Abstract
Objective:
Reproductive factors, including parity, may contribute to dementia risk, due to hormonal, physiological, social, and demographic factors. We hypothesized that higher parity would be associated with increased dementia risk.
Materials and Methods:
We utilized data from the Atherosclerosis Risk in Communities (ARIC) community-based cohort study. Participants were recruited in 1987–1989 and followed through 2017. Participants, all born between 1921 and 1945, were from four U.S. communities in Forsyth County, NC; Jackson, MS; Minneapolis, MN; and Washington County, MD. We included all female participants seen at ARIC visit three or five for whom parity and dementia outcomes were available (N = 7,921). The primary exposure was self-reported number of live births. Our primary outcome was dementia, diagnosed via neurocognitive assessments, informant interviews, and expert adjudication. We created Cox proportional hazards models to evaluate the association between parity and incident dementia, adjusting for demographic factors, education level, apolipoprotein E allele status, and vascular risk factors. We tested for interactions by race and birth cohort.
Results:
The adjusted hazard ratio was 0.82 (95% confidence intervals [CI] 0.69–0.99) for dementia in women with 0–1 births and 0.85 (95% CI 0.72–0.99) for women with 5+ births, compared to women with 2 births (reference group). This association was present in women born from 1924 to 1934, but not in women born in 1935 or later (p-interaction <0.001).
Conclusion:
We found an inverted U-shaped association of parity with dementia risk. This effect was modified by birth cohort, suggesting that the association may depend on demographic and sociocultural factors.
Keywords: dementia, parity, reproduction, women, cognition
Introduction
Women are disproportionately affected by Alzheimer's disease (AD) and related dementias. Globally, women have 80% more dementia deaths and 70% more dementia-related disability-adjusted life years, compared to men.1 While women's longer life spans may partially account for this,2 women with one copy of the apolipoprotein E ɛ4 (APOEɛ4) allele have higher likelihood of AD diagnosis than men with two copies,3 and neuropsychological studies suggest that women experience faster cognitive decline than men.4 Sex-related biological factors may also influence dementia presentation: postmenopausal women perform worse than men on verbal fluency and verbal memory tests.5
Parity is a sex-specific factor which might have either protective or deleterious effects on dementia risk. Hypothesized mechanisms for these effects include increased exposure to estrogen over the life course,6 short- and long-term immunological changes,7 physiological stress of pregnancy,2 and gendered aspects of caregiving that reflect social and cultural experiences.2,8–10
However, the relationship between parity and dementia risk in women remains incompletely understood. Having more pregnancies results in higher lifetime estrogen exposure, but studies of dementia risk and parity as a proxy for estrogen exposure have yielded conflicting results. In a cohort of British women, longer reproductive life span (time from menarche to menopause) and more months spent pregnant had a protective effect against AD risk.11 Similar associations have been observed between higher parity and higher gray matter volume, slower cognitive decline, and lower dementia risk.12,13 However, other studies showed opposite effects, with higher risk of cognitive impairment and dementia in those with higher parity.14,15 A neuropathological study showed that having more children correlated with higher proportion of AD pathology in postmortem brains of women; this finding was not present in men, suggesting a sex specific effect of either childbearing or child rearing.16 Another study showed that while having children was associated with AD in women, the number of children did not impact the risk.17
We investigated the relationship of parity to dementia risk in women within a large prospective cohort, the community-based Atherosclerosis Risk in Communities (ARIC) study. We hypothesized that higher parity is associated with higher risk of incident dementia, independent of demographic characteristics and risk factors.
Materials and Methods
Study design and population
Participants were enrolled through the ongoing ARIC prospective cohort study. Detailed methods of the ARIC study protocol have been previously described.18–20 Study participants comprise people living in four U.S. communities: Forsyth County, NC; Jackson, MS; suburbs of Minneapolis, MN; and Washington County, MD. Beginning in 1987, 4,000 individuals aged 45–64 were randomly recruited and enrolled from each community. A total of 15,792 participants received a baseline examination in 1987–1989, with medical, social, and demographic data recorded. ARIC participants were mostly White in the Washington County and Minneapolis centers, only Black in the Jackson center, and included both races in Forsyth County. Non-Black and non-White participants, as well as Black participants in Minneapolis or Washington County and White participants in Jackson, were excluded from this analysis due to small sample size.21
Participants have been reexamined periodically, with Visit 2 between 1990 and 1992; Visit 3 in 1993–1995; Visit 4 in 1996–1998; Visit 5 in 2011–2013; Visit 6 in 2016–2017; Visit 7 in 2018–2019; and Visit 8 in 2020. Data collection for the ARIC study is ongoing, with ongoing in-person visits and telephone interviews carried out annually (semiannual since 2012) to maintain contact and collect information regarding interim hospitalizations.
Neurocognitive assessments
All ARIC participants underwent a three-instrument cognitive assessment (delayed word recall task, digit symbol substitution from the Wechsler Adult Intelligence Scale-Revised [WAIS-R], and a letter fluency task) at ARIC Visit 2 (1990–1992).
The ancillary ARIC Neurocognitive Study (ARIC-NCS) began in June 2011 at Visit 5, when all surviving ARIC participants were invited to undergo in-person cognitive testing covering multiple cognitive domains, including memory, psychomotor speed/executive functioning, language, and visuospatial.18,22–24 Test scores within each domain were used to derive domain-specific, as well global composite Z-scores. Participants with evidence of cognitive impairment, defined as either a low absolute mini-mental state examination (<21 for White participants; <19 for Black participants) or a Z-score < −1.5 relative to age, race, and education-specific normative data in any of five cognitive domains (memory, language, attention, executive function, or visuospatial), or who showed definite cognitive decline based on prior scores administered at ARIC visits 2 and 4, underwent additional evaluation with informant interviews, including the Clinical Dementia Rating interview and the Functional Activities Questionnaire. A random sample of 10% of those who did not meet these criteria (i.e., those who were presumed cognitively normal) underwent the same evaluation.18 Visit 6 incorporated the same cognitive assessment and informant interviews in a subset. Full details of procedures completed in each study visit and cognitive outcome measures have been previously published18,24 and are summarized in our Supplementary Methods in Supplementary Data. Female participants from ARIC without dementia at Visit 1 were included in this analysis (Supplementary Fig. S1), and our analysis included all data collected through the end of the Visit 6 cycle (end of 2017).
Standard protocol approvals, registrations, and patient consents
All institutional review boards affiliated with each ARIC field center approved this study, and all participants provided written informed consent before enrollment.
Exposures of interest
The primary exposure was number of live births, self-reported by questionnaire at Visit 3, when participants reported “number of liveborn children.” We considered parity both as a dichotomous variable (≥1 live birth/no live births) and as a categorical variable with categories based on the distribution in the study population: 0–1 live births, 2 live births (reference), 3–4 live births, ≥5 live births. As a secondary exposure of interest, we considered age at first live birth, self-reported at visit 3.
Missing data
A total of 737 (8.5%) participants were missing data on key variables such as parity or important covariates (education, leisure index, or vascular risk factors). The breakdown of participants excluded for missing data is shown in the Supplementary Figure S1.
Primary outcome
Our primary outcome was incident dementia after visit 1, categorized as a dichotomous variable (dementia vs. no dementia). Cognitive outcomes, including dementia diagnoses, were assessed in ARIC-NCS using in-person assessments,18 with expert adjudication of cognitive status based on criteria from the National Institute on Aging-Alzheimer's Association workgroups25,26 and Statistical Manual of Mental Disorders, fifth edition (DSM-5).27 Additional dementia cases were identified via telephone interviews if participants were unwilling or unable to be examined in person, telephone interviews of informants, and surveillance of hospitalization codes and death certificate codes between visits.18
Dementia diagnoses in ARIC are classified as follows: Level 1 includes adjudicated dementia from complete evaluation at either of the ARIC-NCS visits, incorporating data from longitudinal cognitive evaluations (visits 2, 4, 5, and 6), complete neuropsychological battery at the ARIC-NCS visit (visits 5 and 6), and the informant interview, with expert classification of cognitive status.24 Level 2 includes level 1 plus (1) participants who did not attend visit 5 or 6 but were classified as having dementia based on predefined criteria from the Telephone Interview for Cognitive Status–Modified or (2) living or deceased persons classified as having dementia based on predefined criteria from informant telephone interviews using a modified version of the Clinical Dementia Rating and the Functional Activities Questionnaire among a subset identified as having suspect dementia, or in an age comparable random sample of 100 participants not otherwise meeting the mentioned criteria. Level 3, the primary outcome in this analysis, also includes dementia cases identified by surveillance based on a prior discharge hospitalization ICD-9 or death certificate code for dementia through the date of last participant contact.18,28,29 For level 1 or level 2 diagnoses, if hospitalization codes were also identified, the earlier date was used as date of onset for the Cox models. Additional details of cognitive assessment and adjudication procedures have been previously published18,24 and are summarized in the Supplementary Methods S1.
Covariates
Covariates of interest included age, a combined race-field center variable, smoking status, alcohol consumption status, body mass index (BMI), APOEɛ4 genotype, total cholesterol, hypertension and diabetes status, leisure index,30,31 education level, and history of stroke before or during ARIC study participation. Covariates were assessed at visit 1; while the primary exposure (parity) was not assessed until visit 3, we assumed that parity did not change significantly between visits 1 and 3 due to the age of the cohort (mean age 53 years at time of recruitment; no participants under age 45 at time of recruitment). Additional details of covariate definitions are in the Supplementary Methods S1.
Secondary analyses
Age at first live birth was considered using the same outcomes as for the primary analysis. We performed planned stratified analyses for both exposures (primary and secondary) by race (Black and White participants), age at baseline enrollment (<55 and ≥55 years), and birth cohort (before and after the median birth year of participants). We performed three sensitivity analyses: the first excluded participants who experienced a stroke between baseline enrollment and time of dementia diagnosis; the second excluded participants who had no live births; and the third included death as a competing risk with dementia diagnosis.
Statistical analysis
Demographics and baseline characteristics were compared between the no dementia and dementia groups using paired t-tests. We generated Kaplan–Meier curves for dementia-free survival and created Cox proportional hazard models to calculate unadjusted and adjusted hazard ratios (HRs) and 95% confidence intervals (95%CI) for the association between parity and dementia. The proportional hazards assumption was checked and met for the primary exposures of interest. Covariates that did not meet the proportional hazards assumption were incorporated into an interaction term with time (for ageXtime), or used as stratification variables (hypertension, diabetes, BMI category, and for some but not all analyses where the proportional hazards assumptions were violated, race-center). Secondary analyses included testing for interactions between the primary exposure and birth cohort (before and after median birth year), as well as interactions between the primary exposure and other key variables (race and education level). We considered an alpha of 0.05 significant for primary analyses and an alpha of 0.1 significant for interaction terms.
Nested adjusted models were constructed sequentially as follows, with covariates selected a priori based on potential confounders for the proposed associations with dementia: Model 1 included age at visit 1 and race/study center. Model 2 added education level to the variables from Model 1. Model 3 included all variables from Model 2, as well as baseline (Visit 1) BMI, hypertension and diabetes status, total cholesterol, smoking and alcohol use, leisure index score category, and APOEɛ4 status. Model 4 included all variables from Model 3 and added history of stroke. We did not correct for multiple comparisons since the final prespecified model included all previous models. Exploratory secondary and sensitivity analyses were not corrected for multiple comparisons. Stata version 17.0 (StataCorp, College Station, TX, USA) was used for all analyses.
Results
There were 7,921 women included in the analyses, with a total of 184,191 person-years of follow-up. Baseline characteristics in the study population are shown in Table 1. The average age of women included in the analysis was 53.8, with birth years ranging from 1921 to 1945. The majority of women (70.2%) were White. Participant education levels varied, with the largest percentage (45%) having a high school education. The most prevalent risk factors were hypertension (35.3%), tobacco use (24.9%), and alcohol use (48.4%).
Table 1.
Baseline Characteristics of Analytic Sample (N = 7,921), Total and by Birth Category
| Characteristic | Total sample (N = 7,921) | 0–1 birth (N = 1,134) | 2 births (N = 2,090) | 3–4 births (N = 3,057) | 5+ births (N = 1,640) |
|---|---|---|---|---|---|
| Age in years | 53.8 (5.7) | 54.1 (6.0) | 53.2 (5.8) | 53.5 (5.7) | 54.8 (5.3) |
| Black race, n (%) | 2,363 (29.8) | 349 (30.8) | 394 (18.9) | 733 (24.0) | 887 (54.1) |
| Educational level | |||||
| <High school, n (%) | 1,897 (24.0) | 238 (21.0) | 332 (15.9) | 618 (20.2) | 709 (43.2) |
| High school/GED, n (%) | 3,550 (44.8) | 506 (44.6) | 977 (46.8) | 1410 (46.1) | 657 (40.1) |
| >High school, n (%) | 2,474 (31.2) | 390 (34.4) | 781 (37.4) | 1029 (33.7) | 274 (16.7) |
| Hypertension, n (%) | 2,799 (35.3) | 407 (35.9) | 622 (29.8) | 986 (32.3) | 784 (47.8) |
| Diabetes, n (%) | 808 (10.2) | 117 (10.3) | 151 (7.2) | 271 (8.9) | 259 (16.4) |
| BMI (kg/m2) | 27.9 (6.1) | 27.2 (6.1) | 26.7 (5.6) | 27.9 (5.9) | 30.1 (6.6) |
| Total cholesterol (mmol/L) | 5.63 (1.12) | 5.60 (1.10) | 5.60 (1.10) | 5.64 (1.13) | 5.69 (1.13) |
| Current smoker, n (%) | 1,968 (24.9) | 331 (29.2) | 529 (25.3) | 713 (23.3) | 395 (24.1) |
| Current alcohol use, n (%) | 3,837 (48.4) | 527 (46.5) | 1121 (53.6) | 1,592 (52.1) | 597 (36.4%) |
| History of stroke (baseline or during follow-up), n (%) | 754 (9.5) | 109 (9.6) | 156 (7.5) | 281 (9.2) | 208 (12.7%) |
| Leisure physical activity score | 2.4 (0.6) | 2.4 (0.6) | 2.4 (0.6) | 2.4 (0.6) | 2.2 (0.6) |
| APOE (1 or 2 ɛ4 alleles), n (%) | 2,332 (29.4) | 380 (33.5) | 694 (33.2) | 1,030 (33.7) | 546 (33.3) |
| Any births, n (%) | 7,760 (98) | 973 (85.8) | 2,090 (100) | 3,057 (100) | 1,640 (100) |
| Age at first birth (years)a | 22.1 (4.2) | 24.9 (5.6) | 23.2 (4.1) | 21.6 (3.3) | 19.6 (2.9) |
| Number of live-born children | 3.3 (2.0) | 0.9 (0.3) | 2.0 (0.0) | 3.4 (0.5) | 6.4 (1.7) |
| Year of birth (median [Q1–Q3]) | 1934 (1929–1939) | 1934 (1928–1939) | 1935 (1930–1940) | 1935 (1930–1939) | 1933 (1929–1937) |
Mean (SD) or N(%) listed. Baseline characteristics collected at Visit 1.
Leisure physical activity score was assessed with a 16-item questionnaire with score ranging from 1 (low) to 5 (high) for each item.
Missing in 699 women, results calculated out of total sample of 7,222 with available data.
APOE, Apolipoprotein E; BMI, body mass index; GED, general educational development; SD, standard deviation.
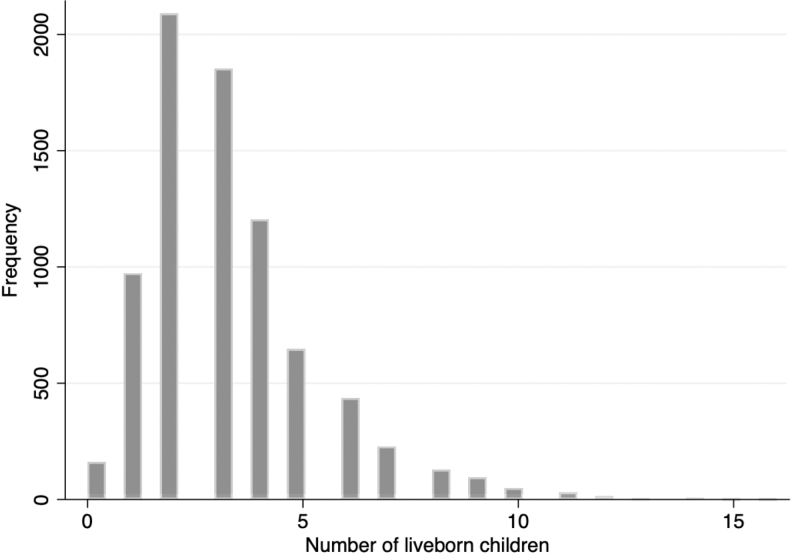
Most women (97.3%) had at least 1 birth with an average of 3.3 live births. The study population demonstrated a positively skewed distribution of number of liveborn children, with two being the most common number of liveborn children (Fig. 1).
FIG. 1.
Distribution of parity in the ARIC cohort. Parity distribution of 7,921 women participants in ARIC included in the analyses. There was an average of 3.3 live births per woman, with 97.3% of women having at least 1 birth. There was a positively skewed distribution of number of liveborn children, with two being the most common. ARIC, Atherosclerosis Risk in Communities.
Primary outcome
A total of 1,368 women were diagnosed with dementia during the study period. Among those diagnosed with dementia, 314 were diagnosed via full in-person assessment (Level 1); 964 diagnosed with combination of telephone interviews of participants and telephone interviews of informants (Level 2); and the remaining 90 were identified from hospitalization records alone (Level 3).
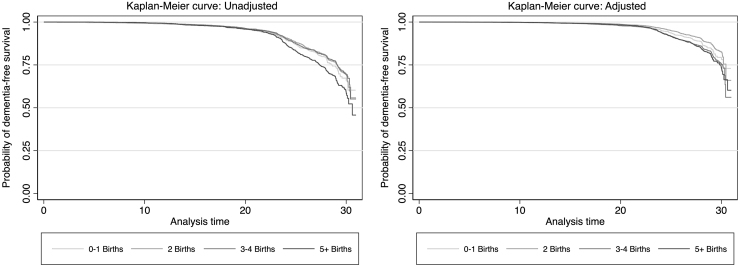
Kaplan–Meier curves for dementia-free survival, categorized by number of births, are shown in Figure 2. Incidence rates and HRs based on number of births in the total sample are shown in Table 2. In unadjusted analysis, having ≥5 live births was associated with a higher risk of dementia (unadjusted HR: 1.41 [95% CI: 1.21–1.64]). However, after controlling for age and race center, this association was no longer significant. In fully adjusted models, women at either extreme—either with 0–1 live birth or ≥5 live births—were less likely to develop dementia than those with 2 live births (the reference group) (adjusted HRs: 0.82 [95% CI: 0.69–0.99]) and (0.85 [95% CI: 0.79–0.99]), respectively. This observation remained significant in sensitivity analyses excluding participants with interval stroke between baseline and dementia onset and excluding women with no live births (Supplementary Tables S1, S2). The effect direction was similar when death was considered as a competing risk (data not shown). A sensitivity analysis, including only Level 1 diagnoses in the outcome, showed a similar protective effect direction for women with 0–1 birth, but no protective effect for women with ≥5 live births (Supplementary Table S3).
FIG. 2.
Kaplan–Meier dementia-free survival curves for women in the ARIC cohort, categorized by number of liveborn children. Adjusted curve accounts for covariates included in Model 4 and shows survival set to median values for age at study onset, education set at high school education, body mass index at median, leisure index at median; center-race variable for a White individual from Forsyth County, and absence of hypertension, diabetes, APOE e4, current smoking, and current alcohol use. APOE, Apolipoprotein E.
Table 2.
Hazard Ratios and Dementia Incidence Rates Based on Number of Births and Age at Birth: Total sample (N = 7,921)
| Dementia incidence rate (per 1,000 person-years) | Unadjusted HR (95% CI) | Model 1 HR (95% CI) | Model 2 HR (95% CI) | Model 3 HR (95% CI) | Model 4 HR (95% CI) | |
|---|---|---|---|---|---|---|
| Number of births | ||||||
| 0–1 | 7.22 | 1.10 (0.92–1.31) | 0.91 (0.76–1.08) | 0.90 (0.75–1.048) | 0.85 (0.71–1.02) | 0.82 (0.69–0.99) |
| 2 (Ref) | 7.07 | 1 | 1 | 1 | 1 | 1 |
| 3–4 | 6.92 | 0.99 (0.86–1.13) | 0.90 (0.78–1.03) | 0.89 (0.77–1.02) | 0.90 (0.78–1.03) | 0.88 (0.77–1.01) |
| 5+ | 9.06 | 1.41 (1.21–1.64) | 0.95 (0.82–1.12) | 0.85 (0.73–1.00) | 0.86 (0.73–1.01) | 0.85 (0.72–0.99) |
| Model 1: age, race-center Model 2: age, race-center, education Model 3: age, race-center, education, smoking (visit 1), cholesterol (visit 1), BMI category, alcohol use (visit 1), leisure index (visit 1), APOE status (stratified by diabetes, hypertension, race-center; included age modeled as age × time interaction, due to violated proportional hazards assumptions) Model 4: model 3 + stroke | ||||||
| Mother's age when first child borna (years) | ||||||
| <20 years | 8.41 | 1.35 (1.20–1.52) | 1.30 (1.15–1.48) | 1.11 (0.97–1.27) | 1.10 (0.96–1.26) | 1.12 (0.97–1.29) |
| 20–29 years (ref/mode) | 6.81 | 1 | 1 | 1 | 1 | 1 |
| 30 years and older | 8.43 | 1.29 (1.03–1.62) | 1.13 (0.90–1.43) | 1.19 (0.95–1.50) | 1.21 (0.96–1.54) | 1.22 (0.96–1.54) |
|
aIn N = 7,222; For age at birth: Model 1: age, stratified by race-center Model 2: age, education, stratified by race-center Model 3: age, race-center, education, smoking (visit 1), cholesterol (visit 1), BMI category, alcohol use (visit 1), leisure index (visit 1), APOE status (stratified by diabetes, hypertension, race-center; included age modeled as age × time interaction, due to violated proportional hazards assumptions) Model 4: model 3 + stroke | ||||||
Bold values indicate confidence interval does not cross 1.
CI, confidence interval; HR, hazard ratio.
Secondary analyses
Age at first birth
Women younger than 20 years old at the time of their first birth or over 30 years of age at the time of their first birth had a higher likelihood of developing dementia in the unadjusted model, but the effect was no longer significant in adjusted models (Table 2).
Race-stratified models
Stratified analyses of parity and dementia risk by race showed that the protective association of higher parity was statistically significant in White, but not Black women. In addition, age <20 years at first birth was associated with higher risk of dementia in White women (adjusted HR 1.21, 95% CI 1.03–1.44), but not in Black women, even adjusting for education (Supplementary Tables S4, S5).
Birth cohort and age
The associations between parity and dementia varied by birth cohort and age. When stratified by birth cohort (born between 1921 and the median birth year of 1934, vs. 1935 or later), there was a significant interaction between cohort and number of children on risk for dementia in the fully adjusted model. In the pre-1935 cohort, having three or more children was protective against risk of dementia compared to the reference category of two children (p-value for interaction = 0.05 for three to four children and p-value for interaction <0.001 for five or more children). In the 1935 or later cohort, this effect was not seen (Table 3). A similar pattern was seen with age at time of study entry (Supplementary Tables S6, S7); older, but not younger women with more children had lower risk of dementia. A breakdown of baseline characteristics of the pre-1935 and 1935 or later cohorts is shown in Supplementary Table S8.
Table 3.
Hazard Ratios and Incidence Rates for Dementia by Birth Cohort
| Pre-1935 birth cohort Year of birth 1921–1934 (N = 4,039); ages 52–66 on study enrollment 1986–1988 | ||||||
|---|---|---|---|---|---|---|
| No. of births | Dementia incidence rate per 1,000 person-years | Unadjusted HR (95% CI) | Model 1 HR (95% CI) | Model 2 HR (95% CI) | Model 3 HR (95% CI) | Model 4 HR (95% CI) |
| 0–1 | 11.49 | 0.97 (0.79–1.19) | 0.87 (0.71–1.07) | 0.86 (0.70–1.6) | 0.81 (0.65–1.00) | 0.77 (0.62–0.96) |
| 2 (Ref) | 12.25 | 1 | 1 | 1 | 1 | 1 |
| 3–4a | 10.72 | 0.85 (0.72–1.00) | 0.84 (0.71–0.98) | 0.83 (0.71–0.98) | 0.81 (0.69–0.96) | 0.79 (0.67–0.93) |
| 5+b | 11.20 | 0.91 (0.76–1.09) | 0.95 (0.80–1.12) | 0.74 (0.62–0.90) | 0.72 (0.59–0.87) | 0.70 (0.57–0.85) |
| 1935 or later birth cohort Year of birth 1935–1945 (N = 3,882); ages 45–54 on study enrollment 1986–1988 | ||||||
|---|---|---|---|---|---|---|
| No. of births | Dementia incidence rate per 1,000 person-years | Unadjusted HR(95% CI) | Model 1 HR(95% CI) | Model 2 HR(95% CI) | Model 3 HR(95% CI) | Model 4 HR(95% CI) |
| 0–1 | 3.29 | 1.14 (0.80–1.64) | 1.06 (0.73–1.52) | 1.06 (0.74–1.52) | 1.05 (0.72–1.52) | 1.03 (0.71–1.51) |
| 2 (Ref) | 3.15 | 1 | 1 | 1 | 1 | 1 |
| 3–4a | 3.70 | 1.21 (0.93–1.57) | 1.08 (0.83–1.41) | 1.03 (0.79–1.35) | 1.04 (0.79–1.37) | 1.02 (0.77–1.35) |
| 5+b | 6.31 | 2.32 (1.74–3.09) | 1.39 (1.03–1.88) | 1.11 (0.82–1.52) | 1.18 (0.86–1.63) | 1.14 (0.82–1.58) |
Bold values indicate confidence interval does not cross 1.
Birth cohorts were divided into 1921–1934 and 1935–1945, based on median year of birth of 1935.
Model 1: age; stratified by race-center.
Model 2: age, education; stratified by race-center.
Model 3: age, education, smoking, cholesterol, alcohol use, leisure index, APOE; stratified by race-center, hypertension, diabetes, BMI category.
Model 4: age, education, smoking, cholesterol, alcohol use, leisure index, APOE; stratified by hypertension, diabetes, BMI category, race-center, stroke.
Models 3 and 4 included age modeled as age × time interaction, due to violated proportional hazards assumptions.
p-value for interaction = 0.05.
p-value for interaction <0.001.
Discussion
In our analysis of women in the community-based ARIC prospective cohort, contrary to our hypothesis, we found an inverted U-shaped relationship between parity and dementia risk. Compared to women with two children, women with zero or one child had 18% lower dementia risk; women with three to four children had 12% nonsignificantly lower risk; and women with five or more children had 17% lower risk. These differences remained significant after adjusting for education, vascular comorbidities, and APOE status.
Number of births could influence future dementia risk in multiple ways.6 Pregnancy results in high exposure to endogenous reproductive hormone levels. Studies conflict regarding whether these hormones have a neuroprotective or harmful effect.32,33 Reproductive life span (time between menarche and menopause) in U.S. women increased significantly from 1959 through 2018, related to both earlier age at menarche and later age at natural menopause.34 In a cohort of over 15,000 U.S. women, lower lifetime endogenous estradiol exposure (later menarche, earlier menopause, or hysterectomy) was associated with higher dementia risk.35 A Korean cohort of over 4.6 million women found a similar effect.36
A longer reproductive life span could correlate with having more children, especially in age groups to whom contraception was not readily available. However, the effect of pregnancy-related endogenous hormone exposure may differ from the effect of cumulative endogenous estradiol due to increased reproductive life span. For example, higher parity was associated with higher rather than lower dementia risk in the Korean cohort.36 Animal studies suggested that pregnancy-related estrogen exposure decreased hippocampal volume in the short-term, but enhanced long-term hippocampal learning plasticity and potentiation through a mechanism of dendritic remodeling.37–39 In humans, grand multiparity (5+ births) was associated with lower hippocampal volume and brain atrophy, and lower score on the mini mental status examination, even after controlling for confounders.40
The association between parity and dementia cannot be reduced to a solely hormonal effect. In our unadjusted model, a high number of births was associated with a higher risk of dementia; however, after adjusting for age, race, and education level, the reverse association was seen and having many children appeared protective. The social support provided by children may help promote healthy cognition as parents age41–45 and increase life expectancy.45 Therefore, having multiple children may have a protective effect on overall, as well as cognitive, health. The associations between parity and dementia remained significant after adjusting for vascular risk factors and excluding those with history of stroke, suggesting that the relationship between parity and dementia risk may depend on social rather than vascular mediators of health.
Number of children among women who survive to older adulthood could also reflect overall biological fitness from an evolutionary perspective, which may also relate to brain health.46 In addition, those who survive more births may represent a particularly robust group of individuals, given patterns of maternal mortality during this cohort's reproductive years.47
We found that the associations between parity and dementia risk differed by birth cohort. The group of women born between 1921 and 1934 had an inverted U-shaped relationship between parity and dementia, with a protective effect of either 0–1 or ≥3 births, compared to women with 2 births. However, in the group of women born between 1935 and 1945, ≥5 births were associated with higher dementia risk; this association was no longer significant after adjusting for education and health characteristics. In the United States, average number of children per woman declined from 3.2 for the 1931–1935 birth cohorts to 2.5 for the 1941–1945 birth cohorts.48 At the same time, median years of education among women age ≥25 years increased from 10.7 years in 1960 to 12.1 years in 1970.49 Thus, our results may reflect shifting roles for women during this time period. Having ≥5 births may protect cognition among older birth cohorts, in a higher fertility environment. For younger cohorts, the benefits of lower fertility may reflect the trade-off between child-rearing and educational and employment opportunities.50
In secondary analyses exploring the relationship between race and dementia risk, we found that higher parity was protective in White, but not Black women. This may be due to being underpowered for subgroup analyses. However, due to systemic inequities in health care rooted in historical and present-day structural racism, Black women experience more adverse pregnancy outcomes, such as preterm birth and hypertensive disorders of pregnancy.51,52 Pregnancy complications are associated with future cardiovascular and cerebrovascular disease,8,53–56 both risk factors for dementia. The protective effect of higher parity may have been attenuated by a higher incidence of adverse pregnancy outcomes in Black women. Unfortunately, ARIC did not collect data regarding pregnancy outcomes.
Our results contradict those of prior studies demonstrating the opposite relationship between parity and age-related diseases. For example, in a Swedish population-based cohort of 1.3 million women, parity was associated with maternal cardiovascular disease in a J-shaped pattern, with 2 births representing the lowest risk category.57 Similarly, the U.S. population-based National Health and Nutrition Examination Survey (NHANES) found a U-shaped relationship between parity and validated biological markers of aging in postmenopausal, but not premenopausal women.58 A cohort study in England demonstrated a similar U-shaped relationship between number of children and dementia risk in both men and women, suggesting a possible parenting rather than pregnancy effect.41 Our results do not align with these prior studies and should be investigated in other cohorts.
Study limitations and strengths
Our study has limitations. Parity and age at first birth were self-reported. While women are likely to accurately recall number of births and age at first birth, early cognitive impairment might affect recall (although we excluded women with baseline dementia). We may have been underpowered to detect significant interactions affecting the relationship between parity and dementia risk. Because of small numbers of women with zero or one birth and five or more births, we combined these groups, respectively, and may not detect differences related to dementia risk for these numbers of births. Important details of the reproductive history that may affect cognitive risk, such as adverse pregnancy outcomes and reproductive life span, were not available. Our study lacks information on key confounders, including caregiving, interactions with children,41–44 and more detailed socioeconomic characteristics such as occupation41; these factors have known gender disparities that might influence parity and dementia risk.59,60
Since some dementia cases were identified by hospital discharge codes, it is possible that vascular risk factors are overrepresented in hospitalized individuals, which may have influenced our results. Indeed, our sensitivity analysis, including only dementia diagnoses identified by in-person assessment, suggested a protective effect for lower, but not higher parity. We also lacked information regarding dementia subtypes (e.g., vascular dementia, Alzheimer's disease). Finally, the original ARIC participants were recruited from the four communities of interest by probability sampling,20 and while the communities were selected to vary by geographic and socioeconomic characteristics (urban, suburban, and rural), as well as racial diversity, they are not nationally representative and our results may not be generalizable to other populations. Our results should be considered hypothesis-generating, interpreted with caution, and verified in other cohorts.
Strengths of our study include the robust diagnoses of dementia, including timing of onset. As a community-based cohort, ARIC is generally representative of these four U.S. communities, and its prospective and longitudinal nature allows ongoing surveillance not only for dementia but also other cardiovascular conditions which could influence survivorship and dementia risk. The wide range of birth years, spanning ∼20 years, allowed for consideration of birth cohort effects.
Conclusions
In a prospective community-based cohort of nearly 8,000 older women, we found an inverted U-shaped relationship between parity and dementia risk. Adjusting for demographic factors, vascular risk factors, and APOE status, women with 0 or 1 births, or ≥5 births, had lower risk of dementia compared to the reference category of women with 2 births. Our results were highly influenced by demographic factors such as birth cohort, education level, and race, suggesting that the relationship between parity and dementia risk may be related to sociocultural factors. Future studies of dementia risk in women must include details of pregnancy history, including number of pregnancies, number of births, and maternal and fetal pregnancy outcomes, as well as gendered aspects of parenting, to better characterize a currently murky relationship between parity and dementia in women.
Supplementary Material
Acknowledgment
The authors thank the staff and participants of the ARIC study for their important contributions.
Disclaimer
This article was partially prepared while R.F.G. was employed at the Johns Hopkins University School of Medicine. The opinions expressed in this article are the author's own and do not reflect the view of the National Institutes of Health, the Department of Health and Human Services, or the U.S. Government.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
E.C.M. receives support from the National Institutes of Health (NIH) National Institute of Neurological Disorders and Stroke (NINDS; Grant Nos. K23NS107645, R01NS122815), the NIH National Institute on Aging (NIA; Grant No. R21AG069111), and the Gerstner Family Foundation (Gerstner Scholars Program); R.F.G. receives support from the NIH NINDS Intramural Research Program; S.E.T. receives support from the NIH NIA (Grant Nos. K01AG050723, R21AG069111, R01AG069109, U19AG066567); K.A.W. receives support from the NIH NIA Intramural Research Program. This study was funded, in part, by the NIA Intramural Research Program. P.L.L. is supported by NIH NHLBI K24 HL159246. The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (Grant Nos. 75N92022D00001, 75N92022D00002, 75N92022D00003, 75N92022D00004, 75N92022D00005). The ARIC Neurocognitive Study is supported by U01HL096812, U01HL096814, U01HL096899, U01HL096902, and U01HL096917 from the NIH (NHLBI, NINDS, NIA, and NIDCD).
Supplementary Material
References
- 1. World Health Organization. Global Health Estimates: Life Expectancy and Leading Causes of Death and Disability. Available from: https://www.who.int/data/gho/data/themes/mortality-and-global-health-estimates [Last accessed: March 8, 2023].
- 2. Miller EC, Wilczek A, Bello NA, et al. Pregnancy, preeclampsia and maternal aging: From epidemiology to functional genomics. Ageing Res Rev 2022;73:101535; doi: 10.1016/j.arr.2021.101535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Riedel BC, Thompson PM, Brinton RD. Age, APOE and sex: Triad of risk of Alzheimer's disease. J Steroid Biochem Mol Biol 2016;160:134–147; doi: 10.1016/j.jsbmb.2016.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Barnes LL, Wilson RS, Schneider JA, et al. Gender, cognitive decline, and risk of AD in older persons. Neurology 2003;60(11):1777–1781; doi: 10.1212/01.wnl.0000065892.67099.2a [DOI] [PubMed] [Google Scholar]
- 5. Weber MT, Maki PM, McDermott MP. Cognition and mood in perimenopause: A systematic review and meta-analysis. J Steroid Biochem Mol Biol 2014;142:90–98; doi: 10.1016/j.jsbmb.2013.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Peterson A, Tom SE. A lifecourse perspective on female sex-specific risk factors for later life cognition. Curr Neurol Neurosci Rep 2021;21(9):46; doi: 10.1007/s11910-021-01133-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Barth C, de Lange AG. Towards an understanding of women's brain aging: the immunology of pregnancy and menopause. Front Neuroendocrinol 2020;58:100850; doi: 10.1016/j.yfrne.2020.100850 [DOI] [PubMed] [Google Scholar]
- 8. Haas DM, Parker CB, Marsh DJ, et al. Association of adverse pregnancy outcomes with hypertension 2 to 7 years postpartum. J Am Heart Assoc 2019;8(19):e013092; doi: 10.1161/JAHA.119.013092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Leon LJ, McCarthy FP, Direk K, et al. Preeclampsia and cardiovascular disease in a large uk pregnancy cohort of linked electronic health records: A CALIBER Study. Circulation 2019;140(13):1050–1060; doi: 10.1161/CIRCULATIONAHA.118.038080 [DOI] [PubMed] [Google Scholar]
- 10. Dayan N, Kaur A, Elharram M, et al. Impact of preeclampsia on long-term cognitive function. Hypertension 2018;72(6):1374–1380; doi: 10.1161/HYPERTENSIONAHA.118.11320 [DOI] [PubMed] [Google Scholar]
- 11. Fox M, Berzuini C, Knapp LA. Cumulative estrogen exposure, number of menstrual cycles, and Alzheimer's risk in a cohort of British women. Psychoneuroendocrinology 2013;38(12):2973–2982; doi: 10.1016/j.psyneuen.2013.08.005 [DOI] [PubMed] [Google Scholar]
- 12. Zhou R, Liu HM, Zou LW, et al. Associations of parity with change in global cognition and incident cognitive impairment in older women. Front Aging Neurosci 2022;14:864128; doi: 10.3389/fnagi.2022.864128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schelbaum E, Loughlin L, Jett S, et al. Association of reproductive history with brain MRI biomarkers of dementia risk in midlife. Neurology 2021;97(23):e2328–e2339; doi: 10.1212/WNL.0000000000012941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Prince MJ, Acosta D, Guerra M, et al. Reproductive period, endogenous estrogen exposure and dementia incidence among women in Latin America and China: A 10/66 population-based cohort study. PLoS One 2018;13(2):e0192889; doi: 10.1371/journal.pone.0192889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Xi H, Gan J, Liu S, et al. Reproductive factors and cognitive impairment in natural menopausal women: A cross-sectional study. Front Endocrinol (Lausanne) 2022;13:893901; doi: 10.3389/fendo.2022.893901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Beeri MS, Rapp M, Schmeidler J, et al. Number of children is associated with neuropathology of Alzheimer's disease in women. Neurobiol Aging 2009;30(8):1184–1191, doi: 10.1016/j.neurobiolaging.2007.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ptok U, Barkow K, Heun R. Fertility and number of children in patients with Alzheimer's disease. Arch Womens Ment Health 2002;5(2):83–86; doi: 10.1007/s00737-002-0142-6 [DOI] [PubMed] [Google Scholar]
- 18. Knopman DS, Gottesman RF, Sharrett AR, et al. Mild cognitive impairment and dementia prevalence: The Atherosclerosis Risk in Communities Neurocognitive Study (ARIC-NCS). Alzheimers Dement (Amst) 2016;2:1–11; doi: 10.1016/j.dadm.2015.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wright JD, Folsom AR, Coresh J, et al. The ARIC (Atherosclerosis Risk In Communities) Study: JACC focus seminar 3/8. J Am Coll Cardiol 2021;77(23):2939–2959; doi: 10.1016/j.jacc.2021.04.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. The Atherosclerosis Risk in Communities (ARIC) Study: Design and objectives. The ARIC investigators. Am J Epidemiol 1989;129(4):687–702. [PubMed] [Google Scholar]
- 21. Norby FL, Chen LY, Soliman EZ, et al. Association of left ventricular hypertrophy with cognitive decline and dementia risk over 20years: The Atherosclerosis Risk In Communities-Neurocognitive Study (ARIC-NCS). Am Heart J 2018;204:58–67; doi: 10.1016/j.ahj.2018.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rawlings AM, Bandeen-Roche K, Gross AL, et al. Factor structure of the ARIC-NCS neuropsychological battery: An evaluation of invariance across vascular factors and demographic characteristics. Psychol Assess 2016;28(12):1674–1683; doi: 10.1037/pas0000293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gottesman RF, Schneider AL, Zhou Y, et al. Association between midlife vascular risk factors and estimated brain amyloid deposition. JAMA 2017;317(14):1443–1450; doi: 10.1001/jama.2017.3090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gottesman RF, Albert MS, Alonso A, et al. Associations between midlife vascular risk factors and 25-year incident dementia in the Atherosclerosis Risk in Communities (ARIC) Cohort. JAMA Neurol 2017;74(10):1246–1254; doi: 10.1001/jamaneurol.2017.1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer's disease: Recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement (Amst) 2011;7(3):263–269; doi: 10.1016/j.jalz.2011.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: Recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement (Amst) 2011;7(3):270–279, doi: 10.1016/j.jalz.2011.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Diagnostic and statistical manual of mental disorders: DSM-5™, 5th ed. American Psychiatric Publishing, Inc.: Arlington, VA, US; 2013. [Google Scholar]
- 28. Alonso A, Mosley TH Jr., Gottesman RF, et al. Risk of dementia hospitalisation associated with cardiovascular risk factors in midlife and older age: The Atherosclerosis Risk in Communities (ARIC) study. J Neurol Neurosurg Psychiatry 2009;80(11):1194–1201; doi: 10.1136/jnnp.2009.176818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schneider AL, Gottesman RF, Mosley T, et al. Cognition and incident dementia hospitalization: Results from the atherosclerosis risk in communities study. Neuroepidemiology 2013;40(2):117–124; doi: 10.1159/000342308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr 1982;36(5):936–942; doi: 10.1093/ajcn/36.5.936 [DOI] [PubMed] [Google Scholar]
- 31. Mediano MFF, Mok Y, Coresh J, et al. Prestroke physical activity and adverse health outcomes after stroke in the Atherosclerosis Risk in Communities Study. Stroke 2021;52(6):2086–2095; doi: 10.1161/STROKEAHA.120.032695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Maki PM, Henderson VW. Hormone therapy, dementia, and cognition: The Women's Health Initiative 10 years on. Climacteric 2012;15(3):256–262; doi: 10.3109/13697137.2012.660613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Brinton RD, Yao J, Yin F, et al. Perimenopause as a neurological transition state. Nat Rev Endocrinol 2015;11(7):393–405; doi: 10.1038/nrendo.2015.82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Appiah D, Nwabuo CC, Ebong IA, et al. Trends in age at natural menopause and reproductive life span among US women, 1959–2018. JAMA 2021;325(13):1328–1330; doi: 10.1001/jama.2021.0278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gilsanz P, Lee C, Corrada MM, et al. Reproductive period and risk of dementia in a diverse cohort of health care members. Neurology 2019;92(17):e2005–e2014; doi: 10.1212/WNL.0000000000007326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yoo JE, Shin DW, Han K, et al. Female reproductive factors and the risk of dementia: A nationwide cohort study. Eur J Neurol 2020;27(8):1448–1458; doi: 10.1111/ene.14315 [DOI] [PubMed] [Google Scholar]
- 37. Pawluski JL, Galea LA. Hippocampal morphology is differentially affected by reproductive experience in the mother. J Neurobiol 2006;66(1):71–81; doi: 10.1002/neu.20194 [DOI] [PubMed] [Google Scholar]
- 38. Kinsley CH, Trainer R, Stafisso-Sandoz G, et al. Motherhood and the hormones of pregnancy modify concentrations of hippocampal neuronal dendritic spines. Horm Behav 2006;49(2):131–142; doi: 10.1016/j.yhbeh.2005.05.017 [DOI] [PubMed] [Google Scholar]
- 39. Li R, Cui J, Jothishankar B, et al. Early reproductive experiences in females make differences in cognitive function later in life. J Alzheimers Dis 2013;34(3):589–594; doi: 10.3233/JAD-122101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jung JH, Lee GW, Lee JH, et al. Multiparity, Brain atrophy, and cognitive decline. Front Aging Neurosci 2020;12:159; doi: 10.3389/fnagi.2020.00159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Read SL, Grundy EMD. Fertility history and cognition in later life. J Gerontol B Psychol Sci Soc Sci 2017;72(6):1021–1031; doi: 10.1093/geronb/gbw013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Furstenberg FF. Banking on families: How families generate and distribute social capital. J Marriage Fam 2005;67:809–821. [Google Scholar]
- 43. Zhou Z, Wang P, Fang Y. Social engagement and its change are associated with dementia risk among chinese older adults: A longitudinal study. Sci Rep 2018;8(1):1551; doi: 10.1038/s41598-017-17879-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Grundy E, Read S. Social contacts and receipt of help among older people in England: Are there benefits of having more children? J Gerontol B Psychol Sci Soc Sci 2012;67(6):742–754; doi: 10.1093/geronb/gbs082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ross CE, Mirowsky J. Family relationships, social support and subjective life expectancy. J Health Soc Behav 2002;43(4):469–489. [PubMed] [Google Scholar]
- 46. Kirkwood TB, Rose MR. Evolution of senescence: Late survival sacrificed for reproduction. Philos Trans R Soc Lond B Biol Sci 1991;332(1262):15–24; doi: 10.1098/rstb.1991.0028 [DOI] [PubMed] [Google Scholar]
- 47. Declercq E, Zephyrin LC. Maternal Mortality in the United States: A Primer [Internet]. New York, USA: The Commonweath Fund; updated December 16, 2020. Available from: https://www.commonwealthfund.org/publications/issue-brief-report/2020/dec/maternal-mortality-united-states-primer [Last accessed: July 25, 2023].
- 48. Centers for Disease Control and Prevention. Fertility Tables for Birth Cohorts by Color 1973. Available from: https://stacks.cdc.gov/view/cdc/21853 [Last accessed; March 11, 2023].
- 49. National Center for Education Statistics. 120 Years of American Education: A Statistical Portrait. Available from: https://nces.ed.gov/pubs93/93442.pdf [Last accessed: March 12, 2023].
- 50. Becker GS. A Treatise on the family. Am J Sociol 1983;89:468–470. [Google Scholar]
- 51. Creanga AA, Bateman BT, Kuklina EV, et al. Racial and ethnic disparities in severe maternal morbidity: A multistate analysis, 2008–2010. Am J Obstet Gynecol 2014;210(5):435 e1–e8; doi: 10.1016/j.ajog.2013.11.039 [DOI] [PubMed] [Google Scholar]
- 52. Gyamfi-Bannerman C, Pandita A, Miller EC, et al. Preeclampsia outcomes at delivery and race. J Matern Fetal Neonatal Med 2020;33(21):3619–3626; doi: 10.1080/14767058.2019.1581522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Grandi SM, Filion KB, Yoon S, et al. Cardiovascular disease-related morbidity and mortality in women with a history of pregnancy complications. Circulation 2019;139(8):1069–1079; doi: 10.1161/CIRCULATIONAHA.118.036748 [DOI] [PubMed] [Google Scholar]
- 54. Wu P, Gulati M, Kwok CS, et al. Preterm delivery and future risk of maternal cardiovascular disease: A systematic review and meta-analysis. J Am Heart Assoc 2018;7(2):e007809; doi: 10.1161/JAHA.117.007809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wu P, Haththotuwa R, Kwok CS, et al. Preeclampsia and future cardiovascular health: A systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes 2017;10(2):e003497; doi: 10.1161/CIRCOUTCOMES.116.003497 [DOI] [PubMed] [Google Scholar]
- 56. Miller EC, Bello NA, Davis R, et al. Women with adverse pregnancy outcomes have higher odds of midlife stroke: The population assessment of tobacco and health study. J Womens Health (Larchmt) 2021;31(4):503–512; doi: 10.1089/jwh.2021.0184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Parikh NI, Cnattingius S, Dickman PW, et al. Parity and risk of later-life maternal cardiovascular disease. Am Heart J 2010;159(2):215–221.e6; doi: 10.1016/j.ahj.2009.11.017 [DOI] [PubMed] [Google Scholar]
- 58. Shirazi TN, Hastings WJ, Rosinger AY, et al. Parity predicts biological age acceleration in post-menopausal, but not pre-menopausal, women. Sci Rep 2020;10(1):20522; doi: 10.1038/s41598-020-77082-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Rote SM, Angel JL. Gender-based pathways to cognitive aging in the Mexican-origin population in the United States: The significance of work and family. J Gerontol B Psychol Sci Soc Sci 2021;76(4):e165–e175; doi: 10.1093/geronb/gbaa189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Fujishiro K, MacDonald LA, Crowe M, et al. The role of occupation in explaining cognitive functioning in later life: Education and occupational complexity in a U.S. National Sample of Black and White Men and Women. J Gerontol B Psychol Sci Soc Sci 2019;74(7):1189–1199; doi: 10.1093/geronb/gbx112 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.