Abstract
Although numerous studies highlight the health benefits of tea, excessive consumption has been linked to toxic conditions. Thus, understanding the optimal consumption of tea is essential to minimize toxicity while maximizing its benefits. In this study, we investigated the effects of eight green tea samples (G1-G8) and eight black tea samples (R1-R8) from Camellia sinensis, the most popular teas in Asian culture, on RSC96 Schwann neural cells and embryonic cardiomyocyte H9c2 cells. The results showed that the IC50 (mg/ml, weight/volume) of both tea types were inversely proportional to their polyphenol content, suggesting a relationship between toxicity and polyphenol levels in both green and black tea. Interestingly, green teas generally have higher polyphenol content than black teas. We also assessed the protective effects of tea in vitro by pretreating cells with the teas at indicated doses of polyphenol and subsequently exposing them to H2O2. Both tea types significantly reduced the decline in cell viability for both cell lines, and there was no significant difference in protective polyphenol concentrations for green (G3 & G7) and black (R3 & R8) teas at effective concentrations (EC20 and EC40). To evaluate the preventative effects of tea in vivo, we examined the impact of two green (G3 & G7) and two black (R3 & R8) teas with varying polyphenol content on dextran sulfate sodium (DSS)-induced inflammatory colitis in mice. Tea-treated groups exhibited significantly lower inflammatory scores (DAI) than the control group. DSS treatment in the control group led to shortened colorectal lengths in mice, while tea co-treatment partially prevented this loss. Histological analysis revealed that G7 and R3 (with a moderate polyphenol content) treatment improved colorectal crypt structure, decreased the severity of inflammatory ulcerative colitis, and significantly reduced histological scores compared to the control group. However, G3 and R8 (with high and low doses of polyphenol content, respectively) did not show these effects, suggesting that a moderate polyphenol level in both tea types is optimal for preventative benefits.
Keywords: black tea, green tea, RSC96 cells, H9c2 cells, toxicity, protective effects, colorectal colitis.
Introduction
Tea has long been an essential aspect of Asian culture, particularly in Japan and China, and is known for its unique physiological benefits 1. Adult and Elderly especially like to drink tea. The most widely consumed teas are green and black tea, both derived from the Camellia sinensis tea tree. The primary difference between these two types of tea is that black tea is fermented before drying and roasting, while green tea remains unfermented. Both teas contain natural polyphenols, such as caffeine and catechins (catechin (C), epicatechin (EC), epicatechin gallate (ECG), epigallocatechin (EGC), and epigallocatechin gallate (EGCG)) 2. Green tea can have up to 5% of EGC and EGCG content, whereas black tea has a lower total polyphenol content. Recent epidemiological studies have demonstrated an inverse association between risks, such as cancer, cardiovascular disease, and psychological dysfunction, and the consumption of polyphenol-rich foods and beverages 3-8, and have been mentioned on their antioxidant and anti-inflammatory properties 9.
However, according to reviews, consuming beyond the recommended safe level of polyphenols in teas can lead to hepatotoxic and gastrointestinal disturbances, such as vomiting and diarrhea 10-15. EGCG exhibits some neurotoxicity 16 and affects human and rat neural progenitor cells (NPCs) development in vitro 17. The underlying reasons remain unclear, but it is possible that polyphenol-rich beverages like green and black tea produce fairly high concentrations of H2O2 during preparation and storage at room temperature 18. As exogenous agents, polyphenols produce nM concentrations of H2O2 in cells and organs, activating signaling factors and increasing cellular Eustress 19. When polyphenols reach high concentrations in the bloodstream, around μM concentrations of H2O2, they induce cytotoxicity and stress due to their exaggerated pro-oxidative effect. High levels of catechin, a major polyphenol in green tea, have been found to enhance carcinogen-induced carcinogenesis 20, 21 and promote DNA cleavage in the presence of Cu2+ in vitro 22. Another study reported that EGCG was protective to DNA at low concentrations but exacerbated DNA oxidative damage at higher concentrations, displaying pro-oxidative effects on DNA 23. Taken together, we theorized that there is margin of effective polyphenol concentration that serves the purpose of maintaining redox homeostasis in the body ideal for conferring better health. Therefore, it is urgent to find the amount of tea that is both safe and conveys adequate protective effect.
Considering that the tea leaf production process, specifically the fermentation level, affects polyphenol content, it is hypothesized that consuming green or black tea at a suitable polyphenol level can generate beneficial H2O2 concentrations and protect the body from environmental toxins. In this research project, the toxic and protective effects of green and black tea were investigated in vitro using RSC96 Schwann neural cells and embryonic cardiomyocyte H9c2 cells to determine their effects on cell viability. Furthermore, the preventive effects of green and black tea were evaluated in vivo using a mouse model, examining their impact on dextran sulfate sodium (DSS)-induced inflammatory colitis.
Materials and Methods
Tea Extract Preparation
Sixteen commercial tea samples, consisting of eight green tea samples (G1-G8) and eight black tea samples (R1-R8) from central Taiwan, were collected, all from the species Camellia sinensis. Each 10 g tea sample was immersed in 100 ml boiling distilled water for 10 min. The leaves were separated using filtration, and the total extracts were adjusted to 100 ml. These final extracts were sterilized at 121 °C for 20 min and stored at 4 °C in a closed container, designated as 100 mg/mL (tea/water) for subsequent experiments.
Polyphenol Content Assessment
The method employed by Singleton et al. 24 was utilized to measure polyphenol content. An extract (1 ml) was mixed with distilled H2O (10 ml) and Folin-Ciocalteu reagent (0.5 ml), shaken, and left at room temperature for 15 min. Around 3 ml of 20% Na2CO3 was added, and the solution was heated at 100°C for 1 min in a water bath. Using a spectrophotometer at 725 nm, the polyphenol content was calibrated to a gallic acid standard, and the percentage of polyphenol in crude leave extracts of both types of tea is shown in Table 1.
Table 1.
The IC50 (mg/ml) toxicity of green and black teas in vitro. The unit of IC50 were adjusted to polyphenolic concentration (pIC50) (% of polyphenol,). Sixteen commercial tea samples belonging to the species of Camellia sinensis including green teas (G1-G8, eight samples) and black teas (R1-R8, eight samples) from central Taiwan were collected. Each tea sample (10 g) was soaked in 100 ml boiled distilled water for 10 min. The polyphenols were detected by Folin-Ciocalteu method.
| Tea | Crude leave extracts (% of polyphenols) |
RSC96 (IC50) (mg/ml) |
RSC96 (pIC50) (% of polyphenols) |
H9c2 (IC50) (mg/ml) |
H9c2 (pIC50) (% of polyphenols) |
|---|---|---|---|---|---|
| G1 | 0.16 | 2.73 | 0.0085 | 1.11 | 0.0035 |
| G2 | 0.15 | 3.24 | 0.0098 | 1.34 | 0.0040 |
| G3 | 0.26 | 2.03 | 0.0104 | 0.90 | 0.0046 |
| G4 | 0.18 | 2.67 | 0.0094 | 1.05 | 0.0037 |
| G5 | 0.25 | 1.87 | 0.0095 | 0.67 | 0.0034 |
| G6 | 0.21 | 2.07 | 0.0088 | 0.99 | 0.0042 |
| G7 | 0.14 | 3.10 | 0.0087 | 1.20 | 0.0034 |
| G8 | 0.26 | 2.10 | 0.0110 | 1.05 | 0.0055 |
| R1 | 0.06 | 5.82 | 0.0075 | 3.34 | 0.0043 |
| R2 | 0.10 | 5.79 | 0.0118 | 1.78 | 0.0036 |
| R3 | 0.07 | 9.49 | 0.0142 | 3.00 | 0.0045 |
| R4 | 0.13 | 3.08 | 0.0083 | 1.13 | 0.0030 |
| R5 | 0.16 | 2.47 | 0.0080 | 1.07 | 0.0035 |
| R6 | 0.17 | 2.43 | 0.0085 | 0.91 | 0.0032 |
| R7 | 0.04 | 10.38 | 0.0091 | 2.37 | 0.0021 |
| R8 | 0.03 | 15.26 | 0.0097 | 3.66 | 0.0023 |
Cell Culture
RSC96 cells were obtained from the Bioresources Collection and Research Center, and H9c2 myocardial cells were acquired from the American Type Culture Collection (ATCC; Rockville, MD). Cells were cultured in Dulbecco's modified Eagle's medium, supplemented with 10% v/v fetal bovine serum (Gibco BRL, Gaithersburg, MD, USA) and 100 μg/mL penicillin/streptomycin (Sigma-Aldrich Chemie, Munich, Germany) at 37◦C in a humidified atmosphere containing 5% CO2. The cells were seeded in 24-well culture plates at an initial density of 2 × 105 cells/mL and grown to around 80% confluence. Oxidative stress was induced using fresh H2O2. The cells were pretreated with tea extracts at the indicated concentrations for 24 h, and the medium containing H2O2 (final concentration at 5.6%) was added and incubated for the indicated time periods. Morphological analysis was performed by observing cell size and number changes under an inverted microscope (Olympus Corp., Japan) at 100× magnification.
MTT Assay
An MTT assay was used to determine cell viability. RSC96 or H9c2 cells were exposed to H2O2, with or without test sample pretreatments (teas). To establish hydrogen peroxide cytotoxicity, RSC96 or H9c2 cells were treated with five H2O2 concentrations, as indicated in a pilot study. This H2O2 concentration resulted in more than 30% cell death after 6 h compared to untreated control cells and was deemed suitable for subsequent experiments. RSC96 or H9c2 cells were starved for 6 h and pretreated with various indicated concentrations of teas for 24 h and treated with H2O2 for 24 h. After treatment, the medium was removed, and RSC96 or H9c2 cells were incubated with MTT 0.5 μg/mL at 37 °C for 4 h. Viable cell numbers were directly proportional to formazan production, dissolved in isopropanol, and determined by measuring absorbance at 570 nm using a microplate reader.
Animals
All animal protocols adhered to animal care guidelines reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) at China Medical University. Male BALB/c mice (18-20 g upon arrival, approximately 5 weeks of age) were procured from the National Laboratory Animal Center (Taipei, Taiwan) and acclimatized for 5-6 days before experimentation. Mice were individually housed in a room with a 12-h dark:light cycle and provided with mouse chow and drinking water ad libitum. Sample size was based on IACUC of China Medical University requirements.
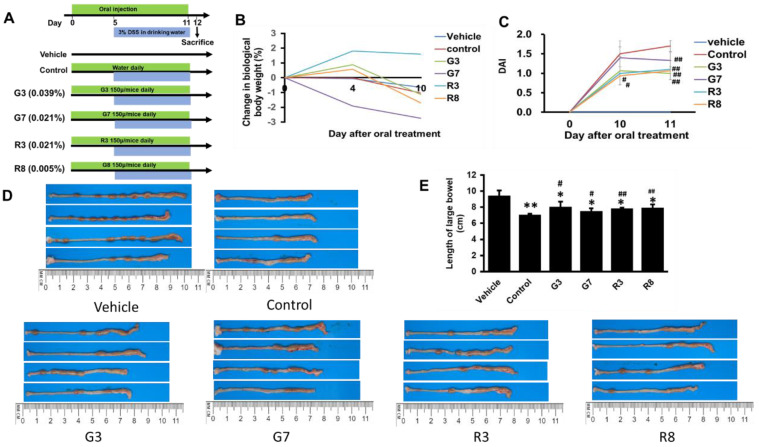
Twenty-four male BALB/c mice (5 weeks old) were maintained under constant conditions (room temperature 23 ± 1°C, 12 h light/dark cycle) with sterilized reverse osmosis water and diet available ad libitum. The mice were divided randomly into six groups: the vehicle group, control DSS group, G3 (G3 + DSS) group, G7 (G7 + DSS) group, R3 (R3 + DSS) group, and R8 (R8 + DSS) group. Starting on day 0, 200 μl of G3, G7, R3, or R8 tea solutions (30, 30, 60, or 30 μl respectively of tea extract + water) (with 3.9, 2.1, 2.1, or 0.5 mg/kg of polyphenol levels, respectively) were administered daily via oral injection until the experiment's end (Fig. 3A) 25. On Day 5, the mice began receiving 3% DSS in drinking water for 7 days, as described in the previous study 26. Body weight, hematochezia, and stool characteristics were monitored according to the disease activity index [DAI = (Weight loss score + Stool characteristics score + Hematochezia score)/3] as described 27. On day 12, all mice were euthanized by CO2 asphyxiation. The colons were removed, and length was measured. The distal colons were fixed in a 10% formalin solution, embedded in paraffin, and stained with H&E according to standard protocols. Histological scoring was performed as described 28. Severe inflammation was diagnosed as ulcerative colitis (UC). A combined score of inflammatory cell infiltration (score, 0 - 3) and tissue damage including the large-scale degeneration and death of epithelial cells in the mucosal layer, fibrous tissue proliferation, goblet cell depletion, and crypt loss (score, 0 - 3) were added to give a histological score of 0 (no changes) to 6 (extensive cell infiltration and tissue damage).
Figure 3.
Teas reduced colitis incidence in DSS-treated mice. (A) Design of the experimental procedure. (B) Quantification of DAI in vehicle, control, G3, G7, R3 and R8 groups. (C) Body weights. (D) Colon Images. (E) Quantification of colon length. *p<0.05; **p<0.01, compared with the vehicle group; #p<0.05; ##p<0.01, compared with the control group.
Hematoxylin-Eosin (HE) Staining and Histological Analysis
Paraffin-embedded colon tissues were sliced into sections (4 μm), which were deparaffinized and rehydrated through a xylene-ethanol-water gradient system. Hematoxylin and eosin (HE) staining was performed, followed by a dehydration process. Histopathological examination was conducted using a light microscope manufactured by Leica (LAS Software Version 4.9).
Statistical Analysis
All parametric data are presented as mean ± SEM. For single time point comparisons, a two-tailed unpaired Student's t-test (two groups) or one-way analysis of variance (ANOVA) with Tukey post-hoc test (more than 3 groups) was conducted. For all statistical tests, a significant difference was considered at p < 0.05.
Results
The toxicity of green and black teas in vitro
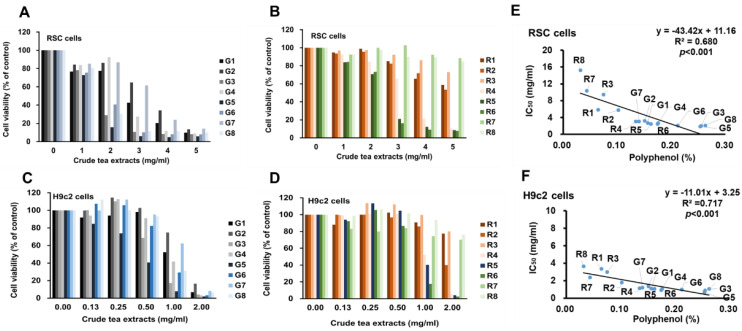
Toxicity test on RSC96 cell viability with 0.0, 1.0, 2.0, 3.0, 4.0, and 5.0 mg/ml of tea extracts for 72 hs showed that all green teas significantly decreased cell viability from 2 mg/ml in a dose-dependent manner (Fig. 1A), and two of the eight black teas resulted in the decrease of cell viability from the dose of 3.0 mg/ml in a dose-dependent manner (Fig. 1B). Toxicity test on H9c2 cell viability with 0.0, 0.125, 0.25, 0.5, 1.0, and 2.0 mg/ml of tea extracts for 72 hs showed significantly decreased cell viability from 0.5 mg/ml in a dose-dependent manner (Fig. 1C), and three of eight black teas significantly decreased cell viability from the dose of 1.0 mg/ml in a dose-dependent manner (Fig. 1D). In the linear regression analysis, the levels of tea polyphenol contents showed significant inverse correlation with each IC50 concentration (mg/ml, weight/volume) in both RSC96 and H9c2 cells (Fig. 1E and 1F). When IC50 was set to polyphenolic concentration (% of polyphenol, pIC50), the values of the green and black teas were at 0.0095 ± 0.0009 and 0.0096 ± 0.0023 for RSC96 cells respectively and 0.0040 ± 0.0007 and 0.0033 ± 0.0009 for H9c2 cells respectively (Table 1). Since reaction of cells to toxicity in the two types of tea were not significantly different (p < 0.05) in either cell types, this indicates the dependence of toxicity on polyphenol content.
Figure 1.
The toxicity of green and black teas in vitro. The RSC96 cells were treated with 1, and 2, 3, 4, and 5 mg/mL of green (A) and black (B) teas and H9c2 cells were treated with 0.125, 0.25, 0.5, 1, and 2 mg/mL of green (C) and black (D) extracts for 72hs. In the linear regression analysis, the levels of tea polyphenol contents in the each tea sample (5 g/100 ml) are significantly correlated with each IC50 concentration in both RSC96 (E) and H9c2 (F).
The protective effects of green and black teas in vitro
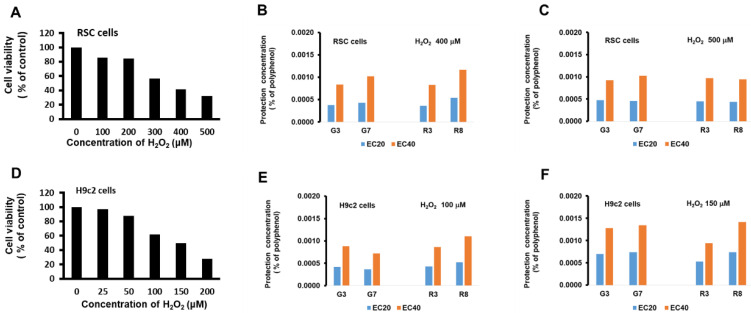
To examine the effects of the teas on H2O2-induced RSC96 cell death, we selected two of each type of teas with the highest and lowest polyphenol content (G3, G7, R3, or R8) for pretreatment for 24 hs before challenging with H2O2 for 72 hs. Administration of H2O2 decreased cell viability in a dose dependent manner, and 400 and 500 μM significantly diminished RSC96 cell viability by 41%, and 32% (Fig. 2A), respectively, and these concentrations were then subjected to EC20 (20% maximal effective concentration) and EC40 (40% maximal effective concentration) tests. The results showed that all tea types, when concentrations were adjusted to 0.0004 ± 0.0001% (Fig. 2B), were able to achieve EC20, and when concentrations were adjusted to 0.0010 ± 0.0001%, able to achieve EC40 (Fig. 2C).
Figure 2.
The protective effects of green and black teas in vitro. (A) RSC96 cells were treated with 0, 100, 200, 300, 400, and 500 μM H2O2 for 72 hs. Cells were pretreated with 0%, 0.0003%, 0.0006%, 0.0012%, and 0.0024 % polyphenol of teas for 24 hs and then challenged with 400 (B) or 500 (C) μM H2O2. The protection concentration of teas reversed the cell decreased viability in the administration of H2O2 by approximately 20% (EC 20) or 40% (EC 40). (D) H9c2 cells were treated with 0, 25, 50, 100, 150, and 200 μM H2O2. Cells were pretreated with 0%, 0.0003%, 0.0006%, 0.0012%, and 0.0024 % polyphenol of teas for 24 h and then challenged with 100 (E) or 150 (F) μM H2O2.
On H9c2 cells, identical treatment also decreased cell viability in a dose dependent manner, and 100 and 150 μM significantly diminished H9c2 cell viability by 62%, and 50%, respectively. EC20 and EC40 tests show that all tea types, when concentrations were adjusted to 0.0006 ± 0.0002% (Fig. 2E), were able to achieve EC20, and when concentrations were adjusted to 0.0011 ± 0.0003%, were able to achieve EC40 (Fig. 2F).
A comparison of the green and black teas (G3, G7, R3 & R8) revealed no significant difference in protective polyphenol concentrations (EC20 and EC40) between these teas in both RSC96 and H9c2 cells. As EC20 and EC40 denote effective concentrations for cell protection, this suggests that the beneficial properties of these teas can be attributed to polyphenol concentrations ranging from 0.0004% to 0.0011%. In an animal study, for instance, 200 μl of G3, G7, R3, or R8 tea solutions (with polyphenol levels of 3.9, 2.1, 2.1, or 0.5 mg/kg, respectively) were administered via oral injection to mice. This study assumed that the effects observed would be similar to those in the previously mentioned study 25. Based on these findings, the investigation of the protective effects in vivo will proceed using the same dosage for subsequent experiments.
The protective effects of green and black teas in vivo
A meta-analysis suggested that tea consumption was inversely associated with UC risk (29). In mice, however, an overdose of green tea polyphenols not only failed to suppress colonic colitis but instead enhanced its formation (30). To determine the effective dosage of polyphenols in tea that confers protective effect against DSS-induced colitis, mice were pretreated with G3, G7, R3, or R8 for four days and then challenged with DSS for seven days (Figure 3A).
In all the DSS-treated groups, G7 resulted in a slight linear decrease in body weight, but all groups experienced negligible changes. (Fig. 3B). The DAI score for the control group was significantly higher at the end of the experiment than that in the tea-treated groups (Fig. 3C). The mice in the tea-treated groups exhibited regular stool consistency compared with mice in the DSS only (control) group. The teas also partially prevented rectal bleeding. Colon shortening is a visual index that reflects the severity of colorectal inflammation 31. The colorectal length of the control groups was shorter than that of the vehicle group (Fig. 3D and 3E). Administration of teas showed a preventive effect against colon shortening, although shortening remained significant. The weight of the other organs exhibited no changes.
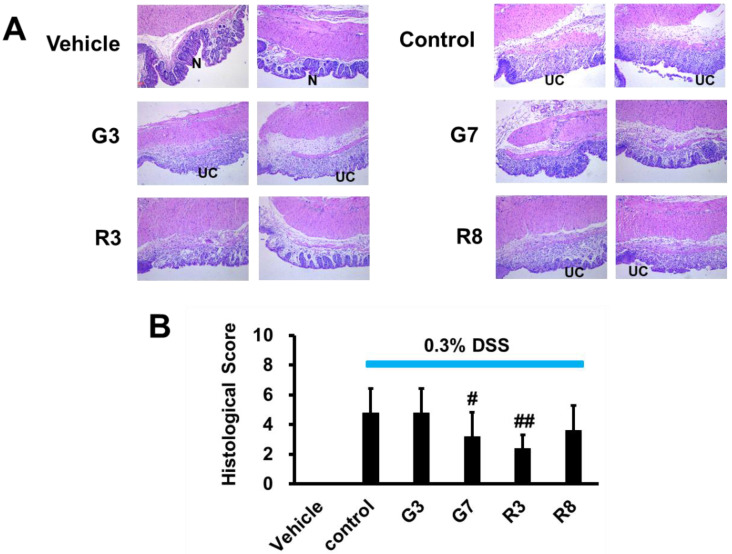
We next analyzed whether teas could block the histopathological changes of the colon tissue. The H&E staining of colon tissues from the vehicle group demonstrated intact mucosal epithelia and a normal number of goblet cells (Figure 4A). In contrast, DSS-treated mice displayed significant epithelial damage, crypt distortion, and inflammatory cell infiltration in both the mucosa and submucosa. The DSS control group exhibited considerable mucosal structure damage, as demonstrated by the histologic score (Figure 4B). Among the four tea samples tested, only G7 and R3, which contained 0.021% polyphenol content, were effective in preserving the colorectal crypt structure and reducing the severity of inflammation associated with ulcerative colitis (UC) when compared to the control group and the other tea samples G3 (0.039%) and R8 (0.005%). The histological scores for G7 and R3 were also significantly lower (Figure 4B), indicating a strong inhibitory effect on DSS-induced inflammation in the distal colonic crypts and prevention of ulcerative progression. These findings suggest that G7 and R3 tea samples, with their optimal polyphenol concentrations, may have potential therapeutic benefits for those suffering from UC.
Figure 4.
Effects of tea on colitis production in DSS-induced mice (magnification 100x). (A) Histological images of normal mucosa (N) and ulcerative colitis (UC) in vehicle, control, G3, G7, R3 and R8 with 2 mice in each group. (B) The statistical analysis of histological score. #p<0.05; ##p<0.01, compared with the control group.
Discussion
In this study, the toxicity and protective effects of 16 black and green teas from Taiwan were assessed using Schwann cells and H9c2 cells as in vitro models for preclinical evaluation of neurological and cardiovascular toxicity. The findings of the study indicated that the degree of toxicity exhibited by the teas relied on their polyphenol content, with certain concentrations of these compounds demonstrating protective effects in the cell culture models. These concentrations were observed to range between 0.0004% and 0.0011% of the total polyphenol content. Polyphenols, when present in low concentrations in the bloodstream, have been observed to generate H2O2 9. This interaction with H2O2 can have implications for various cellular processes, including cell signaling, adaptation, and overall cell survival. It is important to note that the consumption of polyphenols in appropriate quantities and at the right timing, particularly during meals, can lead to a synergistic effect in maintaining a balanced redox state within the body.
However, it is worth noting that when the concentration of polyphenols in the blood system reaches high levels, they generate a relatively high concentration of H2O2 and potentially other derivatives. Previous research has shown that beverages rich in polyphenols, including green tea, black tea, and coffee, generate H2O2 under most physiological conditions, which may induce mutagenicity in vitro 18. Additionally, studies involving healthy volunteers have shown that coffee consumption leads to elevated H2O2 levels in urine 32, 33. However, the total excreted H2O2 equivalent levels in urine are estimated to account for only 0.5-10% of the consumed coffee, suggesting that the remaining H2O2 may be retained or consumed within the body 34, 35, and given that H2O2 in the body is associated with carcinogenicity and genotoxicity 36, reducing H2O2 formation in beverages is desirable.
In H9C2 cardiomyocytes, it was observed EGCG displayed a time- and dose-dependent inhibition of proliferation and induction of apoptosis 37, in which the involvement of SIRT1, a protein associated with cellular processes, was identified. It was suggested that high doses of tea polyphenols, such as EGCG, may be toxic for cardiomyocytes and nerve cells, and related to the inhibition of the SIRT protein. The results from this study demonstrates the effects of oxidative stress from polyphenols which contain H2O2-generating properties.
Colorectal cancer (CRC) is highly associated with environmental factors and geographical cultural conditions (e.g., gender, race, and lifestyle) and is currently the fifth leading cause of cancer-related deaths worldwide 38, 39. Epidemiological research has identified diet as a major factor influencing CRC risk 40. Diets high in protein, fat, and refined carbohydrates may disrupt the balance of microbes in the colon, promoting the progression of inflammatory bowel disease (IBD) and increasing CRC risk 41-43. Therefore, suppressing inflammation is an important strategy in cancer prevention. Dextran sulfate sodium (DSS) is known to induce ulcerative colitis (UC) by damaging cell membranes, increasing colonic epithelial permeability, and altering tight junction protein expression 44, 45. Murakami reported that diets containing low doses (0.01-0.1%) of green tea polyphenols reduced DSS-induced colitis in experimental animal studies, however, diets containing high doses (0.5-1%) of green tea polyphenols aggravated the colitis formation 46. Therefore, this study utilized DSS-induced UC as an in vivo model. The results showed that although tea extracts did not restore colon length, G7 and R3 alleviated inflammation, while G3 and R8 did not, being at an ineffective dosage. This observation, combined with the known antioxidant and anti-inflammatory properties of polyphenols, led researchers to conclude that tea extracts may not inhibit DSS-induced damage to the colonic mucosa but can prevent DSS-induced colitis inflammation in the moderate dosage.
To determine the optimal tea consumption, in vivo and in vitro studies were conducted, emphasizing the importance of polyphenol content in black and green teas. For example, if the polyphenol content in G7 or R3 (10g/100ml) is 0.14% or 0.07% respectively, the ideal consumption for a 50 kg person would be 500 ml of hot water (containing 0.7 g G7 or 1.6 g R3) to achieve a protective prophylactic concentration of polyphenols (0.2 mg/kg) 47. These emphasize the importance of determining the polyphenol content in black and green teas to establish the most beneficial consumption amounts 48.
Conclusion
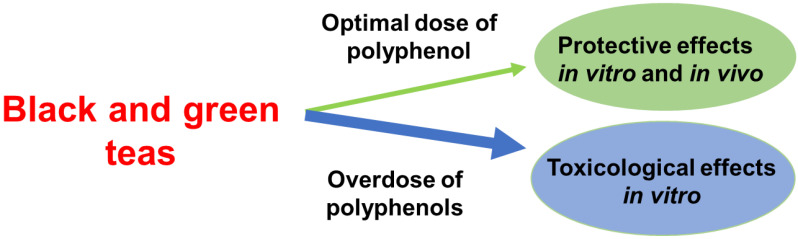
This research highlights the potential oxidative effects of polyphenols both in vitro and in vivo, as well as their role in mitigating inflammation in DSS-induced colitis, and shows that, to account for the toxicity as well as protective effect of black and green tea, the optimal concentration in cell culture models range between 0.0004% and 0.0011% of polyphenol content (Figure 5). These research findings can serve as guidelines for determining the optimal tea consumption based on polyphenol content and individual body weight, ultimately promoting health benefits and reducing potential risks associated with tea consumption.
Figure 5.
The protective and toxicity effects of black and green teas. Consuming an optimal polyphenol dose of teas was found to be more protective effects in vivo and in vitro, as well as consuming an overdose of polyphenols to be toxicological effects. This suggests that the efficacy of teas in treating inflammation-induced diseaases is influenced by the amount of polyphenols consumed.
Acknowledgments
The authors appreciate Andy Poon for English modification of manuscript.
Funding
This work was supported by grants from the Ministry of Science and Technology, Republic of China (MOST 107-2320-B-039-025-MY3 and MOST 106-2320-B-039-022), as well as in part by the China Medical University (CMU109-MF-87 and CMU109-S-29), Taiwan.
References
- 1.Khan N, Hasan Mukhtar H. Tea Polyphenols in Promotion of Human Health. Nutrients. 2018;11:39.. doi: 10.3390/nu11010039. doi: 10.3390/nu11010039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fernández PL, Martín MJ, González AG. et al. HPLC determination of catechins and caffeine in tea. Differentiation of green, black and instant teas. Analyst. 2000;125:421–425. doi: 10.1039/a909219f. doi: 10.1039/a909219f. [DOI] [PubMed] [Google Scholar]
- 3.Shin S, Lee JE, Loftfield E. et al. Coffee and tea consumption and mortality from all causes, cardiovascular disease and cancer: a pooled analysis of prospective studies from the Asia Cohort Consortium. Int J Epidemiol. 2022;51:626–640. doi: 10.1093/ije/dyab161. doi: 10.1093/ije/dyab161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marx W, Kelly J, Marshall S. et al. The Effect of Polyphenol-Rich Interventions on Cardiovascular Risk Factors in Haemodialysis: A Systematic Review and Meta-Analysis. Nutrients. 2017;9:1345.. doi: 10.3390/nu9121345. doi: 10.3390/nu9121345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giacco R, Costabile G, Fatati G. et al. Effects of polyphenols on cardio-metabolic risk factors and risk of type 2 diabetes. A joint position statement of the Diabetes and Nutrition Study Group of the Italian Society of Diabetology (SID), the Italian Association of Dietetics and Clinical Nutrition (ADI) and the Italian Association of Medical Diabetologists (AMD) Nutr Metab Cardiovasc Dis. 2020;30:355–367. doi: 10.1016/j.numecd.2019.11.015. doi: 10.1016/j.numecd.2019.11.015. [DOI] [PubMed] [Google Scholar]
- 6.Bedrood Z, Rameshrad M, Hosseinzadeh H. Toxicological effects of Camellia sinensis (green tea): A review. Phytother Res. 2018;32:1163–1180. doi: 10.1002/ptr.6063. doi: 10.1002/ptr.6. [DOI] [PubMed] [Google Scholar]
- 7.Bhardwaj K, Najda A, Sharma R, Fruit and Vegetable Peel-Enriched Functional Foods: Potential Avenues and Health Perspectives. 2022; 2022: 8543881. doi: 10.1155/2022/8543881. [DOI] [PMC free article] [PubMed]
- 8.Wang W, Ige OO, Ding Y. et al. Insights into the potential benefits of triphala polyphenols toward the promotion of resilience against stress-induced depression and cognitive impairment. Curr Res Food Sci. 2023;6:100527.. doi: 10.1016/j.crfs.2023.100527. doi: 10.1016/j.crfs.2023.100527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kanner J. Polyphenols by Generating H2O2, Affect Cell Redox Signaling, Inhibit PTPs and Activate Nrf2 Axis for Adaptation and Cell Surviving: In vitro, In vivo and Human Health. Antioxidants. 2020;9:797. doi: 10.3390/antiox9090797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keller A, Wallace TC. Tea intake and cardiovascular disease: an umbrella review. Ann Med. 2021;53:929–944. doi: 10.1080/07853890.2021.1933164. doi: 10.1080/07853890.2021.1933164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gabriela Mazzanti G, Francesca Menniti-Ippolito F, Paola Angela Moro PA. et al. Hepatotoxicity from green tea: a review of the literature and two unpublished cases. Eur J Clin Pharmacol. 2009;65:331–341. doi: 10.1007/s00228-008-0610-7. doi: 10.1007/s00228-008-0610-7. [DOI] [PubMed] [Google Scholar]
- 12.Mazzanti G, Sotto AD, Vitalone A. Hepatotoxicity of green tea: an update. Arch Toxicol. 2015;89:1175–1191. doi: 10.1007/s00204-015-1521-x. doi: 10.1007/s00204-015-1521-x. [DOI] [PubMed] [Google Scholar]
- 13.Inoue H, Akiyama S, Maeda-Yamamoto M. et al. High-dose green tea polyphenols induce nephrotoxicity in dextran sulfate sodium-induced colitis mice by down-regulation of antioxidant enzymes and heat-shock protein expressions. Cell Stress Chaperones. 2011;16:653–662. doi: 10.1007/s12192-011-0280-8. doi: 10.1007/s12192-011-0280-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu J, Webster D, Cao J. et al. The safety of green tea and green tea extract consumption in adults - Results of a systematic review. Regul Toxicol Pharmacol. 2018;95:412–433. doi: 10.1016/j.yrtph.2018.03.019. doi: 10.1016/j.yrtph.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 15.Lambert JD, Kennett MJ, Sang S. et al. Hepatotoxicity of high oral dose (-)-epigallocatechin-3-gallate in mice. Food Chem Toxicol. 2010;48:409–416. doi: 10.1016/j.fct.2009.10.030. doi: 10.1016/j.fct.2009.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Silva RFM, Pogačnik L. Polyphenols from Food and Natural Products: Neuroprotection and Safety. Antioxidants. 2020;9:61.. doi: 10.3390/antiox9010061. doi: 10.3390/antiox9010061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barenys M, Gassmann K, Baksmeier C. Epigallocatechin gallate (EGCG) inhibits adhesion and migration of neural progenitor cells in vitro. Arch Toxicol. 2017;91:827–837. doi: 10.1007/s00204-016-1709-8. doi: 10.1007/s00204-016-1709-8. [DOI] [PubMed] [Google Scholar]
- 18.Akagawa M, Shigemitsu T, Suyama K. Production of hydrogen peroxide by polyphenols and polyphenol-rich beverages under quasi-physiological conditions. Biosci Biotechnol Biochem. 2003;67:2632–2640. doi: 10.1271/bbb.67.2632. doi: 10.1271/bbb.67.2632. [DOI] [PubMed] [Google Scholar]
- 19.Cao G, Sofic E, Prior RL. Antioxidant and prooxidant behavior of flavonoids: structure-activity relationships. Free Radic Biol Med. 1997;22:749–760. doi: 10.1016/s0891-5849(96)00351-6. doi: 10.1016/s0891-5849(96)00351-6. [DOI] [PubMed] [Google Scholar]
- 20.Guan F, Liu AB, Li G. et al. Deleterious effects of high concentrations of (-)-epigallocatechin-3-gallate and atorvastatin in mice with colon inflammation. Nutr Cancer. 2012;64:847–855. doi: 10.1080/01635581.2012.695424. doi: 10.1080/01635581.2012.695424. [DOI] [PubMed] [Google Scholar]
- 21.Hirose M, Hoshiya T, Akagi K. et al. Effects of green tea catechins in a rat multi-organ carcinogenesis model. Carcinogenesis. 1993;14:1549–1553. doi: 10.1093/carcin/14.8.1549. doi: 10.1093/carcin/14.8.1549. [DOI] [PubMed] [Google Scholar]
- 22.Hayakawa F, Kimura T, Maeda T. et al. DNA cleavage reaction and linoleic acid peroxidation induced by tea catechins in the presence of cupric ion. Biochim Biophys Acta. 1997;1336:123–131. doi: 10.1016/s0304-4165(97)00019-6. doi: 10.1016/s0304-4165(97)00019-6. [DOI] [PubMed] [Google Scholar]
- 23.Tian B, Sun Z, Xu Z. et al. Chemiluminescence analysis of the prooxidant and antioxidant effects of epigallocatechin-3-gallate. Asia-Pac J Clin Nutr. 2007;16(Suppl. 1):153–157. [PubMed] [Google Scholar]
- 24.Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic. 1965;16:144–158. [Google Scholar]
- 25.Markus Brückner M, Sabine Westphal S, Wolfram Domschke W. et al. Green tea polyphenol epigallocatechin-3-gallate shows therapeutic antioxidative effects in a murine model of colitis. J Crohns Colitis. 2012;6:226–235. doi: 10.1016/j.crohns.2011.08.012. doi: 10.1016/j.crohns.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 26.He XQ, Liu D, Liu HY. et al. Prevention of Ulcerative Colitis in Mice by Sweet Tea ( Lithocarpus litseifolius) via the Regulation of Gut Microbiota and Butyric-Acid-Mediated Anti-Inflammatory Signaling. Nutrients. 2022;14:2208.. doi: 10.3390/nu14112208. doi: 10.3390/nu14112208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murano M, Maemura K, Hirata I, Therapeutic effect of intracolonically administered nuclear factor kappa B (p65) antisense oligonucleotide on mouse dextran sulphate sodium (DSS)-induced colitis, Clin Exp Immunol. 2000; 120: 51-58. doi: 10.1046/j.1365-2249. 2000. 01183.x. [DOI] [PMC free article] [PubMed]
- 28.Haiyan Liu H, Ruohong Chen R, Shuai Wen S. et al. Tea (Camellia sinensis) ameliorates DSS-induced colitis and liver injury by inhibiting TLR4/NF-κB/NLRP3 inflammasome in mice. Biomed Pharmacother. 2023;158:114136.. doi: 10.1016/j.biopha.2022.114136. doi: 10.1016/j.biopha.2022.114136. [DOI] [PubMed] [Google Scholar]
- 29.Nie JY, Zhao Q. Beverage consumption and risk of ulcerative colitis: Systematic review and meta-analysis of epidemiological studies. Medicine (Baltimore) 2017;96:e9070.. doi: 10.1097/MD.0000000000009070. doi: 10.1097/MD.0000000000009070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim M, Murakami A, Miyamoto S. et al. The modifying effects of green tea polyphenols on acute colitis and inflammation-associated colon carcinogenesis in male ICR mice. Biofactors. 2010;36:43–51. doi: 10.1002/biof.69. doi: 10.1002/biof.69. [DOI] [PubMed] [Google Scholar]
- 31.Melgar S, Karlsson L, Rehnström E. et al. Validation of murine dextran sulfate sodium-induced colitis using four therapeutic agents for human inflammatory bowel disease. Int Immunopharmacol. 2008;8:836–844. doi: 10.1016/j.intimp.2008.01.036. doi: 10.1016/j.intimp.2008.01.036. [DOI] [PubMed] [Google Scholar]
- 32.Long LH, Halliwell B. Coffee drinking increases levels of urinary hydrogen peroxide detected in healthy human volunteers. Free Radic Res. 2000;32:463–467. doi: 10.1080/10715760000300461. doi: 10.1080/10715760000300461. [DOI] [PubMed] [Google Scholar]
- 33.Hiramoto K, Kida T, Kikugawa K. Increased urinary hydrogen peroxide levels caused by coffee drinking. Biol Pharm Bull. 2002;25:1467–1471. doi: 10.1248/bpb.25.1467. doi: 10.1248/bpb.25.1467. [DOI] [PubMed] [Google Scholar]
- 34.Suganuma M, Okabe S, Oniyama M. et al. Wide distribution of [3H](-)-epigallocatechin gallate, a cancer preventive tea polyphenol, in mouse tissue. Carcinogenesis. 1998;19:1771–1776. doi: 10.1093/carcin/19.10.1771. doi: 10.1093/carcin/19.10.1771. [DOI] [PubMed] [Google Scholar]
- 35.Morrice PC, Wood SG, Duthie GG. High-performance liquid chromatographic determination of quercetin and isorhamnetin in rat tissues using β-glucuronidase and acid hydrolysis. J Chromatogr B Biomed Sci Appl. 2000;738:413–417. doi: 10.1016/s0378-4347(99)00520-4. doi: 10.1016/s0378-4347(99)00520-4. [DOI] [PubMed] [Google Scholar]
- 36.DeSesso JM, Lavin AL, Hsia SM. et al. Assessment of the carcinogenicity associated with oral exposures to hydrogen peroxide. Food Chem Toxicol. 2000;38:1021–1041. doi: 10.1016/s0278-6915(00)00098-3. doi: 10.1016/s0278-6915(00)00098-3. [DOI] [PubMed] [Google Scholar]
- 37.Cai Y, Zhao L, Qin Y. et al. High dose of epigallocatechin-3-gallate inhibits proliferation and induces apoptosis of H9C2 cardiomyocytes through down-regulation of SIRT1. Pharmazie. 2015;70:12–16. [PubMed] [Google Scholar]
- 38.Sung H, Ferlay J, Siege RL. et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 39.Terzi´c J, Grivennikov S, Karin E. et al. Inflammation and colon cancer. Gastroenterology. 2010;138:2101–2114.e5. doi: 10.1053/j.gastro.2010.01.058. doi: 10.1053/j.gastro.2010.01.058. [DOI] [PubMed] [Google Scholar]
- 40.Cao SY, Li Y, Meng X, Plant foods for the prevention and management of colon cancer, J Funct Foods. 2018; 42: 95-110.
- 41.Yan J, Wang L, Gu Y. et al. Dietary Patterns and Gut Microbiota Changes in Inflammatory Bowel Disease: Current Insights and Future Challenges. Nutrients. 2022;14:4003.. doi: 10.3390/nu14194003. doi: 10.3390/nu14194003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rubin DC, Shaker A, Levin MS, Chronic intestinal inflammation. inflammatory bowel disease and colitis-associated colon cancer. Front Immunol. 2012;3:107.. doi: 10.3389/fimmu.2012.00107. doi: 10.3389/fimmu.2012.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.A. Ekbom, C. Helmick, M. Zack, H.O. Adami, Ulcerative colitis and colorectal cancer- A population-based study, N Engl J Med. 323 (1990) 1228-1233. [DOI] [PubMed]
- 44.Poritz LS, Garver KI, Green C. et al. Loss of the tight junction protein ZO-1 in dextran sulfate sodium induced colitis. J Surg Res. 2007;140:12–19. doi: 10.1016/j.jss.2006.07.050. doi: 10.1016/j.jss.2006.07.050. [DOI] [PubMed] [Google Scholar]
- 45.Yan Y, Kolachala V, Dalmasso G, Temporal and spatial analysis of clinical and molecular parameters in dextran sodium sulfate induced colitis. PLoS One. 2009: 4: e6073. doi: 10.1371/journal.pone.0006073. [DOI] [PMC free article] [PubMed]
- 46.Murakami A. Dose-dependent functionality and toxicity of green tea polyphenols in experimental rodents. Arch Biochem Biophys. 2014;557:3–10. doi: 10.1016/j.abb.2014.04.018. doi: 10.1016/j.abb.2014.04.018. [DOI] [PubMed] [Google Scholar]
- 47.Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22:659–661. doi: 10.1096/fj.07-9574LSF. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- 48.Cladis DP, Weaver CM, Ferruzzi MG. (Poly)phenol toxicity in vivo following oral administration: A targeted narrative review of (poly)phenols from green tea, grape, and anthocyanin-rich extracts. Phytother Res. 2022;36:323–335. doi: 10.1002/ptr.7323. doi: 10. [DOI] [PubMed] [Google Scholar]