Abstract
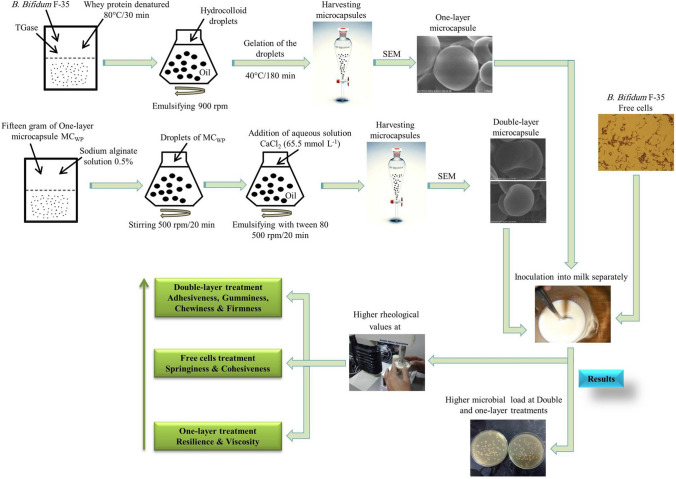
Microencapsulation of B. bifidum F-35 was carried out through emulsification technique in order to increase the microbial load while maintaining the rheological functions of set-yogurt. To produce single-layer (SL) microcapsules of whey protein, the pH was adjusted to 6.4 within Transglutaminase-induced gelation. Sodium alginate was processed as the external layer using calcium-induced gelation (pH 5.5) to produce the double-layer (DL) microcapsule. Scanning electron microscopy revealed that SL and DL microcapsules had sizes of 10 and 280 μm, respectively. The highest microbial load was clearly visible in the DL sample. According to texture profile analysis, the DL sample had the highest levels of gumminess, chewiness, and adhesiveness. The free sample outperformed the encapsulated samples in terms of springiness and cohesiveness. Although the SL sample had the highest viscosity, it produced a deformed gel when firmness was measured. In terms of firmness, the DL sample performed quite well. The viability of encapsulated B. bifidum F-35 in DL was higher than SL microcapsules during storage. Microencapsulation of B. bifidum F-35 with whey protein and sodium alginate is a promising technique that could improve the rheological properties of set-yogurt as a popular vehicle for bioactive ingredients.
Graphical abstract
Keywords: Bifidobacterium bifidum, Sodium alginate, Whey protein, Emulsification technique, Set-yogurt, Microencapsulation
Introduction
Fermented dairy products, such as yogurt, have been used as the most popular vehicle for incorporating probiotic organisms, mainly Bifidobacterium since they are considered live microorganisms that own beneficial belongings for health (Sofyan et al. 2022). Protection of probiotic cells is an inevitable process against adverse conditions of the host environment (e.g. dairy products). Hence, microencapsulation has been reported as a crucial technique that can act as a protective shield (Bakry et al. 2015; Obradović et al. 2022). Moreover, the microencapsulation technique improved the manufacture and storage of dairy products such as yogurt (Cai et al. 2014; Bakry et al. 2015; Iqbal et al. 2022). The microencapsulation process relies heavily on choosing the right physical barrier, which can have both positive and negative effects on the rheological properties of the host product. Consequently, it’s crucial to maintain the stability of microcapsules by utilizing hydrocolloid agents that can influence the mechanical characteristics of the product while minimizing any negative impact on viscosity and texture (Eghbal et al. 2022). In principle, we devoted this study for biopolymers of sodium alginate and whey protein isolates that could recover structurally by using induced-gelation ingredients in such calcium chloride and Transglutaminase (TGase) enzyme (Mousa et al. 2014). Sodium alginate has been implemented widely for encapsulation as a hydrocolloid agent, due to its edible, biodegradable, and ease of handling. Furthermore, sodium alginate could present a strong shell, with few ranges of diameter not exceeding 1 mm (Yuan et al. 2022). Sodium alginate is considered a polysaccharide that contains 1, 4-linked α-L-guluronic acid and β-D-mannuronic acid. The cross-linking between the calcium ions and carboxylate anions of guluronic acid induces the gelation phenomena among the alginate polymers. Because alginate is a negatively charged polysaccharide, it was used in the emulsification technique to produce multiphase microcapsules (Hu et al. 2021). The pH level is a key factor in enabling the implementation of multiphase microcapsules by facilitating the interaction between biopolymers in an opposing manner, such as the combination of protein (positive charge) and sodium alginate (negative charge) (Xi et al. 2022). Traditionally, whey protein isolate is not categorized as a hydrocolloid agent despite its classifiable glutinous performance and its interaction with the polysaccharides somewhat. At that point, Zou et al. (2012) has been investigated successfully the role of TGase-induced gelation method to linkage of whey protein polymers and exhibiting advanced aggregation of the protein. Hence, the emulsification technique in this study could be easily scaled up with few modifications and effectively control of particle size that was developed to produce DL microspheres below 300 μm in size. This technique will be in facing the obstacle of orifice clogging at case of extrusion scheme (Burgain et al. 2011), that in turn, led to not acceptable texture in food products (Krasaekoopt et al. 2003). Iqbal et al. (2022) used the single- and double-coated microbeads of polysaccharide-protein matrices to immobilize B. bifidum ATCC 35,914 and studied only the effect of their viability in yogurt. Theoretically, forming of protein-polysaccharide mixtures is inhibited once the pH exceeds the isoelectric point of whey protein which could lead to the segregative phase separation (Sittikijyothin et al. 2010). To avert this condition in our study, we adjusted the pH of the double-phase emulsion to be higher than the isoelectric point and achieve the associative phase separation (oppositely charged biopolymer mixtures) between the polymers of whey protein and sodium alginate. Accordingly, the current study aims to operate and perceive the macromolecular interfaces between the hydrocolloid shells and set-yogurt matrix through detecting the rheological characteristics of the set-yogurt samples. Also, the microbiological assays did not be neglected. That would be an advanced step in the philosophy of novel food technology.
Materials and methods
Materials
Milk powder was purchased from Guang Ming company (Wenzhou, China). Whey protein isolate (HilmarTM 9400) was brought from Milky Way Trade Co. (Beijing, China). Freeze-dried Direct Vat Set (DVS) cultures contains the starter culture bacteria of Streptococcus thermophiles and Lactobacillus bulgaricus were purchased from Hansen Co Inc, Shanghai, China. B. bifidum F-35 strain (Jiangnan University, Wuxi, China) was employed as a core material in the used microcapsules. The TPY-dicloxacillin medium (Guang Ming Company, Wenzhou, China) was utilized to enumerate B. bifidum F-35 strain. Whereas Elliker’s lactic agar medium (HiMedia Laboratories LLC, Pennsylvania, USA) was used to cultivate lactic acid bacteria in set-yogurt. MacConkey broth (Guangdong Huankai Microbial Sci. & Tech. Co., Ltd., China) was used to detect coliform bacteria. Whereas, Yeast Extract Glucose Chloramphenicol Agar (Sigma-Aldrich, USA) was used to enumerate yeast and molds (Kaminarides et al. 2007). Filter paper, Whatman No. 4 was purchased from Fisher Scientific, Loughborough, UK. Pure soy oil and other materials were supplied from the culture store of Jiangnan University, Wuxi, China.
Preparation of cells for microencapsulation technique
Pure lyophilized B. bifidum F-35 was streaked on MRS agar plate containing a 0.1% filter sterilized L-cysteine hydrochloride (L-Cysteine. HCl) and was then incubated for 24 h at 37 °C anaerobically. A 20 mL of MRS broth was inoculated by one colony and then incubated at 37 °C for 18 h. The culture was centrifuged at 8000 rpm for 10 min at 4 °C. After that, the supernatant was discarded (Nag et al. 2011).
Production of whey protein microcapsules (SL)
TGase-induced technique was adapted for B. bifidum F-35 encapsulation (Zou et al. 2012). The aqueous solution was prepared by rehydrating whey protein isolates powder into deionized water at a concentration of 10 g/100 mL and under thermal process (80 °C/30 min). After that, pH was adjusted to 6.4 after cooling at 40 °C, and then 30 mL of denatured whey protein isolate suspension was mixed with 2 mL of fresh concentrated cell slurry and 10 U of TGase/g. The accomplishment of the TGase/whey protein cross-linking reaction was endorsed by the emulsification of the mixture into soy oil (40 °C) and was stirred at a constant speeding 900 rpm/3 hr. Finally, the microcapsules were harvested by centrifugation (500 × g/1 min) and rinsed twice to remove the oil phase using 1/2 strength Ringer’s solution. The microcapsules were stored at 4 °C till ready to use.
Production of whey protein-sodium alginate microcapsules (DL)
This process was performed according to the method of Mokarram et al. (2009) with some modifications. Fifteen grams of the filtered SL microcapsules were added in 100 mL of 0.5 g/100 mL of alginate solution (pH 5.5), and then stirred (500 rpm) to disperse the beads. The mixture was stirred for 20 min before filtration, collected, and re-suspended into 75 g of oil containing 5 g L−1 of Span 80 alongside 65.5 mmol L−1 of calcium chloride for 20 min. The aforementioned process was implemented to form the sodium alginate gel by initiating the external Ca2+ cross-linking of the peripheral alginate layer. To recover the whey protein-sodium alginate microcapsules, 45 mL of 0.05 mol L−1 calcium chloride solution was mixed into 0.1% peptone solution, added, and transferred to a separation funnel. The final mixture was rinsed twice with a 0.1% sodium chloride solution to remove the remaining oil phase and prevent an unacceptable taste in yogurt.
Morphological and particle size analyses of microcapsules
Scanning electron microscopy (SEM) (Hitachi S-4800 type, the Netherlands FEI Company) was used to observe the size and shape of the microcapsules. Film samples were freeze-dried (0.1 mBar, − 70 °C for 24 h) and cut into squares with a surgical blade. The film squares were mounted around stubs with aided of double-sided copper tape and coated with a thin gold layer under vacuum. The magnification value used was X25.0 K.
Production of set-yogurt
Manufacture was carried out and modified to get a set-type yogurt (Adhikari et al. 2003). The mixture of milk and probiotic was divided separately into four samples; the first sample was considered a control (without B. bifidum F-35) (MCC); the second sample was named a free sample and inoculated with 4 mL/100 mL of free cells B. bifidum F-35 (MCF), the third sample was named a SL sample and inoculated with 10 g/100 mL of whey protein microcapsules (MCWP); the fourth sample was named a DL sample and inoculated with 10 g/100 mL of whey protein/sodium alginate microcapsules (MCWP−AL). All of samples were packaged into 200 mL-capped glass jars and then incubated at 42–43 °C for six hours and then stored at 4 °C overnight. Analyses of samples were carried out prior to (0 day), and during storage (7 & 14 days).
Microbiological assays
Re-suspending 1 g of each sample into 9 mL of physiological solution (0.2 mol/ l phosphate buffer NaH2PO4, pH 8.0) was done. The aqueous solution was then homogenized with a high-speed digital homogenizer (Ultra-Turrax, Model T25- S1; Ika-Werke, Staufen im Breisgau, Germany) for 45 s at approximately 20,000 rpm and deactivated mechanically in a stomacher blender at 4 °C for 20 min in order to give the green light to release the cells of probiotic from capsules (Pavunc et al. 2011). The mixture was then diluted serially by peptone salt solution (1 g/1 L distilled water), and the resulting dilutions were cast on Elliker’s agar medium to enumerate total viable lactic acid bacteria with incubation for 48 h at 37 °C. While, the TPY-dicloxacillin medium was used to enumerate B. bifidum F-35 as a selective medium for Bifidobacterium. The cultures of Bifidobacterium were subjected to anaerobic incubation at a temperature of 37 °C for 48 h. Molds and Yeast were counted through pouring the dilutions on the Extract Glucose Chloramphenicol Agar medium and incubated at 25 °C for 4 days. Coliform bacteria were detected by using MacConkey broth and incubated at 37 °C for 2 days. All the counts were expressed as the logarithm of colony-forming units per gram of yogurt (log CFU g −1).
Texture profile analysis (TPA) of set-yogurt
A TA-XT22 texture analyzer (Stable Micro Systems, UK) was calibrated by a 5 kg load cell. The diameter of the stainless-steel probe was 35 mm and the ratio of probe diameter to yogurt cup diameter was 1.0: 3.5. The used probe was inserted 15 mm into the sample. Two cycles were applied at a constant crosshead velocity (1.0 mm s−1) both downwards and upwards with a sample depth of 15 mm below the set-yogurt surface. The parameters of gumminess, adhesiveness, springiness, cohesiveness, chewiness, and resilience were detected. The test was implemented after immediate refrigeration and three yogurt duplicates were examined at 6 ± 2 °C for each trial and average measurements were calculated.
Set-yogurt firmness
Determination of firmness was carried out by using A TA-XT22 texture analyzer (Stable Micro Systems, UK) with a 25 kg load cell. The diameter probe was 20 mm and the probe velocity was 1.0 mm s−1 during the penetration and relaxation. Three duplicates were taken on each type of sample to get meaningful results, and the test was implemented after picking up the samples from the refrigerator directly.
Viscometry measurement of set-yogurt
All sample formulations were brought to room temperature (21 ± 2 °C) for 2 h before analysis and mixed gently prior to measurement. Viscometry was carried out by using Digital viscometer NDJ-5 S (Guangzhou Brookfield Viscometers & Texture Instruments Service Co. Ltd. China). Conditions of measurement were adjusted as 0.01–80 s−1 of share rate (γ), Spindle No. 3/100 rpm, the temperature of 4 °C, and data was stated in mPa·s (Zhang et al. 2015). Measurement was demonstrated by immersing the spindle into the sample and the rotary transducer expressed the spindle deflection through viscous dragging of sample versus constant spindle rotation. The spindle was rinsed using water and wiped out gently after each test. All of samples were examined on intervals of 0, 7, and 14 days.
Statistical analysis
All of the results of the current study were expressed as means ± SD of three duplicates analysis by using SPSS 19.0 statistical software (Mousa et al. 2014). The significant variances (P < 0.05) were carried out based on the one-way analysis of variance (ANOVA) and Duncan’s multiple-range tests.
Results and discussion
Morphological analyses of microcapsules
Figure 1 showed the dense structure of the SL microcapsule (MCWP) whilst the DL microcapsule exhibited a robust surface (Fig. 2). Both microcapsules were displayed under the lenses of an electronic microscope in spherical shapes and smooth surfaces. The spherical shapes and smooth surfaces of microcapsules might be attributed to the behavior of whey protein in preventing any prospective deterioration or damage to the core material of microcapsules (Rajam et al. 2012). Additionally, there was diversity in the microcapsules diameter that was recorded a 10 μm at SL microcapsule (MCWP) and 280 μm at DL microcapsule (MCWP−AL). The diameter of DL microcapsule (MCWP−AL) was slightly above the optimum range of 100–200 μm (McMaster and Kokott 2005). Generally, the alteration of microcapsules diameter due to the variance in water solubility between sodium alginate and whey protein (Cai et al. 2014). Using whey protein individually for encapsulation of B. bifidum F-35 caused in limiting the diameter of SL microcapsule (MCWP) to 10 μm. Following this further, the duration length of the emulsification process for production SL microcapsules led to distribution and formation of small microcapsule comparatively. The sharp increase in the diameter of the DL microcapsule (MCWP−AL) might be attributed to the use of sodium alginate as an external layer, which was employed to cover the internal whey protein layer of the microcapsule. As it is illustrated in Fig. 1, the dense structure of SL microcapsule MCWP might be due to the role of induced gelation by TGase enzyme in forming a dense gel-like structure in the interior capsules. Hence, the TGase helped in the maintenance of the spherical shape during the freeze-drying process (Zou et al. 2012).
Fig. 1.
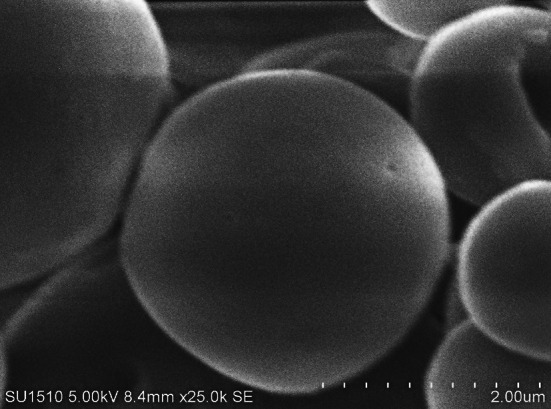
Scanning electron photomicrograph of whey protein microcapsule MCWP (SL)
Fig. 2.
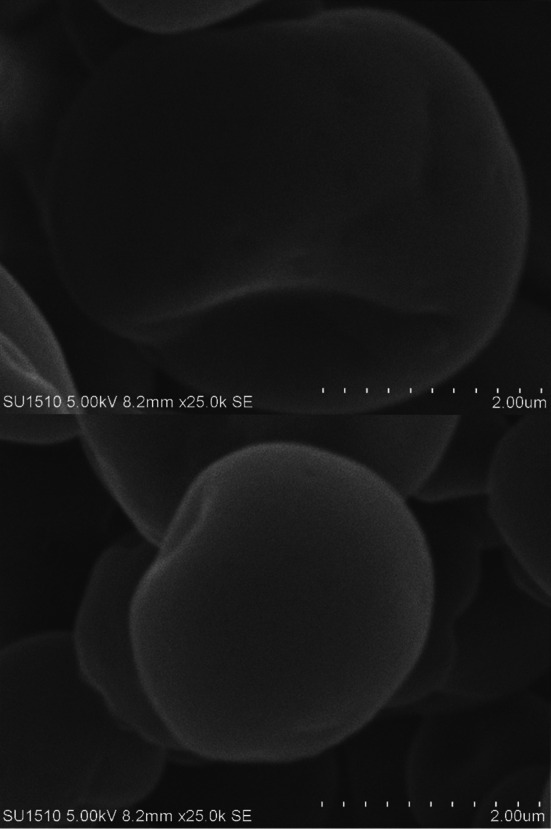
Scanning electron photomicrograph of whey protein-sodium alginate microcapsule MCWP−AL (DL)
As well, whey protein isolates could represent an excellent spherical shell at SL microcapsule MCWP and realize an alliance within B. bifidum F-35 cells (Doherty et al. 2011). The stiff crust of DL microcapsule MCWP−AL might be attributed to the substantial role of guluronate-fractions as one of sodium alginate polymers and its labor in promoting the calcium-induced gelation resulted in coherent microcapsule (Nakauma et al. 2016). Furthermore, the smooth surface of DL microcapsules (MCWP−AL) might be caused by the hydrated agent (Ca+2 ions) that cross-linked the alginate polymers during the emulsification process (Zou et al. 2011).
Total viable lactic acid bacteria, coliforms, molds, and yeast analyses
As exhibited in Fig. 3, non-significant differences were noticed among all the samples prior to storage, while the significant differences (P < 0.05) among values of each sample individually were observed up to the 14th day. Correspondingly, the samples of SL and DL were relatively kept holding over the highest values up to the 14th day. Indubitably, the non-inoculation of B. bifidum F-35 cells into the control sample led to the decline of microbial load significantly after the 14th day and the 7th day compared with the DL and other samples, respectively. There is no shortage of disagreements regarding the high microbial load of DL sample. It might be due to the extra protection of B. bifidum F-35 cells using an extra shell of sodium alginate which could bar the chemical degradation of microcapsules during the period storage of set-yogurt (Zhang et al. 2016). In general, the substantial drop (P < 0.05) in total count for each sample till the end of storage might be due to the normal sensitivity of LAB toward the acidic environment of yogurt (El-Dieb et al. 2012). In the current study, coliform bacteria, molds, and yeast were not found in all the samples. This result was in agreement with El-Shenawy et al. (2012), who reported that the bactericidal effects of the starter culture and B. bifidum F-35 could successfully inhibit the undesirable growths of coliforms, molds, and yeast. Another significant factor exists in the thermal heating of base milk and the sanitary conditions during yogurt manufacture, packing, preservation, and storage that were in agreement with the results of Kaminarides et al. (2007).
Fig. 3.
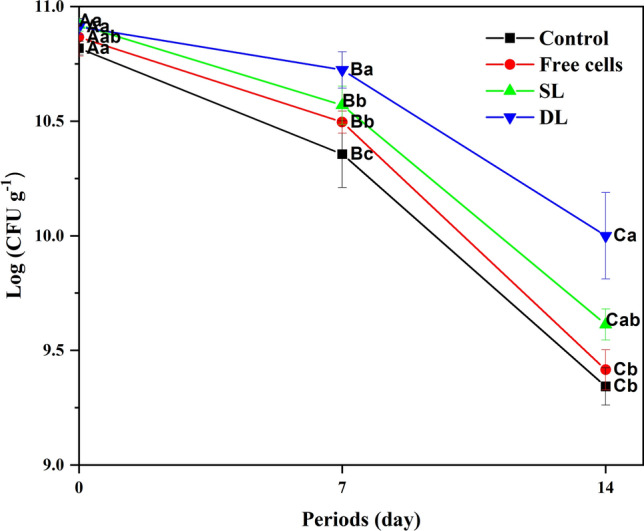
Total viable counts of lactic acid bacteria (log CFU g−1) in set-yogurt during 14 days at 4 ± 1 °C
Survival of B. bifidum F-35 during two weeks of refrigerated storage
Figure 4 illustrates the viability of B. bifidum F-35, both in its free cell and encapsulated forms (SL & DL), during a 14-day storage period in set-yogurt. Initial numbers of B. bifidum F-35 in yogurt samples were 10.38, 10.71 and 10.73 log CFU g−1 and declined to 9.03, 9.05 and 9.36 log CFU g−1 at the end of the cooled storage for yogurt samples containing free cells, SL and DL encapsulated bacteria, respectively. The decline in the viability of B. bifidum F-35 during storage can be attributed to the lower pH of the yogurt as well as a subsequent decrease in the pH during the post-acidification period (Iqbal et al. 2022). As shown in Fig. 4, the encapsulated B. bifidum F-35 samples (SL and DL) were presented in higher viability than free cells sample during storage. Iqbal et al. (2022) reported that the encapsulation of B. bifidum ATCC 35,914 using milk proteins and polysaccharides increased viable cell counts when compared to the non-encapsulated B. bifidum. Our results showed that the difference in population between SL and DL samples prior to storage was not significant (P > 0.05). While a notable distinction was observed when comparing free cells with encapsulated cells (SL and DL). After four days of storage, a rapid reduction in free cells sample numbers from 9.82 to 9.31 log CFU g−1 was detected till the 6th day. However, no significant decrease was observed in the SL and DL samples, indicating the protective role of whey protein as a coating material for cells in the SL sample and the additional layer provided by sodium alginate in the DL sample. During the period of storage from 10th to 14th day, the viability of DL samples was significantly higher (P < 0.05) than SL and free cells samples (Fig. 4). Despite that, the SL sample stilled higher than free cells sample without significant differences (P > 0.05). Our results were consistent with Afzaal et al. (2019) and Iqbal et al. (2022), who documented a significant difference between the encapsulated B. longum and B. bifidum ATCC 35,914 samples respectively compared to the unencapsulated cells in yogurt. Overall, our present study clearly showed that the encapsulation of B. bifidum F-35 cells with double layer of whey protein isolate and sodium alginate led to improvement of their viability in set-yogurt in comparison with the samples of SL and free cells.
Fig. 4.
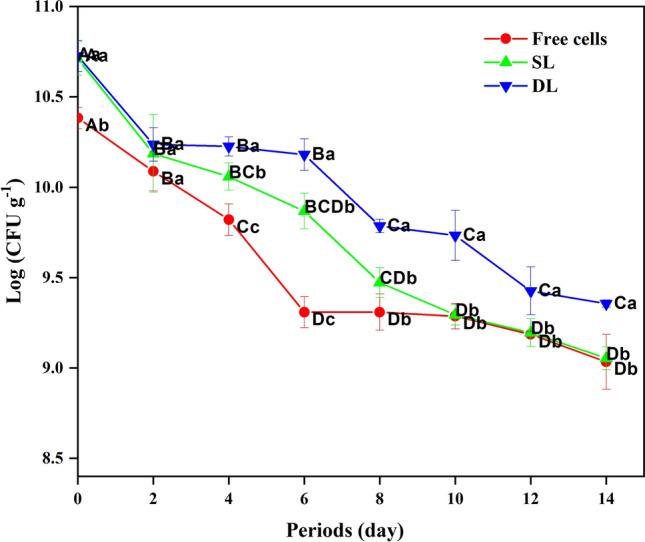
Total viable counts of B. bifidum F-35 (log CFU g−1) in set-yogurt during 14 days at 4 ± 1 °C
Texture profile analysis “TPA”
All the dissimilarities among TPA values of set-yogurt samples were shown in Table 1. Plainly, there was a significantly decreasing (P < 0.05) in gumminess and chewiness values of free and SL samples in comparison to the control prior to storage and after the 14th day. Conversely, the resilience values of the SL sample increased non-significantly (P > 0.05) compared with the other samples prior to storage till the 14th day. Contrariwise, DL sample was distinguished by the highest values of adhesiveness, gumminess, and chewiness compared with the other sample after 7 days up to the end of storage. Moreover, the highest adhesiveness values at DL sample were compared significantly (P < 0.05) with the control via statistical analysis. Prior to storage, values of springiness and cohesiveness were shown as higher values (P > 0.05) at the free sample than at SL and DL samples. Admittedly, the cells of B. bifidum F-35 lack the proteolysis action whilst the yogurt starter culture organisms possess this metabolic act.
Table 1.
Characteristic of the texture profile analysis (TPA) in set-yogurt during 14 days at 4 ± 1 °C
| Periods (days) | Treatments* | Gumminess (n) | Adhesiveness (mJ) | Springiness (mm) | Cohesiveness (mm) | Chewiness (J) | Resilience (mJ) |
|---|---|---|---|---|---|---|---|
| 0 | Control | 63.44 ± 4.98Ba | −252.416 ± 59.030Bb | 0.938 ± 0.031Ba | 0.440 ± 0.030ABa | 59.601 ± 6.508Ba | 0.0137 ± 0.002Ab |
| Free cell | 55.47 ± 1.68Bb | −117.844 ± 33.510Aa | 0.967 ± 0.009Ba | 0.521 ± 0.077Aa | 53.619 ± 1.413Bab | 0.0193 ± 0.004Aab | |
| SL | 52.40 ± 3.30Bb | −118.432 ± 2.899Aa | 0.958 ± 0.010Aa | 0.476 ± 0.018Aa | 50.198 ± 3.359Bb | 0.0223 ± 0.002Aa | |
| DL | 54.32 ± 2.75Ab | −83.532 ± 19.292Aa | 0.962 ± 0.013Aa | 0.460 ± 0.039Aa | 52.275 ± 2.654Aab | 0.0210 ± 0.005Aa | |
| 7 | Control | 78.16 ± 3.77Aa | −147.472 ± 14.583Ab | 0.982 ± 0.005Aa | 0.471 ± 0.007Aa | 76.746 ± 3.980Aa | 0.0137 ± 0.001Aa |
| Free cell | 67.39 ± 1.86Aa | −107.665 ± 9.234Aa | 0.989 ± 0.008Aa | 0.456 ± 0.009Aa | 66.645 ± 2.342Aa | 0.0153 ± 0.003Aa | |
| SL | 59.23 ± 1.14Aa | −102.394 ± 4.774Aa | 0.976 ± 0.005Aa | 0.455 ± 0.003Aa | 57.807 ± 1.356Aa | 0.0177 ± 0.004Aa | |
| DL | 93.02 ± 5.86Aa | −96.228 ± 2.143Aa | 0.973 ± 0.032Aa | 0.451 ± 0.020Aa | 89.647 ± 35.544Aa | 0.0157 ± 0.005Aa | |
| 14 | Control | 72.23 ± 3.78Aa | −366.668 ± 52.844Cb | 0.953 ± 0.015ABa | 0.431 ± 0.011Ba | 68.842 ± 4.634ABa | 0.0130 ± 0.002Aa |
| Free cell | 57.55 ± 3.45Bb | −205.424 ± 14.827Ba | 0.970 ± 0.011Ba | 0.442 ± 0.014Aa | 55.804 ± 3.756Bb | 0.0143 ± 0.002Aa | |
| SL | 55.37 ± 1.71ABb | −174.234 ± 39.196Ba | 0.965 ± 0.021Aa | 0.469 ± 0.013Aa | 53.462 ± 2.492ABb | 0.0177 ± 0.003Aa | |
| DL | 72.16 ± 8.97Aa | −192.708 ± 63.223Ba | 0.967 ± 0.021Aa | 0.440 ± 0.033Aa | 69.908 ± 10.236Aa | 0.0137 ± 0.003Aa |
* Control is considered a yogurt without any supplementations; Free cell is a yogurt that was supplemented with 4% (v/v) of free B. bifidum F-35 cells; SL is a yogurt that was supplemented with 10% (w/v) of whey protein microcapsules “MCWP”; DL is a yogurt that was supplemented with 10% (w/v) of whey protein-sodium alginate microcapsules “MCWP−AL”.
Data are expressed as the mean ± standard deviation (SD) of four treatments (triplicate samples) that were analyzed in each treatment. A−C means the same column superscript uppercase letters differ significantly (P < 0.05) among different storage periods for each treatment. a−b means the same column superscript lowercase letters differ significantly (P < 0.05) among different treatments for the same storage periods.
The comparable capability of the proteolysis process manifested through the flexible protein network in increasing of resilience values at SL sample and decreasing the gumminess and chewiness at free and one-layer samples. It is quite predictable that the highest values of gumminess and chewiness at DL samples due to the incident collapse after 7 days of storage that resulted through partial crumbling of sodium alginate layer and reinforcing the gumminess and chewiness of yogurt samples. Equally important, the higher values of chewiness at the DL sample might be due to the depletion flocculation phenomena that occur through undergoing the hydrocolloid shells an osmotic pressure by casein fragments into milk during storage (McClements 2015).
Haque et al. (2001) reported that the higher gumminess values at DL samples were likely due to the shifts upwards at acid production during storage that could promote the hydrophobic interaction between protein milk micelles and colloid shell fragments. Embedding of alginate and whey protein into double shell led the DL sample to possess the highest values of adhesiveness. Indeed, the findings of Uprit and Mishra (2004) confirmed our results in considering the adhesiveness character as a necessary force to eliminate the material which adheres to the mouth during eating. It was commonly calculated as the field surface of a negative peak.
There was an obvious increase of springiness and cohesiveness values in the free sample. It might be due to the metabolic activity of free B. bifidum cells in increasing exopolysaccharides production (EPS) that conferred desirable rheological attributes (Xu et al. 2019). As well, surrounding casein surface by the produced polysaccharide chains at free sample could be an obstructive performance for the accumulation of casein fragments and increase springiness values (Kiani et al. 2010).
Firmness and viscosity analyses
The divergence among all firmness and viscosity values was shown in Table 2. Evidently, there was a gradual increase in the firmness values of the control sample up to the end of the storage period in comparison to the other samples. Treatment of free cells was shown at unsettled values (P > 0.05) whilst the SL sample values reduced gradually. In contrast, the firmness values of DL sample were increased non-significantly (P > 0.05) compared with SL samples up to the end of the storage period. All of the viscosity values were not differed significantly (P > 0.05) among all of the samples till the end of the storage period. Patently, a gradual decrease occurred in all the viscosity values for each sample except probiotic samples which distinguished through an abrupt decline of approximately 10% from pre-storage.
Table 2.
Firmness (N) and viscosity (P) values of set-yogurt during 14 days at 4 ± 1 °C
| Periods (days) | Treatments* | Firmness | Viscosity |
|---|---|---|---|
| 0 | Control | 7.79 ± 0.76Aa | 34.25 ± 0.002Ab |
| Free cell | 8.48 ± 0.49Aa | 48.25 ± 0.004Aab | |
| SL | 8.37 ± 1.43Aa | 55.75 ± 0.002Aa | |
| DL | 8.80 ± 0.13Aa | 52.50 ± 0.005Aa | |
| 7 | Control | 8.80 ± 1.52Aa | 34.25 ± 0.001Aa |
| Free cell | 7.93 ± 1.05Aa | 38.25 ± 0.003Aa | |
| SL | 8.08 ± 1.25Aa | 44.25 ± 0.004Aa | |
| DL | 8.40 ± 1.08Aa | 39.25 ± 0.005Aa | |
| 14 | Control | 9.63 ± 3.48Aa | 32.50 ± 0.002Aa |
| Free cell | 8.87 ± 1.32Aa | 35.75 ± 0.002Aa | |
| SL | 7.14 ± 1.36Aa | 44.25 ± 0.003Aa | |
| DL | 8.69 ± 2.71Aa | 34.25 ± 0.003Aa |
* Control is considered a yogurt without any supplementations; Free cell is a yogurt that was supplemented with 4% (v/v) of free B. bifidum F-35 cells; SL is a yogurt that was supplemented with 10% (w/v) of whey protein microcapsules “MCWP”; DL is a yogurt that was supplemented with 10% (w/v) of whey protein-sodium alginate microcapsules “MCWP−AL”.
Data are expressed as the mean ± standard deviation (SD) of four treatments (triplicate samples) that were analyzed in each treatment. A−C means the same column superscript uppercase letters differ significantly (P < 0.05) among different storage periods for each treatment. a−b means the same column superscript lowercase letters differ significantly (P < 0.05) among different treatments for the same storage periods.
The clearly increasing of firmness values in the control treatment might be due to the slight evaporation of yogurt moisture (Salvador and Fiszman 2004). Moreover, the absence of the encapsulated cells could hinder any potential negative effects on the evaporation of moisture during storage. At the treatment of free cells, the various metabolic levels of B. bifidum F-35 cells in producing exopolysaccharides could change the firmness values irregularly (Xu et al. 2019). The existence of whey protein as individual coating material at the SL sample could cause a loose structure in yogurt that was observed during the gel deformation by probe compression before the penetrating act. While the higher concentration of sodium alginate 10% at DL sample could reinforce non-significantly the yogurt structure compared with the SL sample (Kaminarides et al. 2007). Moreover, it is reasonable to consider the observable resistance of the DL network to smash during penetration of probe (Serra et al. 2009).
Referring to the dramatic results, the lowest viscosity values of the control treatment were far fewer values compared with other samples. Probably, acceleration at the activity of starter culture and B. bifidum F-35 into yogurt might accelerate the interaction of exopolysaccharides polymers with casein-based micelle into the acidic environment that could diminish the aggregation of casein fragments and declines the viscosity values at control sample (Koksoy and Kilic 2004). SL sample possessed the highest values of viscosity through looking at the original data presented in Table 2. Investiture of whey protein as a shell of SL microcapsule could alter the elasticity behavior of the yogurt network and exhibit an increment in viscosity values compared with other samples (Zhang et al. 2015). Moreover, the presence of TGase enzyme in the hydrocolloid shells could result in a thixotropic demeanor at both of SL and DL samples (Iličić et al. 2008). In general, the stable gelation of encapsulated sample is due to the heating process of milk as confirmed by Xu et al. (2008). As well, the possession of yogurt matrix an electrostatic attraction could create an oversensitive interface to the associative phase separation (coacervation mechanism).
Conclusion
The emulsification process had the potential to significantly alter the size and morphological structure of SL (MCWP) and DL (MCWP−AL) microcapsules. The incorporation of encapsulated B. bifidum F-35 into yogurt increased the total count of lactic acid bacteria in comparison to the free cells. The DL microcapsules prepared with sodium alginate and whey protein isolate (SL) showed higher viability of B. bifidum F-35 than free cells in set yogurt during the refrigerated storage. The encapsulation of B. bifidum F-35 using DLs of sodium alginate and whey protein isolate exhibited perfect results in terms of increasing gumminess, chewiness, adhesiveness, and acceptable firmness values, which led to the strength of yogurt texture during storage periods. Mechanically, using TGase enzyme in cross-linking whey protein polymers enhanced the thixotropic demeanor of the yogurt network. Furthermore, whey protein played a significant role in increasing viscosity and resilience during SL sample and turbulence the elasticity behavior of the yogurt matrix.
Abbreviations
- DL
double-layer
- SL
single-layer
- Bifidobacterium bifidum
B. bifidum
- DVS
Freeze-dried Direct Vat Set
- TGase
Transglutaminase
- SEM
Scanning electron microscopy
Funding
This work was supported by the National Science Fund for Distinguished Young Scholars [G.No. 31125021]; and the National High Technology Research & Development Program of China 863 [G.Nos. 2011AA100901, 2011AA100902].
Declarations
Conflict of interest
The authors have not disclosed any competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ahmed H. Mousa, Email: ahassan@aru.edu.eg
Amr M. Bakry, Email: amr.bakry@agr.nvu.edu.eg, Email: bakryamr@ymail.com
References
- Adhikari K, Mustapha A, Grün IU. Survival and metabolic activity of microencapsulated bifidobacterium longum in stirred yogurt. J Food Sci. 2003;68:275–280. doi: 10.1111/j.1365-2621.2003.tb14152.x. [DOI] [Google Scholar]
- Afzaal M, et al. Functional exploration of free and encapsulated probiotic bacteria in yogurt and simulated gastrointestinal conditions. Food Sci Nutr. 2019;7:3931–3940. doi: 10.1002/fsn3.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakry AM, et al. Microencapsulation of oils: a comprehensive review of benefits, techniques, and applications. Compr Rev Food Sci Food Saf. 2015;62:12179. doi: 10.1111/1541-4337.12179. [DOI] [PubMed] [Google Scholar]
- Burgain J, Gaiani C, Linder M, Scher J. Encapsulation of probiotic living cells: from laboratory scale to industrial applications. J Food Eng. 2011;104:467–483. doi: 10.1016/j.jfoodeng.2010.12.031. [DOI] [Google Scholar]
- Cai S, Zhao M, Fang Y, Nishinari K, Phillips GO, Jiang F. Microencapsulation of lactobacillus acidophilus CGMCC1.2686 via emulsification/internal gelation of alginate using Ca-EDTA and CaCO3 as calcium sources. Food Hydrocoll. 2014;39:295–300. doi: 10.1016/j.foodhyd.2014.01.021. [DOI] [Google Scholar]
- Doherty SB, Gee VL, Ross RP, Stanton C, Fitzgerald GF, Brodkorb A. Development and characterisation of whey protein micro-beads as potential matrices for probiotic protection. Food Hydrocoll. 2011;25:1604–1617. doi: 10.1016/j.foodhyd.2010.12.012. [DOI] [Google Scholar]
- Eghbal N, Viton C, Gharsallaoui A. Nano and microencapsulation of bacteriocins for food applications: a review. Food Biosci. 2022;50:1021. doi: 10.1016/j.fbio.2022.102173. [DOI] [Google Scholar]
- El-Dieb SM, Abd Rabo FHR, Badran SM, Abd El-Fattah AM, Elshaghabee FMF. The growth behaviour and enhancement of probiotic viability in bioyoghurt. Int Dairy J. 2012;22:44–47. doi: 10.1016/j.idairyj.2011.08.003. [DOI] [Google Scholar]
- El-Shenawy M, El-Aziz MA, El-kholy WI, MT F. Probiotic yogurt manufactured with tiger-nut extract (Cyperus Escuilentus) as a functional dairy food. J Agric Res Nat Resour. 2012;1:20–31. [Google Scholar]
- Haque A, Richardson RK, Morris ER. Effect of fermentation temperature on the rheology of set and stirred yogurt. Food Hydrocoll. 2001;15:593–602. doi: 10.1016/S0268-005X(01)00090-X. [DOI] [Google Scholar]
- Hu X, et al. In vitro digestion of sodium alginate/pectin co-encapsulated Lactobacillus bulgaricus and its application in yogurt bilayer beads. Int J Biol Macromol. 2021;193:1050–1058. doi: 10.1016/j.ijbiomac.2021.11.076. [DOI] [PubMed] [Google Scholar]
- Iličić M, et al. Viscosity changes of probiotic yoghurt with transglutaminase during storage. Acta Period Technol. 2008;68:11–19. doi: 10.2298/APT0839011I. [DOI] [Google Scholar]
- Iqbal R, et al. Double layered encapsulation to immobilize Bifidobacterium bifidum ATCC 35914 in polysaccharide-protein matrices and their viability in set type yoghurt. J Food Process Preserv. 2022;46:16748. doi: 10.1111/jfpp.16748. [DOI] [Google Scholar]
- Kaminarides S, Stamou P, Massouras T. Comparison of the characteristics of set type yoghurt made from ovine milk of different fat content. Int J Food Sci Technol. 2007;42:1019–1028. doi: 10.1111/j.1365-2621.2006.01320.x. [DOI] [Google Scholar]
- Kiani H, Mousavi ME, Razavi H, Morris ER. Effect of gellan, alone and in combination with high-methoxy pectin, on the structure and stability of doogh, a yogurt-based iranian drink. Food Hydrocoll. 2010;24:744–754. doi: 10.1016/j.foodhyd.2010.03.016. [DOI] [Google Scholar]
- Koksoy A, Kilic M. Use of hydrocolloids in textural stabilization of a yoghurt drink, ayran. Food Hydrocoll. 2004;18:593–600. doi: 10.1016/j.foodhyd.2003.10.002. [DOI] [Google Scholar]
- Krasaekoopt W, Bhandari B, Deeth H. Evaluation of encapsulation techniques of probiotics for yoghurt. Int Dairy J. 2003;13:3–13. doi: 10.1016/s0958-6946(02)00155-3. [DOI] [Google Scholar]
- McClements DJ. Food emulsions: principles, practices, and techniques. 3. Florida: CRC Press; 2015. [Google Scholar]
- McMaster LD, Kokott SA. Micro-encapsulation of bifidobacterium lactis for incorporation into soft foods. World J Microbiol Biotechnol. 2005;21:723–728. doi: 10.1007/s11274-004-4798-0. [DOI] [Google Scholar]
- Mokarram RR, Mortazavi SA, Najafi MBH, Shahidi F. The influence of multi stage alginate coating on survivability of potential probiotic bacteria in simulated gastric and intestinal juice. Food Res Int. 2009;42:1040–1045. doi: 10.1016/j.foodres.2009.04.023. [DOI] [Google Scholar]
- Mousa A, Liu X-m, Chen Y-q, Zhang H, Chen W. Evaluation of physiochemical, textural, microbiological and sensory characteristics in set yogurt reinforced by microencapsulated Bifidobacterium bifidum F-35. Int J Food Sci Technol. 2014;49:1673–1679. doi: 10.1111/ijfs.12473. [DOI] [Google Scholar]
- Nag A, Han K-S, Singh H. Microencapsulation of probiotic bacteria using pH-induced gelation of sodium caseinate and gellan gum. Int Dairy J. 2011;21:247–253. doi: 10.1016/j.idairyj.2010.11.002. [DOI] [Google Scholar]
- Nakauma M, Funami T, Fang Y, Nishinari K, Draget KI, Phillips GO. Calcium binding and calcium-induced gelation of sodium alginate modified by low molecular-weight polyuronate. Food Hydrocoll. 2016;55:65–76. doi: 10.1016/j.foodhyd.2015.10.021. [DOI] [Google Scholar]
- Obradović N, Volić M, Nedović V, Rakin M, Bugarski B. Microencapsulation of probiotic starter culture in protein–carbohydrate carriers using spray and freeze-drying processes: implementation in whey-based beverages. J Food Eng. 2022;321:110948. doi: 10.1016/j.jfoodeng.2022.110948. [DOI] [Google Scholar]
- Pavunc AL, BeganoviĆ J, Kos B, Buneta ANA, Beluhan S, ŠUŠKoviĆ J. Influence of microencapsulation and transglutaminase on viability of probiotic strain lactobacillus helveticus M92 and consistency of set yoghurt. Int J Dairy Technol. 2011;64:254–261. doi: 10.1111/j.1471-0307.2010.00647.x. [DOI] [Google Scholar]
- Rajam R, Karthik P, Parthasarathi S, Joseph GS, Anandharamakrishnan C. Effect of whey protein – alginate wall systems on survival of microencapsulated Lactobacillus plantarum in simulated gastrointestinal conditions. J Funct Foods. 2012;4:891–898. doi: 10.1016/j.jff.2012.06.006. [DOI] [Google Scholar]
- Salvador A, Fiszman SM. Textural and sensory characteristics of whole and skimmed flavored set-type yogurt during long storage. J Dairy Sci. 2004;87:4033–4041. doi: 10.3168/jds.S0022-0302(04)73544-4. [DOI] [PubMed] [Google Scholar]
- Serra M, Trujillo AJ, Guamis B, Ferragut V. Evaluation of physical properties during storage of set and stirred yogurts made from ultra-high pressure homogenization-treated milk. Food Hydrocoll. 2009;23:82–91. doi: 10.1016/j.foodhyd.2007.11.015. [DOI] [Google Scholar]
- Sittikijyothin W, Sampaio P, Gonçalves MP. Microstructure and rheology of β-lactoglobulin–galactomannan aqueous mixtures. Food Hydrocoll. 2010;24:726–734. doi: 10.1016/j.foodhyd.2010.03.014. [DOI] [Google Scholar]
- Sofyan A, Ikhsani AY, Purwani E, Hasanah LEN, Febriyadin F. The effect of suweg (Amorphophallus paeoniifolius) flour and incubation temperature on characteristics of yogurt with the addition of Bifidobacterium bifidum as probiotic. Mater Today Proc. 2022;63:S507–S512. doi: 10.1016/j.matpr.2022.04.538. [DOI] [Google Scholar]
- Uprit S, Mishra HN. Instrumental textural profile analysis of soy fortified pressed chilled acid coagulated curd (paneer) Int J Food Prop. 2004;7:367–378. doi: 10.1081/JFP-200032918. [DOI] [Google Scholar]
- Xi CY, Sun ZW, Chen X, Ding X, Zhang TH (2022) Characterization of coacervation behavior between whey protein isolate and propylene glycol alginate: a morphology, spectroscopy, and thermodynamics study. Food Chem X 15 [DOI] [PMC free article] [PubMed]
- Xu ZM, Emmanouelidou DG, Raphaelides SN, Antoniou KD. Effects of heating temperature and fat content on the structure development of set yogurt. J Food Eng. 2008;85:590–597. doi: 10.1016/j.jfoodeng.2007.08.021. [DOI] [Google Scholar]
- Xu Y, et al. Exopolysaccharides produced by lactic acid bacteria and Bifidobacteria: structures, physiochemical functions and applications in the food industry. Food Hydrocoll. 2019;94:475–499. doi: 10.1016/j.foodhyd.2019.03.032. [DOI] [Google Scholar]
- Yuan Y, Yin M, Chen L, Liu F, Chen M, Zhong F. Effect of calcium ions on the freeze-drying survival of probiotic encapsulated in sodium alginate. Food Hydrocoll. 2022;130:107668. doi: 10.1016/j.foodhyd.2022.107668. [DOI] [Google Scholar]
- Zhang TH, McCarthy J, Wang GR, Liu YY, Guo MR. Physiochemical properties, microstructure, and probiotic survivability of nonfat goats’ milk yogurt using heat-treated whey protein concentrate as fat replacer. J Food Sci. 2015;80:M788–M794. doi: 10.1111/1750-3841.12834. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Zhang R, McClements DJ. Encapsulation of β-carotene in alginate-based hydrogel beads: impact on physicochemical stability and bioaccessibility. Food Hydrocoll. 2016;61:1–10. doi: 10.1016/j.foodhyd.2016.04.036. [DOI] [Google Scholar]
- Zou Q, et al. Microencapsulation of Bifidobacterium bifidum F-35 in reinforced alginate microspheres prepared by emulsification/internal gelation. Int J Food Sci Technol. 2011;46:1672–1678. doi: 10.1111/j.1365-2621.2011.02685.x. [DOI] [Google Scholar]
- Zou Q, et al. Microencapsulation of bifidobacterium bifidum F-35 in whey protein-based microcapsules by transglutaminase-induced gelation. J Food Sci. 2012;77:M270–M277. doi: 10.1111/j.1750-3841.2012.02673.x. [DOI] [PubMed] [Google Scholar]