Abstract
Background
Despite reduced infectious disease mortality and improved survival, infectious diseases continue to pose health threats due to their contagiousness, societal harm, and morbidity. Empiric antibiotic therapy, often prescribed without knowledge of the causative pathogen, faces challenges from rising antibiotic resistance. This study explores the potential of prior positive culture results to guide empiric antibiotic therapy.
Methods
Data from King Abdullah University Hospital (Jan 2014–Dec 2019) included adult patients with recurrent bacterial infections (pneumonia, sepsis, UTIs, wounds). Excluded cases included: mixed infections, transfers, <14 days or >12 months between episodes. The study compared bacterial growth and sensitivity patterns between previous and recent cultures.
Results
The study included 970 episodes from 650 patients, mainly UTIs (60.3%) and gram-negative bacteria (77.9%). The study found that (65.1%) of culture pairs matched. Empirical therapy was accurate in (71.8%) of cases. Further, accuracy of selected empiric antibiotic therapy was significantly predicted (p < 0.001) by: type of infection, type of antibiotics, and concordance with prior microbiologic data. Multivariate logistic analysis showed blood culture as less predictive of pending identity (OR: 0.234, P < 0.001) compared to urine culture; and prior affirmed gram negative bacterial culture was less predictive (OR: 0.606, P = 0.021) compared to gram positive bacterial culture.
Conclusion
This study underscores the potential of prior positive culture results in guiding empiric antibiotic therapy, enhancing accuracy and identity agreement. Future research should explore this approach in different infection contexts and across multiple centers. Reducing the indiscriminate use of broad-spectrum antibiotics is essential to combat antibiotic resistance.
Keywords: Empiric, Antibiotic, Infection, Culture identity, Jordan
1. Introduction
In the 20th century, despite reduced deaths from infectious diseases and improved survival rates, these illnesses continue to pose a significant health threat due to their contagiousness, potential societal harm, and morbidity [[1], [2], [3]]. Infectious diseases differ from non-communicable ones as they evolve with changing pathogenic strains and the emergence of antibiotic resistance. Ongoing research aims to counteract the acquisition of resistance genes in new bacteria and adapt to shifting susceptibility patterns [4].
Many antibiotics are prescribed empirically, without knowing the causing pathogen or its susceptibility [[5], [6], [7]]. Rising antibiotic resistance calls for more careful antibiotic use, especially in empiric cases [8]. Thus, choosing empiric antibiotics should rely on trusted guidelines, considering probable pathogens and local susceptibility patterns [9,10].
Using prior positive culture results to guide current antibiotic choices is a promising tactic, predicting upcoming culture findings and enabling precise treatment for the current infection [[10], [11], [12], [13], [14]]. Inadequate initial antibiotic treatment and delayed use of the right anti-infective agent can heighten the risk of bloodstream bacterial invasion, prolong hospital stays, raise the chance of hospital-acquired infections, increase in-hospital mortality rates, and boost treatment costs [[15], [16], [17]]. On the flip side, matching empiric antibiotics to the bacteria currently growing, based on previous positive culture results, effectively reduces treatment failures and halts infection progression [17]. Improving the diagnostic process in antibiotic prescribing is a key component of antimicrobial stewardship, which can help restrain antibiotic resistance development [18]. This study assesses the appropriateness of using prior positive cultures for the same type of infection to guide current empirical antimicrobial therapy.
2. Methods
2.1. Study setting
This retrospective study was conducted at King Abdullah University Hospital (KAUH) to collect patients' data from January 1st, 2014 to December 30th, 2019. Ethical approval was obtained from the Institutional Review Board in Jordan University of Science and Technology.
2.2. Study subjects
Eligible cases were identified by accessing hospital databases between July 2019 and January 2020. The study included adult patients from various wards at KAUH who had received diagnoses of specific bacterial infections, namely pneumonia, sepsis, urinary tract infection, or wound infection. These patients had been admitted to the hospital twice with the same type of infection within a 12-month period. If a patient experienced more than two infections during the study period, only the most recent ones were considered. Patients who had been transferred from other hospitals and those with mixed microbial infections (mixed growth) were excluded. Additionally, we excluded cases with a duration of less than 14 days or more than 12 months between the two episodes or encounters. This research compared bacterial growth and sensitivity patterns between the previous culture and the most recent encounter.
2.3. Data collection
Patient information was acquired through file reviews and computerized laboratory results. Infection confirmation relied on positive culture (sputum, urine, blood, or wound) and/or chest x-ray, as appropriate. The hospital's microbiology laboratory identified in vitro susceptibility of current and previous causative bacteria. We gathered demographic and clinical/medical data, including age, gender, comorbidities, drug allergies, recent antibiotic exposure (within 90 days of the latest admission), infection type, isolated bacteria type, and empirical therapy. Refer to Supplementary Document (S1) to access the data collection sheet used in this study.
To explore factors influencing the identification of microorganisms in the latest episode, all episodes were categorized as follows: (1) episodes with matching microorganisms (MO) and (2) episodes with non-matching MO. To evaluate the precision of the most recent empirical treatment, we considered only episodes where empirical therapy was administered and susceptibility data were available. Accuracy was defined as the in vitro susceptibility of empirical therapy. We determined concordance by examining empirical therapy, which was considered concordant if it adhered to guidelines and previous microbiological data. The identification of the causative pathogen and antibiotic susceptibility followed the recommendations of the Clinical and Laboratory Standards Institutes (CLSI) guidelines [19].
2.4. Statistical analysis
After data collection, responses were coded and imported into SPSS (version 23). Descriptive statistics were utilized to summarize the data for the entire sample, presenting categorical variables as numbers (percentages) and continuous variables as medians (interquartile range). Differences in variables were scrutinized using a Chi-square test (χ2) for categorical variables and a Mann-Whitney U test for continuous variables. Logistic regression was employed to evaluate predictors for culture identity and the accuracy of empirical therapy, with odds ratios (OR) and 95% confidence intervals (95% CI%) calculated. Statistical significance was defined as a p-value below 0.05.
3. Results
The current study encompassed 970 episodes from 650 unique patients. Cases had an average age of 63.5 years, with females comprising half (51.6%) of the sample. The majority of cases (87.1%) had comorbid conditions, while only 4.1% had documented antibiotic allergies. Among the most recent admissions, urinary tract infections (UTIs) were the most prevalent (60.3%), followed by sepsis (23.5%). Gram-negative bacteria were the primary causative agents (77.9%), with a notable proportion attributed to Extended Spectrum Beta-Lactamases (ESBL) producing bacteria (34.6%), E. coli (15.2%), and Pseudomonas aeruginosa (12.8%). The average time between isolated pairs was 70.5 days, and the majority of cultures (74.2%) were in agreement with previous microbiological data. You can find detailed demographic and clinical data in Supplementary 1.
4. Culture identity results
Approximately two-thirds of culture pairs (65.1%, n = 631) displayed a matching microorganism (MO) between the most recent episode and the preceding one, while one-third exhibited non-matching MO (34.9%, N = 339). Table 1 provides the results of multivariate analysis regarding factors influencing culture identity.
Table 1.
Predictors of current culture identity.
| Variable | Univariate analysis |
Multivariate analysis |
|||
|---|---|---|---|---|---|
| Not Matching N = 339 (34.9) |
Matching N = 631 (65.1) |
P value | OR (95%CI) | P value | |
| Age, median [IQR] | 65[52–72] | 63[47–73] | 0.248 | 0.997 (0.988–1.006) | 0.560 |
| Gender | 0.135 | Ref | 0.090 | ||
|
164 (48.4%) | 337 (53.4%) | 0.783 (0.590–1.039) | ||
|
175 (51.6%) | 294 (46.6%) | |||
| Comorbidity | 0.006 | 0.470 | |||
|
30 (8.8%) | 95 (15.1%) | Ref | ||
|
309 (91.2% | 536 (84.9%) | 0.836 (0.515–1.359) | ||
| Type of culture | <0.001 | ||||
|
149 (44.0%) | 436 (69.1%) | Ref | <0.001 | |
|
34 (10.0%) | 72 (11.4%) | 0.705(0.442–1.127) | 0.144 | |
|
35 (10.3%) | 16 (2.5%) | 0.155(0.080–0.286) | <0.001 | |
|
121 (35.7%) | 107 (17%) | 0.234(0.153–0.356) | <0.001 | |
| Prior antibiotic use | 0.217 | 0.606 | |||
|
45 (13.3%) | 67 (10.6%) | Ref | ||
|
294 (86.7%) | 564 (89.4%) | 1.126 (0.718–1.765) | ||
| Type of bacteria-current | 0.014 | 0.021 | |||
|
90 (26.5%) | 124 (19.7%) | Ref | ||
|
249 (73.5%) | 507 (80.3%) | 0.606 (0.395–0.928) | ||
| Interval between isolated pairs –days, median [IQR] | 81[41–170] | 65[35–136] | 0.001 | 0.998 (0.996–1) | 0.014 |
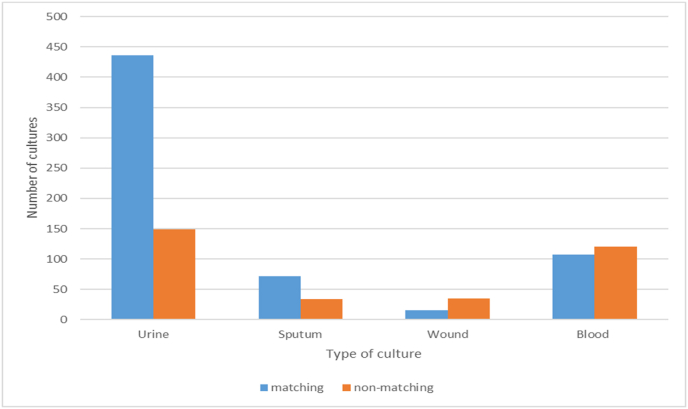
Having a pending urine culture result was a stronger predictor of growing the same previous MO in the prior culture compared to pending blood (OR = 0.234, 95% CI = 0.153–0.356, p < 0.001) or wound (OR = 0.155, 95% CI = 0.080–0.286, p < 0.001) cultures. Furthermore, a prior positive culture with gram-positive bacteria was a better predictor of having the same MO in the most recent culture compared to gram-negative cultures (OR = 0.606, 95% CI = 0.395–0.928, p = 0.021). Additionally, a shorter interval between two isolated cultures was a stronger predictor of culture identity in the most recent culture (OR = 0.998, 95% CI = 0.996–1, p = 0.014). However, the statistical results for both OR (approaching 1) and CI (including 1) concerning the interval between cultures do not have direct clinical implications. Fig. 1 illustrates the graphical representation of culture identity agreement among different types of cultures (Fig. 1: Culture Identity Agreement Among Cultures).
Fig. 1.
Culture identity agreement among cultures.
5. Accuracy of therapy results
Among the 970 culture pairs, 317 were excluded for two reasons: 1) 46 cases with known culture results before admission (from outpatient settings); and 2) 271 cases lacked susceptibility data for the given empirical therapy. Among the remaining 653 cases analyzed for accuracy, 71.8% (n = 469) received accurate empirical therapy. Table 2 presents the results of multivariate analysis concerning factors affecting therapy accuracy in the most recent episode.
Table 2.
Predictors of the accuracy of current therapy.
| variable | Univariate |
Multivariate |
|||
|---|---|---|---|---|---|
| Not accurate N = 184 (28.2) |
Accurate n = 469 (71.8) | P value | OR (95%CI) | P value | |
| Age, median [IQR] | 65[50–77] | 63[48–73] | 0.129 | 0.988 (0.976–1.001) | 0.067 |
| Gender | 0.413 | ||||
|
88 (47.8%) | 241 (51.4%) | |||
|
96 (52.2%) | 228 (48.6%) | |||
| Comorbidity | 0.340 | ||||
|
20 (10.9% | 64 (13.6%) | |||
|
164 (89.1% | 405 (86.4%) | |||
| Type of Infection | <0.001 | ||||
|
110 (59.8%) | 350 (74.6%) | Ref | <0.001 | |
|
38 (20.7%) | 45 (9.6%) | 0.203 (0.111–0.373) | <0.001 | |
|
9 (4.9%) | 23 (4.9%) | 0.642 (0.248–1.662) | 0.361 | |
|
27 (14.7%) | 51 (10.9%) | 0.208 (0.107–0.405) | <0.001 | |
| Prior antibiotic use | 0.597 | ||||
|
16 (8.7%) | 35 (7.5%) | |||
|
168 (91.3%) | 434 (92.5%) | |||
| Type of bacteria-current | 0.578 | ||||
|
14 (7.6%) | 30 (6.4%) | |||
|
170 (92.4%) | 439 (93.6%) | |||
| Interval between isolated pairs –days, median [IQR] | 61[32–149] | 73[37.50–149.5] | 0.336 | ||
| Type of antibiotics | <0.001 | ||||
|
102 (55.4%) | 351 (74.8%) | Ref | <0.001 | |
|
8 (4.3%) | 7 (1.5%) | 0.376 (0.107–1.313) | 0.125 | |
|
10 (5.4%) | 27 (5.8%) | 1.252 (0.512–3.064) | 0.622 | |
|
58 (31.5%) | 60 (12.8%) | 0.291 (0.172–0.493) | <0.001 | |
|
6 (3.3%) | 24 (5.1%) | 1.056 (0.365–3.131) | 0.922 | |
| Concordance | <0.001 | <0.001 | |||
|
106 (57.6%) | 72 (15.4%) | Ref | ||
|
78 (42.4%) | 397 (84.6%) | 7.219 (4.675–11.148) | ||
UTI: urinary tract infection.
MRSA: Methicillin-resistant Staphylococcus aureus.
The results indicated that treating UTIs was more accurate than addressing other infection types such as pneumonia (OR = 0.203, 95% CI = 0.111–0.373, p < 0.001) and sepsis (OR = 0.208, 95% CI = 0.107–0.405, p < 0.001). Additionally, using antipseudomonal therapy was more accurate compared to narrower-spectrum non-antipseudomonal/non-anti-Methicillin-resistant Staphylococcus aureus (MRSA) therapy (OR = 0.291, 95% CI = 0.172–0.493, p < 0.001). Furthermore, using concordant therapy based on prior microbiological data was seven times more likely to predict accuracy (OR = 7.180, 95% CI = 4.675–11.148, p < 0.001).
6. Discussion
The present study investigated the feasibility of using previous positive culture results from various infection types to inform the selection of empirical antibiotics for the most recent episode. Our findings suggest the importance of considering prior culture results when making choices regarding empirical antibiotic therapy. Specifically, factors influencing culture identity included urinary cultures, as opposed to other types, and infections caused by gram-positive bacterial species. Additionally, positive predictors for the accuracy of empirical antibiotic therapy included UTIs, the utilization of antipseudomonal agents for empirical coverage, and alignment with previous culture results.
Regarding the most recent culture's infecting identity, our study revealed that a pending urine culture was more likely to yield the same microorganism (MO) as found in the previous culture, compared to blood, sputum, or wound cultures. This finding reinforces the results reported by Linsemeyer and MacFadden, which demonstrated the utility of a prior positive urine culture in predicting the potential identity and susceptibility of the current positive urine culture [11,13]. The higher odds of cultural identity agreement in urine culture in our study, in comparison to other types of cultures, can be attributed to the higher recurrence potential observed with urinary tract infections (UTIs) after the initial episode [20].
Furthermore, antecedent positive culture with gram positive bacteria was more predictive to having the same MO in the most recent culture compared to gram negative culture. A potential explanation is that infections implicated by gram negative bacterial species have higher odds of mortality compared to gram positive bacterial counterpart, in addition to delayed healing, and prevalent antibiotic resistance [[21], [22], [23]]. In addition, the survival of patients with prior infective episodes caused by gram positive species increases the likelihood of subsequent infection, particularly by gram positive strains [24], as aligned by the results of the present study.
Regarding antibiotic selection accuracy, two-thirds of the patients received appropriate antibiotic therapy. Treating a UTI episode, as opposed to other types of infections, was predictive of accurate empirical antibiotic therapy. As mentioned earlier, the higher prevalence of UTIs and the increased likelihood of UTI recurrence enhance healthcare providers' familiarity with UTI susceptibility patterns and treatment options compared to other types of infections [[25], [26], [27]].
In addition to the type of infection, the spectrum of antibiotic activity is another factor that can influence the accuracy of therapy. Antipseudomonal antibiotics provided more accurate coverage compared to narrower-spectrum options, such as non-antipseudomonal/non-anti-MRSA antimicrobials. Empirically, it is common practice to start with broad-spectrum antibiotics to increase the likelihood of targeting potential pathogens causing the infection [9,28]. Antipseudomonal antibiotics, in this context, are considered broader-spectrum antibiotics that can effectively combat a wider range of pathogens. When initiated empirically, antipseudomonal agents offer higher chances of targeting the infecting strains in a given episode, resulting in superior accuracy rates.
It's crucial to distinguish between “accurate” and “appropriate” empiric therapy. Broad-spectrum antibiotics are often an accurate choice because they are likely to be effective against the causative pathogen. However, they may not always be the “appropriate” choice, as they might not align with the local epidemiological context and the specific clinical condition of the infected patient.
Furthermore, concordance with previous culture results emerged as another noteworthy predictor of accuracy. Opting for empirical antibiotic therapy that aligned with prior microbiological data increased the accuracy rate by approximately sevenfold compared to selecting therapy that didn't match previous microbiological findings. This aligns with findings from a study by Linsenmeyer et al. which reported a doubling of the accuracy rate when using a concordant anti-infective agent to treat subsequent UTI episodes [11].
As evident, our study delved into a novel concept that had not been explored in previous research. Our approach involved an extensive review of patient encounters rather than relying solely on admission codes, thereby enhancing the reliability of our results. However, there are a few limitations to our study. Firstly, given the limited number of studies addressing factors affecting the accuracy of empiric antibiotic selection, comparing our findings to similar ones proved challenging. Furthermore, the comprehensive inclusion of various types of infections may have influenced the interrelationships between different variables. Lastly, our study was conducted at a single center, which could potentially limit the generalizability of our results.
The insights gleaned from this study could have implications for antibiotic stewardship if applied to guide the selection of empiric antibiotic therapy, particularly in light of the significant clinical characteristics identified in infectious episodes. Future research with a similar focus should encompass multiple centers and ensure a more even distribution of different types of infections.
7. Conclusion
Selecting empiric antibiotics presents a challenge as it involves a delicate balance between potential benefits and associated risks, considering the likely infecting species and local susceptibility patterns. This study has laid the groundwork for enhancing the use of empiric antibiotics by highlighting the role of prior microbiological data in guiding healthcare providers' choices for treating the current episode, thereby improving both accuracy and agreement in identity.
We strongly recommend further prospective studies that consider classifying different infections based on factors such as acquisition site, severity, or complexity (e.g., primary vs. relapse, complicated vs. uncomplicated). This would help establish a more accurate relationship, as suggested by our findings.
There is an urgent need to curb the practice of indiscriminately prescribing broad-spectrum antibiotics, as it leads to excessive use of these antibiotics, resulting in antibiotic resistance, societal risks, secondary infections, and increased costs.
Funding source
This study was supported by the Deanship of Research at Jordan University of Science and Technology, Irbid, Jordan (grant number 411/2019).
Key summary
Inadequate empirical antibiotic therapy significantly contributes to morbidity and mortality related to infections. This study has emphasized factors that enhance the ability to predict pending culture results, a crucial step in optimizing antibiotic therapy. Predicting the growth of bacteria helps improve outcomes, reduce costs, and mitigate public health concerns linked to therapy failure and antibiotic resistance.
Ethics statement
The ethical approval to conduct this study was obtained from the Institutional Review Board (IRB) in Jordan University of Science and Technology (reference number: 2/125/2019). All methods were performed in accordance with the guidelines and regulations as aligned by IRB committee. This study was deemed exempt from written informed consent for participation as promulgated by IRB committee's letter of approval (reference number: 2/125/2019).
Authors contributions
Conceptualization: RK, BM. Data curation: RK, SS, LA, BM. Formal analysis: SS, BM. Funding acquisition: RK. Methodology: RK, LA, SS, BM. Project administration: RK, LA, SS, BM. Visualization: RK, BM. Writing–original draft: RK, SS, BM. Writing–review & editing: RK, SS, LA, BM.
Declaration of generative AI and AI-assisted technologies in the writing process
During the preparation of this work the author(s) used [ChatGPT] in order to [improve language and readability of the manuscript]. After using this tool/service, the author(s) reviewed and edited the content as needed and take(s) full responsibility for the content of the publication.
Declaration of competing interest
The Author(s) declare(s) that there is no conflict of interest.
Acknowledgment
The authors would like to acknowledge the deanship of research at Jordan University of Science and Technology for supporting this research (Grant Number: 411/2019).
Handling Editor: Patricia Schlagenhauf
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nmni.2023.101182.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Kwong J.C., Ratnasingham S., Campitelli M.A., Daneman N., Deeks S.L., Manuel D.G., et al. The impact of infection on population health: results of the ontario burden of infectious diseases study. PLoS One. 2012;7 doi: 10.1371/journal.pone.0044103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.El Bcheraoui C., Mokdad A.H., Dwyer-Lindgren L., Bertozzi-Villa A., Wstubbs R., Morozoff C., et al. Trends and patterns of differences in infectious disease mortality among US Counties. 1980-2014. JAMA - J Am Med Assoc. 2018;319 doi: 10.1001/jama.2018.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schmid D., Leitzmann M.F. Physical activity epidemiology. Handb. Epidemiol. 2014 doi: 10.1007/978-0-387-09834-0_67. Second Ed. [DOI] [Google Scholar]
- 4.Shuman E.K., Malani P.N. Infectious diseases mortality in the United States ongoing investment needed for continued progress. JAMA, J Am Med Assoc. 2018;319 doi: 10.1001/jama.2018.1525. [DOI] [PubMed] [Google Scholar]
- 5.Chem E.D., Anong D.N., Akoachere J.F.K.T. Prescribing patterns and associated factors of antibiotic prescription in primary health care facilities of Kumbo East and Kumbo West Health Districts, North West Cameroon. PLoS One. 2018;13 doi: 10.1371/journal.pone.0193353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klinker K.P., Hidayat L.K., DeRyke C.A., DePestel D.D., Motyl M., Bauer K.A. Antimicrobial stewardship and antibiograms: importance of moving beyond traditional antibiograms. Therapeut Adv infect Dis. 2021;8 doi: 10.1177/20499361211011373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bielicki J.A., Sharland M., Johnson A.P., Henderson K.L., Cromwell D.A., Berger C., et al. Selecting appropriate empirical antibiotic regimens for paediatric bloodstream infections: application of a Bayesian decision model to local and pooled antimicrobial resistance surveillance data. J Antimicrob Chemother. 2016;71 doi: 10.1093/jac/dkv397. [DOI] [PubMed] [Google Scholar]
- 8.Claridge J.A., Pang P., Leukhardt W.H., Golob J.F., Carter J.W., Fadlalla A.M. Critical analysis of empiric antibiotic utilization: establishing benchmarks. Surg Infect. 2010;11 doi: 10.1089/sur.2009.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leekha S., Terrell C.L., Edson R.S. General principles of antimicrobial therapy. Mayo Clin Proc. 2011;86 doi: 10.4065/mcp.2010.0639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.MacFadden D.R., Coburn B., Shah N., Robicsek A., Savage R., Elligsen M., et al. Decision-support models for empiric antibiotic selection in Gram-negative bloodstream infections. Clin Microbiol Infect. 2019;25 doi: 10.1016/j.cmi.2018.03.029. [DOI] [PubMed] [Google Scholar]
- 11.Linsenmeyer K., Strymish J., Gupta K. Two simple rules for improving the accuracy of empiric treatment of multidrug-resistant urinary tract infections. Antimicrob Agents Chemother. 2015;59 doi: 10.1128/AAC.01638-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tuon F.F., Rocha J.L., Leite T.M., Dias C. A simple mathematical model to determine the ideal empirical antibiotic therapy for bacteremic patients. Braz J Infect Dis. 2014;18 doi: 10.1016/j.bjid.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.MacFadden D.R., Ridgway J.P., Robicsek A., Elligsen M., Daneman N. Predictive utility of prior positive urine cultures. Clin Infect Dis. 2014;59 doi: 10.1093/cid/ciu588. [DOI] [PubMed] [Google Scholar]
- 14.Ahmed H., Farewell D., Francis N.A., Paranjothy S., Butler C.C. Choice of empirical antibiotic therapy and adverse outcomes in older adults with suspected urinary tract infection: cohort study. Open Forum Infect Dis. 2019;6 doi: 10.1093/ofid/ofz039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Savage R.D., Fowler R.A., Rishu A.H., Bagshaw S.M., Cook D., Dodek P., et al. The effect of inadequate initial empiric antimicrobial treatment on mortality in critically ill patients with bloodstream infections: a multi-centre retrospective cohort study. PLoS One. 2016;11 doi: 10.1371/journal.pone.0154944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ott S.R., Hauptmeier B.M., Ernen C., Lepper P.M., Nüesch E., Pletz M.W., et al. Treatment failure in pneumonia: impact of antibiotic treatment and cost analysis. Eur Respir J. 2012;39 doi: 10.1183/09031936.00098411. [DOI] [PubMed] [Google Scholar]
- 17.Peeters P., Ryan K., Karve S., Potter D., Baelen E., Rojas-Farreras S., et al. The impact of initial antibiotic treatment failure: real-world insights in patients with complicated, health care-associated intra-abdominal infection. Infect Drug Resist. 2019;12 doi: 10.2147/IDR.S184116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dyar O.J., Moran-Gilad J., Greub G., Pulcini C. Diagnostic stewardship: are we using the right term? Clin Microbiol Infect. 2019;25 doi: 10.1016/j.cmi.2018.12.011. [DOI] [PubMed] [Google Scholar]
- 19.Omoya Funmilola Oluyemi, Ajayi Kehinde Oluyemi. Clinical and laboratory Standards institute (CLSI) (2014) performance Standards for antimicrobial susceptibility testing. 2016. https://www.scirp.org/(S(351jmbntvnsjt1aadkposzje))/reference/ReferencesPapers.aspx?ReferenceID=1939363 Twenty-Fourth Informational Supplement. CLSI Document M100-S24, Wayne, 34(1). - References - Scientific Research Publishing. Adv Microbiol.
- 20.Glover M., Moreira C.G., Sperandio V., Zimmern P. Recurrent urinary tract infections in healthy and nonpregnant women. Urol Sci. 2014;25 doi: 10.1016/j.urols.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tabah A., Koulenti D., Laupland K., Misset B., Valles J., Bruzzi De Carvalho F., et al. Characteristics and determinants of outcome of hospital-acquired bloodstream infections in intensive care units: the EUROBACT International Cohort Study. Intensive Care Med. 2012;38 doi: 10.1007/s00134-012-2695-9. [DOI] [PubMed] [Google Scholar]
- 22.Rutter L. Identifying and managing wound infection in the community. Br J Community Nurs. 2018;23(Sup3):S6–S14. doi: 10.12968/bjcn.2018.23.Sup3.S6. [DOI] [PubMed] [Google Scholar]
- 23.Tseng W.P., Chen Y.C., Chen S.Y., Chen S.Y., Chang S.C. Risk for subsequent infection and mortality after hospitalization among patients with multidrug-resistant gram-negative bacteria colonization or infection. Antimicrob Resist Infect Control. 2018;7 doi: 10.1186/s13756-018-0388-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dang T.T., Eurich D.T., Weir D.L., Marrie T.J., Majumdar S.R. Rates and risk factors for recurrent pneumonia in patients hospitalized with community-acquired pneumonia: population-based prospective cohort study with 5 years of follow-up. Clin Infect Dis. 2014;59 doi: 10.1093/cid/ciu247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Disler R.T., Gallagher R.D., Davidson P.M., Sun S.-W., Chen L.-C., Zhou M., et al. Factors impairing the postural balance in COPD patients and its influence upon activities of daily living. Eur Respir J. 2019;15 [Google Scholar]
- 26.Cowan S.L., Holland J.A., Frost I., Kane A.D. Recognition and management of sepsis by junior doctors. Future Hosp J. 2016;3 doi: 10.7861/futurehosp.3-2-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Isberg H.K., Hedin K., Melander E., Mölstad S., Beckman A. Increased adherence to treatment guidelines in patients with urinary tract infection in primary care: a retrospective study. PLoS One. 2019;14 doi: 10.1371/journal.pone.0214572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Strich J.R., Heil E.L., Masur H. Considerations for empiric antimicrobial therapy in sepsis and septic shock in an era of antimicrobial resistance. J Infect Dis. 2021:222. doi: 10.1093/INFDIS/JIAA221. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.