Abstract
Heterotrophic fast-growing thraustochytrids have been identified as promising candidates for the bioconversion of organic sources into industrially important valuable products. Marine thraustochytrids exhibit remarkable potential for high-value polyunsaturated fatty acids (PUFAs) production however their potential is recently discovered for high-value carotenoids and terpenoids which also have a role as a dietary supplement and health promotion. Primarily, omega-3 and 6 PUFAs (DHA, EPA, and ARA) from thraustochytrids are emerging sources of nutrient supplements for vegetarians replacing animal sources and active pharmaceutical ingredients due to excellent bioactivities. Additionally, thraustochytrids produce reasonable amounts of squalene (terpenoid) and carotenoids which are also high-value products with great market potential. Hence, these can be coextracted as a byproduct with PUFAs under the biorefinery concept. There is still quite a few printed information on bioprocess conditions for decent (co)-production of squalene and carotenoid from selective protists such as lutein, astaxanthin, canthaxanthin, and lycopene. The current review seeks to provide a concise overview of the coproduction and application of PUFAs, carotenoids, and terpenoids from oleaginous thraustochytrids and their application to human health.
Keywords: Thraustochytrid, Polyunsaturated fatty acids, Terpenoids, Carotenoids, PUFA
Introduction
Several oleaginous microorganisms have been identified as promising sources of saturated fatty acids (SFAs) and polyunsaturated fatty acids (PUFAs), such as microalgae, protists, bacteria, fungi, and yeast among them, protists are highly oleaginous. Moreover, they are also capable of producing a reasonable quantity of carotenoids and terpenoids under the same bioprocess conditions. The biorefinery concept advocates for the sustainable production of multiple products under the same bioprocess to meet economic, environmental, and social goals. Thraustochytrids are heterotrophic marine protists such as Thraustochytrium, Aurantiochytrium, and Schizochytrium are known as promising producers of lipids because of their excellent ability of substrate utilization for their rapid growth rates and lipid accumulation under stress (Bai et al. 2022). Thraustochytrids are non-photosynthetic heterotrophic groups of microbes that are located in estuarine and marine habitats but are mostly found in water upscale in sediments and organic substances (Patel et al. 2022c). Essential fatty acids like polyunsaturated fatty acids (PUFA), have been classified in omega-3 (ω-3) such as alpha-linolenic acid (ALA, C18:3), eicosapentaenoic acid (EPA, C20:5), docosapentaenoic acid (DPA, C22:5), and docosahexaenoic acid (DHA, C22:6), and omega-6 (ω-6) arachidonic acid (ARA, C20:4ω-6), linoleic acid (LA), and gamma-linolenic acid (GLA) are reported in thraustochytrids (Patel et al. 2022c; Leyton et al. 2022). Fatty acids are more prominent in thraustochytrids than in photosynthetic microorganisms such as microalgae, cyanobacteria, and plants due to the activity of dual pathways. First, the synthase pathway of fatty acids, provides the lower chain (C-16) saturated fatty acids which are identical to the ones located in mammals and plants FAs, palmitic acid (C16:0), which is a popular fraction for biodiesel production. The second synthase pathway includes the production of omega PUFA species such as DHA, EPA, and DPA (Tran et al. 2020). Additionally, thraustochytrids are explored in two emerging sectors of industrial biotechnology as the latest resource medium for lipid and biofuel bio-factories. Habitually, thraustochytrids have been extracted from a range of aquatic sources like seagrasses, sediments, coastal waters, algae, and fallen mangrove leaves.
Omega PUFAs are a vital fatty acids that need to be derived from food supplements. It cannot be made by humans and different mammals due to a lack of the endogenous enzyme that is responsible for the desaturation of omega-3. A balanced ratio of omega-6 and omega-3 is essential for fitness and the prevention and control of obesity. The beneficial ingestion ratio of omega-3 to omega-6 (ω-6) FAs was likely close to 1:1 beyond 100 to 150 years before nutritional routines changed. The Ministry of National Health and Welfare Canada prescribed an omega-3/omega-6 ratio of 1:4 in human foods (Patel et al. 2022c). The purpose behind choosing thraustochytrids for crucial fatty acid manufacturing is rapid growth, low-charge sustainable supply, ease to domesticate, managed fermentation situations, productiveness, higher yield than distinct sources, oxidative balance, and lessened dependency on animal and plant sources (Patel et al. 2022c).
To stay healthy and maintain proper growth and protection, humans are remarkably dependent on omega-rich food. ALA is transformed into EPA and DHA in the human body, but at a low level; hence, it should be complemented through food to fulfil each day’s dietary essentials. DHA is an important structural lipid that may be observed as a constituent of triacylglycerols or phospholipids, or as an unfastened fatty acid in animals. It plays an essential key in cell interaction, membrane fluidity, and cell signaling (Patel et al. 2021). DHA and EPA fatty acids have received growing attention over the years attributable to their capability to reduce the risk of coronary heart disease, schizophrenia, Alzheimer's, bipolar disorder, and type-2 diabetes (Patel et al. 2021). DHA plays an important role in retinal and neural development, memory formation, photoreceptor biogenesis and ageing, and the function of synaptic membrane and vision. Presently, fish oils and fish are the essential sources of DHA production. Although fish oil includes a minimal quantity of DHA and the large-scale manufacturing of DHA from fish oil is tough within the context of over-utilizing fish inventory. On this behalf, thraustochytrids should be considered (Kalidasan et al. 2021).
Microbial oils are gradually becoming popular because of their various advantages over fish oils in providing long-chain PUFAs (Rollin et al. 2022). Microbial lipids obtained from oleaginous microbes vary between 20 and 70%. Marine thraustochytrids are especially known for their capacity to produce DHA which is observed dispersed in cellular membrane lipids and impartial lipids or triacylglycerols (Ma et al. 2021). Thraustochytrids play a necessary role as oddment scavengers, utilizing extracellular enzymes to fodder on the substrates that exist in the decaying sediments. Besides, thraustochytrids provide feed for zooplankton and may accumulate as much as 30% to 50% of their lipid content in their dry weight, with DHA showing half of this content material (Colonia et al. 2021).
Recently, thraustochytrids have also been identified as a potential source of carotenoids production, categorized as ketocarotenoids including astaxanthin, canthaxanthin, phoenicoxanthin, and echinenone as well as non-ketocarotenoids like β-carotene and zeaxanthin (Song et al. 2022). However, carotenoid production is well known for photosynthetic organisms, but the function is not clear in non-photosynthetic organisms; hence diverse bioactivity is anticipated from protist carotenoids. Commonly, the antioxidative ability of carotenoids helps to protect the cells in harsh habitats. In fact, studies on specific Antarctic heterotrophic bacteria have demonstrated that carotenoids have a protective effect against freeze–thaw stress and sun radiation (Dieser et al. 2010). The total carotenoid from thraustochytrid has been reported in the range of 5.7 to 450 μg g−1 biomass. Carotenoid content and composition depend on producing species and their growth conditions. The yellow and red colored pigments are soluble in lipids observed in numerous organisms. Carotenoids are crucial precursors of vitamin A, which is beneficial for human immunity and maintains eye function (Watanabe et al. 2018). It shows effective and beneficial effects on human health, and consumption of carotenoids decreases the risk of cardiovascular disease, macular degeneration, and different types of cancer. Thraustochytrids are potential cell factories for producing terpenoids like squalene and carotenoids in addition to lipids. Microorganisms produce terpenoids via the 2-C-methyl-D-erythritol-4-phosphate (MEP) pathway and the mevalonate (MVA) pathway. According to reports, the MVA route is how terpenoid buildup occurs in thraustochytrids. Both pathways, lipid biosynthesis and terpenoid biosynthesis use the common precursor acetyl-CoA (Du et al. 2021).
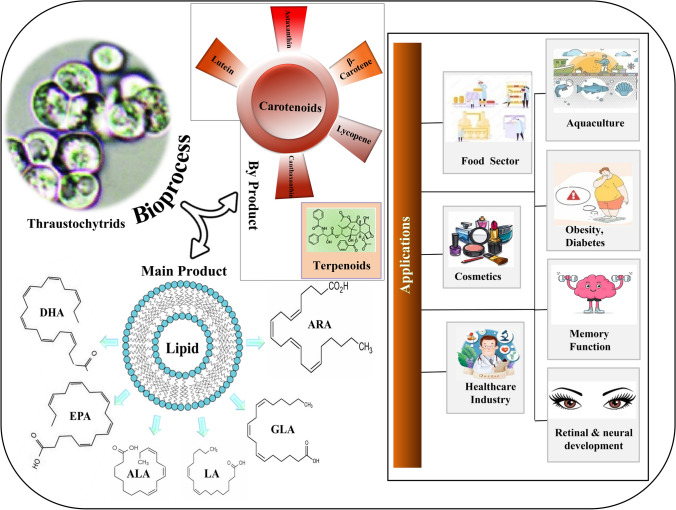
The current review article aims to provide a concise overview of the coproduction of PUFAs, carotenoids, and terpenoids from oleaginous thraustochytrids and their emerging health applications in humans. There are still limited studies on the (co)-production of terpenoids and carotenoid from thraustochytrid groups which are already recognized as an emerging source of PUFAs; however, co-extraction of other two high-value products from the same bioprocess can lead to a sustainable bioprocess. There is no recent review covering advances in the coproduction of the above high-value products from thraustochytrids under the biorefinery concept and discussing their commercial potential. Figure 1 illustrates the thraustochytrid potential for PUFA, carotenoids, and terpenoid production and emerging health applications.
Fig. 1.
Thraustochytrid potential for PUFA, carotenoids and terpenoid production and emerging health applications
Polyunsaturated fatty acids
The most extensively researched bioactive compound for human health and metabolism is fatty acids. Due to the lack of enzymes, the human body cannot synthesize some fatty acids known as essential fatty acids (Brenna et al. 2018). PUFA plays a crucial role in homeostasis as fatty acids have a double bond between C-3 and C-4, whereas ω-6 fatty acids have a double bond between C-6 and C-7 (Patel et al. 2022c). The polyketide-like synthase pathway and the elongase-desaturase pathway are two well-known pathways for the generation of PUFAs in thraustochytrids. The Polyketide-Like Synthase (PKS) pathway is more efficient than the elongase-desaturase system since it produces fatty acids with just 14 NADPH molecules compared to 26 NADP molecules with the elongase-desaturase pathway (Du et al. 2021).
Maintaining a suitable ratio of ω-6 and ω-3 PUFA not only increases performance but can also decrease health issues such as type-2 diabetes, coronary artery diseases, and obesity. ω-6 fatty acids are rich in soybean, sunflower, palm, and rapeseed oils whereas nuts, plant, and fish oils are known for these fatty acids (Alagawany et al. 2019). Flaxseeds and walnuts are mostly very general plant sources of omega-3 polyunsaturated fatty acids (especially ALA) and function on extreme scientific grounds in the clinical trial, whereas Brazil nuts and seeds of passion fruit contain a high content of PUFAs with a richness of LA (Santos et al. 2020). Microbial sources of PUFAs including filamentous, yeast, fungi, bacteria, marine heterokonts, and microalgae, as well as transgenic plants have been considered as second sources for the assembly of polyunsaturated fatty acids. Microalgae are probable sources of rich-value lipids, with vital fatty acids that endow health benefits, such as omega-3 PUFAs. Oleaginous yeast and microalgae are the prime favorable sources of lipids and assemble higher content of lipids within their bodies (Arora et al. 2019). The form of an oleaginous genus of microalgae allows for simultaneous lipid manufacturing. Single-celled eukaryotic thraustochytrids such as Thraustochytrium, Aurantiochytrium, and Schizochytrium were found to be potential microbial sources for obtaining high PUFA. Most of the research has said that oleaginous thraustochytrids contain a high amount of lipids between 30 and 75% of their total dry weight (Leyton et al. 2022). The most important aspect of thraustochytrid’s ability to effectively synthesize DHA which is high-yielding among other oleaginous microorganisms. The elongase-desaturase pathway and the polyketide-like synthase (PKS) pathway are two recognized PUFA production mechanisms in thraustochytrids. The targeted PUFA must be produced from SFAs using a variety of desaturases and elongases, then from different intermediate PUFAs (Chauhan et al. 2023). The PUFA-rich thraustochytrids can be used to produce a variety of bioactive chemicals as a supplement for formulations of animal feed, antimicrobial, antioxidant, biodiesel, enzymes, carotenoids, squalene, exopolysaccharides, vaccines, cancer medicines, and nanoparticles (Kalidasan et al. 2021).
Omega 3 fatty acid: DHA and EPA
The intake of omega-3 PUFAs has increased worldwide. Both long-chain and short-chain omega-3 PUFAs are vital and provide health benefits, having distinct functions in the body (Colonia et al. 2020). Within the years 2003–2008 Humans in the US fed on, a diet of approx. 0.17 g/d (median consumption of 0.11 g/d) of omega-3 acids (DHA, EPA, and EPA similar (33% from alteration of SDA, 5% from alteration of ALA), i.e., below the suggested 0.5 g/day. Scientists have been specifically interested in omega-3 fatty acids because of their importance to constructing the cell membrane. Cereal products have been the main essential source, which provides 36% of omega-3 fatty acids (Cholewski et al. 2018). However, mostly intakes of EPA and DHA for most humans are properly below the endorsed doses, and there are developing concerns about the supply which may be often regarded as unsustainable and no longer capable of fulfilling l future demands for human consumption, wild fish considers as beneficial source of DHA and EPA. The requirement for wild fish on a worldwide level is now a lot more massive than the oceans can maintain. Recently, studies have been directed in the direction of a more sustainable supply. Those consist of aquaculture with plant-primarily based feeds, marine microalgae, krill, genetically modified plants, and microalgae-like protists (Adarme-Vega et al. 2014). Between their microbial origins, marine water microalgae and heterokonts have acquired much attention for commercial manufacture because of their higher omega-3 fatty acids than fungi and bacteria (approx. 1–2 times more). EPA and DHA are mainly obtained from marine sources (mostly algae and fish). Noticeably, those compounds are not honestly synthesized by fishes themselves, but they collected by ingesting small fishes or microalgae that have already gathered them from their tissue. DHA, EPA, and ALA are the most crucial form of omega-3 (Amjad Khan et al. 2017). Extensive studies on the cardiovascular advantages of omega-3 PUFAs (polyunsaturated fatty acids) are geared in the direction of fish-rich foods and fish oil supplementation. However, vegetarianism and veganism are getting more popular across all parts of society, because of logic as varied as non-public, ethical, and spiritual values, people possibilities, and environment-related standards, amongst others. Due to the importance of polyunsaturated FAs, plant sources of omega-3 polyunsaturated FAs warrant added consideration (Santos et al. 2020). DHA is a long-chain PUFA of aquatic origin that is essential for the function and formation of the nervous system, especially the retina and the brain of humans. DHA is regarded as a vital nutrient during pregnancy and breastfeeding because of its active contribution to the growth of the nervous system in babyhood (Quilodrán et al. 2020).
Large amounts of ω-3 extremely unsaturated FAS, particularly docosahexaenoic acid, which is crucial for the development and physiological tasks of marine organisms, are present in thraustochytrids. The Thraustochytrids' large-scale cultivation is simple, steady, and systematic since they are heterotrophic and grow quickly in artificially regulated settings (Sato et al. 2018). The thraustochytrids, one of the two major Labyrinthulea groups, are a recognized and widely used alternative source for long-chain (LC, C20) omega-3-containing oils. The fatty acid profiles of the thraustochytrid oil obtained from Aurantiochytrium sp. strain TC 20 were examined using GC and GC–MS, DHA 22:63 emerged as the dominant PUFA, accounting for up to 40% of the total FA (Lee-Chang et al. 2021).
Omega 6 fatty acid
In the western food plan, the omega-6 PUFAs, ARA, and GLA make a considerable contribution to the FAs present within the phospholipid membrane of cells taking part in inflammation. ARA is a precursor of some powerful pro-inflammatory intermediates, which include well-described leukotrienes and prostaglandins, which led to the evolution of anti-inflammatory medications that target the ARA path for successfully managing inflammation. As a biogenetic precursor to the biologically active prostaglandins, thromboxane, prostacyclins, and leukotrienes, ARA carries out several essential physiological tasks. GLA has also been linked to reports of anti-cancer effects, particularly against malignant glioma and breast cancer (Shah et al. 2022). Therefore, it is normally accepted that improving dietary consumption of the omega-6 FAs ARA or its precursor LA (linoleic acid) will raise inflammation. The biological benefits of ARA and GLA are extensive and include decreasing blood cholesterol and cardiovascular mortality (Jin et al. 2019). ω-6 fatty acids are a very precious element of cell membranes and are essential for normal physiological function and the health of the human being. Foods that contain omega-6 include palm, sunflower, soybean, and rapeseed oils (Alagawany et al. 2019). The ω-6 fatty acid can help in regulating proliferation and cell migration, the productivity of inflammatory mediators, and phagocytic due to this capacity, omega-6 gets massive attention among fatty acid. Mainly, the intake of polyunsaturated fatty acid ω-6 as the alternative to saturated fat can have a great effect on the management of blood lipids, leading to a reduction in whole cholesterol and a rise in HDL cholesterol (Jang and Park, 2020). Aurantiochytrium sp. accumulates large content of DHA and DPA, while Schizochytrium sp., Thraustochytrium sp., and Parietichytrium sp. produce small amounts of EPA and ARA (Kikukawa et al. 2022). Table 1 summarizes the yield range of ω-3 and ω-6 PUFAs from various thraustochytrids strains by recent studies.
Table 1.
Total lipid and ω-3 and ω-6 content in potential thraustochytrids on optimum growth conditions
| Thraustochytrids | Growth conditions | Total lipid content (%) | ω-3 and 6% in total lipid |
Yield (g/L) | References |
|---|---|---|---|---|---|
| Aurantiochytrium sp. |
Glucose—30 g L−1 Yeast extract—3 g L−1 Peptone—3 g L−1 Natural Sea Water—50% Temperature—26 °C |
62.4 ± 1.81 |
DHA—33.49 ± 2.9 EPA—0.62 ± 0.16 ARA—0.58 ± 0.01 |
DHA—1.34 ± 0.32 EPA—0.04 ± 0.22 ARA—0.04 ± 0.03 |
Chauhan et al. (2023) |
| Schizochytrium mangrovei TB17 |
Glucose—30 g L−1 Yeast extract—10 g/L, Salt conc.—15%, Temperature—28 °C |
34.17 ± 1.92 |
DHA 36.66 ± 1.63 EPA 0.48 ± 0.01 |
DHA—1.57 ± 7.25 EPA—0.02 ± 0.01% |
Thom et al. (2022a) |
| Aurantiochytrium sp. ICTED5 |
Glucose—40 g L−1, Yeast extract—10 g L−1 Temperature—25 °C |
61.09 | DHA 45.24 | DHA—6.88 | Bagul et al. (2021a) |
| Thraustochytrium sp. RT2316-16 |
Glucose—20 g L−1 Yeast extract—6 g L−1 Monosodium glutamate—0.6 g L−1 Temperature—15 °C |
34 ± 3 |
EPA + DHA 14.0–26.9% |
Leyton et al. (2022) | |
| Thraustochytrium sp. |
Glucose—40 g L−1 Yeast extract—10 g L−1 Artificial seawater—18 g L−1 Temperature—25 °C |
34.98—58.86 |
DHA- 23.10–47.12% EPA— < 2% ARA— < 2% |
DHA—2.49 ± 1.43 EPA- < 0.17 ARA- < 0.17 |
Bagul et al. (2021b) |
| Thraustochytrium kinnei |
Glucose—3 g L−1 Peptone—1.25 g L−1 Yeast extract—1.25 g L−1 Temperature—28 °C |
41.33 |
DHA—39.16% EPA—7.76% |
DHA—2.19 EPA—0.43 |
Kalidasan et al. (2021) |
| Aurantiochytrium sp. |
Glucose—20 g L−1 Yeast extract—5 g L−1 Peptone—5 g L−1 Natural sea salt—50% Temperature—25 °C |
40–57.2 | DHA—4.87–16.5% | Abdel-Wahab et al. (2021) | |
| Schizochytrium sp. SHG104 |
Glucose—50 g L−1 Yeast extract—10 g L−1 Artificial sea salt—g L−1 KH2PO4—9 g.L−1 Temperature—28 °C |
45.8 | DHA—32.1% | DHA—1.62 | Park et al. (2018) |
Sustainable production of carotenoids
Carotenoids are a huge group of lipophilic compounds that are synthesized by many photosynthetic organisms and also synthesized by some non-photosynthetic fungi and bacteria. Chlorophylls and carotenoids are necessary complexes involved in photo-protection and photosynthesis. In most plants and bacteria, carotenoids are synthesized by two synthase pathways; the plastidial (MEP) 2 C-methyl-D-erythritol 4-phosphate pathway and the cytosolic (MVA) mevalonate pathway (Song et al. 2022). Some animals, which cannot produce carotenoid de novo, essentially include them in their food supplements to deliver essential antioxidants, provitamins, and coloring requirements. Over 600 carotenoids occur in the body: 20 are dietary carotenoids, such as lutein, astaxanthin, canthaxanthin, lycopene, zeaxanthin, cryptoxanthin, and meso-zeaxanthin (Johra et al. 2020). Carotenoids have different applications in the food, pharmaceutical, flavor, and feed industries. Due to this applications, there are high demand for carotenoids, although the synthesis and extraction of these compounds can be technically challenging and expensive (Li et al. 2020). The carotenoid profiles such as those found in β-carotene, astaxanthin, canthaxanthin, phoenicoxanthin, and echinenone in Schizochytrium SH104 and SHG104 were confirmed by HPLC and LC/APCI–MS. Furthermore, a genomic study of protists Schizochytrium and Aurantiochytrium sp. demonstrated the presence of genes and enzymes involved in the carotenogenesis pathway, such as geranylgeranyl diphosphate (GGPP), phytoene synthase, lycopene cyclase, and cytochrome P450 hydroxylase that are required for the production of antioxidants through the biosynthetic KEGG pathway (Liu et al. 2022). Cyanobacteria and plants have mostly been studied for carotenoid production pathways and associated enzymes among oxygenic phototrophs. Phytoene desaturase endured a sequence of reactions to transform the phytoene produced from GGPP into lycopene. Then, starting from lycopene via and β-carotene, respectively, the route was separated into the lutein synthesis pathway and the astaxanthin synthesis pathway. Only astaxanthin synthesis, where astaxanthin is generated via β-carotene, echinenone, and canthaxanthin, has been documented in thraustochytrid strains (Du et al. 2021).
More than a thousand natural carotenoids have been reported in fungi, bacteria, plants, microalgae, and thraustochytrids. Marine water microalgae and macroalgae (seaweeds) constitute a sustainable supply of several bioactive natural carotenoids, including β-carotene, astaxanthin, zeaxanthin, lutein, fucoxanthin, and violaxanthin (Patel et al. 2022a). Nowadays, thraustochytrids are considered as emerging sources for oxygenated carotenoids such as canthaxanthin, astaxanthin, xanthophylls, lutein, β-carotene, etc. By scavenging damaging free oxygen radicals, these significant antioxidants serve a key role in maintaining human health (Aasen et al. 2016). Carotenoids accumulated in a different strain of thraustochytrids are mentioned in Table 2.
Table 2.
Optimal condition and carotenoid yields from thraustochytrids
| Thraustochytrids (strain name) |
Growth conditions | total carotenoids | Carotenoid yield | References |
|---|---|---|---|---|
| Schizochytrium mangrovei TB17 |
Glucose—3%, Yeast extract—1%, ASC—15%, Temperature—28 °C |
35.86 ± 1.52 µg g−1 | Astaxanthin—7.18 ± 0.21 µg g−1 | Thom et al. (2022a) |
| Aurantiochytrium sp. SZU445 |
Glucose—20 g/L, Peptone—1.50 g/L Yeast extract—1.00 g L−1 KH2PO4—0.025 g L−1, NSW salinity—30.8–33.2% Temperature—23 ℃ |
42.39 ± 5.28 µg g−1 |
β-carotene—37.96 µg g−1 Phytoene—1.55 µg g−1 β-cryptoxanthin—1.57 µg g−1 Antheraxanthin—0.02 µg g−1 α-cryptoxanthin—0.25 µg g−1 Echinenone—0.72 µg g−1 |
Song et al. (2022) |
| Aurantiochytrium sp. TZ209 |
Glucose—20 g L−1 Yeast extract—10 g L−1, Monosod. glutamate—15 g L−1, Artificial seawater—21.25 g L−1 Temperature—28 ℃ |
– |
β-carotene—386 µg g−1 Astaxanthin—221 µg g−1 |
Yin et al. (2022) |
| Schizochytrium sp. | – | Astaxanthin—8100 µg L−1 | Liu et al. (2022) | |
| Schizochytrium mangrovei PQ6 |
Glucose—90 g L−1, Yeast extract—10 g L−1, ASW—17.5 g L−1 Temperature—28 °C |
– |
Astaxanthin—10 mg g−1 Canthaxanthin—6100 µg g−1 |
Xie et al. (2017) |
| Thraustochytrium pachydermum TSL10 |
Glucose—9% Yeast extract—1% Temperature—25–28 °C |
178.00 ± 2.43 µg L−1 |
Astaxanthin—8.30 ± 0.28 µg L−1 |
Thom et al. (2022b) |
| Schizochytrium sp. SHG104 |
Glucose—50 g L−1 Yeast extract—10 g L−1 Artificial sea salt—5 g L−1 KH2PO4—9 g L−1 Temperature—28 °C |
– | Astaxanthin—4600 µg L−1 | Park et al. (2018) |
ASC: artificial salt concentration; ASW: artificial seawater, NSW: Natural seawater
Lutein
Lutein is a form of xanthophyll carotenoid with noticeable applications in the healthcare industry because of its anti-inflammatory, ocular-protective, and anti-oxidative activities. It is commonly used as a colorant in the textile industry (Patel et al. 2022a). Moreover, several studies have revealed that lutein can also have positive effects on various clinical conditions, such as ameliorating cognitive function, reducing the chance of cancer, and enhancing measures of heart health (Buscemi et al. 2018). There is rising proof that lutein may play a major role in decreasing oxidative injury in the retina. Consequently, ingestion of lutein as a supplement may decrease the prevalence or development of age-related macular degeneration. It is common in fruits and vegetables such as oranges, grapes, kiwi fruits, peaches, zucchini, squash, spinach, kale, corn, carrots, peas, egg yolks, and marigolds (Chauhan et al. 2022). Harvesting terrestrial plants and extracting lutein are labor-intensive processes; therefore, microbial sources are promising sources for the production of lutein. Current data have recommended that species of microalgae and thraustochytrids under suitable cultivation conditions can have considerably higher productivity rates of lutein than marigold flowers. Microalgae produce 3 to 6 times more lutein compared to marigold flowers. Eukaryotic non-photosynthetic thraustochytrids are reported as a cost-effective way to produce lutein due to their ease of cultivation, rapid growth, and high accumulation of lutein in deficient conditions (Zheng et al. 2022). At present, Chlorella sorokiniana Kh12 is found to have high lutein production from microalgae up to 13.69 to 17 mg g−1 mg g−1 respectively, with advanced bioprocess engineering and important parameter optimization (Patel et al. 2022b; Vadrale et al. 2023). Lutein has been reported in Thraustochytrium sp. RT2316-16, where the total carotenoids were 168 ± 7 μg g−1 dry weight. In thraustochytrid, it could be further enhanced by different lutein induction strategies (Leyton et al. 2022).
Astaxanthin
Xanthophyll astaxanthin is the most vital and expensive industrial pigment and a very valuable carotenoid used in the cosmetic, food, aquaculture, and pharmaceutical industries on a high level (Patel et al. 2022c). Astaxanthin plays a major role in the colorant of some marine creatures, such as ornamental fish and rainbow trout (Patel et al. 2022a; c). Animals cannot produce astaxanthin by themselves, but they can obtain it through their diet. Astaxanthin can also involve in-directly in our daily food supplement by including crustaceans (e.g., krill, copepods, and shrimp) and species of Salmonidae (e.g., rainbow trout, salmon), whose foodstuffs contain a natural resource of astaxanthin (Donoso et al. 2021). It has significant antioxidant properties produced by some marine and fresh water micro-organisms, including yeast, fungi, bacteria, and microalgae. Although, in the market, there is mostly chemically synthesized astaxanthin, which is inefficient and structurally heterogeneous for biological uptake (Ren et al. 2021). The utilization of petrochemical based raw substances in the manufacturing of synthetic astaxanthin and associated side effects, and the increasing desire for natural outcomes, have raised the search for natural resources of astaxanthin. In the current year, great efforts had been made to decrease the production charge of natural astaxanthin by using microbial. However, Aurantiochytrium sp., Schizochytrium sp., Thraustochytrium sp., and Ulkenia sp. are reported thraustochytrids to generate efficient astaxanthin (Aasen et al. 2016). Microalgae such as Haematococcus pluvialis, Chlorella zofingiensis, Chlamydomonas reinhardtii, Ettlia carotinosa (Neochloris wimmeri), Chlorococcum sp. and Scenedesmus sp. found to accumulate and produce a range of enriched astaxanthin concentration which are necessary for the reproduction and growth of some animals (Fang et al. 2019). The different strategy such as high light treatment, butylated hydroxytoluene treatment, 60Co-γ irradiation, and magnesium aminoclay nanoparticles has opted for the enhancement of astaxanthin production in microalgae. The maximum yield of astaxanthin in microalgae achieved up to 47.21 mg g−1 which is enhanced by three-stage mutagenesis using ethyl methane sulphonate, UV irradiation, diphenylamine (Patel et al. 2022c). These strategies can be applied during thraustochytrids fermentation and enhance astaxanthin production.
β-carotene and canthaxanthin
The β-carotene and canthaxanthin mainly found in fungi, plants, and algae is a very stable and common pigment present in nature (Bogacz et al. 2018). β-carotenoid can be changed into vitamin A in the human being, where it applies anticancer activity, oxidation resistance, and anti-cardiovascular properties. β-carotene has consequently been extensively used in medication, food, and in the nutrition industry (Patel et al. 2022a). β-carotene converts into vitamin A were first discovered in 1930 by Moore after he noticed that animals with vitamin-A deficiency recovered when fed β-carotene-rich food. In animals, carotenoids are not the sole source of vitamin-A. After carotenoids are consumed and converted into vitamin A, this vitamin which is fat soluble are stored in the form of retinyl ester in distinct organs, such as the adipose tissue and the liver. So, the consumption of definite organs such as the liver from another animal can be a sizeable supply of vitamin A for humans. Vitamin A likewise present in milk, providing these essential supplements to the newborn (Coronel, et al. 2019). β-Carotene contains 40 carbon atoms and a type of C40 carotenoid. There are various processes for uniting 2 or 3 smaller particles for the formation of the C40 chain and fabricating β-Carotene. The most precious source of bio-production for β-Carotene is microalgae (Patel et al. 2022a). Scientists have virtue many functions of carotenoids, maximum of which are helpful for human health, debate in the scientific community, however, some noticeable exceptions have sparked extreme debate within the scientific community (Coronel et al. 2019).
During the stationary phase, Aurantiochytrium synthesized substantial levels of triacylglycerols (DHA) as well as canthaxanthin, while squalene and free cholesterol rose during the early exponential phase. canthaxanthin are synthesized from farnesyl pyrophosphate and successfully found and quantified in Thraustochytrids A. limacinum and Hondaea fermentalgiana (Dellero et al. 2018). The strain RT2316-16 of Thraustochytrium sp. reported for canthaxanthin, β-carotene, and astaxanthin. From 4-day cultivated biomass, canthaxanthin levels were on average 13% higher in the dark than in the light conditions. Moreover, the carotenoid produced depended on the nature of the nutrient consumption and conditions: biomass fed with yeast extract primarily contained canthaxanthin, whereas biomass fed with glucose enhance the β-carotene. The light's adverse effects on the hydroxylation and ketolation events required for converting β-carotene to canthaxanthin may have contributed to the increased β-carotene in total carotenoids. Despite the fact that Nostoc punctiforme PCC 73,102 and other cyanobacteria are known to upregulate the expression of carotenogenic genes to boost canthaxanthin production. In thraustochytrids, there is no evidence that light has any impact on the expression of any particular carotenogenesis gene. Nevertheless, Schizochytrium sp. SH104 has been given white light to stimulate the production of carotenoids (Leyton et al. 2022).
Lycopene
Lycopene is a form of natural carotenoid pigment with a variant of consumption on public inquiries. However, lycopene exists in human beings is mainly obtained from tomatoes, other vegetables, and fruits also contain this red-colored compound (Caseiro et al. 2020). The consumption of lycopene was commonly safe and favorable for several health effects in human beings. But the virtue of the result was not so high (Li et al. 2021). Lycopene is a tetraterpene complex that includes eight units of isoprene and eleven double linear bonds. It is a carotenoid that is not capable to provide vitamin A but it is described as an interposition of carotenoid origin in plants. It must be noted that the human body cannot synthesize lycopene. So, it should be an intake in a daily food plan (Caseiro et al. 2020). Lycopene has been used in the long term as functional food, cosmetic, nutraceutical, and pharmaceutical because of its anti-cancer and anti-oxidative activities. Even though, research present that lycopene can reduce a wide variety of chronic state, how lycopene applies this bioactivity is still relatively unknown (Arballo et al. 2021).
Promising cell factories for squalene
Terpenoids are the most extensive and varied types of biological products, with more than 80 thousand different structures recognized in plants, microorganisms, and aquatic organisms (Jin et al. 2022). Terpenoids show a wide variety of primary metabolites and secondary metabolites synthesized by animals, plants, and microbes. Thraustochytrids can serve as cell factories for the manufacture of terpenoids like squalene and carotenoids in addition to lipids. Microorganisms often produce terpenoids via the 2-C-methyl-D-erythritol-4-phosphate (MEP) route and the mevalonate (MVA) pathway (Du et al. 2021). Squalene (triterpenoid) is a potent antioxidant and a bio-product in very high demand because of its applications in numerous industries, such as pharmaceuticals, food industries, and cosmetics. It is significantly used as a food supplement, moisturizer, vaccine adjuvant, cardio-defensive agent, and anti-tumor agent. It is the precursor of countless sterols and hormones in plants and animals respectively, commonly derived from the liver oil of deep-sea Squalus sp. (shark) (Lozano-Grande et al. 2018).
Many studies have demonstrated that Aurantiochytrium sp. SD116 expresses a number of sterol 24-C-methyltransferase, cycloartenol synthase, and squalene synthetase-related enzymes that help in squalene and sterol synthesis. It has also been observed that in the presence of NADPH and Mg2+, the squalene synthase of Aurantiochytrium sp. KRS101 leads to the conversion of two molecules of FPP into squalene. However, squalene levels as high as 6.6% of total lipid were found in strains that have been preferentially opted for their TAG and/or DHA concentrations. Squalene was accumulated in Aurantiochytrium mangrovei PQ6 (formerly Schizochytrium mangrovei PQ6) during the lipid accumulation phase, reaching a high of 33 mg g−1 of CDW which is 6% of total lipid (Aasen et al. 2016). Besides, exogenous compounds like mannitol and biotin are known to increase the yield of squalene generated by microalgae and thraustochytrids when added to media. It has been reported that a Thraustochytrium sp. ATCC 26,185 culture produced the highest yield 642 ± 13.6 mg L−1 and content 72.9 ± 9.6 mg g−1 of squalene with mannitol 1 g L−1 which is the most promising source among microbes (Ali et al. 2022). Similarly, biomass and squalene productions were 459 ± 2.9 g L−1 and 55.7 ± 3.2 mg g−1 respectively with 0.15 mg L−1 biotin addition (Ali et al. 2022). As compared to thraustochytrid, other microbes are also reasonable producers of squalene. Among various fungi and yeast (CDW basis), it has been demonstrated that Saccharomyces cerevisiae, Torulaspora delbrueckii, Aspergillus nidulans, and Saccharomyces uvarum were able to accumulate 1.6, 0.24, 0.3, and 14.3 mg/g squalene, respectively. Currently, microalgae signify an alternative resource of squalene for industrial application. Based on dry biomass, it was revealed that microalgae Botryococcus braunii Abt02 contained 43.75% hydrocarbons, of which 87.54% were squalene and its derivatives. During heterotrophic growth on a variety of carbon sources from the abattoir, cassava, and brewery wastewaters, the cyanobacterial strain Phormidium autumnale produced 0.2–0.6 mg kg−1 of squalene (Patel et al. 2022). Researchers also made many efforts to increase squalene production through genetic modification of these microorganisms have concentrated basically on (i) increasing the amount of the native rate-limiting enzyme, 3-hydroxy-3-methylglutaryl-CoA reductase (HMGR), which is encoded by the HMG1 gene, (ii) optimizing the native metabolic pathway, (iii) increasing the availability of NADPH and acetyl-CoA (Liu et al. 2020).
Latest extraction methods of bioactive compounds from thraustochytrids
Several extraction techniques have been developed, including chemical extraction (solvents), mechanical extraction (cell disruption using mortar-pestle, homogenization, sonication, bead milling, etc.), followed by lipid extraction by solvent and/or enzyme-assisted methods (Byreddy and Barrow 2015). Moreover, some toxic solvents like chloroform and methanol are used in chemical extraction techniques. To completely liberate the lipid droplets from the thraustochytrids cells into solvent, effective cell disruption techniques (mechanical disruption) are necessary (Byreddy and Barrow 2015). Usually, 100% acetone is used to extract freeze-dried thraustochytrids biomass, and carbon dioxide to evaporate the acetone phase that contained the total carotenoids. An acetone/methanol solution (7:3, v/v) is used to dissolve the carotenoid. With the ratio of 7:2:1, v/v, acetonitrile, dichloromethane, and methanol, the carotenoids are eluted and quantified (Khumrangsee et al. 2022).
Alternative extraction technologies, such as supercritical fluid extraction (De et al. 2020) and enzymatic extraction methods (Lin et al. 2018), can circumvent the issues of high energy consumption and the use of toxic solvents, however, these procedures are expensive. Surfactant-assisted extraction (SAE), microwave-assisted extraction (MAE), ultrasound-assisted extraction (UAE), and the use of alternative green solvents including deep eutectic solvents are emerging techniques that may be effective for lipid extraction from thraustochytrids (Pätzold et al. 2019). Recent studies have found the effective recovery (72–74%) and purification (70%) of PUFAs from freeze-dried thraustochytrid biomass using a straightforward combination of potassium hydroxide treatment in ethanol. Nevertheless, the recovery cost was reduced to half when using wet biomass (Mirmehrabi and Brar 2020).
Biorefinery and bioeconomy of marine thraustochytrids
To obtain useful bioactive and biochemicals for human nutrition, thraustochytrids are the most fascinating source among the numerous marine microorganisms. This section focuses on industrially relevant bioproducts made from thraustochytrids and how it can be used to produce bioactive compounds under a biorefinery system or implement in the framework of a circular economy. The major advantage of the thraustochytrids is the ability to control innate metabolic pathways by adjusting the fermentation conditions to produce high levels of desired lipids, PUFA, and other bioactive compounds such as carotenoids and squalene. These fast-growing thraustochytrids are able to accumulate a significant amount of lipids in their dry cell weight along with other potential bioproducts. It contains promising amounts of SFAs and MUFAs (SFAs + MUFAs, 76% of TFA) in their cells. Due to shorter-chain SFAs and MUFAs being thermally stable and less susceptible to oxidation than PUFA, therefore it can be used as a source for the production of biodiesel. The capability of thraustochytrids for large-scale biodiesel synthesis was recently demonstrated in a scale-up study with Thraustochytrium sp. BM2 for biodiesel production employing semi-batch fermentation and pre-treatment crude glycerol. Thus, thraustochytrids are considered an emerging microbial platform for the biorefinery system (Gupta et al. 2022).
Thraustochytrids have been used as a substitute for common fishmeal (plant-based meal), which can be used as a sustainable and affordable alternative feed due to the accumulation of numerous significant bioactive molecules, including ω-PUFA. Thraustochytrids have been considered an aquaculture feed because of their ability to produce a large amount of lipids rich in DHA (> 60% of TFA). The size of the global aquafeed market was evaluated at US $56.92 billion in 2019 and is predicted to increase at a CAGR of 5.3% to reach US $80.05 billion by 2027 (Fortune business insights 2022). Although it has been suggested that blended oil (vegetable oil and thraustochytrid oil) could be used as a source of DHA in commercial aquafeeds, it has been reported that the oil from thraustochytrids could effectively replace fish oil in diets for juvenile salmon, thereby reducing the overfishing (Wei et al. 2021).
Thraustochytrids are rich in different bioactive carotenoids, which confer several advantageous biological properties, including antioxidant, anti-inflammatory, anti-obesity, and antiangiogenic properties (Miranda et al. 2020). These carotenoids are also employed as feed additives, which can reflect the pink color of farmed fish, food colors (in dairy products, beverages, etc.), and as a component of cosmetics and medications. The global carotenoid market is projected to grow at a CAGR of 5.75% from US$1.45 billion in 2019 to reach 2.04 billion by 2025 (Gupta et al. 2022).
Squalene is a polyunsaturated triterpene hydrocarbon that both plants and animals use to make bile acids and steroids. Most thraustochytrids also have significant levels of naturally occurring antioxidants such as squalene in their cellular compartments to guard ω-3 fatty acids from oxidative stress (Senbagalakshmi et al. 2019). It also serves as an intermediary metabolite in cholesterol formation. These substances have antioxidant capabilities, which shield skin cells from free radicals and reactive oxygen species and make them useful as moisturizing and calming agents in the cosmetics industry (Nakazawa et al. 2014). For the delivery of medications, vaccines, and other medical substances, squalene has been widely employed in pharmaceutical emulsions. Squalene's global market size was estimated to be $129 million USD in 2020 and grow at a CAGR of 7.3% to $184 million (Markets-and-markets, 2022).
Current challenges and prospects of thraustochytrids
How have these microbial platforms evolved to successfully replace the present PUFA and carotenoid-producing plant and animal sources? Will ω-PUFA and carotenoids offer the same quantity of bioactivities and health benefits, aside from that? These types of research questions are always raised at research meetings or conferences. In addition to determining whether microbial PUFA and carotenoids would be a suitable alternative source for a vegetarian diet, it is also important to figure out which production yield would be more cost-effective. All those are hypothetical questions that will depend on the preliminary results of their production and yield within the acceptable range. Research has shown that one type of microbe cannot manufacture all types of PUFAs and carotenoids and that the genetic capacity of an organism to make a certain type of PUFA and carotenoids depends largely on dietary factors. Over the past few decades, several articles have been published covering the enhancement of PUFA and carotenoid-producing techniques from thraustochytrids. None of the studies demonstrate a significant improvement in carotenoids and ω-6 PUFAs, whereas ω-6 PUFAs' are intermediates in the metabolic route of ω-3 PUFAs' (Patel et al. 2020). Prior to using omics (genomics, proteomics, miRNAomics, transcriptomics, nutrigenomics, etc.) and mutation techniques to increase the ω-6 fraction in microbial cells, it is necessary to target increased production of ω-6 PUFAs from these strains (Kumar et al. 2020). Furthermore, recent research also tested a few exogenous supplementation techniques to increase ω-PUFA and carotenoid production above its baseline range. Thraustochytrid biomass was significantly improved via fed-batch fermentation. One of the prospects focuses on the development of thraustochytrids bioprocess for carotenoid production using fed-batch mode. Moreover, the stepwise aeration method enhances docosahexaenoic acid and carotenoid content. In these studies, the technique began with batch cultivation and then switched to a fed-batch mode. In which, the first fed-batch phase operated with a medium comprising a high C:N (5:2) ratio, and the latter fed-batch used an excessive glucose-containing medium for the enhancement of PUFA and carotenoids (Khumrangsee et al. 2022).
Conclusions
A larger bioeconomy is supported by marine biotechnology, which is essential in offering options for sustainable and renewable food, and dietary supplement production. Thraustochytrids are a promising source of SFAs and PUFAs containing lipid production with potential implications in various product domains including biodiesel and functional foods with defined nutritional value for human health. Thraustochytrids are rich sources of omega PUFA which are an emerging alternative platform of the commercialized PUFAs market replacing the marigold. One of the most effective utilizations of marine biotechnology during the last decade was the industrial production of carotenoids and terpenoids. Mixotrophic microalgae are fascinating for carotenoids as the main product with lipids however heterotrophic thraustochytrids are more fascinating with lipids (PUFA) as the main product with carotenoids and terpenoids. There is evidence that the market demand for naturally occurring carotenoids is rising. It is necessary to boost their production from sustainable and renewable sources as an emerging platform to make it more cost-effective and sustainable production from microbial sources.
Acknowledgements
The authors acknowledge National Science and Technology Council, Taiwan for this study
Author contributions
ASC: writing-original draft, literature review; C-WC: supervision, draft preparation; HY: literature review, draft preparation; BP: literature review, draft preparation; supervision.; RRS: supervision, writing—review, and editing.; C-DD: supervision, writing—review and editing. AKP: supervision, writing—review and editing.
Funding
The Project is funded by AKP is grateful to NSTC, Taiwan for funding support (Ref. No. NSTC 111-2222-E-992-006).
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Consent for publication
All authors have given their consent for the publication of review article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ajeet Singh Chauhan and Chiu-Wen Chen have contributed equally to this work.
Contributor Information
Cheng-Di Dong, Email: cddong@nkust.edu.tw.
Anil Kumar Patel, Email: anilkpatel22@nkust.edu.tw.
References
- Aasen IM, Ertesvåg H, Heggeset TM, Liu B, Brautaset T, Vadstein O, Ellingsen TE. Thraustochytrids as production organisms for docosahexaenoic acid (DHA), squalene, and carotenoids. Appl Microbiol Biotechnol. 2016;100(10):4309–4321. doi: 10.1007/s00253-016-7498-4. [DOI] [PubMed] [Google Scholar]
- Abdel-Wahab MA, El-Samawaty AERMA, Elgorban AM, Bahkali AH. Thraustochytrids from the Red Sea mangroves in Saudi Arabia and their abilities to produce docosahexaenoic acid. Bot Mar. 2021;64(6):489–501. doi: 10.1515/bot-2021-0061. [DOI] [Google Scholar]
- Adarme-Vega TC, Thomas-Hall SR, Schenk PM. Towards sustainable sources for omega-3 fatty acids production. Curr Opin Biotechnol. 2014;26:14. doi: 10.1016/j.copbio.2013.08.003. [DOI] [PubMed] [Google Scholar]
- Alagawany M, Elnesr SS, Farag MR, Abd El-Hack ME, Khafaga AF, Taha AE, Tiwari R, Yatoo MI, Bhatt P, Khurana SK, Dhama K. Omega-3 and omega-6 fatty acids in poultry nutrition: effect on production performance and health. Animals. 2019;9(8):573. doi: 10.3390/ani9080573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali MK, Sen B, He Y, Bai M, Wang G. Media supplementation with mannitol and biotin enhances squalene production of Thraustochytrium ATCC 26185 through increased glucose uptake and antioxidative mechanisms. Molecules. 2022;27(8):2449. doi: 10.3390/molecules27082449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amjad Khan W, Chun-Mei H, Khan N, Iqbal A, Lyu SW, Shah F. Bioengineered plants can be a useful source of omega-3 fatty acids. BioMed Res Int. 2017;2017:7348919. doi: 10.1155/2017/7348919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arballo J, Amengual J, Erdman Jr JW. Lycopene (2021) A critical review of digestion, absorption, metabolism, and excretion. Antioxidants 10(3):342 [DOI] [PMC free article] [PubMed]
- Arora N, Patel A, Mehtani J, Pruthi PA, Pruthi V, Poluri KM. Co-culturing of oleaginous microalgae and yeast: paradigm shift towards enhanced lipid productivity. Environ Sci Pollut Res. 2019;26(17):16952–16973. doi: 10.1007/s11356-019-05138-6. [DOI] [PubMed] [Google Scholar]
- Bagul VP, Annapurna US. Isolation and characterization of docosahexaenoic acid-producing novel strain Aurantiochytrium sp. ICTFD5: a sterol with vitamin d-cholecalciferol, and cellulase and lipase producing thraustochytrid. Bioresour Technol. 2021;14:100688. [Google Scholar]
- Bagul VP, Annapurna US. Isolation of fast-growing thraustochytrids and seasonal variation on the fatty acid composition of thraustochytrids from mangrove regions of Navi Mumbai. India J Environ Manage. 2021;290:112597. doi: 10.1016/j.jenvman.2021.112597. [DOI] [PubMed] [Google Scholar]
- Bai M, Sen B, Wen S, Ye H, He Y, Zhang X, Wang G. Culturable diversity of thraustochytrids from coastal waters of Qingdao and their fatty acids. Mar Drug. 2022;20(4):229. doi: 10.3390/md20040229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buscemi S, Corleo D, Di Pace F, Petroni ML, Satriano A, Marchesini G. The effect of lutein on eye and extra-eye health. Nutrient. 2018;10(9):1321. doi: 10.3390/nu10091321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byreddy AR, Gupta A, Barrow CJ, Puri M. Comparison of cell disruption methods for improving lipid extraction from thraustochytrid strains. Mar Drugs. 2015;13(8):5111–27. doi: 10.3390/md13085111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caseiro M, Ascenso A, Costa A, Creagh-Flynn J, Johnson M, Simões S. Lycopene in human health. Lwt. 2020;127:109323. doi: 10.1016/j.lwt.2020.109323. [DOI] [Google Scholar]
- Chauhan AS, Chen CW, Singhania RR, Tiwari M, Sartale RG, Dong CD, Patel AK. Valorizations of marigold waste for high-value products and their commercial importance: a comprehensive review. Resources. 2022;11:91. doi: 10.3390/resources11100091. [DOI] [Google Scholar]
- Chauhan AS, Patel AK, Chen CW, Chang JS, Michaud P, Dong CD, Singhania RR. Enhanced production of high-value polyunsaturated fatty acids (PUFA) from potential thraustochytrid Aurantiochytrium sp. Bioresour Technol. 2023;370:128536. doi: 10.1016/j.biortech.2022.128536. [DOI] [PubMed] [Google Scholar]
- Cholewski M, Tomczykowa M, Tomczyk M. A comprehensive review of chemistry, sources and bioavailability of omega-3 fatty acids. Nutrients. 2018;10(11):1662. doi: 10.3390/nu10111662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonia BSO, de Melo Pereira GV, Soccol CR. Omega-3 microbial oils from marine thraustochytrids as a sustainable and technological solution: a review and patent landscape. Trends Food Sci Technol. 2020;99:244–256. doi: 10.1016/j.tifs.2020.03.007. [DOI] [Google Scholar]
- Colonia BSO, de Melo Pereira GV, Rodrigues FM, Miranda Muynarsk EDS, Vale ADS, Carvalho JCD, Soccol VT, Penha RDO, Soccol RC. Integrating metagenetics and high-throughput screening for bioprospecting marine thraustochytrids producers of long-chain polyunsaturated fatty acids. Bioresour Technol. 2021;333:125176. doi: 10.1016/j.biortech.2021.125176. [DOI] [PubMed] [Google Scholar]
- Coronel J, Pinos I, Amengual J. β-carotene in obesity research: technical considerations and current status of the field. Nutrients. 2019;11(4):842. doi: 10.3390/nu11040842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellero Y, Cagnac O, Rose S, Seddiki K, Cussac M, Morabito C, Lupette J, Cigliano RA, Sanseverino W, Kuntz M, Jouhet J. Proposal of a new thraustochytrid genus Hondaea gen. nov. and comparison of its lipid dynamics with the closely related pseudo-cryptic genus Aurantiochytrium. Algal Res. 2018;35:125–141. doi: 10.1016/j.algal.2018.08.018. [DOI] [Google Scholar]
- Dieser M, Greenwood M, Foreman CM. Carotenoid pigmentation in Antarctic heterotrophic bacteria as a strategy to withstand environmental stresses. Arct Antarct Alp Res. 2010;42(4):396–405. doi: 10.1657/1938-4246-42.4.396. [DOI] [Google Scholar]
- Donoso A, González-Durán J, Muñoz AA, González PA, Agurto-Munoz C. Therapeutic uses of natural astaxanthin: an evidence-based review focused on human clinical trials. Pharmacol Res. 2021;166:105479. doi: 10.1016/j.phrs.2021.105479. [DOI] [PubMed] [Google Scholar]
- Du F, Wang YZ, Xu YS, Shi TQ, Liu WZ, Sun XM, Huang H. Biotechnological production of lipid and terpenoid from thraustochytrids. Biotechnol Adv. 2021;48:107725. doi: 10.1016/j.biotechadv.2021.107725. [DOI] [PubMed] [Google Scholar]
- Fang N, Wang C, Liu X, Zhao X, Liu Y, Liu X, Du Y, Zhang Z, Zhang H. De novo synthesis of astaxanthin: from organisms to genes. Trends Food Sci Technol. 2019;92:162–171. doi: 10.1016/j.tifs.2019.08.016. [DOI] [Google Scholar]
- Fortune business insights (2022) https://www.fortunebusinessinsights.com/industry-reports/aquafeed-market-100698. Last accessed 08/03/2023
- Gupta A, Barrow CJ, Puri M. Multiproduct biorefinery from marine thraustochytrids towards a circular bioeconomy. Trends Biotechnol. 2022;40(4):448–62. doi: 10.1016/j.tibtech.2021.09.003. [DOI] [PubMed] [Google Scholar]
- Jang H, Park K. Omega-3 and omega-6 polyunsaturated fatty acids and metabolic syndrome: a systematic review and meta-analysis. Clin Nutr. 2020;39(3):765–773. doi: 10.1016/j.clnu.2019.03.032. [DOI] [PubMed] [Google Scholar]
- Jin M, Zhai R, Xu Z, Wen Z. Microbial lipid production. New York: Humana; 2019. Production of high-value polyunsaturated fatty acids using microbial cultures; pp. 229–248. [DOI] [PubMed] [Google Scholar]
- Jin K, Xia H, Liu Y, Li J, Du G, Lv X, Liu L. Compartmentalization and transporter engineering strategies for terpenoid synthesis. Microb Cell Fact. 2022;21(1):1–12. doi: 10.1186/s12934-022-01819-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalidasan K, Vinithkumar NV, Peter DM, Dharani G, Dufossé L. Thraustochytrids of mangrove habitats from Andaman Islands: species diversity, PUFA profiles and biotechnological potential. Mar Drug. 2021;19(10):571. doi: 10.3390/md19100571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khumrangsee K, Charoenrat T, Praiboon J, Chittapun S. Development of a fed-batch fermentation with stepwise aeration to enhance docosahexaenoic acid and carotenoid content in Aurantiochytrium sp. FIKU018. J. Appl. Phycol. 2022;34(3):1243–53. doi: 10.1007/s10811-022-02726-x. [DOI] [Google Scholar]
- Kikukawa H, Watanabe K, Kishino S, Takeuchi M, Ando A, Izumi Y, Sakuradani E. Recent trends in the field of lipid engineering. J Biosci Bioeng. 2022;133(5):405–413. doi: 10.1016/j.jbiosc.2022.02.001. [DOI] [PubMed] [Google Scholar]
- Kumar G, Shekh A, Jakhu S, Sharma Y, Kapoor R, Sharma TR. Bioengineering of microalgae: recent advances, perspectives, and regulatory challenges for industrial application. Front Bioeng Biotechnol. 2020;8:914. doi: 10.3389/fbioe.2020.00914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee-Chang KJ, Taylor MC, Drummond G, Mulder RJ, Mansour MP, Brock M, Nichols PD. Docosahexaenoic acid is naturally concentrated at the sn-2 position in triacylglycerols of the Australian thraustochytrid Aurantiochytrium sp. Strain TC 20. Mar Drug. 2021;19(7):382. doi: 10.3390/md19070382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyton A, Shene C, Chisti Y, Asenjo JA. Production of carotenoids and phospholipids by Thraustochytrium sp in Batch and repeated-batch culture. Mar Drug. 2022;20:416. doi: 10.3390/md20070416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Swofford CA, Sinskey AJ. Modular engineering for microbial production of carotenoids. Metab Eng Commun. 2020;10:e00118. doi: 10.1016/j.mec.2019.e00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Wu X, Zhuang W, Xia L, Chen Y, Wu C, Rao Z, Du L, Zhao R, Yi M, Wan Q, Zhou Y. Tomato and lycopene and multiple health outcomes: umbrella review. Food Chem. 2021;343:128396. doi: 10.1016/j.foodchem.2020.128396. [DOI] [PubMed] [Google Scholar]
- Lin Y, Xie X, Yuan B, Fu J, Liu L, Tian H, Chen T, He D. Optimization of enzymatic cell disruption for improving lipid extraction from Schizochytrium sp. through response surface methodology. J Oleo Sci. 2018;67(2):215–24. doi: 10.5650/jos.ess17166. [DOI] [PubMed] [Google Scholar]
- Liu H, Wang F, Deng L, Xu P. Genetic and bioprocess engineering to improve squalene production in Yarrowia lipolytica. Bioresour Technol. 2020;317:123991. doi: 10.1016/j.biortech.2020.123991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu PY, Li G, Lin CB, Wu JJ, Jiang S, Huang FH, Wan X. Modulating DHA-producing Schizochytrium sp. toward astaxanthin biosynthesis via a seamless genome editing system. ACS Synth Biol. 2022;11(12):4171–4183. doi: 10.1021/acssynbio.2c00490. [DOI] [PubMed] [Google Scholar]
- Lozano-Grande AM, Gorinstein S, Espitia-Rangel E, Dávila-Ortiz G, Martínez-Ayala AL. Plant sources, extraction methods, and uses of squalene. Int J Agro. 2018;2018:1829160. [Google Scholar]
- Ma W, Wang YZ, Nong FT, Du F, Xu YS, Huang PW, Sun XM. An emerging simple and effective approach to increase the productivity of thraustochytrids microbial lipids by regulating glycolysis process and triacylglycerols decomposition. Biotechnol Biofuel. 2021;14(1):1–14. doi: 10.1186/s13068-021-02097-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda AF, Tran TL, Abramov T, Jehalee F, Miglani M, Liu Z, Rochfort S, Gupta A, Cheirsilp B, Adhikari B, Puri M. Marine protists and Rhodotorula yeast as bio-convertors of marine waste into nutrient-rich deposits for mangrove ecosystems. Protist. 2020;171(3):125738. doi: 10.1016/j.protis.2020.125738. [DOI] [PubMed] [Google Scholar]
- Mirmehrabi M, Brar SK. A novel process for isolation and purification of polyunsaturated fatty acids from a thraustochytrid. Algal Res. 2020;1(46):101806. [Google Scholar]
- Nakazawa A, Kokubun Y, Matsuura H, Yonezawa N, Kose R, Yoshida M, Tanabe Y, Kusuda E, Van Thang D, Ueda M, Honda D. TLC screening of thraustochytrid strains for squalene production. J Appl Phycol. 2014;26:29–41. doi: 10.1007/s10811-013-0080-x. [DOI] [Google Scholar]
- Park H, Kwak M, Seo JW, Ju JH, Heo SY, Park SM, Hong WK. Enhanced production of carotenoids using a Thraustochytrid microalgal strain containing high levels of docosahexaenoic acid-rich oil. Bioprocess Biosyst Eng. 2018;41(9):1355–1370. doi: 10.1007/s00449-018-1963-7. [DOI] [PubMed] [Google Scholar]
- Patel A, Karageorgou D, Rova E, Katapodis P, Rova U, Christakopoulos P. An overview of potential oleaginous microorganisms and their role in biodiesel and omega-3 fatty acid-based industries. Microorganisms. 2020;8(3):434. doi: 10.3390/microorganisms8030434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel A, Karageorgou D, Katapodis P, Sharma A, Rova U, Christakopoulos P, Matsakas L. Bioprospecting of thraustochytrids for omega-3 fatty acids: a sustainable approach to reduce dependency on animal sources. Trends Food Sci Technol. 2021;115(2021):433–444. doi: 10.1016/j.tifs.2021.06.044. [DOI] [Google Scholar]
- Patel A, Bettiga M, Rova U, Christakopoulos P, Matsakas L. Microbial genetic engineering approach to replace shark livering for squalene. Trend Biotechnol. 2022;40(10):1261–1273. doi: 10.1016/j.tibtech.2022.03.008. [DOI] [PubMed] [Google Scholar]
- Patel AK, Albarico FPJB, Perumal PK, Vadrale AP, et al. Algae as an emerging source of bioactive pigments. Bioresour Technol. 2022;351:126910. doi: 10.1016/j.biortech.2022.126910. [DOI] [PubMed] [Google Scholar]
- Patel AK, Tambat VS, Chen CW, Chauhan AS, Kumar P, Vadrale AP, Huang CY, Dong CD, Singhania RR. Recent advancements in astaxanthin production from microalgae: a review. Bioresour Technol. 2022;26:128030. doi: 10.1016/j.biortech.2022.128030. [DOI] [PubMed] [Google Scholar]
- Patel AK, Chauhan AS, Kumar P, Michaud P, Gupta VK, Chang JS, Chen CW, Dong CD, Singhania RR. Emerging prospects of microbial production of omega fatty acids: Recent updates. Bioresour Technol. 2022;360:127534. doi: 10.1016/j.biortech.2022.127534. [DOI] [PubMed] [Google Scholar]
- Patel AK, Vadrale AP, Tseng YS, Chen CW, Dong CD, Singhania RR. Bioprospecting of marine microalgae from Kaohsiung seacoast for lutein and lipid production. Bioresour Technol. 2022;351:126928. doi: 10.1016/j.biortech.2022.126928. [DOI] [PubMed] [Google Scholar]
- Pätzold M, Siebenhaller S, Kara S, Liese A, Syldatk C, Holtmann D. Deep eutectic solvents as efficient solvents in biocatalysis. Trends Biotechnol. 2019;37(9):943–59. doi: 10.1016/j.tibtech.2019.03.007. [DOI] [PubMed] [Google Scholar]
- Quilodrán B, Cortinez G, Bravo A, Silva D. Characterization and comparison of lipid and PUFA production by native thraustochytrid strains using complex carbon sources. Heliyon. 2020;6(11):e05404. doi: 10.1016/j.heliyon.2020.e05404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Y, Deng J, Huang J, Wu Z, Yi L, Bi Y, Chen F. Using green alga Haematococcus pluvialis for astaxanthin and lipid co-production: advances and outlook. Bioresour Technol. 2021;340:125736. doi: 10.1016/j.biortech.2021.125736. [DOI] [PubMed] [Google Scholar]
- Rollin S, Gupta A, Puri M. Optimising pineapple filtrate assisted cell disruption of wet thraustochytrid biomass for improved lipid extraction. J Clean Prod. 2022;378:134393. doi: 10.1016/j.jclepro.2022.134393. [DOI] [Google Scholar]
- Santos HO, Price JC, Bueno AA. Beyond fish oil supplementation: the effects of alternative plant sources of omega-3 polyunsaturated fatty acids upon lipid indexes and cardiometabolic biomarkers-an overview. Nutrients. 2020;12(10):3159. doi: 10.3390/nu12103159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Ishihara K, Shimizu T, Aoya J, Yoshida M. Laboratory scale culture of early-stage kuruma shrimp Marsupenaeus japonicus larvae fed on thraustochytrids Aurantiochytrium and Parietichytrium. J Shellfish Res. 2018;37(3):571–579. doi: 10.2983/035.037.0310. [DOI] [Google Scholar]
- Senbagalakshmi P, Muthukrishnan S, Jebasingh T, Kumar TS, Rao M, Kumar TS, Rao MV. Squalene, biosynthesis and its role in production of bioactive compounds, a proper scientific challenge—a review. J Emerg Technol Innov Res. 2019;6:505–526. [Google Scholar]
- Shah AM, Yang W, Mohamed H, Zhang Y, Song Y. Microbes: a hidden treasure of polyunsaturated fatty acids. Front Nutr. 2022;17(9):827837. doi: 10.3389/fnut.2022.827837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Zhu X, Wang B, Ibrar M, Hu Z, Li S, Yang X. Overexpression of the KAS III-like gene YxwZ3 increases carotenoids production in Aurantiochytrium sp. SZU445. Ind Crop Prod. 2022;187:115435. doi: 10.1016/j.indcrop.2022.115435. [DOI] [Google Scholar]
- Thom LT, Ha NC, Hien HTM, Thu NTH, Loan VT, Dan NT, Hai TB, Hong DD. Biological characteristics of the heterotrophic marine microalgae Thraustochytrium pachydermum TSL10 isolated from the sea area of Truong Sa Archipelago. Vietnam Viet J Biotechnol. 2022;20(3):545–563. doi: 10.15625/1811-4989/16835. [DOI] [Google Scholar]
- Thom LT, Ha NC, Hong DD. Optimization of cultural conditions for omega 3–6 fatty acids and carotenoids production by Schizochytrium mangrovei TB17. Acad J Biol. 2022;44(1):11–28. doi: 10.15625/2615-9023/16208. [DOI] [Google Scholar]
- Thomas BJ, Plourde M, Stark KD, Jones PJ, Lin YH. Best practices for the design, laboratory analysis, and reporting of trials involving fatty acids. The Am J Clin Nutr. 2018;108(2):211–227. doi: 10.1093/ajcn/nqy089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran TLN, Miranda AF, Gupta A, Puri M, Ball AS, Adhikari B, Mouradov A. The nutritional and pharmacological potential of new Australian thraustochytrids isolated from mangrove sediments. Mar Drug. 2020;18(3):151. doi: 10.3390/md18030151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johra FT, Bepari AK, Bristy AT, Reza HM. A mechanistic review of β-carotene, lutein, and zeaxanthin in eye health and disease. Antioxidants. 2020;9(11):1046. doi: 10.3390/antiox9111046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K, Arafiles KHV, Higashi R, Okamura Y, Tajima T, Matsumura Y, Nakashimada Y, Matsuyama K, Aki T. Isolation of high carotenoid-producing Aurantiochytrium sp. mutants and improvement of astaxanthin productivity using metabolic information. J. Oleo Sci. 2018;67(5):571–578. doi: 10.5650/jos.ess17230. [DOI] [PubMed] [Google Scholar]
- Wei M, Parrish CC, Guerra NI, Armenta RE, Colombo SM. Extracted microbial oil from a novel Schizochytrium sp. (T18) as a sustainable high DHA source for Atlantic salmon feed: impacts on growth and tissue lipids. Aquaculture. 2021;15(534):736249. doi: 10.1016/j.aquaculture.2020.736249. [DOI] [Google Scholar]
- Xie Y, Sen B, Wang G. Mining terpenoids production and biosynthetic pathway in thraustochytrids. Bioresour Technol. 2017;244:1269–1280. doi: 10.1016/j.biortech.2017.05.002. [DOI] [PubMed] [Google Scholar]
- Yin FW, Zhan CT, Huang J, Sun XL, Yin LF, Zheng WL, Luo X, Zhang YY, Fu YQ. Efficient co-production of docosahexaenoic acid oil and carotenoids in Aurantiochytrium sp. using a light intensity gradient strategy. Appl Biochem Biotechnol. 2023;195(1):623–638. doi: 10.1007/s12010-022-04134-w. [DOI] [PubMed] [Google Scholar]