Abstract
Thalassemia is among the most common hereditary disorders in the world. Approximately 5% of the world’s population are carriers of hemoglobinopathies, and 2.9% are carriers of beta thalassemia. Haemoglobin A2 (HbA2) constitutes less than 3% of the total hemoglobin (Hb) in adults, and the determination of Hb A2 levels is important to diagnose the beta thalassemia trait (BTT). In some cases, the level of HbA2 is not typically elevated, and some difficulties may arise in making the diagnosis. Cation exchange high-performance liquid chromatography (HPLC) and HbCZE (haemoglobin capillary zone electrophoresis) are considered acceptable methods to diagnose BTT, but these vary in their accuracy and cut-offs. In this study, we attempted to compare HbA2 values using two methods, HPLC and HbCZE, in 536 whole blood samples sent by physician-ordered hemoglobinopathy screening over two years. This included antenatal women, patients with anemia not responding to iron, and cases of familial screening where either a child or a sibling had been diagnosed with hemoglobinopathy or thalassemia. The performance characteristics of both machines were compared for the detection of the 5 most common hemoglobin variants: Hb A, HbF, HbS, Hb C, and HbE. On comparing the HbA2 values, the HPLC showed higher values for HbA2 as compared to HbCZE, while the HbF and HbS measurement agreement was good between both methods. Normal ranges and mean normal values of HbA2 differ between different methods and different manufacturers; hence, each institute using these machines should validate its cutoffs.
Keywords: Haemoglobinopathies, HbA2, Haemoglobin capillary zone electrophoresis, High-performance liquid chromatography
Introduction
Hb A2 is a hemoglobin tetramer composed of two alpha and two delta globin chains (a2d2) [1]. The levels of HbA2 are elevated in most BTT cases, and therefore, correct quantification of HbA2 is of paramount importance [1–4]. The increase in HbA2 in carriers of beta thalassemia may be due to transcriptional and sometimes post-translational effects [5].
The different types of beta-thalassemia mutation produce clinical and hematological phenotypes that vary in severity even in the carrier state. In India, the incidence of beta thalassemia among Sindhis, Punjabi Hindus, Lohanas, and Jat Sikhs varies from 1.5 to 3.6% in different states across the country. [4] The carriers of either b0 where no beta chains are produced, or severe b + + mutations have a high total red blood cell count (TRBC), while mean corpuscular volume (MCV = hematocrit/RBC number) and mean corpuscular hemoglobin (MCH = Hb/RBC number) are markedly reduced (MCV: 60–70 fl, MCH: 19–23 pg). The Hb levels are variable and may range from normal to up to 2 g/dL. The most widely used cutoff values of MCV and MCH for indicating thalassemia are 79 fl and 27 pg, respectively. Reticulocyte counts may be normal or mildly increased and have no diagnostic value as such. In the presence of microcytic hypochromic anemia parameters as shown by the complete blood count (CBC), HbA2 must be accurately and precisely quantified because the difference in HbA2 concentrations between people with and without β-thalassemia trait is narrow. Most laboratories consider HbA2 of ≥ 4.0% diagnostic of beta thalassemia trait, some others consider HbA2 ≥ 3.3% or HbA2 ≥ 3.5% as the cut-off for establishing β thalassemia carrier diagnosis. The “borderline HbA2 levels”, though not deleterious to health, are important in β thalassemia carrier diagnosis because they can lead to misinterpretation of the results. (In our laboratory, the upper limit of the reference range for HbA2 is 3.5%; we consider HbA2 in β-thalassemia trait of being > 4.0%) [6, 7]. When laboratory findings are discordant for a diagnosis of β thalassemia trait other factors that may influence HbA2 levels and the corresponding red cell indices must be kept in mind. Two most common causes of this are megaloblastic anemia and drugs that inhibit nucleic acid synthesis such as those given in HIV that is the nucleoside reverse transcriptase inhibitors category both of these lead to a raised HbA2 level. The presence of a megaloblastic anemia with a β thalassemia, disguises the anemia and the RBC indices could be normocytic or even microcytic instead of being macrocytic and the HbA2 levels may decrease to borderline levels. Mean HbA2 values may remain lower than normal in untreated hypothyroid cases while in hyperthroid cases HbA2 levels are higher and bceome normal post treatment. Fluctuations in HbA2 levels may also be influenced by mechanical or technical factors and therefore quality assurance is an important consideration. At laboratory level all individuals with a borderline level of HbA2 must have at least two repeated HbA2 measurements, with instrument validation and quality assurance [QA]/quality checks [QC] compliance in place.
Material and methods
From March 2019 to December 2020, data from 536 whole blood samples sent to our institute for physician-ordered hemoglobinopathy screening was studied. The samples included patients being evaluated for microcytic hypochromic anemia, suspected iron deficiency anemia unresponsive to iron therapy, prenatal/antenatal screening, or sickle cell disease treatment and monitoring. HPLC was performed using the Bio-Rad Variant II—Thalassemia Short Program (Bio-Rad, Hercules, CA), while HbCZE was performed using the Mini Flex Piercing Analyzer (Sebia trivitron). The performance characteristics of both machines were compared for the detection of the five most common hemoglobin variants. (Hb A, HbF, HbS, HbC, and HbE) and a few other rare variants that were picked up. Cases of human immunodeficiency virus infection (HIV) on highly active human antiretroviral therapy (HAART), bone marrow failure syndromes (BMFS), and myelodysplastic syndromes (MDS) were excluded.
High-Pressure Liquid Chromatography
HPLC analysis was performed using the manufacturer’s instructions for the Bio-Rad Variant II -thalassemia Short Program (Bio-Rad, Hercules, CA), which separates hemoglobin variants by cation exchange chromatography using a salt gradient. The Variant II is a fully automated HPLC system that can separate and determine area percentages for hemoglobins A2 and F and provide qualitative determinations of abnormal hemoglobins. The identification of these hemoglobin variants is made using retention time windows such as a “D-window,” “S-window,” and “C-window.” In the Variant II—thalassemia Short Program, the samples are automatically mixed and diluted on the Variant II Sampling Station (VSS) and injected into the analytical cartridge. Different hemoglobins vary in their retention times, i.e., the time from application to elution from the column, and their characteristics. Each sample run takes about six and a half minutes, and nine samples may be run in one hour [8].
Hemoglobin Capillary Zone Electrophoresis
Hemoglobin electrophoresis is in many ways an intermediary technique between classical zone electrophoresis and liquid chromatography that separates analytes according to the pH of the electrolyte solution and electroosmotic flow. For this study, the capillary Mini Flex Piercing analyzer, Sebia trivitron, was used. This instrument uses the principle of capillary electrophoresis in a free solution. This instrument is equipped to resuspend, lyse, separate, and analyze whole blood samples for variants collected in EDTA tubes. The hemoglobin bands are detected by absorption photometry and optical density measurements and converted to a migration image. This is then displayed as an electropherogram. The migration position for each hemoglobin in each sample is standardized relative to the position of the hemoglobin A and A2 bands and is measured in arbitrary units between 0 and 300. The distinct peaks in the migration image are assigned to one of 15 zones and quantified as percentage hemoglobins: HbA, HbF, HbA2, and HbC are provisionally identified and color-coded. Twenty eight samples can be processed in one go, and eight samples are run per hour [8].
Results
The comparison of quantitative variables was analyzed between two groups using the Mann–Whitney U test and the independent t-test. A paired t-test or Wilcoxon signed-rank test was used for comparison between the two techniques. A total of 536 patients with suspected hemoglobinopathy were studied. The age group of the study population ranged from 0.2 to 73 years, with a mean age of 26.43 years. There were more females in the study group: 377 females (70.34%) and 159 males (29.66%), owing to the large number of antenatal cases that were screened. The most common subset was patients referred for antenatal checkups (41.79%). Clinically, pallor had been documented in 84 (15.67%) patients, and 112 (20.90%) cases had been sent as family screening cases. 17 cases out of 536 (3.17%) presented had jaundice on presentation, while a history of transfusion was documented in 53 cases. Out of a total of 536 patients that were screened, 141 patients were detected to have underlying thalassemia or hemoglobinopathy. The majority of these patients were BTT 97 (68.79%), followed by compound heterozygous Hb E-beta-thalassemia 15 (10.64%) and heterozygous E-state 12 (8.51%). There were two (1.42%) homozygous beta-thalassemia patients and three (2.13%) with homozygous HbS and heterozygous sickle cell states, respectively. One each (0.71%) of compound heterozygous HbS beta-thalassemia, compound heterozygous delta beta-thalassemia, HbD Iran, heterozygous HbO-Indonesia, and hemoglobin G Sriraj/O Arab were also identified. Three samples had a previous history of transfusion, and one sample showed a carryover of HbD of 2.7%. A repeat HPLC/HbCZE was advised for these patients, but they were lost to follow-up. The distribution of subgroups is depicted in Table 1. The erythrocyte indices of the commonest subgroups are shown in Table 2.
Table 1.
Distribution of subgroups of study subjects
| Subgroups | Frequency | Percentage |
|---|---|---|
| Homozygous beta thalassemia | 2 | 1.42 |
| Heterozygous beta thalassemia | 97 | 68.79 |
| Heterozygous E state | 12 | 8.51 |
| Compound heterozygous Hb E -beta-thalassemia | 15 | 10.64 |
| Homozygous HbS | 3 | 2.13 |
| Heterozygous sickle cell state | 3 | 2.13 |
| Compound heterozygous HbS beta-thalassemia | 1 | 0.71 |
| Compound heterozygous delta beta /beta-thalassemia | 1 | 0.71 |
| HbD Iran | 1 | 0.71 |
| Heterozygous HbO-Indonesia | 1 | 0.71 |
| Haemoglobin G Sriraj / O Arab | 1 | 0.71 |
| Post transfusion sample /carry over samples | 4 | 2.84 |
| Total | 141 | 100.00 |
Table 2.
Erythrocyte indices of the commonest subgroups heterozygous beta-thalassemia, compound heterozygous Hb E beta-thalassemia and Heterozygous E state
| Erythrocyte indices | Heterozygous beta thalassemia(n = 97) | Compound heterozygous Hb E beta-thalassemia(n = 15) | Heterozygous E state(n = 12) |
|---|---|---|---|
| Haemoglobin(g/dL) | 11.3 ± 2.14 | 8.13 ± 3.46 | 11.61 ± 1.63 |
| TRBC(× 1012/l) | 5.57 ± 1.11 | 4.52 ± 1.15 | 4.62 ± 1.1 |
| HCT | 36.62 ± 6.32 | 29.11 ± 11.02 | 35.93 ± 4.5 |
| Mean corpuscular haemoglobin (fl) | 55.3 ± 85.3 | 61.32 ± 8.32 | 73.79 ± 4.38 |
| Mean corpuscular haemoglobin (pg) | 20.67 ± 3.21 | 17.83 ± 3.7 | 23.81 ± 1.97 |
| Mean corpuscular haemoglobin concentration (g/dL) | 32.67 ± 2 | 28.79 ± 2.6 | 31.48 ± 2.39 |
On HbCZE the Hb A values in the study group ranged from 3.2 to 99% with a mean HbA of 93.74 ± 12.66%. For the sake of statistical analysis HbA2 values were divided into the following groups for ease of comparison < 2%, 2.0–3.3%, 3.4–3.7%, 3.8–7.0%, and > 7% with HbA2 < 2% indicative of IDA, alpha thalassemia, HbH disease δβ thalassemia (if hemoglobin F was also elevated), 2.0–3.3% was essentially the normal values for HbA2, HbA2 of 3.4–3.7% was indicative of a borderline elevated HbA2, or severe iron deficiency in β thalassemia trait. Haemoglobin A2 values of > 7.0% are rare and a structural variant needs exclusion after repeating HbA2 estimation. On the HbCZE majority of the patients 340 (63.43%) had levels of HbA2 within the normal range i.e. 2.0–3.3% while the second commonest was HbA2 levels of 3.8–7.0% totaling to 149 pts (27.80%). Six pts had borderline values of between 3.4% and 3.7% only two pts (0.37%) had values over 7%. This is depicted in Table 3.
Table 3.
Comparison of HbA2 values obtained by HPLC and HbCZE
| HBCZE | HPLC | |
|---|---|---|
| < 2 | 39 (7.28%) | 8 (1.49%) |
| 2.0–3.3 | 340 (63.43%) | 352 (65.67%) |
| 3.4–3.7 | 6 (1.12%) | 9 (1.68%) |
| 3.8–7.0 | 149 (27.80%) | 136 (25.37%) |
| > 7.0 | 2 (0.37%) | 31 (5.78%) |
| Mean ± SD | 3.21 ± 1.35 | 5.96 ± 11.75 |
As per the HPLC 352 (65.67%) pts had normal HbA2 values, and 136(25.37%) pts had HbA2 which fell into the range of 3.8–7.0%. Thirty one pts had values that were more than 7% and these were later sub-grouped based on the percentage of HbA2 present as Hb Lepore, HbE, and HbD Iran as all of these co-eluate in this window. Values of HbA2 between 10 and 14% are indicative of Hb Lepore, 25–40% of Hb E heterozygous, 44–48% of HbD Iran and 70–90% of E homozygous. The P2 window is indicative of glycated Hb in the P3 window values up to 6% are acceptable, values of 6–12% are indicative of sample deterioration and values of 15–25% are indicative of Hb J Meerut which is an alpha chain variant. In our study mean P2 values were 3.52 ± 1.05 while mean P3 values were 4.67 ± 1.43. No abnormal variant was detected in the P3 window.
Comparison of HbA2 HbCZE Versus HPLC
On comparing the HbA2 values between HbCZE and HPLC, HPLC showed higher values for HbA2 with a median value of 2.9% as compared to HbCZE where the value was 2.65%. The mean HbA2 for HbCZE was 3.21 ± 1.35%. In the case of cumulative HbA2 for HPLC, it was higher because the HbE, HbD Iran, and glycated HbS also fell in the HbA2 window thereby raising the value. When HbA2 values were compared by HbCZE in patients with HbS and without HbS the mean HbA2 was higher in patients with HbS being 3.44 ± 1.25% in patients with HbS while in patients without HbS it was 3.2 ± 1.35%. These were not statistically significant. When the same values were compared on the HPLC machine the mean for patients with HbS was 4.07 ± 1.22% while for those without HbS (also excluding HbE/HbC/HbD-Iran) it was 3.38 ± 1.18% and this difference was statistically significant. We know that glycated adducts have a retention time similar to HbA2 thereby falsely elevating the percentage of HbA2 in the case of HbS on the HPLC machine and this was the reason for these elevated values.
Comparison of HbA2 by HBCZE and HPLC Cases When HbE Cases Were Excluded
While comparing HbA2 values between HBCZE and HPLC, when HbE cases were excluded the mean HbA2 by CZE was 3.15 ± 1.27% while by HPLC it was 3.47 ± 2.06% and this difference was statistically significant. Further, if both HbS and HbE cases were excluded the mean HbA2 by CZE was 3.15 ± 1.27% while by HPLC it was 3.47 ± 2.07% this difference was again statistically significant. On correlating HbA2 between HPLC and HbCZE values in patients with HbA2 < 3.5% the correlation coefficient was 0.867 with a significant p-value of < 0.001 while in patients with HbA2 > 3.5%the correlation coefficient was 0.369 with a p-value of < 0.001 thereby implying the poor correlation between HbA2 values between CZE and HPLC at values over 3.5%. As Hb E, HbD Iran also fell into the HbA2 window in the HPLC this may lead to elevated values as already discussed. The bias for low HbA2 < 3.5% was − 0.2882 while for higher values > 3.5% it was 8.0012. This is depicted in Fig. 1.
Fig. 1.
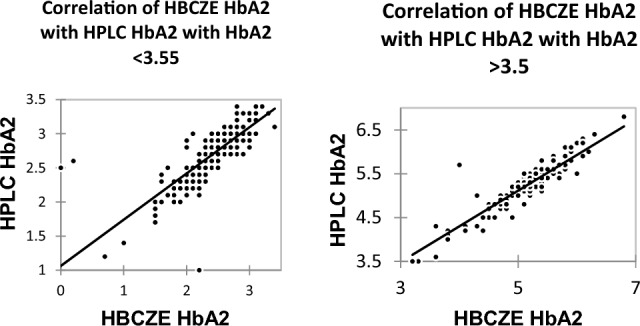
Correlation of HBCZE HbA2 and HPLC HbA2 in patients without HbS, HbE, HbD and HbC with HbA2 < 3.5% and > 3.5%
HbA2 by HBCZE and HPLC Cases When HbE, HbC, HbS, and Hb D Iran Cases Were Excluded
Once the HbE, HbC, HbS, and Hb D Iran cases are removed at values < 3.5% the correlation coefficient was 0.879 while at higher values of HbA2 > 3.5% the correlation coefficient was 0.870. This implies that the correlation is better at lower HbA2 values than at higher values. The bias was − 0.2808 at HbA2 < 3.5 and it was − 0.09851at HbA2 > 3.5.
Discussion
On comparing the HbA2 values the HPLC showed higher values for HbA2 with a median value of 2.9% as compared to HbCZE where the value was 2.65%.The mean HbA2 for HbCZE was 3.21 ± 1.35. The total values for HbA2 for HPLC were higher because the HbE and HbD Iran cases also fell in the HbA2 window and glycated HbS adducts also tend to increase the value of HbA2 on the HPLC. We thereafter compared HbA2 values of less than 3.5% and more than 3.5% and there was a good correlation at lower values of HbA2 and an extremely poor correlation at higher values of HbA2. For HbA2 at levels < 3.5% and values of over 3.5% the r values are 0.867 and 0.369 respectively. Once the HbE, HbC, HbS, and HbD Iran were excluded the cases with HbA2 values < 3.5% to the correlation coefficient was 0.879 while at higher values of HbA2 > 3.5% the correlation coefficient value was 0.870. This implies that the correlation is better at lower HbA2 values than at higher values and the correlation improves once the HbE, HbC, and HbS are excluded. The bias at lower values of HbA2 is − 0.2808 while at higher values it is − 0.09851. In the study by Greene et al. [9] they found a higher bias at higher concentrations and lower bias and lower concentration of HbA2 which was similar to our study. In studies by Van Delft et al.[10], Higgins et al.[11], Keren et al.[12] Hafiza et al.[13] also the HPLC gave higher values for HbA2 as compared to the HbCZE.In our study on HPLC, we obtained higher values of HbA2 with coexisting HbS than without HbS (other variants in the HbA2 window excluded) on the HbCZE no significant variation was obtained between HbA2 with or without HbS. This was similar to studies by Van Delft et al. [10] and Keren et al. [12]. However, on the HBCZE there was no significant difference between the HbA2 values in cases with and without HbS. This is so because acetylated hemoglobin F and other post-translationally modified adducts are not separated in HbCZE this being an advantage of HbCZE over HPLC.
In our study similar to the survey by Keren et al. [12] and Greene et al. [9] the HbA and HbF values correlated well with both HPLC and HbCZE.
A tabulation of the studies comparing HbCZE and HPLC is shown in the table below Table 4. Table 5 depicts the differences between HPLC and HbCZE while diagnosing thalassemia and haemoglobinopathies.
Table 4.
Various studies comparing HPLC and HBCZE performance in the diagnosis of hemoglobinopathies
| Study | Salient findings |
|---|---|
| Higgins, Khajuria & Mack [11] |
HbA2 is lower with HbCZE(variant II) as compared to HPLC HbA2 in pts with HbS, heterozygous and homozygous similar by both HbCZE better quantifying HbA2 in HbE, HbD Punjab traits HbA2 upper reference range to be 3.1% by CE as compared to 3.6% by HPLC |
| Hafiza Alauddin et al. [13] |
Normal ranges for HbA2 and HbF by HbCZE lower than HPLC HbA2 levels for HbE heterozygotes are higher than that of normal but lower than beta thal heterozygotes |
| Mais et al. [14] |
HbA2 of patients with the HbE trait was higher than normal HbA2 of HbE homozygotes was higher than heterozygotes HbE level was 24.28% by CE which was much lower than that of HPLC |
| Van Delft et al. [10] |
HbA2 higher( 2.67) with Variant II(2.51) as compared to Capillarys Elevated or overestimated HbA2 fractions are measured in the presence of HbS |
| Keren D.F, et al. [12] |
Hb A levels were similar on both HPLC and Sebia HBCZE HbA2levels were higher by CE than by HPLC In cases with HbS HbA2 levels were greater by HPLC than HbCZE HbA2 was occasionally not separated from HbC by CE but did separate from HbE by CE |
| Greene DN et al. [9] |
Hb A measurements agreed between HPLC (Variant II) and HbCZE(Sebia) Hb F measurement agreement was also good If no variant was present Hb A2concentrations showed excellent agreement Hb A2 bias was concentration-dependent: at low concentrations, a low bias was observed; at high concentrations, a high bias was observed |
| Our Study |
Hb F, HbS measurement agreement was good between HPLC and HbCZE HbA2levels were higher by HbCZE than by HPLC In cases with HbS HbA2 levels were higher on HPLC than in those without HbA2, no significant variation on HbCZE Once variants are excluded the correlation is better at lower HbA2 values than at a higher value |
Table 5.
Comparison of High performance liquid chromatography and Haemoglobin capillary zone electrophoresis in diagnosing thalassemias and haemoglobinopathies
| High performance liquid chromatography | Haemoglobin capillary zone electrophoresis |
|---|---|
| Mixture of molecules separated on basis of their adsorption, detected optically by retention time and area under the curve | Mixture of molecules separated by means of charge( mass/charge ratio) when electric current is passed through supporting media |
| Quantification of normal and variant Hb for each sample is seen as retention time and area under the curve | Migration position of each Hb variant is standardized relative to HbA and HbA2 bands in arbitrary units between 0–300, 15 zones assigned |
| Larger range of variants can be identified | – |
| Glycosylated and acetylated variant have different elution times on HPLC–HbA2 falsely elevated with HbS, glycosylated HbS may have same RT as HbA, glycosylated HbA can falsely elevate HbF | No separation of glycosylated and acetylated variants |
| Decreased HbA2 levels in HbD Punjab trait increased HbA2 levels with HbS | No such variation |
| Hb E,Hb Lepore, Hb D Iran,Hb Coushatta, Hb Christianborg, Hb Korle Blu co elute with HbA2 | HbE,Hb Lepore,Hb D Iran, Hb Coushatta, Hb Christianborg, Hb Korle Blu separated |
| Hb variants with RT less than 0.63 min not indicated on report must be seen on chromatogram eg. HbH | No such interference |
| Bilirubin appears as a pre-integration peak with RT less than 0.63 min | Bilirubinemia has no interference |
| HbA2 and HbC are clearly separated | HbA2 is not separated from HbC |
Conclusion
Being an important cause of morbidity and mortality hemoglobinopathies impose a heavy burden on families and the health sector in our country. The identity of a hemoglobin variant is generally inferred from its electrophoretic mobility, its quantity, and the patient’s ethnic background however definite identification can be achieved only by DNA analysis or amino acid sequencing. [15, 16]. HPLC has the advantage of quantifying Hb F and Hb A2 along with hemoglobin variant screening in a single, highly reproducible system. It will accurately quantify Hb A2 in the presence of Hb C, but not in the presence of Hb E or glycated Hb S. The disadvantages are that it cannot separate HbE, Hb Lepore, HbD Iran, as these elute in the HbA2 window. Similarly, glycosylated Hb S can elute in the HbA2 window falsely elevating it. HbCZE is a relatively newer technique. It can measure HbA2 in the presence of HbE but not in the presence of HbC. HbCZE patterns can be read-only if HbA and HbA2 are present in the sample and therefore mixing studies have to be carried out if it is absent. In the presence of HbS measured HbA2 is higher on HPLC while in Hb D HbA2 is lower on HPLC due to an integration problem leading to an incomplete return to baseline. For BTT detection in Hb D heterozygotes or in Hb S heterozygotes HbCZE is suitable which in turn is not suitable in the presence of Hb C, Hb E, or Hb O.
We have found HPLC and HbCZE to be complementary techniques and use both routinely. Normal ranges and means normal values differ between different methods and different manufacturers. This study his illustrates that each laboratory should have an alternative method adapted to each case and keep in mind the performance characteristics of each method.It appears that an extensive molecular work up of the β globin gene is the only definite method to detect borderline HbA2 β thalassemia carriers, more so in populations that have a high prevalence.
Author Contributions
Conceptualisation—GK, ST, TS, MM. Methodology: ST, TS, JD, GKV. Data Curation: GK, RH. Writing: GK, AS.
Funding
This research received no external funding.
Declarations
Conflicts of interest
NIL.
Informed Consent
Informed consent was obtained from all subjects.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Gurpreet Kaur, Email: gurpreet.sagoo37@gmail.com.
Seema Tyagi, Email: drseematyagi@hotmail.com.
Tulika Seth, Email: drtulikaseth@gmail.com.
Manoranjan Mahapatra, Email: mrmahapatra@hotmail.com.
Ganesh Kumar Viswananthan, Email: ganeshpgi@gmail.com.
Jasmita Dass, Email: drjasmita@gmail.com.
Rama Hariharan, Email: rama.siddarth@gmail.com.
Arijit Sen, Email: aseniaf@gmail.com.
References
- 1.Williams TN, Weatherall DJ. World distribution, population genetics and health burden of the hemoglobinopathies. Cold Spring Harb Prospects Med. 2012;2:011692. doi: 10.1101/cshperspect.a011692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bain B, Bates I, Laffan M, editors. Dacie and Lewis practical haematology. 12. UK: Elsevier; 2017. [Google Scholar]
- 3.International Committee For Standardization In Haematology Recommendations for selected methods for quantitative estimation of Hb A2 and for Hb A2 reference preparation. Br J Haematol. 1978;38:573–578. doi: 10.1111/j.1365-2141.1978.tb01082.x. [DOI] [PubMed] [Google Scholar]
- 4.Stephens AD, International Council for Standardization in Haematology (ICSH] et al. ICSH recommendations for assessing automated high-performance liquid chromatography and capillary electrophoresis equipment for the quantitation of HbA2. Int J Lab Hematol. 2015;37(5):577–82. doi: 10.1111/ijlh.12413. [DOI] [PubMed] [Google Scholar]
- 5.Nadkarni AH, Gorakshakar AC, Sawant PM, et al. The phenotypic and molecular diversity of hemoglobinopathies in India: a revie wof 15 years at a referral center. Int J Lab Hematol. 2018;41:218–226. doi: 10.1111/ijlh.12948. [DOI] [PubMed] [Google Scholar]
- 6.Bain BJ, Wild BJ, Stephens AD, et al. Variant haemoglobins: a guide to identification. 1. West Sussex: Wiley Blackwell; 2010. [Google Scholar]
- 7.Joutovsky A, Hadzi-Nesic J, Nardi MA. HPLC retention time as a diagnostic tool for hemoglobin variants and hemoglobinopathies: a study of 60000 samples in a clinical diagnostic laboratory. Clin Chem. 2004;50:1736–1747. doi: 10.1373/clinchem.2004.034991. [DOI] [PubMed] [Google Scholar]
- 8.VARIANT II b thalassemia short program instruction manual
- 9.Greene DN, et al. Comparison of sebia capillarys Flex capillary electrophoresis with the biorad variant II high pressure liquid chromatography in the evaluation of hemoglobinopathies. Clin Chim Acta. 2012;413(15–16):1232–1238. doi: 10.1016/j.cca.2012.03.027. [DOI] [PubMed] [Google Scholar]
- 10.Van Delft P, Lenters E, Bakker-Verweij M, de Korte M, Baylan U, Harteveld CL, Giordano PC. Evaluating five dedicated automatic devices for haemoglobinopathydiagnostics in multi-ethnic populations. Int J Lab Hematol. 2009;31:484–495. doi: 10.1111/j.1751-553X.2009.01158.x. [DOI] [PubMed] [Google Scholar]
- 11.Higgins TN, Khajuria A, Mack M. Quantification of HbA2 in patients with and without b-thalassemia and in the presence of HbS, HbC, HbE and HbD Punjab haemoglobin variants. Comparison of two systems. Am J Clin Pathol. 2009;131:357–362. doi: 10.1309/AJCP28QKSOPHYOBC. [DOI] [PubMed] [Google Scholar]
- 12.Keren DF et al. Comparison of Sebia Capillarys capillary electrophoresis with the primus high-pressure liquid chromatography in the evaluation of hemoglobinopathies. Am J Clini Pathol 130: 824–831 [DOI] [PubMed]
- 13.Hafiza A, et al. HbA2 levels in normal, beta-thalassaemia and haemoglobin E carriers by capillary electrophoresis. Malays J Pathol. 2012;34(2):161–164. [PubMed] [Google Scholar]
- 14.Mais D, et al. The Range of hemoglobin A(2) in hemoglobin E heterozygotes as determined by capillary electrophoresis. Am J Clini Pathol. 2009;132(34–8):10. doi: 10.1309/AJCPP50JIXXZVLSS. [DOI] [PubMed] [Google Scholar]
- 15.Galanello R, Cao A. Relationship between genotype and phenotype: thalassemia intermedia. Ann NY Acad Sci. 1998;850:325–333. doi: 10.1111/j.1749-6632.1998.tb10489.x. [DOI] [PubMed] [Google Scholar]
- 16.Paleari R, Gulbis B, Cotton F, Mosca A. Interlaboratory comparison of current high-performance methods for HbA2. Int J Lab Hematol. 2012;34(4):362–368. doi: 10.1111/j.1751-553X.2012.01403.x. [DOI] [PubMed] [Google Scholar]


