Abstract
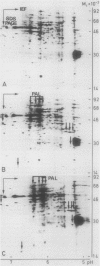
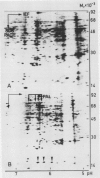
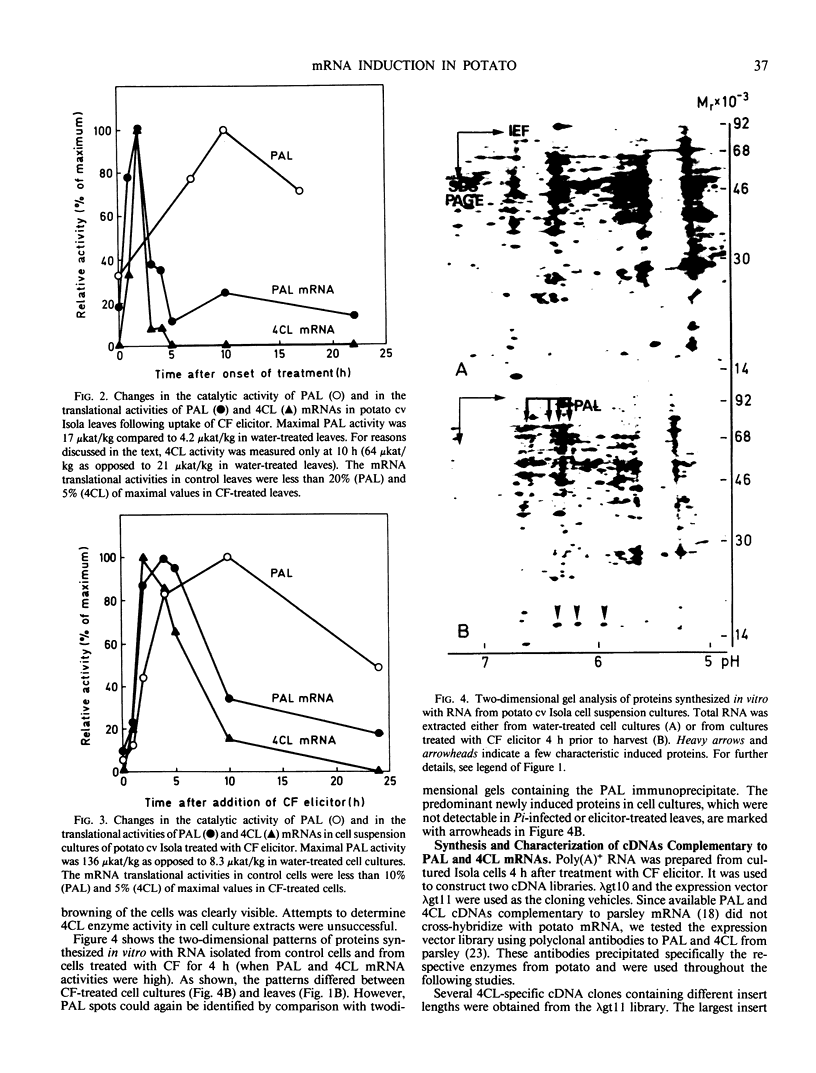
Infection of potato leaves with the fungal pathogen Phytophthora infestans (Pi) resulted in the rapid stimulation of phenylpropanoid metabolism. Increases in the activities of several mRNAs, including those encoding phenylalanine ammonia-lyase (PAL) and 4-coumarate:CoA ligase (4CL), were detectable within a few hours postinoculation, as demonstrated by two-dimensional gel electrophoresis of proteins synthesized in vitro. This effect was closely mimicked by application of Pi culture filtrate through cut leaf stems. PAL and 4CL mRNA activities were also rapidly and transiently induced in potato cell suspension cultures by treatments with Pi culture filtrate or arachidonic acid. This induction was exploited to generate cDNA probes complementary to PAL and 4CL mRNAs. Blot hybridizations using these probes revealed almost immediate, transient and coordinate increases in the transcription rates and subsequent changes in the amounts of PAL and 4CL mRNAs in leaves treated with Pi culture filtrate. Similar changes in the mRNA amounts were found in infected leaves of potato cultivars carrying resistance genes R1 (cv Datura) or R4 (cv Isola), independent of whether a virulent or an avirulent Pi pathotype was used for inoculation. These results are discussed in relation to recent cytological observations with the same potato cultivars and Pi pathotypes.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Bostock R. M., Laine R. A., Kuć J. A. Factors affecting the elicitation of sesquiterpenoid phytoalexin accumulation by eicosapentaenoic and arachidonic acids in potato. Plant Physiol. 1982 Nov;70(5):1417–1424. doi: 10.1104/pp.70.5.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cramer C. L., Bell J. N., Ryder T. B., Bailey J. A., Schuch W., Bolwell G. P., Robbins M. P., Dixon R. A., Lamb C. J. Co-ordinated synthesis of phytoalexin biosynthetic enzymes in biologically-stressed cells of bean (Phaseolus vulgaris L.). EMBO J. 1985 Feb;4(2):285–289. doi: 10.1002/j.1460-2075.1985.tb03627.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards K., Cramer C. L., Bolwell G. P., Dixon R. A., Schuch W., Lamb C. J. Rapid transient induction of phenylalanine ammonia-lyase mRNA in elicitor-treated bean cells. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6731–6735. doi: 10.1073/pnas.82.20.6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher T. F., Ellis R. J. Light-stimulated transcription of genes for two chloroplast polypeptides in isolated pea leaf nuclei. EMBO J. 1982;1(12):1493–1498. doi: 10.1002/j.1460-2075.1982.tb01345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Johnson L. M., Snyder M., Chang L. M., Davis R. W., Campbell J. L. Isolation of the gene encoding yeast DNA polymerase I. Cell. 1985 Nov;43(1):369–377. doi: 10.1016/0092-8674(85)90042-x. [DOI] [PubMed] [Google Scholar]
- Knobloch K. H., Hahlbrock K. 4-Coumarate:CoA ligase from cell suspension cultures of Petroselinum hortense Hoffm. Partial purification, substrate specificity, and further properties. Arch Biochem Biophys. 1977 Nov;184(1):237–248. doi: 10.1016/0003-9861(77)90347-2. [DOI] [PubMed] [Google Scholar]
- Kuhn D. N., Chappell J., Boudet A., Hahlbrock K. Induction of phenylalanine ammonia-lyase and 4-coumarate:CoA ligase mRNAs in cultured plant cells by UV light or fungal elicitor. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1102–1106. doi: 10.1073/pnas.81.4.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuć J., Rush J. S. Phytoalexins. Arch Biochem Biophys. 1985 Feb 1;236(2):455–472. doi: 10.1016/0003-9861(85)90648-4. [DOI] [PubMed] [Google Scholar]
- Lapeyre B., Amalric F. A powerful method for the preparation of cDNA libraries: isolation of cDNA encoding a 100-kDal nucleolar protein. Gene. 1985;37(1-3):215–220. doi: 10.1016/0378-1119(85)90275-6. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Peters B. M., Cribbs D. H., Stelzig D. A. Agglutination of plant protoplasts by fungal cell wall glucans. Science. 1978 Jul 28;201(4353):364–365. doi: 10.1126/science.201.4353.364. [DOI] [PubMed] [Google Scholar]
- Ragg H., Kuhn D. N., Hahlbrock K. Coordinated regulation of 4-coumarate:CoA ligase and phenylalanine ammonia-lyase mRNAs in cultured plant cells. J Biol Chem. 1981 Oct 10;256(19):10061–10065. [PubMed] [Google Scholar]
- Somssich I. E., Schmelzer E., Bollmann J., Hahlbrock K. Rapid activation by fungal elicitor of genes encoding "pathogenesis-related" proteins in cultured parsley cells. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2427–2430. doi: 10.1073/pnas.83.8.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]