtained by the volunteer.
At each moment, 80% of the MVICs were calculated, and through visual biofeedback, a horizontal line representing this value was positioned on a monitor in front of the volunteer. When reaching the line of 80% MVIC, endurance timing was started, considering the entire period of sustained muscle contraction for as long as possible above the 80% MVIC point.18,19 What was considered the state of muscle fatigue was reached when the individual was not able to maintain the level of MVIC above the horizontal line of the monitor.18,19 This moment was used to assess the MDF of the EMG signal.
EXE protocol and Modified Borg Scale
Before initiating the EXE interventions, the volunteers were submitted to a static balance evaluation using a FootWork Pro version 3.2.2.0 baropodometer (IST Informatique, France). The volunteers were positioned standing on a platform (dimensions: 57.5 cm long × 45 cm wide × 2.5 cm high), with the feet positioned 10 cm of intermalleolar distance, to ensure safe conditions for the practice of the EXE.
The EXE protocol was performed in the Human Movement Analysis Laboratory of the Federal University of Alfenas (LAM), which is equipped with eight 42-inch LED TVs and Xbox 360 Kinect® consoles (Microsoft), under the supervision of the physiotherapist who had no contact with the evaluations.
The study used the game “Your Shape Fitness Evolved 2012” (Ubisoft, Canada). All cancer volunteers participated in EXE sessions and followed the recommendations proposed by the American College of Sports Medicine for prescribing physical exercise in individuals with cancer, requiring at least 150 minutes of moderate-intensity exercise, or an equivalent combination of both per week, but also recommends that the activity be individualized to each patient. In addition, this study also sought to meet the individual needs of each volunteer.1,6,7
The chosen games within “Your Shape Fitness Evolved 2012” were “Stomp It,” “Run the World,” and “Wall Breaker.”6,7 The goal of the game Stomp It is to step on the lights that appear around the participant, performing anterior and lateral movements of the lower limbs. The Run the World game simulates a 400-meter walk, a condition determined by the game, performing knee and hip flexion movements. The game Wall Breaker aims to break blocks, allowing upper limb movement. This game was added to increase the workload and minimize any possible disinterest from the participants.
During the EXE intervention period, the perceived effort was measured using the Modified Borg Scale. Each participant was instructed to indicate their perceived level of effort in the table at the end of each session. The intensity of perceived exertion was reported on a scale of 0 to 10 points (0, as no shortness of breath; 10, as maximum shortness of breath).20 This scale determines that the lowest score indicates the best effort condition.
Statistical analysis
The sample size and power were previously calculated using a pilot study. The calculation (G*Power 3.1.7; Franz Paul, Universität Kiel, Germany) sampling power and effect size was obtained by means of the fatigue subscale scores, using the following parameters: Test family: t-tests > Statistical test: means: difference between two independent means (two groups) > type of power analysis: a priori: compute required sample size—given α, power, and effect size. The calculated sample presented the following results: (EXE = 30.14 ± 9.67; WI = 40.71 ± 8.88; d = 1.031; power = 0.839), requiring a minimum of 12 volunteers.
The Statistical Package for Social Sciences (SPSS; IBM Corp., Chicago), version 20.0 was used for the statistical analysis of the data. To test the normality of the data, the Shapiro–Wilk test was used. The analysis of the EXE (pre and post) and WI (pre and post) moments were submitted to the t-paired test when the normal distribution was reached. When normality was not achieved, the Wilcoxon test was used as the nonparametric alternative. Furthermore, the analysis of the differences in means obtained between the EXE and WI moments were subjected to the independent t-test if the sample had a normal distribution, and when normality was not achieved, the Mann–Whitney test was used as a nonparametric alternative.
The effect size (Cohen's d) between the EXE and WI moments was obtained using the G*Power 3.1.7 software, obeying the following parameters: values equal to and greater than 0.8 represent a large effect size; values between 0.8 and 0.2 represent medium effect size values; and values below 0.2 represent small effect size.21 In all analyses, a significance level of 5% was considered.
Results
The sociodemographic and clinical characteristics of the sample of the present study are presented in Table 1.
Table 1.
Sociodemographic and Clinical Characteristics of Participants at Baseline
| Variables | N | Results |
|---|---|---|
| Age (years old) | 60.07 ± 12.10 | |
| Weight (kg) | 68.37 ± 13.63 | |
| Height (m) | 1.59 ± 0.08 | |
| BMI (kg/m2) | 26.79 ± 5.33 | |
| Sex (%) | ||
| M | 14 | 36.84 |
| F | 24 | 63.16 |
| Cancer diagnostic (months) | 22.37 ± 23.62 | |
| Cancer diagnostic sites (%) | ||
| Gastrointestinal tract | 6 | 15.79 |
| Breast | 13 | 34.21 |
| Abdominal and pelvic | 10 | 26.31 |
| Oropharyngeal | 4 | 10.53 |
| Others | 5 | 13.16 |
| Stage (%) | ||
| 0 | 2 | 5.26 |
| I | 11 | 28.94 |
| II | 16 | 42.11 |
| III | 9 | 23.69 |
| IV | — | — |
| Chemotherapeutic drugs (%) | ||
| Taxanes | 13 | 34.21 |
| Platinum | 7 | 18.42 |
| Alkaloids | 7 | 18.42 |
| Others | 11 | 28.95 |
| Types of treatments (cycles) | 10.32 ± 9.41 | |
| Chemotherapy sessions | ||
| EXE protocol | 2.72 ± 0.46 | |
| Session (times per week) | 109.36 ± 21.23 | |
BMI; Other (Leukemia, lymphoma, bone cancer, brain cancer, lymphoma chronic myelogenous); other medications: CMF, carboplatin–methotrexate–fluorouracil; ABVD, adriamycin, bleomycin, vinblastine, and dacarbazine; kg: kilogram; m: meter; kg/m2: kilogram per square meter; n = sample number; %: percentage.
BMI, body mass index.
The domain scores of the FACIT-F questionnaire are presented in Table 2. At the EXE moment, after 20 EXE sessions, significant increases in the scores of the Subscale Fatigue, Fact-G domains, FACIT-F domains, and reduced scores in the modified Borg Scale were observed. These results indicate a reduction in the CRF and an improvement in the quality of life and reduced perceived exertion of the participants. At the moment WI, no significant differences were observed for the FACIT-F domains. At the EXE moment, a reduction in the CRF and an improvement in the quality of life were observed when compared with the WI moment. In addition, the EXE moment showed a large effect size for the analyzed variables (Table 2).
Table 2.
Comparative Analysis of the Median, Standard Error, Difference Means ± SD, and 95% CI Values Obtained in the Fatigue Subscale, Fact-G, and Facit-F Domains of the FACIT-F Questionnaire and Scale Modified of Borg Analyzed at EXE Intervention and WI Times
| |
EXE (n = 38) |
WI (n = 38) |
EXE vs. WI |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variables | Pre | Post | Difference ± SD | P a | Pre | Post | Difference ± SD | P a | P b | ES |
| FS | 39.00 (1.32) | 48.00 (0.78) | 10.15 ± 6.40 (8.07–12.22) | <0.001b | 41.00 (1.67) | 38.00 (1.50) | −1.41 ± 8.98 (−4.32–1.50) | 0.391 | <0.001a | 1.482 |
| FACT-G | 82.00 (2.26) | 91.00 (2.01) | 7.04 ± 11.22 (3.40–10.67) | <0.001b | 83.57 (2.57) | 77.66 (2.46) | −9.50 ± 9.16 (−1.13–8.06) | 0.129 | <0.001a | 1.615 |
| FACIT-F | 121.00 (3.09) | 138.00 (2.60) | 17.90 ± 12.12 (12.92–21.44) | <0.001b | 127.66 (3.89) | 115.16 (3.62) | −12.77 ± 15.77 (−11.17–1.41) | 0.097 | 0.034a | 2.181 |
| BORG | 7.00 (0.32) | 4.00 (0.23) | −2.95 ± 2.50 (−3.76− −2.14) | <0.001b | — | — | — | — | — | 1.694 |
Wilcoxon test (Pre vs. Post).
Mann–Whitney test (EXE vs. WI).
95% CI, 95% confidence interval; ES, effect size; EXE, exergaming; FACIT-F, Functional Assessment of Chronic Illness Therapy-Fatigue; FACT-G, Functional Assessment of Cancer Therapy-General; FS, fatigue subscale; SD, standard deviation; WI, without intervention.
Table 3 shows the results obtained from MVIC and endurance time of the plantar dorsiflexor and flexor muscles of volunteers with cancer, in the EXE and WI moments.
Table 3.
Comparative Analysis of the Median, Standard Error, Difference Means ± SD, and 95% CI Values of Strength (Kgf) and Endurance Time (s) of Dorsiflexor and Plantar Flexor Muscles of Cancer Volunteers at EXE Intervention and WI Times
| Muscle group | EXE (n = 38) |
WI (n = 38) |
EXE vs. WI |
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Difference ± SD | P | Pre | Post | Difference ± SD | P | P | ES | |
| LDF | ||||||||||
| Kgf | 13.97 (1.39) | 18.98 (2.46) | 10.04 ± 13.61 (5.26–14.49) | <0.001a | 16.56 (2.04) | 15.42 (1.55) | 3.22 ± 7.79 (0.69–5.74) | 0.014a | <0.001b | 1.195 |
| s | 8.09 (1.06) | 34.74 (2.42) | 27.62 ± 18.12 (21.74–33.49) | <0.001a | 18.38 (2.38) | 17.78 (1.94) | 3.72 ± 11.99 (−0.17–7.60) | 0.046a | <0.001b | 2.039 |
| RDF | ||||||||||
| Kgf | 17.17 (1.37) | 18.94 (1.50) | 3.68 ± 5.89 (1.77–5.59) | 0.008a | 17.82 (1.48) | 16.18 (1.28) | 1.09 ± 3.54 (−0.06–2.23) | 0.063 | <0.001b | 0.981 |
| s | 16.97 (1.96) | 28.55 (2.59) | 13.00 ± 16.10 (7.78–18.22) | 0.001a | 17.03 (2.53) | 15.96 (1.77) | 3.66 ± 10.69 (0.20–7.13) | 0.039a | <0.001b | 2.580 |
| LPF | ||||||||||
| Kgf | 31.18 (1.88) | 33.49 (2.52) | 4.16 ± 10.98 (0.60–7.72) | 0.029a | 29.14 (1.91) | 27.84 (1.30) | 3.74 ± 8.11 (1.11–6.37) | 0.006a | 0.001b | 0.818 |
| s | 20.41 (1.91) | 42.29 (3.10) | 20.83 ± 21.48 (13.86–27.79) | 0.031a | 27.80 (2.15) | 27.74 (1.89) | 1.29 ± 16.79 (−4.15–6.73) | 0.633 | <0.001b | 1.147 |
| RPF | ||||||||||
| Kgf | 32.07 (2.79) | 33.54 (2.44) | 1.86 ± 17.81 (−3.91–7.63) | 0.308 | 35.51 (2.08) | 34.54 (2.11) | 0.59 ± 10.50 (−2.81–3.99) | 0.726 | 0.092 | 0.167 |
| s | 26.94 (2.98) | 40.71 (3.17) | 12.47 ± 22.87 (5.05–19.88) | 0.308 | 27.49 (2.67) | 30.08 (3.72) | −4.59 ± 22.68 (−11.94–2.75) | 0.561 | 0.039b | 0.635 |
Wilcoxon test (Pre vs. Post).
Mann–Whitney test (EXE vs. WI).
ES, effect size; LDF, left dorsiflexion; LPF, left plantar flexion; RDF, right dorsiflexion; RPF, right plantar flexion.
In the EXE moment after 20 EXE sessions, there were significant increases in MVIC values to left dorsiflexion (LDF), right dorsiflexion (RDF), and left plantar flexion indicating increased muscle strength and endurance time. At the WI moment, there is a significant reduction in the MVIC values to LDF and RDF indicating decreased muscle strength and endurance time. The results indicate that EXE contributed to increased muscle strength and endurance, with a large effect size.
Table 4 shows the MDF values of the electromyographic signal of the dorsiflexor and plantar flexor muscles, as well as the differences in means between the EXE and WI moments.
Table 4.
Comparative Analysis of the Median, Standard Error, Difference Means ± SD, and 95% CI Values of the Median Frequency (Hz) of the Electromyographic Signal of the Anterior Tibial Muscles and Medial and Lateral Gastrocnemius of Both Lower Limbs of Cancer Volunteers at EXE Intervention and WI times
| |
EXE (n = 38) |
WI (n = 38) |
|
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Muscles | Pre (0 session) | Post (20th session) | difference ± SD | P a | Pre (0 session) | Post (20th session) | Mean difference ± SD | P b | EXE vs. WI P-value | ES |
| LAT | 111.49 (3.50) | 112.32 (3.92) | 6.71 ± 20.97 (−0.85–14.27) | 0.107 | 108.84 (4.84) | 110.41 (4.10) | 6.18 ± 30.92 (4.96–17.33) | 0.771 | 0.225 | 0.249 |
| RAT | 121.83 (4.20) | 131.71 (4.47) | 5.37 ± 18.31 (−1.23–11.97) | 0.018a | 123.71 (4.22) | 121.12 (4.08) | 1.78 ± 36.16 (−11.25–14.82) | 0.617 | 0.187 | 0.305 |
| LLG | 160.00 (5.44) | 172.49 (4.30) | 10.30 ± 31.61 (−1.09–21.70) | 0.104 | 163.69 (5.30) | 162.92 (4.04) | −1.41 ± 30.39 (−12.37–9.54) | 0.796 | 0.166 | 0.320 |
| LMG | 161.94 (4.18) | 176.49 (4.20) | 13.49 ± 26.23 (4.04–22.95) | 0.002a | 173.10 (5.19) | 160.04 (4.14) | 15.74 ± 33.60 (3.63–27.86) | 0.016a | 0.983 | 0.922 |
| RLG | 162.10 (4.29) | 167.71 (3.58) | 10.31 ± 21.02 (2.73–17.88) | 0.007a | 163.39 (4.60) | 166.02 (4.76) | 0.33 ± 18.74 (−6.43–7.09) | 0.634 | 0.017b | 0.564 |
| RMG | 156.00 (4.21) | 163.67 (3.99) | −6.71 ± 31.30 (−17.99–4.57) | 0.113 | 152.71 (4.42) | 157.08 (4.31) | −3.54 ± 29.49 (−14.17–7.09) | 0.063 | 0.295 | 0.149 |
Paired t-test.
Independent t-test.
ES, effect size; LAT, left anterior tibial; LLG, left lateral gastrocnemius; LMG, left medial gastrocnemius; RAT, right anterior tibial; RLG, right lateral gastrocnemius; RMG, right medial gastrocnemius.
At the EXE moment, there was a significant increase in the MDF values for the right tibial anterior, left medial gastrocnemius, and right lateral gastrocnemius (RLG) muscles. At WI time, there was no significant reduction in the electromyographic signal, except for the muscle (LMG: P = 0.016).
At the EXE moment, no significant differences were found in the electromyographic signal of the evaluated muscles compared with the WI moment, except for the RLG muscle (P = 0.017), indicating low and inconsistent signs of myoelectric fatigue, with small-to-medium effect size between the moments evaluated, except for the LMG.
Discussion
Cancer patients experience signs of fatigue and often a low quality of life7,10 during and after treatment.22,23 Studies show that the associated chemotherapy and/or radiotherapy protocols can lead to different manifestations of reported fatigue.3,6,7 These results are in agreement with other studies, which indicate that the low scores are obtained in the subscale domains of fatigue, FACT-G, and FACIT-F, suggesting higher levels of CRF and a low quality of life.6,7,10,17
Another factor that seems to influence the state of fatigue and quality of life of cancer patients is medical advice to remain at rest after diagnosis.22,24 Therefore, this study proposed an exercise program through the game, “Your Shape Fitness Evolved 2012” that would contribute to improving the quality of life and reducing the reported fatigue of volunteers with cancer while undergoing chemotherapy. The use of this type of therapy includes the practice of different activities that facilitate motor learning6 and encourage players to constantly improve their performance.12,13
In addition, EXE provides feedback on the physical fitness of the participants through scoring and verbal command, and the subjective perception of effort was measured using the modified Borg Scale. After 20 EXE sessions, there was a reduction in CRF, an improvement in the quality of life, and an improvement in muscle function associated with a reduced perception of effort by the modified Borg Scale. Although it is important to pay attention to the prescription parameters of physical activities for people with cancer,1 the intensity of EXE was self-adjusted as the volunteers progressed in their ability to play the game. In the FACIT-F questionnaire, the differences in the mean scores of the EXE and WI moments reflect the magnitude of the impact of EXE on the quality of life and CRF. Studies suggest that mean differences of 2.55 points for FACIT-F have important clinical implications.25,26 In the present study, the mean difference values found were high at the time of intervention, suggesting that EXE can contribute to improving the health conditions of the assessed volunteers.
Chemotherapy is able to change the speed of conduction of the nervous impulse, changing the MDF of the EMG signal.6,7,27 It has affected the action potential,18,19 leading to changes in the muscular environment that compromise the neuromuscular system.27 However, our MDF findings for the evaluated muscles suggest that volunteers with cancer tend to exhibit less peripheral fatigue and higher levels of central fatigue,19,27 although some muscle groups appear to be more susceptible to treatment-induced changes.
In a previous study, there was an increase in MVIC of both the dorsiflexor and left plantar flexor muscles, in addition to significantly increased endurance time of all evaluated muscles. It is plausible that during the intervention period, neuromuscular adjustments such as improved motor unit recruitment and firing rates28 may have occurred, even if the changes in the conduction velocity of the nerve impulse evaluated by MDF were not significant during evaluation. With EXE, an improvement in fatigue resistance and MVIC levels was observed, with no changes in the conduction speed of the action potential.6
The muscle contraction force exerted is an essential parameter in experimental fatigue research and can be easily measured with a force transducer. The decline in the level of strength is generally used to reflect the severity of fatigue.19,29 In addition, nerve fibers carry information through the dorsal region of the spinal cord, which communicates directly or indirectly with areas linked to central fatigue. In the presence of chemotherapy-induced toxicity30 and muscle fatigue, these afferent signals restrict the output of impulses from motor neurons, as well as muscle activation. Under these conditions, there is a downward limitation of voluntary upstream movement of the motor cortex, reducing the excitability of the corticospinal pathway.31 Therefore, this reflects the ability to reach the MVIC and the endurance time in the dorsiflexor and plantar flexor muscles between the evaluated moments.
The study model proved to be efficient in evaluating the effects of interventions in two moments.16 In addition, a washout period was important to help eliminate any possible residual effects of an intervention.16 It is noted at the moment when physical exercises were interrupted that there was a reduction in the values of the analyzed variables, indicating the appearance of fatigue. Studies show that the absence of physical activity for 1 to 2 weeks seems to contribute to a reduction in muscle performance, such as muscle strength and endurance.32,33 Therefore, the results of this study suggest the maintenance of a continuous program of physical activity practice for individuals with cancer.
This study has some limitations. First, as we were not in a referral center for cancer treatment during the study period, there was difficulty in recruiting, and due to the losses that occurred during the study, the result was a greater number of women than men. Second was the heterogeneity of the cancers obtained in the cancer group, as well as the types of treatment performed on these volunteers. It is worthy of note that the effect of EXE for upper limbs was not evaluated, which could provide other important information for the study. Although the Modified Borg Scale was used for the perception of reported exertion, other methods are needed to ensure the highest accuracy of the results, due to the subjectivity of the assessment of this scale. In addition, other possibilities of fatigue symptoms, such as hematological factors and corticosteroid use were not evaluated, associated with the level of physical activity of the volunteers before the study began. Future studies are needed to better elucidate these factors that have not yet been fully clarified.
Conclusion
The EXE protocol used proved to be capable of promoting improvement in reported fatigue and quality of life, restoring strength and endurance time of the dorsiflexor and plantar flexor muscles and the MDF values in a crossover intervention model. Therefore, 20 consultations with EXE improved quality of life and reduced reported fatigue and muscle fatigue in volunteers with cancer undergoing chemotherapy.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This work was supported by the Foundation for Research of the State of Minas Gerais-FAPEMIG, Brazil [grant number: APQ: 03580-13]; the Oncology Network of the State of Minas Gerais-FAPEMIG, Brazil [grant number: RED-11-14].
References
- 1. Campbell KL, Winters-Stone KM, Wiskemann J, et al. . Exercise guidelines for cancer survivors: Consensus statement from international multidisciplinary Roundtable. Med Sci Sports Exerc 2019;51(11):2375–2390; doi: 10.1249/MSS.0000000000002116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Adamsen L, Andersen C, Midtgaard J, et al. . Struggling with cancer and treatment: Young athletes recapture body control and identity through exercise: Qualitative findings from a supervised group exercise program in cancer patients of mixed gender undergoing chemotherapy. Scand J Med Sci Sports 2008;19(1):55–66; doi: 10.1111/j.1600-0838.2007.00767.x [DOI] [PubMed] [Google Scholar]
- 3. Dimeo F, Schwartz S, Wesel N, et al. . Effects of an endurance and resistance exercise program on persistent cancer-related fatigue after treatment. Ann Oncol 2008;19(8):1495–1499; doi: 10.1093/annonc/mdn068 [DOI] [PubMed] [Google Scholar]
- 4. Puetz TW, Herring MP. Differential effects of exercise on cancer-related fatigue during and following treatment: A meta-analysis. Am J Prev Med 2012;43(2):e1; doi: 10.1016/j.amepre.2012.04.027 [DOI] [PubMed] [Google Scholar]
- 5. Irwin ML, McTiernan A, Manson JE, et al. . Physical activity and survival in postmenopausal women with breast cancer: Results from the women's health initiative. Cancer Prev Res (Phila) 2011;4(4):522; doi: 10.1158/1940-6207.CAPR-10-0295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Da Silva Alves R, Iunes DH, Pereira IC, et al. . Influence of exergaming on the perception of cancer-related fatigue. Games Health J 2017;6(2):119–126; doi: 10.1089/g4h.2016.0051 [DOI] [PubMed] [Google Scholar]
- 7. Oliveira PF de, Iunes DH, Alves RS, et al. . Effects of exergaming in cancer related fatigue in the quality of life and electromyography of the middle deltoid of people with cancer in treatment: A Controlled Trial. Asian Pac J Cancer Prev 2018;19(9):2591; doi: 10.22034/APJCP.2018.19.9.2591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vernadakis N, Derri V, Tsitskari E, et al. . The effect of Xbox Kinect intervention on balance ability for previously injured young competitive male athletes: A preliminary study. Phys Ther Sport 2014;15(3):148–155; doi: 10.1016/j.ptsp.2013.08.004 [DOI] [PubMed] [Google Scholar]
- 9. Oliveira PF de, Alves R da S, Iunes DH, et al. . Effect of exergaming on muscle strength, pain, and functionality of shoulders in cancer patients. Games Health J 2020;20(4):1–7; doi: 10.1089/g4h.2019.0113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Da Silva Alves R, Iunes DH, De Carvalho JM, et al. . Effects of exergaming on quality of life in cancer patients. Games Health J 2018;7(6):385–392; doi: 10.1089/g4h.2017.0174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Comello MLG, Francis DiB, Marshall LH, et al. . Cancer survivors who play recreational computer games: Motivations for playing and associations with beneficial psychological outcomes. Games Health J 2016;5(4):286–292; doi: 10.1089/g4h.2016.0003 [DOI] [PubMed] [Google Scholar]
- 12. Rhodes RE, Warburton DER, Bredin SSD. Predicting the effect of interactive video bikes on exercise adherence: An efficacy trial. Psychol Health Med 2009;14(6):631–640; doi: 10.1080/13548500903281088 [DOI] [PubMed] [Google Scholar]
- 13. Tough D, Robinson J, Gowling S, et al. . The feasibility, acceptability and outcomes of exergaming among individuals with cancer: A systematic review. BMC Cancer 2018;18(1):1151; doi: 10.1186/s12885-018-5068-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ho SS, Lwin MO, Sng JRH, et al. . Escaping through exergames: Presence, enjoyment, and mood experience in predicting children's attitude toward exergames. Comput Human Behav 2017;72:381–389; doi: 10.1016/j.chb.2017.03.001 [DOI] [Google Scholar]
- 15. Senn S. Cross-Over Trials in Clinical Research, 2nd ed. John Wiley & Sons, Ltd.: Hoboken, NJ, USA; 2002; doi: 10.1002/0470854596 [DOI] [Google Scholar]
- 16. Nolan SJ, Hambleton I, Dwan K. The use and reporting of the cross-over study design in clinical trials and systematic reviews: A systematic assessment. PLoS One 2016;11(7); doi: 10.1371/journal.pone.0159014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Webster K, Cella D, Yost K. The Functional Assessment of Chronic Illness Therapy (FACIT) measurement system: Properties, applications, and interpretation. Health Qual Life Outcomes 2003;1:79; doi: 10.1186/1477-7525-1-79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Monga U, Jaweed M, Kerrigan AJ, et al. . Neuromuscular fatigue in prostate cancer patients undergoing radiation therapy. Arch Phys Med Rehabil 1997;78(9):961–966; doi: 10.1016/S0003-9993(97)90058-7 [DOI] [PubMed] [Google Scholar]
- 19. Kisiel-Sajewicz K, Davis MP, Siemionow V, et al. . Lack of muscle contractile property changes at the time of perceived physical exhaustion suggests central mechanisms contributing to early motor task failure in patients with cancer-related fatigue. J Pain Symptom Manage 2012;44(3):351–361; doi: 10.1016/j.jpainsymman.2011.08.007 [DOI] [PubMed] [Google Scholar]
- 20. Burdon JGW, Juniper EF, Killian KJ, et al. . The perception of breathlessness in asthma. Am Rev Respir Dis 1982;126(5):825–828; doi: 10.1164/arrd.1982.126.5.825 [DOI] [PubMed] [Google Scholar]
- 21. Cohen J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed. Lawrence Erlbaum Associates, Publishers: New York, NY; 2013; doi: 10.4324/9780203771587 [DOI] [Google Scholar]
- 22. Savina S, Zaydiner B. Cancer-related fatigue: Some clinical aspects. Asia-Pacific J Oncol Nurs 2019;6:7–9; doi: 10.4103/APJON.APJON_45_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Borneman T. Assessment and management of cancer-related fatigue. J Hosp Palliat Nurs 2013;15(2):77–86; doi: 10.1097/NJH.0b013e318286dc19 [DOI] [Google Scholar]
- 24. Irwin ML, Smith AW, McTiernan A, et al. . Influence of pre- and postdiagnosis physical activity on mortality in breast cancer survivors: The health, eating, activity, and lifestyle study. J Clin Oncol 2008;26(24):3958–3964; doi: 10.1200/JCO.2007.15.9822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cella D, Lai J, Chang C-H, et al. . Fatigue in cancer patients compared with fatigue in the general United States population. Cancer 2002;94(2):528–538; doi: 10.1002/cncr.10245 [DOI] [PubMed] [Google Scholar]
- 26. Santana MJ, Au HJ, Dharma-Wardene M, et al. . Health-related quality of life measures in routine clinical care: Can FACT-Fatigue help to assess the management of fatigue in cancer patients? Int J Technol Assess Health Care 2009;25(1):90–96; doi: 10.1017/S0266462309090126 [DOI] [PubMed] [Google Scholar]
- 27. Prinsen H, Van Dijk JP, Zwarts MJ, et al. . The role of central and peripheral muscle fatigue in postcancer fatigue: A randomized controlled trial. J Pain Symptom Manage 2015;49(2):173–182; doi: 10.1016/j.jpainsymman.2014.06.020 [DOI] [PubMed] [Google Scholar]
- 28. Häkkinen K, Alen M, Kraemer WJ, et al. . Neuromuscular adaptations during concurrent strength and endurance training versus strength training. Eur J Appl Physiol 2003;89(1):42–52; doi: 10.1007/s00421-002-0751-9 [DOI] [PubMed] [Google Scholar]
- 29. Zwarts MJ, Bleijenberg G, van Engelen BGM. Clinical neurophysiology of fatigue. Clin Neurophysiol 2008;119(1):2–10; doi: 10.1016/j.clinph.2007.09.126 [DOI] [PubMed] [Google Scholar]
- 30. Argyriou AA, Bruna J, Marmiroli P, et al. . Chemotherapy-induced peripheral neurotoxicity (CIPN): An update. Crit Rev Oncol Hematol 2012;82(1):51–77; doi: 10.1016/j.critrevonc.2011.04.012 [DOI] [PubMed] [Google Scholar]
- 31. Sidhu SK, Weavil JC, Mangum TS, et al. . Group III/IV locomotor muscle afferents alter motor cortical and corticospinal excitability and promote central fatigue during cycling exercise. Clin Neurophysiol 2017;128(1):44–55; doi: 10.1016/j.clinph.2016.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kraemer, WJ; Ratamess, NA; French D. Resistance training for health and performance. Curr Sports Med Rep 2002;1:165–171. [DOI] [PubMed] [Google Scholar]
- 33. Mujika I, Padilla S. Muscular characteristics of detraining in humans. Med Sci Sports Exerc 2001;33(8):1297–1303; doi: 10.1097/00005768-200108000-00009 [DOI] [PubMed] [Google Scholar]