Dear Editor,
Chronic kidney disease (CKD) can be found in approximately one-quarter to one-third of patients with BCR-ABL1-negative myeloproliferative neoplasms (MPNs), potentially as a manifestation of MPN-related glomerulopathy [1, 2]. More importantly, the presence of CKD has been associated with inferior outcomes in MPNs [1]. The exact pathophysiological mechanisms behind CKD development in MPNs are unknown; however, blood hyperviscosity has been proposed as one important contributor to kidney damage in MPNs. This view was mainly supported by the fact that some polycythemia vera (PV) patients may show improvements in estimated glomerular filtration rate (eGFR) over time; the effect was attributed to microcirculatory improvement with phlebotomies and the use of aspirin [1]. On the other hand, the prevalence of CKD in the elderly may also be quite high [3]. Therefore, considering that calculation of eGFR is age-dependent [4], the increased prevalence of CKD in MPNs may be confounded by an advanced age. In order to tackle these important issues, we have investigated the prevalences of CKD in a case-matched cohort of PV and secondary polycythemia (SP) patients.
We have retrospectively evaluated a multicentric cohort of patients from three community hospitals in Croatia, evaluated in the period from 2002 to 2021; PV diagnosis was reassessed according to 2016 World Health Organization (WHO) criteria, whereas SP required the presence of a presumed cause of SP, the diagnostic exclusion of PV, and the laboratory thresholds for PV defined by the WHO criteria (hemoglobin > 165 g/L for males and > 160 g/L for females, and/or hematocrit > 49% for males and > 48% for females, measured on at least two occasions). Clinical and laboratory data were recorded at the time of diagnosis. Kidney function was estimated using the Modification of Diet in Renal Disease formula [4] whereas CKD was defined as eGFR < 60 mL/min/1.73 m2 ≥ 3 months.
Statistics were performed with MedCalc Statistical Software® (Ostend, Belgium, version 20.112). Case-matching procedure in a 1:1 ratio aimed to adjust for sex, age (± 1 year), and the presence of arterial hypertension and/or diabetes mellitus, as these two cardiovascular risk-factors are the main causes of CKD in the general population. The chi-squared and the Mann–Whitney U tests were used for post-hoc comparisons between the matched cohorts.
Out of a total of 101 and 100 patients with PV and SP, respectively, 50 PV and 50 SP patients (smoking was found in 21, chronic obstructive pulmonary disease/asthma in 17, kidney cysts in 13, and sleep apnea in 7 patients; 23 patients had ≥ 2 reasons for SP) were successfully matched. As presented in Supplementary Table 1., PV patients more often had higher total leukocyte, granulocyte, erythrocyte, and platelet counts, lower lymphocyte count, lower eGFR, prior thrombosis, and were less likely to smoke (p < 0.050 for all analyses). These associations are in line with our previously published report [5]. More interestingly, despite the comparable hemoglobin and hematocrit levels between the two case-matched groups, PV patients more often had CKD at diagnosis (14/50, 28%, vs. 4/50, 8%; p = 0.009), as shown in Fig. 1.
Fig. 1.
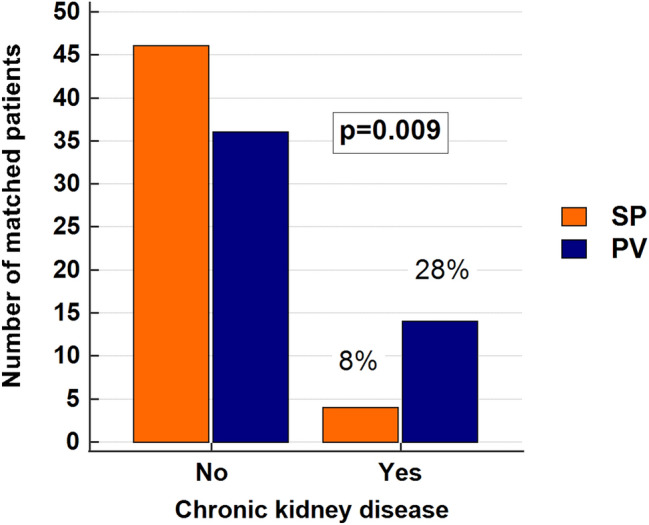
The prevalence of chronic kidney disease (CKD) was significantly higher in polycythemia vera (PV) than in secondary polycythemia (SP) patients. The chi-square test was used
Our findings suggest that the increased blood viscosity (indirectly measured through elevated hematocrit) may not be the predominant pathophysiological mechanism of kidney damage in PV. In fact, presented results suggest that PV as a clonal myeloproliferation per se may be a risk factor for CKD development. Limitations of this work are the retrospective design of the study and the limited number of patients included. In addition, due to missing data, we were unable to systematically collect informations regarding the urinary tract infections and ultrasonographic findings in both patient groups. Nevertheless, our results further highlight the need for additional studies elucidating the underlying pathophysiologic mechanisms of kidney damage in MPNs. It should be pointed out that prior reports on kidney biopsies in MPN patients with CKD and/or proteinuria have already shown that thrombotic microangiopathy, intracapillary platelet aggregates, podocyte and endothelial damage, mesangial sclerosis, and extramedullary hematopoesis were the most common findings [1]. However, considering the high risk of periprocedural bleeding in MPN patients, the establishment of appropriate surrogate biomarkers of kidney damage in MPNs is highly needed. Finally, as CKD has been recently recognized as a risk factor for thrombosis in MPNs [1], prospective studies are also needed to determine whether there may be a role for cytoreductive treatment in MPN patients with CKD who are not otherwise considered to be candidates for disease-specific treatment, and whether cytoreductive agents, phlebotomies and antithrombotic treatment may have a beneficial impact on kidney function in MPNs.
Supplementary Information
Below is the link to the electronic supplementary material.
Declaration
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
The study was approved by the Ethics Committees from the three participating centers; General Hospital of Šibenik-Knin County (Reference No. 01-129/1-21), General Hospital Zadar (Reference No. 02-2025/20-6/20), and “Dr. Josip Benčević” General Hospital (Reference No. 04000000/20-37). Due to the retrospective design of the study, informed consent was not required.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lucijanic M, Krecak I, Kusec R. Renal disease associated with chronic myeloproliferative neoplasms. Expert Rev Hematol. 2022;15(2):93–96. doi: 10.1080/17474086.2022.2039117. [DOI] [PubMed] [Google Scholar]
- 2.Krečak I, Holik H, Lucijanić M. The prevalence of simple kidney cysts in polycythemia vera and its clinical associations. Indian J Hematol Blood Transfus. 2022;38(2):429–431. doi: 10.1007/s12288-021-01515-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang QL, Rothenbacher D. Prevalence of chronic kidney disease in population-based studies: systematic review. BMC Public Health. 2008;8:117. doi: 10.1186/1471-2458-8-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130(6):461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 5.Krečak I, Holik H, Morić Perić M, Zekanović I, Coha B, Gverić-Krečak V, et al. High platelet-to-lymphocyte ratio may differentiate polycythemia vera from secondary polycythemia. Wien Klin Wochenschr. 2022;134(11–12):483–486. doi: 10.1007/s00508-022-02027-w. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


