Summary
Background
In acute myeloid leukaemia (AML), interleukin-6 (IL-6) promotes chemo-resistance and its levels correlate with poor prognosis. IL-6 blockade may represent a promising therapeutic strategy. We aimed to test, tocilizumab, an anti-IL-6 receptor (R) monoclonal antibody in combination with standard intensive AML induction chemotherapy.
Methods
This investigator-initiated single-centre phase 1 trial was conducted at Nantes University Hospital in France. According to a continual reassessment method, three escalating doses were tested of intravenous (IV) tocilizumab (4, 6, and 8 mg/kg) administered at day (d) 8 of a standard AML induction chemotherapy (IV idarubicine 8 mg/m2 d1 to d5 + IV cytarabine 100 mg/m2 d1 to d7). All adults (aged ≥ 18 years) with an Eastern Cooperative Oncology Group performance status of 0–2 and with a newly diagnosed (excluding patients with a favourable risk according to ELN-2017 classification if <60 year-old) or a relapsed/refractory AML were eligible. The primary objective was to determine the maximum tolerated dose of tocilizumab to administrate with a standard intensive AML induction. Safety outcomes were continuously monitored for at each participant contact. This trial is registered with ClinicalTrials.gov, NCT04547062.
Findings
Between Dec 29, 2020 and Dec 1, 2022, 12 patients were enrolled, of whom 75% had an ELN-2017 high-risk profile, and were treated with tocilizumab— two patients at 4 mg/kg, two at 6 mg/kg and eight at 8 mg/kg of tocilizumab. No dose-limiting toxicity related to tocilizumab was documented. There were nine serious adverse events, none of which were related to tocilizumab, and there was no treatment-related deaths. MTD was thus not reached. Two deaths occurred during induction. In the remaining ten evaluable patients, nine responded to treatment.
Interpretation
The combination of tocilizumab with standard AML intensive induction appears to be safe and resulting responses are encouraging. A dose of 8 mg/kg of tocilizumab given at day 8 of induction could be used for further phase 2/3 studies.
Funding
The Leucémie Espoir Atlantique Famille (LEAF)—“Tous avec Fabien” association.
Keywords: Acute myeloid leukaemia, Induction chemotherapy, IL-6, Tocilizumab
Research in context.
Evidence before this study
PubMed and Clinicaltrials.gov were searched from database inception to March 29, 2023, for studies investigating the combination of tocilizumab with intensive chemotherapy for patients with AML with no language or study type restrictions. The search terms “AML” and “TOCILIZUMAB” and “induction chemotherapy” but also “AML” and “INTERLEUKIN-6” and “induction chemotherapy” were used. No study was identified. Inflammation is one of the hallmarks of cancer. In AML, inflammation has been linked to progression from myelodysplastic syndrome to AML. Several studies have already shown that in a subset of patients with AML, unique inflammation gene signatures are associated with inferior outcomes. Interleukin-6 (IL-6) is a pro-inflammatory cytokine the level of which is found to be significantly higher all along induction chemotherapy in patients with AML compared with healthy controls. Remission rates after induction chemotherapy still need to be improved, particularly in the subset of unfavourable-risk AML often associated with elderly patients, a population exposed to age-induced inflammation.
Added value of this study
Our study findings show that intending to lower the inflammation present during AML induction through IL-6 targeting using tocilizumab in addition to induction chemotherapy appears to be safe. No dose-limiting toxicity related to tocilizumab was documented. Median plasma levels of tocilizumab were higher using 6 or 8 mg/kg of tocilizumab compared with a 4 mg/kg dosage, even if the size of the population precludes any statistical analyses. Furthermore, in this small population of high-risk patients with AML, the overall remission rate, defined by the combination of complete remission i.e., CR, CR with incomplete haematological recovery (Cri) and morphologic leukaemia-free state (MLFS), according to ELN 2017 classification for evaluable patients, was high: CR 4/10 (40%), CRi 4/10 (40%) and MLFS 1/10 (10%)—i.e., 90% (9/10 evaluable patients). Finally, IL-6 plasma levels increased significantly after tocilizumab injection, suggesting at least a partial saturation of IL6-R expressing cells, including leukaemic cells.
Implications of all the available evidence
The results of this phase 1 study suggest that the addition of a 8 mg/kg dose of tocilizumab in combination with standard intensive AML induction chemotherapy is safe, providing both a high response rate and an expected biological effect through the saturation of IL-6R and high soluble IL-6 concentrations. This study provides a strong rationale for targeting the IL-6 pathway during intensive induction chemotherapy in AML in future trials. An 8 mg/kg dose of tocilizumab would be recommended to be added after intensive induction chemotherapy, at day 8 of induction, for phase 2/3 studies in patients with AML.
Introduction
Intensive induction chemotherapy combining a continuous-infusion of cytarabine together with an anthracycline has remained the standard of care for more than 40 years in eligible patients with acute myeloid leukemia (AML).1 New therapeutic options have emerged in recent years, such as the liposomal formulation containing cytarabine and daunorubicin (CPX-351), FLT3 and isocitrate dehydrogenase 1/2 inhibitors or venetoclax given in combination with hypomethylating agents.1 Intensive induction remains a valid option in patients with AML fit enough to receive such a therapy. However, in both younger and older patients, complete remission (CR) rates and survivals are still disappointing with, for example in the latter, around 40%–60% CR achievement and less than 15% of long-term survival.1,2 Thus, new therapeutic approaches are definitely needed to increase outcomes in patients with AML.
Inflammation is one of the hallmarks of cancer.3 Among pro-inflammatory cytokines, interleukin-6 (IL-6), which is produced mainly by immune cells, has been described as implicated in a wide variety of pathologies ranging from chronic inflammatory conditions to cancer.4 The IL-6 signalling pathway plays a significant role in cancer biology, particularly through its involvement in metastasis formation.5 IL-6 has also been proven, together with other cytokines, to contribute to blast proliferation, treatment-resistance and prognosis in AML.6, 7, 8 AML blasts are exposed to microenvironment-derived and self-produced circulating IL-6 derived from both autocrine and paracrine mechanisms.9 If IL-6-induced STAT3 signalling has been associated with chemo-resistance in AML,10 how IL-6 advantages AML blast survival remains to be elucidated.
We have recently and prospectively evaluated the impact of peripheral IL-6 levels at various times during first-line intensive induction in 62 patients with AML showing that those with a higher plasma IL-6 level at day 22 had a worse outcome compared with others.11
As a consequence, targeting the IL-6 signalling pathway appears to be an interesting approach in AML to overcome bad prognosis, especially in patients at high-risk of relapse.
Tocilizumab is an IgG1 humanised monoclonal antibody that inhibits soluble and membrane IL-6 receptors (IL-6R). It is indicated for the treatment of certain forms of rheumatoid arthritis, COVID-19 virus infection and for the treatment of chimeric antigen receptor (CAR) T cell-induced severe or life-threatening cytokine release syndrome (CRS). For the latter, a single dose of 8 mg/kg (for adults with a weight of 30 kg or more) is recommended, and up to 3 additional doses can be considered with an interval of at least 8 h between each injection in some cases.12
Here, the feasibility to add a single dose of tocilizumab (up to 8 mg/kg) to a standard intensive AML induction chemotherapy was evaluated in a population of patients with high-risk AML in a prospective, monocentric, open-label, phase 1 study with three escalating doses of tocilizumab tested in combination with standard AML intensive induction.
Methods
Study design and participants
This study was performed as an investigator-initiated trial at Nantes University Hospital in France. This trial is registered with ClinicalTrials.gov, NCT04547062.
The main eligibility criteria were: (i) age ≥ 18 year-old (ii) newly diagnosed (excluding patients with a favourable risk according to ELN-2017 classification13 if less than 60 year-old or relapsed/refractory AML; see protocol in Appendix).
Post-remission or salvage treatments after induction were at the discretion of investigators. Allogeneic stem cell transplantation (Allo-SCT) was allowed in responders after induction or any consolidation course.
Dose escalation steps were determined using a continual reassessment method14 with a targeting experimental drug-related toxicity level <30%. Patients had to be included two by two. A maximum of twelve patients had to be included. Patients were removed from the study in case of screen failure or consent withdrawal. Only patients receiving tocilizumab were considered for analyses, and a patient included but having not received tocilizumab had to be replaced by another one.
Ethics
The trial was approved by an ethics committee (Comité de Protection des Personnes CPP Sud-Ouest et Outre-Mer 1: CPP 1-20-060 ID 9103) and the French Health Agency (ANSM: MEDAECNAT-2020-07-00044 2020-003209-77). All patients provided written informed consent.
Interventions
Tocilizumab was tested as a single dose at 4, 6, and 8 mg/kg administered intravenously (IV) over 1 h at day 8 of a standard intensive AML induction chemotherapy. This consisted of IV idarubicin 8 mg/m2 (from day 1 to day 5) + IV cytarabine 100 mg/m2 (from day 1 to day 7). A prophylaxis of infusion-related reactions (IRR) using 1 g of IV acetaminophen and 5 mg of IV dexchlorpheniramine was given before tocilizumab administration. This sequential design (chemotherapy then tocilizumab) was chosen in order to minimise interactions between both treatments with the hope to avoid a potential toxicity of the combination and to better discriminate between adverse events related to chemotherapy vs tocilizumab. Criteria for discontinuation were: inpvestigator choice if he/she believed that a change of treatment was in the patient’s best interest, occurrence of a significant medical event which, from the investigator’s or patient’s point of view, prevented continuation of the study treatment, and consent withdrawal.
Primary objective and safety assessment
The primary objective of the study was to determine the maximum tolerated dose (MTD) of one single dose of tocilizumab administered at day 8 of a standard intensive induction chemotherapy in patients with higher-risk AML. MTD was determined as the dose closest to the target toxicity level of 30%.
Thus, targeting an experimental drug-related toxicity level <30%, a one-parameter empirical model was used to describe the relationship between the dose of tocilizumab and the probability of observing a dose-limiting toxicity (DLT). DLT was defined by the appearance of grade 3–4 adverse events (AE) related to tocilizumab and not reversible after 7 days, except for haematological AEs, or by the absence of haematological recovery by week six after induction (in the absence of treatment failure). The occurrence of tuberculosis or grade 3–4 diverticulitis were also considered as DLT. Toxicity was evaluated according to NCI-CTCAE criteria version 5. Search for a DLT was considered until day +45 of induction.
Investigators were requested to continuously monitor for and report any AE, eliciting AE information at each participant contact. Reports were continuously monitored by the sponsor. An independent safety committee was involved to assess safety data.
Haematopoietic recoveries after induction plus tocilizumab
Neutrophil recovery was defined by the time between day 1 of induction and the first of 3 days with polynuclear neutrophils >1 × 109/L.
Platelet recovery was defined by the time between day 1 of induction and the first of 3 consecutive days with platelets >100 × 109/L.
Responses assessment after induction plus tocilizumab
Hematologic responses, including complete remission (CR), CR with incomplete haematological recovery (CRi) and morphologic leukaemia-free state (MLFS), were considered according to ELN 2017 criteria; The overall response rate (ORR) was defined by the rate of patients achieving at least <5% of blasts in bone marrow with no blasts in the peripheral blood, whatever the haematopoietic recovery. ORR was considered according to the whole cohort but also for patients evaluable for response (i.e., excluding those who died during induction). Responses were evaluated between day +30 and +45 after the beginning of induction. Bone marrow (BM) evaluation was also performed at day 15 for patients under 60 years.
Minimal residual disease (MRD) for patients achieving CR/CRi/MLFS was assessed in multiparameter flow cytometry using an eight-colour panel derived from the European LeukaemiaNet recommendations.15 Briefly, this included the following conjugated antibodies: FITC (fluorescein isothiocyanate)-CD34, PE (Phycoerythrin)-CD13, PerCP (Peridinin-Chlorophyll-Protein) 5.5-CD7, PE-Cy7 (PE-Cyanine 7)-CD33, APC (Allophycocyanin)-CD56, APC-Cy7 (Allophycocyanin-Cyanin 7)-CD117, V450-DR, and V569-CD45. One hundred microliters of whole BM were incubated with the antibody mixture followed by lysis-no-wash. Blast immunophenotypic expression of these markers at diagnosis was compared with follow-up medullary subsets in a combined LAIP (Leukaemia associated immunophenotypic pattern) and different-from normal (DfN) approach according to published data.16 Data were expressed as percentages of identified residual blast cells among viable CD45+ leukocytes.
Cytokines assessment
A cytokine multiplex assay from Milliplex (MILLIPLEX® Map Kit, Human Cytokine/Chemokine/Growth Factor Panel A—Immunology Multiplex Assay HCYTO-60K, Millipore-Sigma Aldrich St Louis, MO) was used to assess Flt-3 ligand and IL-6 concentrations in plasma samples collected at d1 (just before induction chemotherapy), d8 (before tocilizumab injection), d15 and d22 of induction. According to our previous publications, patients having a low level of Flt-3 ligand (<100 pg/mL) all along the induction were considered as having a “low level profile”, which has been reported as being correlated with worse survival, compared with patients who had increasing Flt-3 ligand levels between d1 and d15 or d22.11,17 The procedure was performed according to manufacturer recommendations. Results are expressed as pg/mL. Briefly, after centrifugation at 100 × g for 10 min at 4 °C to remove debris, 25 μL of each plasma was incubated in duplicate overnight at 4 °C on a plate shaker with antibody-conjugated magnetic beads. After 3 washes, bead-complexes were incubated with 25 μL of biotinylated detection antibody for 1 h, followed by a 30 min incubation with 25 μL of streptavidin-phycoerythrin. Both incubations were performed on a plate shaker at room temperature. After 3 washes, 150 μL of sheath fluid was added to all wells. Plates were read on a Bio-Plex® 200 and analysed by Bio-Plex Manager™ Software (Bio-Rad, Hercules, CA) Concentrations below the detection limit of any assay were reported as 0.
Tocilizumab concentrations assessment
Tocilizumab plasma concentrations were evaluated at d15 and d22. Tocilizumab concentrations were quantified in plasma samples using an enzyme-linked immunoassay (ELISA), the LISA-TRACKER Tocilizumab kit (Ref. LTT 002-48, Theradiag, Marne la Vallée, France). Results were expressed in μg/mL. The assay detection limit was 1 μg/mL and the lower and upper limits of quantification were 1 and 50 μg/mL respectively. In accordance with the manufacturer protocol, samples were diluted to 1:1001, then 100 μL of diluted plasma were added in the pre-coated plate. Controls and standards were also included. After 1 h of incubation, the plate was washed, then the biotinylated detection antibody was added for 1 h. The plate was washed 3 times, then a streptavidin-peroxidase solution was added for 30 min, followed by washes. The tetramethylbenzidine substrate was added for 15 min. The reaction was stopped with 100 μL of H2SO4 and the optical density was measured at 450 nm. A four-parameter logistic (4 PL) curve was applied to obtain tocilizumab concentrations.
Statistical analyses
This is a phase 1 monocentric study, sample size was defined by the capacity of recruitment in a 2 years’ period and with regard of the continual reassessment method used for dose escalation steps.
Various outcomes were prespecified during the follow-up: overall survival (OS), event-free survival (EFS), leukaemia free survival (LFS), cumulative incidence of relapse (CIR). OS was defined as the time between day 1 of induction and the date of death, censored by the date of last follow-up if still alive. EFS was defined as the time between day 1 of induction and the date of death, failure to treatment or relapse, censored by the date of last follow-up if still alive. LFS was defined by the time between the day of CR/CRi/MLFS documentation and the date of relapse or death, censored by the date of last follow-up if still alive. CIR was defined by the time between the day of CR/CRi/MLFS documentation until the date of relapse. Patients not known to have relapsed were censored on the date they were last examined. Patients who died without relapse were counted as a competing cause of failure. Relapse was defined according to ELN-2017 criteria.
OS, EFS, and LFS were analysed using the Kaplan–Meier method. CIR was analysed using Kalbfleisch et Prentice method. Median survival and survival rates were reported with a 95% confidence interval. All included patients having received tocilizumab were considered evaluable for toxicity and efficacy. The data collection cut-off was March 29, 2023. Wilcoxon signed rank exact test was used for comparisons of cytokine plasma levels. Fisher's exact test was used to evaluated the impact of the ‘low level profile’ of FLT3 ligand.
No post hoc nor sensitivity analysis were made.
All statistical analyses were performed using R version 4.2.3. A p value < 0.05 was considered as statistically significant.
Role of the funding source
The funder was not involved in protocol writing, data collection, data analysis, data interpretation, nor manuscript writing.
Results
Participants
Between Dec 29, 2020 and Dec 1, 2022, 12 patients were enrolled (Fig. 1). There no screening failures or events of consent withdrawal. All 12 patients received tocilizumab. At inclusion (Table 1), their median age was 66 years (inter quartile range [IQR] 65.0–70.5). The ECOG score was 0 for eight patients (67%), 1 for three patients (25%), and 2 for one patient (8%). All patients (n = 12) were newly diagnosed with AML. The ELN 2017 risk was intermediate in 3/12 (25%) cases and unfavourable in 9/12 (75%).
Fig. 1.
Trial profile. pt: patient; CR: complete remission; CRi: complete remission with incomplete haematological recovery; MLFS: morphological leukaemia free state; MRD: measurable residual disease; Allo-HCST: allogeneic stem cell transplantation; MOF: multi organ failure.
Table 1.
Patient characteristics.
| Level 1 (Tocilizumab 4 mg/kg) | Level 2 (Tocilizumab 6 mg/kg) | Level 3 (Tocilizumab 8 mg/kg) | All patients | |
|---|---|---|---|---|
| Number of patients | 2 | 2 | 8 | 12 |
| Age (median, IQR) | 65.5 (65.2–65.8) | 69.0 (67.0–71.0) | 66.5 (65.0–70.5) | 66.0 (65.0–70.5) |
| Gender (n, %) (male/female) | 1/1 (50%/50%) | 0/2 (0%/100%) | 4/4 (50%/50%) | 5/7 (42%/58%) |
| ECOG | ||||
| 0 | 1 (50%) | 2 (100%) | 5 (62%) | 8 (67%) |
| 1 | 1 (50%) | 0 (0%) | 2 (25%) | 3 (25%) |
| 2 | 0 (0%) | 0 (0%) | 1 (12%) | 1 (8.3%) |
| ELN-2017 risk profile | ||||
| Intermediate | 1 (50%) | 1 (50%) | 1 (12%) | 3 (25%) |
| Defavorable | 1 (50%) | 1 (50%) | 7 (88%) | 9 (75%) |
| De novo AML | 1 (50%) | 2 (100%) | 5 (62%) | 8 (67%) |
| Secondary AML | 1 (50%) | 0 (0%) | 3 (38%) | 4 (33%) |
| First line | 2 (100%) | 2 (100%) | 8 (100%) | 12 (100%) |
Primary objective and safety
Among enrolled patients, two received tocilizumab at 4 mg/kg, two at 6 mg/kg and eight at 8 mg/kg. No DLT related to tocilizumab was observed, thus, the maximum tolerated dose was not reached. There was no IRR after tocilizumab infusion, all patients received their treatment as required at day 8 of induction. No adverse event neither serious adverse event related to tocilizumab was documented at time of infusion or thereafter up to day +45.
Considering the combination of chemotherapy plus tocilizumab, the most common grade 3/4 AE were bacteraemia in 8/12 (67%) patients and pneumonia in 2/12 (16%). Finally, there were 9 serious AE, according to investigators and the data safety monitoring board, yet none of them were related to tocilizumab and there was no treatment-related death (Table 2).
Table 2.
Adverse events (AE) related to the combination of chemotherapy plus tocilizumab.
| Number of patients with AE median AE/patients | Grade 1 |
Grade 2 |
Grade 3 |
Grade 4 |
Grade 5 |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| 8 |
12 |
10 |
6 |
2 |
||||||
| 2 |
6 |
3 |
1 |
1 |
||||||
| Before injection (D1–D7) | From Tocilizumab injection (from D8) | Before injection (D1–D7) | From Tocilizumab injection (from D8) | Before injection (D1–D7) | From Tocilizumab injection (from D8) | Before injection (D1–D7) | From Tocilizumab injection (from D8) | Before injection (D1–D7) | From Tocilizumab injection (from D8) | |
| Blood and lymphatic system disorders | 0 | 0 | 0 | 1 | 4 | 4 | 0 | 4 | 0 | 0 |
| Anaemia | 0 | 0 | 0 | 0 | 3 | 1 | 0 | 0 | 0 | 0 |
| Febrile bone marrow aplasia | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Lymphadenopathy | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Neutropenia | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 2 | 0 | 0 |
| Thrombocytopenia | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 2 | 0 | 0 |
| Ear and labyrinth disorders | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Vertigo | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Eye disorders | 0 | 0 | 0 | 2 | 0 | 1 | 0 | 0 | 0 | 0 |
| Lacrimation decreased | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Periorbital oedema | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Photophobia | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Gastrointestinal disorders | 3 | 4 | 8 | 18 | 0 | 3 | 0 | 1 | 0 | 0 |
| Abdominal pain | 0 | 2 | 2 | 1 | 0 | 2 | 0 | 0 | 0 | 0 |
| Abdominal pain upper | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Colitis | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Constipation | 1 | 0 | 2 | 4 | 0 | 0 | 0 | 0 | 0 | 0 |
| Diarrhoea | 0 | 1 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 0 |
| Haemorrhoids | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Large intestine perforation | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Lip dry | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Nausea | 2 | 0 | 3 | 6 | 0 | 0 | 0 | 0 | 0 | 0 |
| Vomiting | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| General disorders and administration site conditions | 0 | 1 | 0 | 3 | 1 | 2 | 0 | 1 | 0 | 0 |
| Mucosal inflammation | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 |
| Pain | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pyrexia | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 |
| Ulcer | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Infections and infestations | 0 | 0 | 0 | 2 | 5 | 15 | 0 | 1 | 0 | 1 |
| Appendicitis | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Bacillus infection | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Bacteraemia | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Bacteroides bacteraemia | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Escherichia bacteraemia | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Escherichia urinary tract infection | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 |
| Fungal infection | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 |
| Klebsiella bacteraemia | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Pneumonia | 0 | 0 | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 0 |
| Pseudomonal bacteraemia | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Sepsis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Septic shock | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Staphylococcal bacteraemia | 0 | 0 | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 0 |
| Staphylococcal infection | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Tooth infection | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 |
| Urinary tract infection enterococcal | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Urinary tract infection staphylococcal | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Injury, poisoning and procedural complications | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 |
| Eschar | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Transfusion-related immunomodulation reaction | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Metabolism and nutrition disorders | 3 | 5 | 2 | 6 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hyperkalaemia | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hypokalaemia | 3 | 3 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 |
| Magnesium deficiency | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Malnutrition | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Starvation | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 |
| Neoplasms benign, malignant and unspecified (incl cysts and polyps) | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Renal cell carcinoma | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Nervous system disorders | 0 | 0 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| Haemorrhage intracranial | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Headache | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Parkinsonism | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Psychiatric disorders | 0 | 0 | 5 | 2 | 0 | 0 | 0 | 0 | 0 | 0 |
| Anxiety | 0 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Insomnia | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 |
| Renal and urinary disorders | 0 | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 |
| Acute kidney injury | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Bladder dilatation | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Urinary retention | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Reproductive system and breast disorders | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Prostatic obstruction | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Respiratory, thoracic and mediastinal disorders | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Dyspnoea | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Skin and subcutaneous tissue disorders | 0 | 1 | 3 | 7 | 0 | 1 | 0 | 0 | 0 | 0 |
| Dry skin | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Erythema | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Rash | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Rash maculo-papular | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Rash morbilliform | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Rash pruritic | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Skin mass | 0 | 0 | 1 | 4 | 0 | 1 | 0 | 0 | 0 | 0 |
| Vascular disorders | 0 | 0 | 2 | 3 | 0 | 0 | 0 | 1 | 0 | 0 |
| Deep vein thrombosis | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hypertension | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 |
| Shock haemorrhagic | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Thrombosis | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total | 6 | 12 | 23 | 50 | 10 | 28 | 0 | 8 | 0 | 2 |
Haematopoietic recoveries after chemotherapy plus tocilizumab
For evaluable patients, the median times for neutrophil and platelets recoveries were 29.0 days (IQR 26.0–30.0) and 36.5 days (IQR 31.8–40.0), respectively.
Responses after induction plus tocilizumab
BM assessment at d15 was performed only in one patient (showing a non-blastic aplasia). All other patients were ≥60-year-old and thus did not have this assessment at d15. Two patients (belonging to the level 3 cohort) (17%) died during induction. One death occurred at d21 due to infection (Pseudomonas aeruginosa) and another one at d15 was related to a probable cerebral haemorrhage in the context of a Bacillus cereus bacteriemia.
Thus, 10 patients out of 12 (83%) were evaluable for response to treatment. CR, CRi, and MLFS were documented in 4, 4, and 1 cases, respectively, leading to an ORR of 75% (9/12) overall and 90% (9/10) in those evaluable. One patient was refractory to the combination.
Among the 3 patients with ELN-2017 intermediate risk profile, the ORR was 66% (2/3) and 100% (2/2) in those evaluable. Among the 9 patients with ELN-2017 high-risk profile, the ORR were 77% (7/9) and 87.5% (7/8) in those evaluable.
MRD was studied in 6 of the 9 responders (66%) showing a negative status in 4 (66%).
Cytokine assessment
A total of 90 cytokine assays (IL-6: n = 45; Flt-3 ligand n = 45) were performed.
Median IL-6 plasma concentrations (pg/mL) increased all along induction, especially after tocilizumab infusion (d+15 and d+22). Indeed, median concentrations were 3 (IQR 1.0–7.0) at d1, 28.0 (IQR 15.0–41.0) at d8, 203.0 (IQR 80.0–1933.0) at d15 and 621.0 (IQR 228.0–1038.0) at d22. The difference was highly significant between d1 and d22 (p < 0.001) (Fig. 2A). Of note, patients documented with very high IL-6 levels (>2000 pg/mL) or those showing an increase of IL-6 level all along the induction did not have more grade 3 or 4 febrile neutropenia compared with others.
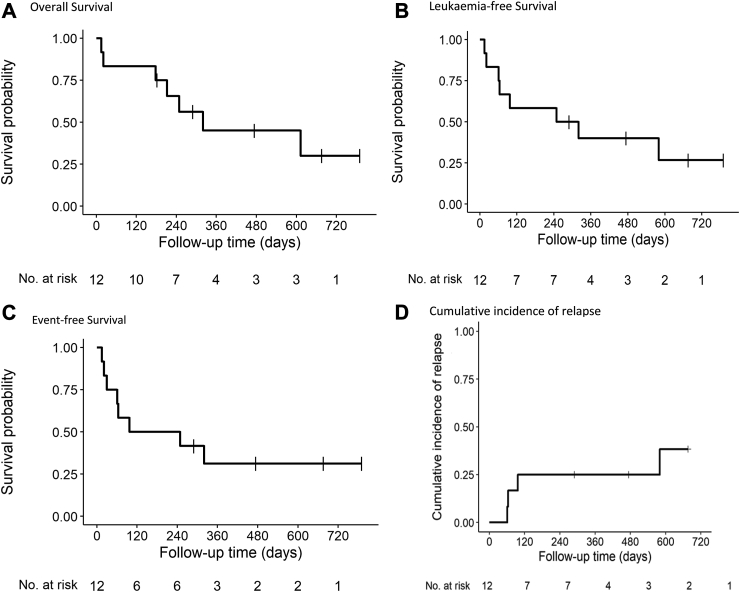
Fig. 3.
Patient outcomes. (A) Overall Survival, (B) Leukaemia-free Survival, (C) Event-free Survival, (D) Cumulative incidence of relapse.
The same was observed for median Flt-3 ligand plasma concentration (pg/mL) which increased all along the induction: 3.4 (IQR 0.60–7.5) at d1, 16.0 (3.0–107.0) at d8, 115.0 (20.0–404.0) at d15, and 216.0 (90.0–298.0) at d22. The difference was also significant between d1 and d22 (p = 0.006). Moreover, three different kinetic profiles were disclosed, one showing a continuous increase of cytokine levels during induction, one showing an increase of Flt-3 ligand until d15 then a decrease at d22 and one showing a low level of Flt-3 ligand concentration all along induction (Fig. 2B). All the patients with a low profile of Flt-3 ligand were refractory or relapsed (R/R) (n = 3/3), compared with the group with increasing Flt-3 ligand levels where there was only 1R/R (n = 1/8; p = 0.024).
Tocilizumab concentration assessment
A total of 21 assays of tocilizumab were available. Median levels of tocilizumab plasma concentration (μg/mL) were higher at d15 and d22 for dose levels 2 and 3 compared with level 1. Indeed, results for level 2 were 35.0 μg/mL at d15 (IQR 33.0–37.0) (n = 2) and 17.0 μg/mL at d22 (IQR 16.0–18.0) (n = 2). For level 3, they were 38.0 μg/mL at d15 (IQR 29.0–43.0) (n = 6) and 13.0 μg/mL at d22 (IQR 12.0–23.0) (n = 7) while they were 11.0 μg/mL at d15 (IQR 11.0–11.0) (n = 2) and 1.0 μg/mL at d22 (IQR 1.0–2.0) (n = 2) for level 1 (Fig. 2C). Nevertheless, due to the low number of patients in levels 1 and 2, no statistical test was made to evaluate whether these differences were significant.
Outcomes after chemotherapy plus tocilizumab
After induction, 7 out of the 9 responders (78%) received a consolidation. The refractory patient received a salvage regimen allowing to obtain CR. Finally, 7 of the 10 patients alive after induction (70%) were allo-transplanted in CR (Table 3).
Table 3.
Patient characteristics and outcomes.
| Patients' N° | Level/dose of Tocilizumab | Gender | ECOG PS | Caryotype | Molecular assessment | ELN2017 classification | Response to treatment according to ELN 2017 classification | MRD | Cytogenetic remission | Treatment received after induction | Allo-HCST | Relapse | Cause of death | Status at last follow-up |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 01 | L1 : 4 mg/kg | M | 1 | Complex with del5q | Flt3 neg/idh1 and 2 neg/npm1 neg | Unfavourable | Primary refractory disease | NA | NA | Salvage with azacitidine = failed; 2nd attempt with low dose arac + venetoclax = CR | yes | Yes (before transplant) | Leukaemia | Dead |
| 02 | L1 : 4 mg/kg | F | 0 | trisomy 11 | Flt3 neg/idh1 neg/IDH2 POS/npm1 neg | Intermediate | CR | neg | not done | Consolidation with azacitidine (1 course) | yes | no | NA | Alive, in CR |
| 03 | L2 : 6 mg/kg | F | 0 | complex | npm1 neg/Flt3 neg | Unfavourable | CRi | neg | yes | Consolidation with Idarubicin (1 day) + cytarabine; 1 course | yes | no | Infection and MOF | Dead, in CR |
| 04 | L2 : 6 mg/kg | F | 0 | Normal | Flt3neg, NPM1neg, IDHneg, CEBP alpha neg; EZH2 pos | intermediate | CR | 0.16% | NA | Consolidation with Idarubicin (1 day) + cytarabine; 2 courses | Yes | No | NA | Alive, in CR |
| 05 | L3 : 8 mg/kg | M | 0 | Normal | RUNX1 pos and ASXL1 pos | unfavourable | CR | Negative | NA | Consolidation with Idarubicin (1 day) + cytarabine; 1 course | Yes | No | GVHD | Dead, in CR |
| 06 | L3 : 8 mg/kg | M | 0 | 2 anomalies including monosomy 7 | Mutations in JAK2, TET2, DNMT3A, MPL and NFE2 | Unfavourable | CRi | Not done | Yes | Consolidation with Idarubicin (1 day) + cytarabine; 1 course | Yes | No | NA | Alive, in CR |
| 07 | L3 : 8 mg/kg | M | 2 | del 5q, monosomy 7 and MECOM rearrangement | 2 mutations of TP53 + 1 mutation of CXCR4 | Unfavourable | CR | 0.6% | Yes | Consolidation with Idarubicin (1 day) + cytarabin; 1 course | No | Yes | Leukaemia | Dead |
| 08 | L3 : 8 mg/kg | F | 0 | Complex with del5q | del TP53 | Unfavourable | CRi | Not done | No | Salvage with azacitidine + venetoclax = failed; 2nd attempt with high dose of cytatabine = failed; sequential Allo-HSCT = CR | Yes | Yes (before transplant) | Engraftment syndrom | Dead, in CR |
| 09 | L3 : 8 mg/kg | M | 1 | normal | Mutation of: IDH2, ASXL1, SRSF2 et RUNX1 | Unfavourable | CRi | Negative | NA | Consolidation with azacitidine (1 course) | Yes | No | NA | Alive, in CR |
| 10 | L3 : 8 mg/kg | F | 1 | Normal | NPM1neg, Flt3neg, IDH1 pos, IDH 2 neg, DNMT3 pos | Intermediate | NA | NA | NA | NA | No | NA | Infection during induction | Dead |
| 11 | L3 : 8 mg/kg | F | 0 | Monosomy 7 | no mutations on a 22 gene NGS panel (including npm1 and Flt3) | Unfavourable | MLFS | Not done | Not done | Salvage with azacitidine + venetoclax = failed; Allo-HSCT with active disease | yes | Yes (before transplant) | NA | Alive, in CR |
| 12 | L3 : 8 mg/kg | F | 0 | Normal | Mutation of ASXL1 | Unfavourable | NA | NA | NA | NA | no | NA | Probable cerebral haemorrhage during induction | Dead |
With a median follow-up of 22.5 months (IQR 9.6-NA), 3 responders have relapsed between 20 and 64 days after reaching CR (n = 1), CRi (n = 1) or MLFS (n = 1). These three relapsed patients had been treated at level 3. CIR was 25% [95% CI 5.3–52] at 120 days (Fig. 3A). At last follow-up, 7 patients have died, including 2 during induction and 5 after induction. Causes of deaths after induction were relapse in 2 and post-transplant related mortality in 3. Five patients (42%) are still alive in CR. One-year OS, LFS and EFS were estimated at 45% (95% CI 23%–89%); 40% (95% CI 20%–82%) and 31% (95% CI 13%–75%), respectively (Fig. 3B–D).
Fig. 2.
Plasma concentrations. (A) IL-6, (B) Flt-3 ligand, (C) Tocilizumab.
Discussion
In this prospective, single-centre trial, targeting the IL-6 pathway through IL-6R blockade by the addition of tocilizumab to a standard intensive induction AML therapy appeared to be very safe. All patients could receive tocilizumab as programmed, the highest level (8 mg/kg) being reached as quickly as the continual reassessment method (CRM) allowed it. It was chosen to use the CRM instead of traditional rule-based approaches such as the 3 + 3 design, since the former has been shown to be more accurate in targeting the MTD and assigning more trial participants at or close to MTD.14 More importantly, none of the AE or SAE were related to tocilizumab, leading to the conclusion that MTD was not reached and that a dose of 8 mg/kg can be used for further phase 2/3 studies.
Intensive standard induction chemotherapy using a combination of anthracycline and cytarabine remains associated with a 5%–16% early death rate, even in large recent studies, in both adults and older patients.18, 19, 20 In this study, 2/12 patients (16%) died before d30. The proportion of early deaths thus appears relatively similar to what has been reported before, but the size of the population is small, which is obviously one of its limitations. Regarding these two patients, one (70 years old) died because of an infection due to a multidrug-resistant P. aeruginosa and the other one (72 years old) because of a probable cerebral haemorrhage in the context of a B. cereus bacteraemia, an infection that has been reported to sometimes cause central nervous system infection.21 Again, these two deaths were not linked to tocilizumab. One could argue that adding tocilizumab could increase the risk of infection. However, the rate of infections reported in this series (67% of bacteriemia and 25% of pneumonia) seems again quite comparable to others.19
All patients in this study were older (≥60-year-old) newly diagnosed AML cases. These older patients frequently present with unfavourable prognostic features, such as poor-risk cytogenetics or oncogenetics, as was the case here since 75% of the patients were documented with an ELN-2017 high-risk profile. Moreover, in this population, the CR rate achieved after induction chemotherapy was lower than that in young adults, and the remission duration was rarely longer than a year.19,20 In two recent studies, CR/CRi rates were comprised between 21,7% (in the conventional arm) and 59% for older patients with unfavourable cytogenetics.19,20 Although no comparison can be made, it is however encouraging to report here a high overall response rate especially for patients with a poor risk AML since 80% of them achieved CR or CRi. Moreover, even if very few patients could be assessed, MRD was negative in most of the cases. Consecutively, most of the patients could receive a consolidation and/or an allogeneic stem cell transplant and the induction combination did not preclude the continuation of the treatment.
Besides safety and efficacy assessments, it was possible to document some biological effects. The assay of plasma IL-6 concentrations showed higher levels at d22, suggesting a good saturation of tocilizumab targets (IL-6-R). This may be of crucial importance as AML cells are known to express IL-6R. This suggests that tocilizumab may participate to a potential anti-leukaemic effect by blocking leukemic cells signalling and proliferation through IL-6R. The concentration of plasma Flt-3 ligand, reported by our group to be associated with responses and outcomes in AML was also assessed here.11,17 We were able to retrieve the different kinetic profiles showing again that patients with a persistent low level of Flt-3 ligand throughout induction were associated to a greater risk of relapse and/or refractory disease, even if these results have to be interpreted with caution, given the low number of patients. To confirm the hypothesis that Flt-3 ligand levels, along with plasma IL-6 concentrations, could be powerful and early biomarkers in patients with AMLreceiving intensive induction, we are currently running another multicentric observational study (ClinicalTrials.gov, NCT04641910). Finally, tocilizumab dosages revealed that higher concentrations at one week and the persistence of the drug in plasma at two weeks after infusion for levels 2 and 3 may also participate to the efficacy of the drug on response.
The question of providing additional doses of tocilizumab during induction could be of interest. However, IL-6 assays showed a constant and consequent increase of median concentrations between day 1 and day 22 with only one dose, indicating the efficacy of IL-6R blockade. This means that adding an earlier dose of tocilizumab would probably not improve these results. Moreover, no IL-6 kinetic profile has been identified predicting response in this series, suggesting that patients for example with a decrease of IL-6 level between day 15 and day 22 would also probably not benefit from another dose of tocilizumab. Therefore, the next step could be a randomised trial comparing Intensive standard induction chemotherapy with or without one infusion of tocilizumab.
In conclusion, this study shows that the combination of tocilizumab to a standard AML intensive induction appears to be safe providing both a high response rate and an expected biological effect through the saturation of IL-6R and high soluble IL-6 concentrations. We now recommend a dose of 8 mg/kg of tocilizumab given at day 8 of induction for further phase 2/3 studies.
Contributors
PP and PC participated in the conception and design of the study. PP, AG, ALB, TG, MJ and PC treated patients and participated in the clinical data collection and assembly. JG performed cytokines assessment, MR performed tocilizumab assessment, YLB, OT, ME, and MCB, performed biological analyses. LP performed statistical analyses. All authors verified the underlying data, participated in data analysis and interpretation, participated in manuscript development, and provided final approval of the submitted version. All authors had full and direct access to all the data in the study, verified the underlying data in this study and had final responsibility for the decision to submit for publication.
Data sharing statemnet
Study data are not publicly available to respect participant confidentiality. Reasonable requests for sharing deidentified data should be directed to the corresponding author.
Declaration of interests
We declare no competing interests.
Acknowledgments
This study was supported by a grant from the Leucémie Espoir Atlantique Famille (LEAF)—“Tous avec Fabien” association. This study has been presented in part at the 2023 ASCO and the 2023 EHA 2023 meetings. The authors would like to thank the association LEAF-Tous avec Fabien, Dr Laurent Flet, Mrs. Bettina Viel, Mrs. Cécile Braudeau and Mrs. Justine Chevreuil for technical expertise with the LISA-TRACKER Tocilizumab, the biological resource centre for biobanking (Nantes Université, CHU Nantes, Centre de ressources biologiques (CRB), F-44000 Nantes, France) (BRIF: BB-0033-00040) and the Medical Affairs and Research Department of Nantes University Hospital, Frédérique Adolphe for technical expertise with multiplex assays, the THERASSAY core facility (SFR Bonamy, UMR 1087, Nantes, France), member of the Scientific Interest Group (GIS) Biogenouest and IBISA, for the use of Bio-Plex® 200.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2023.102254.
Appendix ASupplementary data
References
- 1.Döhner H., Wei A.H., Appelbaum F.R., et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood. 2022;140(12):1345–1377. doi: 10.1182/blood.2022016867. [DOI] [PubMed] [Google Scholar]
- 2.Döhner H., Weisdorf D.J., Bloomfield C.D. Acute myeloid leukemia. N Engl J Med. 2015;373(12):1136–1152. doi: 10.1056/NEJMra1406184. [DOI] [PubMed] [Google Scholar]
- 3.Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 4.Hirano T., Yasukawa K., Harada H., et al. Complementary DNA for a novel human interleukin (BSF-2) that induces B lymphocytes to produce immunoglobulin. Nature. 1986;324(6092):73–76. doi: 10.1038/324073a0. [DOI] [PubMed] [Google Scholar]
- 5.Rašková M., Lacina L., Kejík Z., et al. The role of IL-6 in cancer cell invasiveness and metastasis—overview and therapeutic opportunities. Cells. 2022;11(22):3698. doi: 10.3390/cells11223698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanchez-Correa B., Bergua J.M., Campos C., et al. Cytokine profiles in acute myeloid leukemia patients at diagnosis: survival is inversely correlated with IL-6 and directly correlated with IL-10 levels. Cytokine. 2013;61(3):885–891. doi: 10.1016/j.cyto.2012.12.023. [DOI] [PubMed] [Google Scholar]
- 7.Stevens A.M., Miller J.M., Munoz J.O., Gaikwad A.S., Redell M.S. Interleukin-6 levels predict event-free survival in pediatric AML and suggest a mechanism of chemotherapy resistance. Blood Adv. 2017;1(18):1387–1397. doi: 10.1182/bloodadvances.2017007856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Binder S., Luciano M., Horejs-Hoeck J. The cytokine network in acute myeloid leukemia (AML): a focus on pro- and anti-inflammatory mediators. Cytokine Growth Factor Rev. 2018;43:8–15. doi: 10.1016/j.cytogfr.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 9.Beauchemin V., Villeneuve L., Rodriguez-Cimadevilla J.C., et al. Interleukin-6 production by the blast cells of acute myeloblastic leukemia: regulation by endogenous interleukin-1 and biological implications. J Cell Physiol. 1991;148(3):353–361. doi: 10.1002/jcp.1041480305. [DOI] [PubMed] [Google Scholar]
- 10.Luciano M., Krenn P.W., Horejs-Hoeck J. The cytokine network in acute myeloid leukemia. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.1000996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peterlin P., Gaschet J., Guillaume T., et al. A new cytokine-based dynamic stratification during induction is highly predictive of survivals in acute myeloid leukemia. Cancer Med. 2021;10(2):642–648. doi: 10.1002/cam4.3648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.European Medicines Agency 2018. https://www.ema.europa.eu/en/medicines/human/EPAR/roactemra EMA. [cité 2 juin 2023]. RoActemra.
- 13.Döhner H., Estey E., Grimwade D., et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129(4):424–447. doi: 10.1182/blood-2016-08-733196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wheeler G.M., Mander A.P., Bedding A., et al. How to design a dose-finding study using the continual reassessment method. BMC Med Res Methodol. 2019;19(1):18. doi: 10.1186/s12874-018-0638-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schuurhuis G.J., Heuser M., Freeman S., et al. Minimal/measurable residual disease in AML: a consensus document from the European LeukemiaNet MRD Working Party. Blood. 2018;131(12):1275–1291. doi: 10.1182/blood-2017-09-801498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lacombe F., Campos L., Allou K., et al. Prognostic value of multicenter flow cytometry harmonized assessment of minimal residual disease in acute myeloblastic leukemia. Hematol Oncol. 2018;36(2):422–428. doi: 10.1002/hon.2488. [DOI] [PubMed] [Google Scholar]
- 17.Peterlin P., Gaschet J., Guillaume T., et al. FLT3 ligand plasma levels in acute myeloid leukemia. Haematologica. 2019;104(6):e240–e243. doi: 10.3324/haematol.2018.209460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stone R.M., Mandrekar S.J., Sanford B.L., et al. Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation. N Engl J Med. 2017;377(5):454–464. doi: 10.1056/NEJMoa1614359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lancet J.E., Uy G.L., Cortes J.E., et al. CPX-351 (cytarabine and daunorubicin) liposome for injection versus conventional cytarabine plus daunorubicin in older patients with newly diagnosed secondary acute myeloid leukemia. J Clin Oncol. 2018;36(26):2684–2692. doi: 10.1200/JCO.2017.77.6112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pigneux A., Béné M.C., Guardiola P., et al. Addition of androgens improves survival in elderly patients with acute myeloid leukemia: a GOELAMS study. J Clin Oncol. 2017;35(4):387–393. doi: 10.1200/JCO.2016.67.6213. [DOI] [PubMed] [Google Scholar]
- 21.Koizumi Y., Okuno T., Minamiguchi H., Hodohara K., Mikamo H., Andoh A. Survival of a case of Bacillus cereus meningitis with brain abscess presenting as immune reconstitution syndrome after febrile neutropenia – a case report and literature review- BMC Infect Dis. 2020;20(1):15. doi: 10.1186/s12879-019-4753-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.