Abstract
Background
Anaemia occurs when blood contains fewer red blood cells and lower haemoglobin levels than normal, and is a common complication among adults with chronic kidney disease (CKD). Although a number of approaches are applied to correct anaemia in adults with CKD, the use of androgen therapy is controversial.
Objectives
The aim of this review was to determine the benefits and harms of androgens for the treatment of anaemia in adult patients with CKD.
Search methods
We searched CENTRAL, the Cochrane Renal Group's Specialised Register, the Chinese Biomedicine Database (CBM), CNKI, VIP and reference lists of articles without language restriction. The most recent search was conducted in August 2014.
Selection criteria
All randomised controlled trials (RCTs) that assessed the use of androgens for treating anaemia of CKD in adults were eligible for inclusion.
Data collection and analysis
Two authors independently extracted data and assessed risk of bias in the included studies. Meta‐analyses were performed using relative risk (RR) for dichotomous outcomes and mean differences (MD) for continuous outcomes, with 95% confidence intervals (CI).
Main results
We included eight studies that reported data from 181 participants. Study quality was assessed as moderate in six studies, one was low quality, and one was high quality. The small number of included studies, and low participant numbers adversely influenced evidence quality overall.
We found limited evidence (1 study, 24 participants) to indicate that oxymetholone can increase haemoglobin (Hb) (MD 1.90 g/dL, 95% CI 1.66 to 2.14), haematocrit (HCT) (MD 27.10%, 95% CI 26.49 to 27.71), change in albumin (MD 4.91 g/L, 95% CI 3.69 to 6.13), alanine aminotransferase (ALT) (MD 54.50 U/L, 95% CI 43.94 to 65.06), and aspartate aminotransferase (AST) (MD 47.33 U/L, 95% CI 37.69 to 56.97); and decrease high‐density lipoprotein (HDL) (MD ‐15.66 mg/dL, 95% CI ‐24.84 to ‐6.48). We also found that compared with erythropoietin alone, nandrolone decanoate plus erythropoietin may increase HCT (3 studies, 73 participants: MD 2.54%, 95% Cl 0.96 to 4.12). Compared with erythropoietin (1 study, 27 participants), limited evidence was found to suggest that nandrolone decanoate can increase plasma total protein (MD 0.40 g/L, 95% CI 0.13 to 0.67), albumin (MD 0.20 g/L, 95% CI 0.01 to 0.39), and transferrin (MD 45.00 mg/dL, 95% CI 12.61 to 77.39) levels. Compared with no therapy (remnant kidney), evidence was found to suggest that nandrolone decanoate can increase Hb (2 studies, 33 participants: MD 1.04 g/dL, 95% Cl 0.66 to 1.41) and HCT (1 study, 24 participants: MD 3.70%, 95% Cl 0.68 to 6.72). Compared with no therapy (anephric), evidence was found (1 study, 5 participants) to suggest that nandrolone decanoate can increase Hb (MD 1.30 g/dL, 95% Cl 0.57 to 2.03), but nandrolone decanoate did not increase HCT (MD 2.00%, 95% Cl ‐0.85 to 4.85).
However, oxymetholone was not found to reduce blood urea nitrogen (BUN), serum creatinine (SCr), cholesterol, or triglycerides; or increase plasma total protein, prealbumin, or transferrin. No evidence was found to indicate that nandrolone decanoate increased prealbumin or decreased BUN, SCr, AST, ALT, cholesterol, triglycerides, HDL or low‐density lipoprotein (LDL). Adverse events associated with androgen therapy were reported infrequently.
Authors' conclusions
We found insufficient evidence to confirm that use of androgens for adults with CKD‐related anaemia is beneficial.
Plain language summary
Androgens for the anaemia of chronic kidney disease in adults
Anaemia, which occurs when red blood cell and haemoglobin levels fall below normal, is a common problem among adults with chronic kidney disease (CKD). Anaemia can cause breathlessness, dizziness and chest pain (angina); reduce ability to think clearly; limits ability to exercise; and contributes to sexual problems, poor appetite and reduced quality of life. Anaemia may also cause longer hospital stays, and sometimes death.
There are several approaches to correct anaemia in people with CKD, including drugs to stimulate red blood cell production, dialysis to remove waste and excess water from the blood, blood transfusions, dietary management, and supplementary iron and folate agents.
Other drugs, such as androgens ‐ which are male steroid hormones ‐ may be given in some settings to help reduce undesirable effects of treatments. Another possible benefit of androgen therapy for people with CKD, especially in regions with limited health resources, is that these drugs have lower costs than some other treatments.
We assessed eight small studies that presented data from 181 adults with CKD‐related anaemia that investigated use of androgen therapy. Limitations and flaws in the evidence lead to our conclusion that androgen therapy for adults with CKD‐related anaemia was not associated with substantial benefits.
Summary of findings
for the main comparison.
| Nandrolone decanoate plus erythropoietin (rHuEPO) versus erythropoietin (rHuEPO) for the anaemia of chronic kidney disease in adults | ||||||
|
Patient or population: adults with the anaemia of chronic kidney disease Intervention: nandrolone decanoate plus rHuEPO versus rHuEPO | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| rHuEPO | Nandrolone decanoate plus rHuEPO | |||||
| Haematocrit (%) | Mean haematocrit in the intervention group was 2.4 higher (1.04 to 3.77 higher) | 73 (3) | ⊕⊕⊝⊝ low¹ | |||
| Haemoglobin (g/dL) | Mean haemoglobin in the intervention group was 0.44 higher (0.17 lower to 1.05 higher) | 32 (1) | ⊕⊕⊝⊝ low² | |||
| Serum albumin (g/L) | Mean serum albumin in the intervention group was 0.09 lower (0.24 lower to 0.06 higher) | 51 (2) | ⊕⊝⊝⊝ very low² | |||
| Adverse events | Study population | RR 7.72 (1.45 to 41.12) | 83 (2) | ⊕⊝⊝⊝ very low³ |
||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).CI: Confidence interval; RR: Risk ratio | ||||||
|
GRADE Working Group grades of evidence
High quality: Further research is very unlikely to change our confidence in the estimate of effect
Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: We are very uncertain about the estimate | ||||||
|
Reduce the evidence quality factors: methodology defect, included in the research results of the inconsistency, indirect evidence, inexactness, publication bias. Increase the level of evidence factor: large effect quantity, confounding factors cannot change effect quantity, or the existing concentration–response relationship. | ||||||
| ¹ Few studies included ² One study included ³ High risk of performance bias Two studies included | ||||||
Background
Description of the condition
Chronic kidney disease (CKD) is a major public health challenge. In the USA, 20 million adults have CKD, and another 20 million are at risk of developing the disease (NKF 2007). Data from NHANES III (1988 to 1994) and USRDS (1998) indicated that prevalence of CKD ranged from 64.3% for stage 1 to 0.2% for stage 5 (NKF 2002).
Anaemia occurs when levels of red blood cells and haemoglobin are reduced. Anaemia is a common complication among people with CKD, and contributes to the burden of disease, causing breathlessness, dizziness, angina, decreased exercise capacity, cognitive and dysfunctions, poor appetite, reduced quality of life, longer hospital stays, and death. Given that numbers of people with CKD are increasing, the public health impact of CKD‐associated anaemia is substantial. Anaemia prevalence increases as kidney function decreases. McClellan 2004 reported that anaemia was present in 47.7% of predialysis patients, and Hsu 2002 estimated that the overall burden of CKD‐associated anaemia (haemoglobin (Hb) ≤ 11 g/dL) was 610,000 women and 230,000 men in the US population. In 2000, five European countries participating in the Dialysis Outcomes and Practice Patterns Study (DOPPS, Combe 2001) reported that 49% of haemodialysis patients had Hb concentrations below the level recommended by the European Best Practice Guidelines (Geddes 2006). Patients with Hb < 10 g/dL were 29% more likely to be hospitalised than those with Hb levels between 11 and 12 g/dL. The risk of hospitalisation was 4% lower for every 1 g/dL increase in Hb concentration, and mortality risk in these patients was 5% lower for each 1 g/dL increase (Locatelli 2004b).
Description of the intervention
Several approaches are commonly applied to correct anaemia in adults with CKD, including erythropoiesis‐stimulating agents (ESAs), dialysis, blood transfusions, nutritional management, iron and folate supplements. ESAs augment erythropoiesis through direct or indirect actions on the erythropoietin receptor, and dialysis can improve anaemia by eliminating some toxins. In the past, iron and folate supplements were mainstay treatments. Although a direct and effective method of treating CKD‐related anaemia, blood transfusion carries risk of infection and inducing histocompatibility leukocyte antigens, which may jeopardise future kidney transplant in eligible patients (Eschbach 1989; Ward 1990).
The human erythropoietin (EPO) gene was characterised in 1983 (Lin 1985), and recombinant human EPO (rHuEPO) treatment efficacy was first demonstrated in dialysis patients in 1986 (Winearls 1986). The advent of rHuEPO revolutionised management for people with CKD‐associated anaemia and contributed to reducing risks associated with blood transfusion (Cody 2005), sensitisation, and preventing iron overload, and enhancing exercise tolerance, cognitive capacity, sexual function, and quality of life. However, three key issues have been associated with rHuEPO use:
Cost: Medicare spending on EPO reached US$1.8 billion in 2004, an increase of 17% from 2003 (USRDS 2006).
Risk of pure red cell aplasia (PRCA): a severe, non‐regenerative form of anaemia, with selective erythroid aplasia of the bone marrow.
Predictable side effects: hypertension, clotting of arteriovenous fistulas, and seizures (Navarro 2003).
Furthermore, rHuEPO may increase cancer mortality risk by stimulating tumour growth (Brian 2005; Henke 2003).
How the intervention might work
Androgens are a class of male steroid hormones that play a major role in the development and maintenance of masculine secondary sexual characters, and which also affect nitrogen metabolism (BOE 2007; Omwancha 2006; Pielecka 2006). Androgens mechanism of action is thought to be due to an increase in EPO synthesis and secretion (Shahani 2009). A positive correlation has been shown between increased Hb and HCT in people treated with androgens (Navarro 2001a; Navarro 2001b; Navarro 2002; Yared 1997).
Adjuvant therapy is often required to help optimise therapeutic response among people with CKD‐associated anaemia who are either unresponsive to erythropoiesis‐stimulating agents (ESA) or require large doses of ESAs (Navarro 2001a). In some settings, androgens may be administered as adjuvant therapies for people with CKD‐related anaemia. Androgens have been reported to increase erythropoiesis, raise plasma EPO concentration in dialysed patients with CKD‐anaemia (Teruel 1996a), and elevate Hb concentration in people with CKD (Teruel 1996b). Androgens have also achieved satisfactory results for treating PRCA (Sanchez 2006). Economic cost benefits have been identified for androgen therapy in resource‐limited settings (Locatelli 2004a).
Why it is important to do this review
Controversy exists about the use of androgens to treat anaemia in people with CKD. The KDOQI guidelines strongly recommend against androgens as adjuvant therapy to ESA treatment in people with CKD‐related anaemia. The rationale is that androgens have been associated with adverse effects including acne, virilisation, priapism, hyperglycaemia, liver dysfunction, injection site pain, risk of peliosis hepatis, hepatocellular carcinoma, risk of prostate cancer, and cancer risk in women. Three small, short‐term RCTs (Berns 1992; Gaughan 1997; Sheashaa 2005a) explored the possible role of adjuvant androgen therapy with ESA for people undergoing haemodialysis. Recommended Hb levels were not achieved; however, these studies did not enrol participants with ESA hyporesponsiveness (NKF 2006).
Objectives
The aim of this review was to determine the benefits and harms of androgens for the treatment of anaemia in adult patients with CKD.
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials (RCTs) assessing the use of androgens for the treatment of CKD‐related anaemia in adults were eligible for inclusion. There was no restriction on language or publication type. The first phases of cross‐over RCTs were also eligible for inclusion.
Types of participants
Inclusion criteria
Adults (aged 18 years or over) with CKD‐related anaemia. All CKD stages were included (predialysis, dialysis, and transplant patients). Anaemia and CKD definitions applied by each study were accepted.
CKD‐related anaemia participants with controlled hypertension and cardiac disorders, left ventricular hypertrophy, hypogonadism, kidney‐related bone disease, or neurological disorders were included.
Exclusion criteria
Pregnancy
Androgen therapy in the six months before study entry
Anaemia related to causes other than kidney disease, such as folate or B deficiency, sickle cell disease, primary haemolytic anaemia, or multiple myeloma
Haematological disorders known to cause anaemia
Iron deficiency (ferritin < 100 ng/mL or transferrin saturation < 20% or both)
Bleeding
Known malignancy such as prostate cancer
Presence of infection or inflammation
Active ischaemic heart disease
Uncontrolled hypertension (systolic blood pressure > 190 mm Hg, diastolic > 105 mm Hg)
Abnormal liver function (positive albumin, bilirubin, alkaline phosphatase (ALP), gamma glutamic transpeptidase (gamma GT), aspartate aminotransferase (AST), alanine aminotransferase (ALT), or prothrombin findings)
Active hepatitis
HIV infection
Study duration less than six months
Studies in which only one arm received dialysis or transplantation.
Types of interventions
Any androgen versus no drug or placebo alone
Any androgen versus routine therapy (e.g. androgens versus EPO)
Any androgen plus routine therapy versus routine therapy (androgens plus EPO versus EPO)
Any androgen plus routine therapy versus placebo plus routine therapy (e.g. androgen plus EPO versus placebo plus EPO).
Types of outcome measures
Mortality
Measures of anaemia correction (e.g. haemoglobin (Hb) and haematocrit (HCT) values)
Participant‐based quality of life assessment using an internationally validated minimum standard checklist (Efficace 2003; Efficace 2006; Efficace 2007)
Liver function (e.g. alanine aminotransferase (ALT) and aspartate aminotransferase (AST) values)
Kidney function (e.g. blood urea nitrogen (BUN) and serum creatinine (SCr) values)
Lipid profile (e.g. cholesterol, triglyceride (TG), high‐density lipoprotein (HDL), and low‐density lipoprotein (LDL) values)
Total protein, albumin, prealbumin, and transferrin values
Adverse events: acne, virilisation, priapism, hyperglycaemia, liver dysfunction, prostate cancer, prostate hypertrophy, peliosis hepatis, hepatocellular carcinoma, rash, amenorrhoea, menstrual disorders, enlarged clitoris, excess body hair, increased appetite, increased sexual desire, depression, mania, delirium, acute schizophrenia, cardiovascular adverse events, and other adverse effects including gastrointestinal bleeding, episodes of peritonitis and hydrothorax.
Primary outcomes
Mortality
Measures of anaemia correction, such as Hb and HCT values.
Secondary outcomes
Quality of life assessment using an internationally validated minimum standard checklist (Efficace 2003; Efficace 2006; Efficace 2007)
Liver function (ALT and AST values)
Kidney functions (BUN and SCr values)
Lipid profile (cholesterol, TG, HDL, and LDL values)
Total protein, albumin, prealbumin, and transferrin values
Adverse events: acne, virilisation, priapism, hyperglycaemia, liver dysfunction, prostate cancer, prostate hypertrophy, peliosis hepatis, hepatocellular carcinoma, rash, amenorrhoea, menstrual disorders, enlarged clitoris, excess body hair, increased appetite, increased sexual desire, depression, mania, delirium, acute schizophrenia, cardiovascular adverse events, and other adverse effects including gastrointestinal bleeding, episodes of peritonitis and hydrothorax.
Search methods for identification of studies
We searched electronic databases and where necessary corresponded with study authors to obtain additional information.
Electronic searches
We searched the Cochrane Renal Group's Specialised Register (to August 2014) through contact with the Trials' Search Co‐ordinator using search terms relevant to this review. The Cochrane Renal Group’s Specialised Register contains studies identified from the following sources.
Monthly searches of the Cochrane Central Register of Controlled Trials CENTRAL
Weekly searches of MEDLINE OVID SP
Handsearching of renal‐related journals and the proceedings of major renal conferences
Searching of the current year of EMBASE OVID SP
Weekly current awareness alerts for selected renal journals
Searches of the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
Studies contained in the Specialised Register are identified through search strategies for CENTRAL, MEDLINE, and EMBASE based on the scope of the Cochrane Renal Group. Details of these strategies as well as a list of handsearched journals, conference proceedings and current awareness alerts are available in the Specialised Register section of information about the Cochrane Renal Group.
We also searched the Chinese Biomedicine Database (CBM), VIP and China National Knowledge Infrastructure (CNKI) (to August 2012).
See Appendix 1 for search terms used in strategies for this review.
Searching other resources
We searched reference lists of identified study reports and the ISI Citation Index database, Science and Social Science Citation Index/Web of Science Services.
We were unable to contact primary investigators of identified studies for details of additional studies and companies or pharmaceutical firms that produce immunosuppressants used for unpublished data they may possess.
Data collection and analysis
Selection of studies
The search strategy described was used to obtain titles and abstracts of studies that may be relevant to the review. Titles and abstracts were screened independently by two authors who discarded studies that were not applicable; however, studies and reviews that might include relevant data or information on studies were retained initially. Two authors independently assessed retrieved abstracts, and if necessary, the full text of these studies to determine which satisfied the inclusion criteria.
Data extraction and management
Data extraction was carried out independently by two authors using a pre‐tested data extraction form. Where more than one publication of one study existed, reports were grouped together and only the publication with the most complete data was used. Where relevant outcomes were only published in earlier versions, these data were used. Any discrepancies between published versions were to be highlighted. Any further information required from the original author was requested by written correspondence. Disagreements were resolved by consensus and with a third author.
Assessment of risk of bias in included studies
The following items were independently assessed by two authors using the risk of bias assessment tool (Higgins 2011) (see Appendix 2).
Was there adequate sequence generation (selection bias)?
Was allocation adequately concealed (selection bias)?
-
Was knowledge of the allocated interventions adequately prevented during the study (detection bias)?
Participants and personnel
Outcome assessors
Were incomplete outcome data adequately addressed (attrition bias)?
Are reports of the study free of suggestion of selective outcome reporting (reporting bias)?
Was the study apparently free of other problems that could put it at a risk of bias?
Measures of treatment effect
We analysed data both dichotomous and continuous data in this review. Results for dichotomous data are expressed as relative risk (RR) with 95% confidence intervals (CI). We showed continuous data as mean differences (MD) with 95% CI.
Dealing with missing data
Where necessary, further information required from the original author was requested by written correspondence or telephone. Evaluation of important numerical data such as screened, randomised patients as well as intention‐to‐treat, as‐treated and per‐protocol population was carefully performed. Attrition rates, such as drop‐outs, losses to follow‐up and withdrawals, were investigated. Issues of missing data and imputation methods were critically appraised (Higgins 2011).
Assessment of heterogeneity
Heterogeneity was analysed using a Chi² test on N‐1 degrees of freedom, with an alpha of 0.05 used for statistical significance and with the I² test (Higgins 2003). I² values of 25%, 50% and 75% correspond to low, medium and high levels of heterogeneity.
Assessment of reporting biases
We planned to assess for reporting bias by plotting data in a funnel plot.
Data synthesis
Data were pooled using the random‐effects model under the assumption that the effects being estimated were not identical throughout, but followed certain distribution patterns. The fixed‐effect model was also analysed to ensure robustness of the model chosen and susceptibility to outliers (Higgins 2011).
Subgroup analysis and investigation of heterogeneity
We planned to conduct subgroup analyses based on androgen type, comparators and outcomes, however there were insufficient studies to do this.
Sensitivity analysis
We planned to perform sensitivity analyses to evaluate evidence stability, however there were insufficient studies to do this.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification.
Results of the search
The search strategy described identified 269 records. After removing 148 duplicates, 121 titles and abstracts were assessed, and 77 records were excluded. After further assessment, 20 studies (23 records) were excluded, and three studies (7 records) could not be obtained and are awaiting assessment (Ganguli 2003; Ota 1986; Suzuki 1986). We included eight studies (14 records) in our review (Aramwit 2010; Cattran 1977; Gaughan 1997; Kim 1999a; Koronis 2000; Navarro 2002; Sheashaa 2005a; Solomon 1988b) (Figure 1).
1.
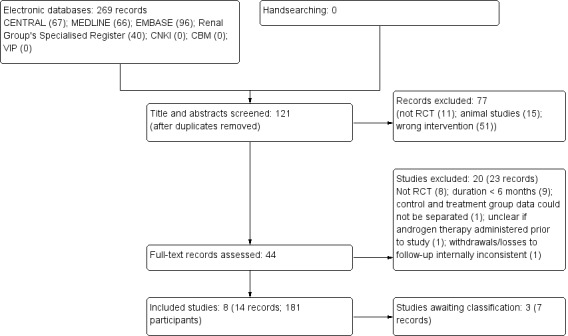
Flow diagram showing study selection
Included studies
We included eight studies that enrolled 194 adults with CKD‐associated anaemia, of whom 181 completed the studies. Study populations were small, ranging from 9 to 37 participants (average = 24.25 participants). Studies were conducted in Thailand, USA, Egypt, Canada, Spain, Korea and Greece.
We found a substantial bias favouring inclusion of males (M/F: 107/24) in the five studies that reported gender (Aramwit 2010; Cattran 1977; Gaughan 1997; Navarro 2002; Sheashaa 2005a). Participants' sex was not reported in three studies (Kim 1999a; Koronis 2000; Solomon 1988b).
Participants' ages ranged from 25 to 78 years. Mean participant age was not reported in three studies (Kim 1999a; Koronis 2000; Solomon 1988b)
All studies recruited participants with CKD‐related anaemia (Aramwit 2010; Cattran 1977; Gaughan 1997; Kim 1999a; Koronis 2000; Navarro 2002; Sheashaa 2005a; Solomon 1988b). Dialysis interventions included peritoneal dialysis for at least three months (Aramwit 2010); continuous peritoneal dialysis for at least six months (Navarro 2002); haemodialysis (Gaughan 1997; Kim 1999a; Koronis 2000; Sheashaa 2005a; Solomon 1988b); or chronic dialysis (Cattran 1977). Blood chemistry requirements varied, but included Hb concentration < 11 g/dL; serum ferritin concentrations of at least 100 ng/mL; serum transferrin saturations of at least 20%. Iron parameters also varied, as did Hb and HCT levels.
All studies were of six months duration. Intervention arm participants received androgens administered as subcutaneous (SC) or intramuscular (IM) injections (nandrolone decanoate), or oral tablets (oxymetholone). Study drug doses and frequencies were: 50 mg tablet twice daily (Aramwit 2010); nandrolone decanoate 200 mg IM once weekly (Cattran 1977; Navarro 2002); nandrolone decanoate 100 mg IM once weekly (Solomon 1988b); nandrolone decanoate 100 mg IM plus rHuEPO 1500 U intravenously (IV) once weekly (Gaughan 1997); nandrolone decanoate 100 mg IM plus rHuEPO 2000 U SC (Kim 1999a); nandrolone decanoate 100 mg IM plus rHuEPO 1000 U SC every 15 days (Koronis 2000); and nandrolone decanoate 50 mg IM plus rHuEPO 1000 U SC twice weekly (Sheashaa 2005a). Only Aramwit 2010 reported three month follow‐up. Four studies investigated nandrolone decanoate plus rHuEPO versus rHuEPO alone as the control (Gaughan 1997; Kim 1999a; Koronis 2000; Sheashaa 2005a); one study investigated nandrolone decanoate versus rHuEPO alone as the control drug (Navarro 2002); two studies investigated no therapy as the control (Cattran 1977; Solomon 1988b); one study investigated oxymetholone plus rHuEPO versus placebo plus rHuEPO as the control drug (Aramwit 2010).
Four studies (Aramwit 2010; Navarro 2002; Sheashaa 2005a; Cattran 1977) reported Hb and HCT levels as indicators of anaemia correction; Kim 1999a and Koronis 2000 reported HCT levels only; Solomon 1988b reported Hb levels only.
Aramwit 2010 and Navarro 2002 reported kidney function (BUN and SCr values). Aramwit 2010 also reported liver function measures (AST and ALT values) and lipid profile indicators (TG, HDL, LDL values); and Gaughan 1997 reported TG only.
Four studies (Aramwit 2010; Gaughan 1997; Navarro 2002; Sheashaa 2005a) reported serum albumin. Aramwit 2010 and Navarro 2002 reported total protein; and Navarro 2002 reported prealbumin and transferrin levels.
Four studies reported adverse events, including: gastrointestinal bleeding (Aramwit 2010); hydrothorax (Navarro 2002); acne (Gaughan 1997); menstrual disorders and excess body hair (Sheashaa 2005a).
Four studies reported no drop‐outs, withdrawals or losses to follow‐up (Kim 1999a; Koronis 2000; Sheashaa 2005a; Solomon 1988b). Gaughan 1997 reported that one participant withdrew after developing an active mycobacterium tuberculosis infection; Aramwit 2010 and Navarro 2002 both reported two participants discontinued treatment. Cattran 1977 reported that eight participants did not complete the study (21.6%).
Excluded studies
We excluded 20 studies; of these eight were not RCTs (Buchwald 1977; Diez 1997; Gascon 1999; Lee 2002; Logan 2005; Mora 2001; Solomon 1987; von Hartitzsch 1976); nine were fewer than six months duration (Aggarwal 2005; Aslanhan 2001; Ballal 1991; Berns 1992; Eiam‐Ong 2007; Hendler 1974; Mirahmadi 1979; Saxena 1997; Williams 1974); treatment and control group data were indistinct in Naik 1978; it was unclear if androgen therapy had been administered to participants during the six months preceding the study (Neff 1981); reported withdrawals/losses were internally inconsistent in Brockenbrough 2006; and Solomon 1988b investigated an intervention outside the scope of this review. See Characteristics of excluded studies.
Risk of bias in included studies
Aramwit 2010 was assessed as a high quality study; six were judged to be of moderate quality (Gaughan 1997; Kim 1999a; Koronis 2000; Navarro 2002; Sheashaa 2005a; Solomon 1988b); and Cattran 1977 was low quality. Summaries of quality assessments are presented in Figure 2 and Figure 3.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study
Allocation
Although all studies reported that participants were randomised, six did not provide sequencing details (Cattran 1977; Gaughan 1997; Kim 1999a; Koronis 2000; Sheashaa 2005a; Solomon 1988b). Aramwit 2010 and Navarro 2002 used computer‐generated random number tables, and although Aramwit 2010 did not report allocation concealment methods, it was likely that concealment was adequate; investigators were not actively involved in treatment, and physicians involved in participants' care were unaware of study outcome parameters. Allocation concealment was not adequately reported by the remaining seven studies (Cattran 1977; Gaughan 1997; Kim 1999a; Koronis 2000; Navarro 2002; Sheashaa 2005aSolomon 1988b).
Blinding
Aramwit 2010 and Cattran 1977 were double‐blinded, placebo‐controlled studies; Gaughan 1997 was an open‐label study. Blinding was not reported in the remaining five studies (Kim 1999a; Koronis 2000; Navarro 2002; Sheashaa 2005a; Solomon 1988b).
Incomplete outcome data
Outcome data were reported clearly in seven studies (Aramwit 2010; Gaughan 1997; Kim 1999a; Koronis 2000; Navarro 2002; Solomon 1988b; Sheashaa 2005a), among which drop‐out rates and losses to follow‐up were lower than 10%; and hence, risk of attrition bias was assessed as low. Cattran 1977 reported a drop‐out and loss to follow‐up rate of 21.6% (8/37 participants), and was assessed at high risk of attrition bias.
Selective reporting
Investigators involved in two studies were contacted to obtain study protocols (Aramwit 2010; Gaughan 1997). Although protocols were not provided, it was clear that the published reports included all expected outcomes, and were assessed at low risk of reporting bias.
Reporting was insufficient in six studies and was assessed as unclear risk of selective reporting (Cattran 1977; Kim 1999a; Koronis 2000; Navarro 2002; Sheashaa 2005a; Solomon 1988b).
Other potential sources of bias
Participant demographic data were comparable and protocols were approved by relevant ethics committees in two studies (Aramwit 2010; Gaughan 1997). It was unclear if a protocol was completed for the study by Navarro 2002, and ethics approval was not reported. Cattran 1977 secured participants' informed consent but neither demographic data nor ethics approval were reported, and was determined to be at high risk of bias.
Participant demographic data were similar between arms in three studies (Kim 1999a; Koronis 2000; Sheashaa 2005a), but informed consent, protocols and ethics approval were not reported. Signed informed consent and demographic data were not reported in Solomon 1988b.
Effects of interventions
See: Table 1
We analysed reported data on androgens plus routine therapy versus routine therapy alone, androgens plus routine therapy versus placebo plus routine therapy, androgens versus routine therapy, and androgens versus no drug. Androgens assessed were nandrolone decanoate and oxymetholone.
Baseline participant characteristics were clearly reported in six studies (Aramwit 2010; Gaughan 1997; Kim 1999a; Koronis 2000; Navarro 2002; Sheashaa 2005a).
None of the included studies reported on death or quality of life.
Nandrolone decanoate plus rHuEPO versus rHuEPO alone
Four studies investigated nandrolone decanoate plus rHuEPO versus rHuEPO alone (Gaughan 1997; Kim 1999a; Koronis 2000; Sheashaa 2005a).
Haemoglobin and haematocrit
Sheashaa 2005a reported no significant difference in Hb (Analysis 1.1 (1 study, 32 participants): MD 0.44 g/dL, 95% CI ‐0.17 to 1.05).
1.1. Analysis.

Comparison 1 Nandrolone decanoate + rHuEPO versus rHuEPO, Outcome 1 Haemoglobin.
Compared to rHuEPO alone nandrolone decanoate plus rHuEPO significantly increased HCT (Analysis 1.2 (3 studies, 73 participants): MD 2.54%, 95% CI 0.96 to 4.12); I² = 17%)
1.2. Analysis.

Comparison 1 Nandrolone decanoate + rHuEPO versus rHuEPO, Outcome 2 Haematocrit.
Albumin and triglycerides
There was no significant difference in serum albumin at the end of treatment (Analysis 1.3 (2 studies, 51 participants): MD ‐0.09 g/dL, 95% CI ‐0.24 to 0.06; I² = 0%).
1.3. Analysis.
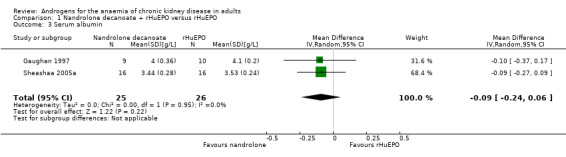
Comparison 1 Nandrolone decanoate + rHuEPO versus rHuEPO, Outcome 3 Serum albumin.
Gaughan 1997 reported no significant difference in serum triglycerides at the end of treatment (Analysis 1.4 (1 study, 19 participants): MD 34.00 mg/dL, 95% CI ‐74.70 to 42.70).
1.4. Analysis.

Comparison 1 Nandrolone decanoate + rHuEPO versus rHuEPO, Outcome 4 Triglycerides.
Adverse events
Gaughan 1997 reported no differences in acne between the groups (Analysis 1.5.1 (1 study, 19 participants): RR 3.30, 95% CI 0.15 to 72.08). Sheashaa 2005a reported no differences in menstrual disorders (Analysis 1.5.2 (1 study, 32 participants): RR 11.0, 95% CI 0.66 to 183.79) or excess body hair (Analysis 1.5.3 (1 study, 32 participants): RR 11.0, 95% CI 0.66 to 183.79) between the groups.
1.5. Analysis.

Comparison 1 Nandrolone decanoate + rHuEPO versus rHuEPO, Outcome 5 Adverse events.
Oxymetholone plus alpha rHuEPO versus placebo plus alpha rHuEPO
Aramwit 2010 investigated oxymetholone plus alpha rHuEPO versus placebo plus alpha rHuEPO.
Haemoglobin and haematocrit
Aramwit 2010 reported oxymetholone plus rHuEPO significantly increased Hb when compared to rHuEPO alone (Analysis 2.1 (1 study, 24 participants): MD 1.90 g/dL, 95% CI 1.66 to 2.14) and HCT (Analysis 2.2 (1 study, 24 participants): MD 27.10%, 95% CI 26.49 to 27.71).
2.1. Analysis.

Comparison 2 Oxymetholone + rHuEPO versus placebo + rHuEPO, Outcome 1 Haemoglobin g/dL.
2.2. Analysis.

Comparison 2 Oxymetholone + rHuEPO versus placebo + rHuEPO, Outcome 2 Haematocrit.
Liver and kidney function
Aramwit 2010 reported oxymetholone plus rHuEPO significantly decreased ALT (Analysis 2.3.1 (1 study, 24 participants): MD 54.50 U/L, 95% CI 43.94 to 65.06 and AST levels (Analysis 2.3.2 (1 study, 24 participants): MD 47.33 U/L, 95% CI 37.69 to 56.97). No improvement in kidney function was apparent with either intervention measured in terms of BUN (Analysis 2.4.1 (1 study, 24 participants): MD ‐0.94 mmol/L, 95% CI ‐4.02 to 2.14) or SCr (Analysis 2.4.2 (1 study, 24 participants): MD 261.60 µmol/L, 95% CI ‐37.76 to 560.96).
2.3. Analysis.

Comparison 2 Oxymetholone + rHuEPO versus placebo + rHuEPO, Outcome 3 Liver function.
2.4. Analysis.

Comparison 2 Oxymetholone + rHuEPO versus placebo + rHuEPO, Outcome 4 Kidney function.
Lipid profile and albumin
Aramwit 2010 reported no significant differences in total cholesterol (Analysis 2.5.1 (1 study, 24 participants): MD ‐26.16 mg/dL, 95% CI ‐63.86 to 11.54), TG (Analysis 2.5.2 (1 study, 24 participants): MD ‐47.80 mg/dL, 95% CI ‐117.41 to 21.81) or LDL (Analysis 2.5.4 (1 study, 24 participants): MD ‐1.11 mg/dL, 95% CI ‐31.80 to 29.58), but a significant decrease was reported for HDL (Analysis 2.5.3 (1 study, 24 participants): MD ‐15.66 mg/dL, 95% CI ‐24.84 to ‐6.48)
2.5. Analysis.

Comparison 2 Oxymetholone + rHuEPO versus placebo + rHuEPO, Outcome 5 Lipid profile.
A significant increase in change in serum albumin was reported for oxymetholone plus rHuEPO (Analysis 2.6 (1 study, 24 participants): MD 4.91 g/L, 95% CI 3.69 to 6.13)
2.6. Analysis.

Comparison 2 Oxymetholone + rHuEPO versus placebo + rHuEPO, Outcome 6 Change in serum albumin.
Adverse events
Aramwit 2010 reported no significant differences in adverse effects (Analysis 2.7 (1 study, 24 participants): RR 1.18, 95% CI 0.08 to 16.78).
2.7. Analysis.
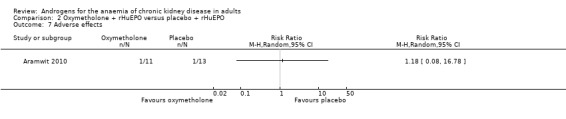
Comparison 2 Oxymetholone + rHuEPO versus placebo + rHuEPO, Outcome 7 Adverse effects.
Nandrolone decanoate versus rHuEPO
Navarro 2002 investigated nandrolone decanoate versus rHuEPO.
Haemoglobin and haematocrit
Navarro 2002 reported no significant change in Hb (Analysis 3.1 (1 study, 27 participants): MD 0.10 g/dL, 95% CI ‐0.28 to 0.48) or HCT (Analysis 3.2 (1 study, 27 participants): MD 0.40%, 95% CI ‐0.77 to 1.57).
3.1. Analysis.

Comparison 3 Nandrolone decanoate versus EPO, Outcome 1 Haemoglobin.
3.2. Analysis.

Comparison 3 Nandrolone decanoate versus EPO, Outcome 2 Haematocrit.
Total protein, pre‐albumin, transferrin and albumin
Navarro 2002 reported nandrolone decanoate significantly increased total protein (Analysis 3.3 (1 study, 27 participants): MD 0.40 g/L, 95% CI 0.13 to 0.67), transferrin (Analysis 3.5 (1 study, 27 participants): MD 45.00 mg/dL, 95% CI 12.61 to 77.39) and albumin (Analysis 3.6 (1 study, 27 participants): MD 0.20/L, 95% CI 0.01 to 0.39); but not prealbumin (Analysis 3.4 (1 study, 27 participants): MD 1.00 mg/dL, 95% CI ‐3.53 to 5.53).
3.3. Analysis.
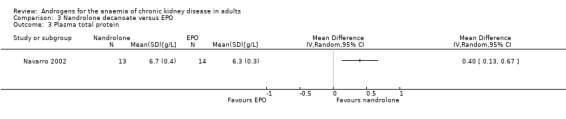
Comparison 3 Nandrolone decanoate versus EPO, Outcome 3 Plasma total protein.
3.5. Analysis.

Comparison 3 Nandrolone decanoate versus EPO, Outcome 5 Transferrin.
3.6. Analysis.
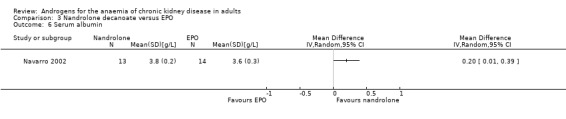
Comparison 3 Nandrolone decanoate versus EPO, Outcome 6 Serum albumin.
3.4. Analysis.

Comparison 3 Nandrolone decanoate versus EPO, Outcome 4 Prealbumin.
Kidney function
Navarro 2002 reported nandrolone decanoate did not significantly decrease BUN (Analysis 3.7.1 (1 study, 27 participants): MD ‐9.00 mg/dL, 95% Cl ‐18.13 to 0.13) or SCr (Analysis 3.7.2 (1 study, 27 participants): MD 0.80 mg/dL, 95% Cl ‐0.88 to 2.48).
3.7. Analysis.

Comparison 3 Nandrolone decanoate versus EPO, Outcome 7 Kidney function.
Adverse events
Navarro 2002 reported that one participant in the nandrolone decanoate group developed peritonitis, and one participant in the rHuEPO group experienced exacerbation of CKD‐related anaemia. There was no statistical difference in adverse effects between groups (Analysis 3.8 (1 study, 27 participants): RR 1.08, 95% CI 0.07 to 15.50).
3.8. Analysis.

Comparison 3 Nandrolone decanoate versus EPO, Outcome 8 Adverse effects.
Nandrolone decanoate versus no therapy
Cattran 1977 and Solomon 1988b investigated nandrolone decanoate versus no drug. Cattran 1977 investigated the effects of nandrolone decanoate versus no drug therapy among remnant kidney patients (15/9 intervention/control) and anephric participants (3/2 intervention/control).
Haemoglobin and haematocrit
Nandrolone decanoate significantly increased Hb (Analysis 4.1 (2 studies, 33 participants): MD 1.04 g/dL, 95% CI 0.66 to 1.41; I² = 0%). Cattran 1977 reported nandrolone decanoate significantly increased HCT (Analysis 4.2 (1 study, 24 participants): MD 3.70%, 95% CI 0.68 to 6.72). Cattran 1977 also reported the effects in anephric participants and reported a statistically significant increase in Hb with nandrolone decanoate (Analysis 5.1 (1 study, 5 participants): MD 1.30 g/dL, 95% CI 0.57 to 2.03), but no significant difference in HCT (Analysis 5.2 (1 study, 5 participants): MD 2.00%, 95% CI ‐0.85 to 4.85).
4.1. Analysis.
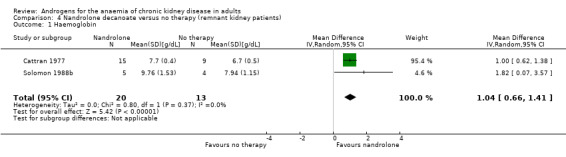
Comparison 4 Nandrolone decanoate versus no therapy (remnant kidney patients), Outcome 1 Haemoglobin.
4.2. Analysis.

Comparison 4 Nandrolone decanoate versus no therapy (remnant kidney patients), Outcome 2 Haematocrit.
5.1. Analysis.

Comparison 5 Nandrolone decanoate versus no therapy (anephric patients), Outcome 1 Haemoglobin.
5.2. Analysis.

Comparison 5 Nandrolone decanoate versus no therapy (anephric patients), Outcome 2 Haematocrit.
Mortality, quality of life and adverse events were not reported.
Discussion
Summary of main results
We found limited evidence to suggest that androgens may confer positive effects to increase Hb, HCT and serum albumin.
Studies by Kim 1999a, Koronis 2000 and Sheashaa 2005a comparing nandrolone decanoate plus rHuEPO with rHuEPO alone reported increased HCT levels. Sheashaa 2005a reported no significant increase in Hb. There was insufficient evidence to discern if nandrolone decanoate plus rHuEPO increased serum albumin and decreased rates of adverse events.
Findings reported by Aramwit 2010 in a comparison of oxymetholone plus rHuEPO with placebo plus rHuEPO indicated significant increases in Hb, HCT, serum albumin, AST and ALT; and a significant decrease in HDL. There was insufficient evidence to discern if oxymetholone plus rHuEPO decreased BUN, SCr, cholesterol, TG, LDL and adverse events.
In a comparative analysis of nandrolone decanoate with rHuEPO, Navarro 2002 reported a statistically significant increase in total protein, transferrin, and serum albumin. BUN was decreased but not significantly. There was insufficient evidence to assess if nandrolone decanoate had an effect in increasing Hb, HCT, prealbumin, and decreasing SCr and adverse events.
Cattran 1977 and Solomon 1988b, comparing nandrolone decanoate with no therapy, reported increased Hb levels. In a subgroup analysis of participants with remnant kidney status. Cattran 1977 reported that compared with no therapy, nandrolone decanoate significantly increased HCT levels. In a further subgroup analysis in anephric participants, a significant increase in Hb was reported, but there was no change in HCT. Evidence was insufficient to demonstrate if nandrolone decanoate increased HCT in anephric patients.
There was limited evidence to indicate that oxymetholone may increase Hb, HCT, plasma albumin, AST, and ALT; and decrease HDL, cholesterol, triglyceride, and LDL. Evidence was insufficient to determine if oxymetholone had an effect in decreasing BUN, SCr, and LDL or in increasing plasma total protein, prealbumin, and transferrin. There was no evidence to demonstrate that androgens decreased BUN or SCr.
Nandrolone decanoate may have an effect in increasing Hb, HCT, plasma total protein, plasma albumin, and transferrin, but there was is insufficient evidence to demonstrate increases prealbumin or decrease in BUN, SCr, AST, ALT, cholesterol, triglyceride, HDL, or to decrease LDL. Reporting was suboptimal, and it remains unclear whether oxymetholone and nandrolone decanoate conferred significant adverse effects among adults with CKD‐related anaemia.
We were unable to determine if:
dose‐effect relationships exist in relation to androgens and CKD‐related anaemia in adults;
time‐effect relationships exist in relation to androgens and CKD‐related anaemia in adults;
androgens‐erythropoietin relationships exist in relation to androgens and CKD‐related anaemia in adults.
Overall completeness and applicability of evidence
Based on the eight small included studies, scant evidence was identified in relation to the efficacy or safety of androgens for adults with CKD‐related anaemia. Evidence was flawed by sparseness, methodological and reporting quality in most studies. More adequately powered and robust study designs would help to clearly determine the role of androgens for adults with CKD‐related anaemia compared with other therapies. There was a marked absence of reporting of mortality and quality of life, and although there was some reporting of adverse effects, the small study cohorts may have meant that studies were underpowered to capture rates accurately. Our findings of adverse events reporting did not equate with those cited in the KDOQI guidelines (NKF 2006).
Quality of the evidence
Evidence quality and reporting were suboptimal (Table 1). Results were not robust, studies inadequately powered, and methodological processes were absent or flawed in many instances. Randomisation was claimed, but methods not reported in six included studies (Cattran 1977; Gaughan 1997; Kim 1999a; Koronis 2000; Sheashaa 2005a; Solomon 1988b). Similarly, allocation concealment was mentioned, but detailed description was not included in those six studies.
Blinding procedures were unclear in five studies (Kim 1999a; Koronis 2000; Navarro 2002; Sheashaa 2005a; Solomon 1988b) and reported as open‐label in Gaughan 1997.
Consequently, there was a high risk of selection, performance and detection biases. Our efforts to clarify and expand on reported data by contacting study authors did not obtain significant or sufficient additional data to enhance our assessments.
Potential biases in the review process
We assessed that six of the eight included studies were of suboptimal methodological quality, although it is possible that these deficits may relate wholly or partly to lack of adequate reporting. Nevertheless, there was significant likelihood of presence of selection, performance, and detection biases in the evidence.
Agreements and disagreements with other studies or reviews
Adamu 2012 conducted a review that compared androgen therapy and EPO use for the treatment of people with CKD‐related anaemia while focusing on implications for developing countries. Similar methodological approaches were applied in both reviews, and both looked at nandrolone decanoate versus rHuEPO and measures of anaemia correction such as haemoglobin, total protein and liver function.
There were differences in study inclusion criteria: we excluded reviews fewer than six months duration, which were eligible for inclusion by Adamu 2012; we included eight studies (181 participants) and Adamu 2012 included four studies (114 participants). Only one study was common to both reviews (Navarro 2002). Interventions investigated also differed: we investigated androgens versus EPO; androgens versus no drug or placebo alone; androgens plus EPO versus EPO; androgens plus EPO versus placebo plus EPO. Adamu 2012 considered nandrolone versus EPO and nandrolone plus EPO versus EPO. There were also differences in outcome measures. We sought kidney function measures (BUN and SCr values), lipid profiles (TG, HDL, LDL values), albumin, prealbumin, and transferrin values; while Adamu 2012 sought only blood pressure as an outcome measure.
Although there were some similarities in reported results, there were also differences. Our finding based on limited evidence suggesting that nandrolone decanoate may increase Hb and HCT was not reported by Adamu 2012, who found that total protein and blood pressure were increased. However, both reviews found that there was significant change in total protein increase associated with nandrolone decanoate versus rHuEPO; there was no change in liver function.
Authors' conclusions
Implications for practice.
Oxymetholone may help to correct anaemia and increase albumin among adults with CKD‐related anaemia by increasing ALT and AST, and decreasing HDL levels. However, evidence quality was poor, and we were unable to confirm if oxymetholone could reduce BUN, SCr, cholesterol, or TG. Furthermore, it could not be established if oxymetholone increased plasma total protein, prealbumin, or transferrin. Limited evidence suggested that nandrolone decanoate may increase HCT, plasma total protein, plasma albumin, and transferrin.
There was no compelling evidence to indicate if nandrolone decanoate increased Hb or prealbumin, or decreased BUN, SCr, AST, ALT, cholesterol, TG, HDL, adverse events, or LDL.
Suboptimal study quality meant that evidence to support the use of androgens in adults with anaemia of CKD was not identified.
Implications for research.
Large, multicentre RCTs investigating androgens for anaemia of CKD in adults are required. Future investigators should ensure that people at all CKD stages are recruited, and assessment of a comprehensive range of targeted androgen therapies is undertaken. Triallists should plan measure changes in symptom relief, quality of life, and rate of CKD progression to end‐stage kidney disease.
Reporting of mortality, quality of life and adverse events outcomes is required. Future studies should explore if occurrence of relative EPO insufficiency is a possible rationale for using androgens for adults with CKD‐related anaemia, and whether prolonged or short courses of androgen therapy is of greater benefit. The most suitable dosages of androgens should also be explored for adults living with CKD‐related anaemia.
History
Protocol first published: Issue 1, 2008 Review first published: Issue 10, 2014
| Date | Event | Description |
|---|---|---|
| 11 September 2008 | Amended | Converted to new review format. |
Acknowledgements
We would like to thank the peer referees for their comments and advice. We also acknowledge the assistance of the Cochrane Renal Group's editorial team, and Dr Margaret Anderson, of the Cochrane Developmental, Psychosocial and Learning Problems Group, for her help in retrieving articles.
Appendices
Appendix 1. Electronic search strategies
| Database | Search terms |
| CENTRAL |
|
| MEDLINE (Ovid SP) |
|
| EMBASE (Ovid SP) |
|
| Chinese databases (CBM, VIP and CNKI) |
|
Appendix 2. Risk of bias assessment tool
| Potential source of bias | Assessment criteria |
|
Random sequence generation Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence |
Low risk of bias: Random number table; computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; minimization (minimization may be implemented without a random element, and this is considered to be equivalent to being random). |
| High risk of bias: Sequence generated by odd or even date of birth; date (or day) of admission; sequence generated by hospital or clinic record number; allocation by judgement of the clinician; by preference of the participant; based on the results of a laboratory test or a series of tests; by availability of the intervention. | |
| Unclear: Insufficient information about the sequence generation process to permit judgement. | |
|
Allocation concealment Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment |
Low risk of bias: Randomisation method described that would not allow investigator/participant to know or influence intervention group before eligible participant entered in the study (e.g. central allocation, including telephone, web‐based, and pharmacy‐controlled, randomisation; sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes). |
| High risk of bias: Using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure. | |
| Unclear: Randomisation stated but no information on method used is available. | |
|
Blinding of participants and personnel Performance bias due to knowledge of the allocated interventions by participants and personnel during the study |
Low risk of bias: No blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding; blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding; blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Blinding of outcome assessment Detection bias due to knowledge of the allocated interventions by outcome assessors. |
Low risk of bias: No blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding; blinding of outcome assessment ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding; blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Incomplete outcome data Attrition bias due to amount, nature or handling of incomplete outcome data. |
Low risk of bias: No missing outcome data; reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias); missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size; missing data have been imputed using appropriate methods. |
| High risk of bias: Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; ‘as‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation; potentially inappropriate application of simple imputation. | |
| Unclear: Insufficient information to permit judgement | |
|
Selective reporting Reporting bias due to selective outcome reporting |
Low risk of bias: The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way; the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon). |
| High risk of bias: Not all of the study’s pre‐specified primary outcomes have been reported; one or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not pre‐specified; one or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; the study report fails to include results for a key outcome that would be expected to have been reported for such a study. | |
| Unclear: Insufficient information to permit judgement. | |
|
Other bias Bias due to problems not covered elsewhere in the table |
Low risk of bias: The study appears to be free of other sources of bias. |
| High risk of bias: Had a potential source of bias related to the specific study design used; stopped early due to some data‐dependent process (including a formal‐stopping rule); had extreme baseline imbalance; has been claimed to have been fraudulent; had some other problem. | |
| Unclear: Insufficient information to assess whether an important risk of bias exists; insufficient rationale or evidence that an identified problem will introduce bias. |
Data and analyses
Comparison 1. Nandrolone decanoate + rHuEPO versus rHuEPO.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Haemoglobin | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2 Haematocrit | 3 | 73 | Mean Difference (IV, Random, 95% CI) | 2.54 [0.96, 4.12] |
| 3 Serum albumin | 2 | 51 | Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.24, 0.06] |
| 4 Triglycerides | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5 Adverse events | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5.1 Acne | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Menstrual disorders | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 Hirsutism | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 2. Oxymetholone + rHuEPO versus placebo + rHuEPO.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Haemoglobin g/dL | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2 Haematocrit | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3 Liver function | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 AST | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 ALT | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Kidney function | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.1 BUN [mmol/L] | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 SCr [µmol/L] | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Lipid profile | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.1 Total cholesterol [mg/dL] | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Triglycerides [mg/dL] | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 HDL [mg/dL] | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.4 LDL [mg/dL] | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Change in serum albumin | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7 Adverse effects | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 3. Nandrolone decanoate versus EPO.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Haemoglobin | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2 Haematocrit | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3 Plasma total protein | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4 Prealbumin | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5 Transferrin | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6 Serum albumin | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7 Kidney function | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7.1 BUN [mg/dL] | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 SCr [mg/dL] | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Adverse effects | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 4. Nandrolone decanoate versus no therapy (remnant kidney patients).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Haemoglobin | 2 | 33 | Mean Difference (IV, Random, 95% CI) | 1.04 [0.66, 1.41] |
| 2 Haematocrit | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 5. Nandrolone decanoate versus no therapy (anephric patients).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Haemoglobin | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2 Haematocrit | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aramwit 2010.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation using computer‐generated random sampling table |
| Allocation concealment (selection bias) | Low risk | Not reported; however, allocation concealment was performed, because the investigators were not actively involved in the treatment of the participants and the physicians who took care of the participants were not informed of the outcome parameters |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Neither physicians nor participants had knowledge of drug allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported detail in the article. An e‐mail was sent to the author, who told us that the outcomes were assessed in a blinded manner |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | After enrolment 2 participants withdrew: one oxymetholone group participant withdrew because of gastrointestinal bleeding; a blood transfusion was administered in the fourth month of the study. One EPO group participant withdrew during the fifth month of the study due to hydrothorax |
| Selective reporting (reporting bias) | Low risk | Investigators reported that although the study protocol was available, we were unable to obtain a copy. However, it was clear that the published reports included all expected outcomes |
| Other bias | Low risk | Signed informed consent obtained from all participants. The study was approved by the Ethics Committee of the Institute Review Board at Phramongkutklao Hospital, Thailand. Overall, participant demographic data were similar in both arms |
Cattran 1977.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes | ALT, AST, BUN, SCr, cholesterol, TG, HDL, LDL, mortality, BUN, SCr, total protein, albumin, pre‐albumin, and transferrin were not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Mentioned randomisation, but not detailed |
| Allocation concealment (selection bias) | Low risk | Allocation concealment mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 8 participants withdrew after enrolment |
| Selective reporting (reporting bias) | Unclear risk | Insufficient reporting to enable assessment |
| Other bias | High risk | Signed informed consent obtained from all subjects. Participant demographic data not reported. No indication that ethics approval was sought or granted |
Gaughan 1997.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation claimed, but detail not provided |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One ND arm participant withdrew: active injection with mycobacterium tuberculosis. It was not reported that skin tuberculin tests were undertaken for study participants |
| Selective reporting (reporting bias) | Low risk | Investigators reported that although the study protocol was available, we were unable to obtain a copy. However, it was clear that the published reports included all expected outcomes |
| Other bias | Low risk | Signed informed consent was obtained from all participants. Study protocol approved by the Institutional Review Board at Thomas Jefferson University Hospital. Overall, participant demographic data were similar between groups |
Kim 1999a.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation reported, but not described |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete outcome data |
| Selective reporting (reporting bias) | Unclear risk | Investigators did not respond to a request for the protocol; Insufficient reporting to enable assessment |
| Other bias | Unclear risk | Signed informed consent not reported. Ethics Committee approval not reported. Demographic data similar between groups |
Koronis 2000.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation reported, but not detailed |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete outcome data reported |
| Selective reporting (reporting bias) | Unclear risk | Investigators did not respond to a request for the protocol; Insufficient reporting to enable assessment |
| Other bias | Unclear risk | Signed informed consent not reported. Demographic data not reported. Ethics Committee approval not reported |
Navarro 2002.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised using a computer‐generated random sampling table |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated clearly |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated clearly |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 participants withdrew (1 each from ND and EPO groups): who respectively developed peritonitis and kidney transplant |
| Selective reporting (reporting bias) | Unclear risk | Insufficient reporting to enable assessment |
| Other bias | Low risk | All participants signed informed consent. Overall, participant demographic data between arms were similar |
Sheashaa 2005a.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for in analysis |
| Selective reporting (reporting bias) | Unclear risk | Insufficient reporting to enable assessment |
| Other bias | Unclear risk | Signed informed consent not reported. Ethics Committee approval not reported. Participant demographic data similar between groups |
Solomon 1988b.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation reported, but not detailed |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not clearly stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not clearly stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete outcome data reported |
| Selective reporting (reporting bias) | Unclear risk | Insufficient reporting to enable assessment |
| Other bias | Unclear risk | Signed informed consent not reported. Demographic data not reported. The study was approved by the Human Studies Subcommittee of the Veterans' Administration Medical Center of West Haven |
ALT ‐ alanine aminotransferase; AST ‐ aspartate aminotransferase; BUN ‐ blood urea nitrogen; CAPD ‐ continuous ambulatory peritoneal dialysis; Hb ‐ haemoglobin; HCT ‐ haematocrit; HD ‐ haemodialysis; HDL ‐ high‐density lipoprotein; EPO ‐ erythropoietin; IM ‐ intramuscular; LDL low‐density lipoprotein; ND ‐ nandrolone decanoate; rHuEPO ‐ recombinant human erythropoietin; SC ‐ subcutaneously; SCr ‐ serum creatinine; TG ‐ triglyceride
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aggarwal 2005 | Study duration less than six months |
| Aslanhan 2001 | Study duration less than six months |
| Ballal 1991 | Study duration less than six months |
| Berns 1992 | Study duration less than six months |
| Brockenbrough 2006 | RCT. Reported withdrawals/losses in the text were not consistent with table 1 and table 2 data |
| Buchwald 1977 | Not RCT |
| Diez 1997 | Not RCT; wrong intervention |
| Eiam‐Ong 2007 | Study duration less than six months |
| Gascon 1999 | Not RCT |
| Hendler 1974 | Study duration less than six months |
| Lee 2002 | Not RCT |
| Logan 2005 | Not RCT |
| Mirahmadi 1979 | Study duration less than six months; wrong intervention |
| Mora 2001 | Not RCT |
| Naik 1978 | Could not separate treatment group and control group data in the report |
| Neff 1981 | Unclear if participants received androgen treatment in the six months before entry into the study |
| Saxena 1997 | Study duration less than six months |
| Solomon 1987 | Not RCT |
| von Hartitzsch 1976 | Not RCT |
| Williams 1974 | Study duration less than six months |
RCT ‐ randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
Ganguli 2003.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Insufficient information to determine relevance to this review |
Ota 1986.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Insufficient information to determine relevance to this review |
Suzuki 1986.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Unable to obtain a copy of this paper |
Differences between protocol and review
In addition to the bibliographic databases listed in the protocol for this review, we also searched the Chinese Biomedicine Database (CBM). This search did not identify any records for assessment.
Contributions of authors
Study selection – Qianchun Yang, Minawaer Abudou
Extraction of data from studies ‐ Qianchun Yang, Taixiang Wu
Quality assessment – Qianchun Yang, Taixiang Wu and Xisheng Xie
Enter data into RevMan – Qianchun Yang, Xisheng Xie
Carried out the analysis – Qianchun Yang, Minawaer Abudou and Taixiang Wu
Interpreted the analysis – Qianchun Yang, Taixiang Wu and Xisheng Xie
Drafted the final review – Qianchun Yang, Minawaer Abudou, Taixiang Wu and Xisheng Xie
Disagreement resolution – Taixiang Wu
Sources of support
Internal sources
The Second Clinical College, Guangzhou University of Chinese Medicine, China.
External sources
Cochrane Renal Group, Australia.
Declarations of interest
None known.
New
References
References to studies included in this review
Aramwit 2010 {published data only}
- Aramwit P, Palapinyo S, Wiwatniwong S, Supasyndh O. The efficacy of oxymetholone in combination with erythropoietin on hematologic parameters and muscle mass in CAPD patients. International Journal of Clinical Pharmacology & Therapeutics 2010;48(12):803‐13. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Supasyndh O, Wiwatniwong S, Aramwit P, Ruangkanchanasetr P, Choovichian P. Short course oral androgenic steroid improved lean body mass in continuous ambulatory peritoneal dialysis patients [abstract no: F‐PO580]. Journal of the American Society of Nephrology 2005;16:461A. [Google Scholar]
- Wiwatniwong S, Supasyndh O, Aramwit P, Ruangkanchanasetr P, Luesuthiviboon L. Oral androgenic steroid therapy for anemia in continuous ambulatory peritoneal dialysis patients [abstract no: F‐PO599]. Journal of the American Society of Nephrology 2005;16:465A. [Google Scholar]
Cattran 1977 {published data only}
- Cattran DC, Fenton SS, Wilson DR, Oreopoulos D, Shimizu A, Richardson RM. A controlled trial of nandrolone decanoate in the treatment of uremic anemia. Kidney International 1977;12(6):430‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gaughan 1997 {published data only}
- Gaughan WJ, Liss KA, Dunn SR, Mangold AM, Buhsmer JP, Michael B, et al. A 6‐month study of low‐dose recombinant human erythropoietin alone and in combination with androgens for the treatment of anemia in chronic hemodialysis patients. American Journal of Kidney Diseases 1997;30(4):495‐500. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Liss KA, Gaughan WJ, Dunn SR, Michael B, Goldman JM, Armenti VT, et al. A six month study of low‐dose recombinant human erythropoietin alone and in combination with androgen for the treatment of anemia in chronic hemodialysis patients [abstract no: A1206]. Journal of the American Society of Nephrology 1996;7(9):1490. [CENTRAL: CN‐00626123] [DOI] [PubMed] [Google Scholar]
Kim 1999a {published data only}
- Kim HY, Earm JH, Seo JC. Effects of low‐dose nandrolone decanoate on anemia and nutritional parameters in chronic hemodialysis patients with low‐dose subcutaneous erythropoietin [abstract no: A1452]. Journal of the American Society of Nephrology 1999;10(Program & Abstracts):287A. [Google Scholar]
Koronis 2000 {published data only}
- Koronis C, Makris F, Stavroulaki E, Lambropoulou A, Orthopoulos V. Combination of low‐dose recombinant human erythropoietin with androgens for the treatment of anaemia in hemodialysis patients [abstract]. 37th Congress European Renal Association. European Dialysis and Transplantation Association; 2000 Sept 17‐20; Nice, France. 2000:235. [CENTRAL: CN‐00461096]
Navarro 2002 {published data only}
- Navarro JF, Mora C, Macia M, Chahin J, Gallego E, Mendez ML, et al. Effects of androgen therapy on hematologic and nutritional parameters in elderly peritoneal dialysis patients [abstract]. International Urology & Nephrology 2001;33(4):715‐6. [CENTRAL: CN‐00615848] [Google Scholar]
- Navarro JF, Mora C, Macia ML, Gallego E, Chahin J, Mendez ML, et al. Prospective comparison between rHuEPO and androgens in CAPD patients: impact on hematologic and nutritional parameters [abstract]. Journal of the American Society of Nephrology 2001;12(Program & Abstracts):436A. [CENTRAL: CN‐00644231] [Google Scholar]
- Navarro JF, Mora F, Macia M, Garcia J. Randomized prospective comparison between erythropoietin and androgens in CAPD patients. Kidney International 2002;61(4):1537‐44. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sheashaa 2005a {published data only}
- Sheashaa H, Abdel‐Razek W, El‐Husseini A, Selim A, Hassan N, Abbas T, et al. Use of nandrolone decanoate as an adjuvant for erythropoietin dose reduction in treating anemia in patients on hemodialysis. Nephron 2005;99(4):c102–6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Solomon 1988b {published data only}
- Hendler ED, Solomon LR. Prospective controlled study of androgen effects on red cell oxygen transport and work capacity in chronic hemodialysis patients. Acta Haematologica 1990;83(1):1‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Solomon LR, Hendler ED. Prospective controlled study of androgen therapy in the anemia of chronic renal disease: effects on iron kinetics. Acta Haematologica 1988;79(1):12‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Aggarwal 2005 {published data only}
- Aggarwal HK, Sehgal R, Singh S, Nand N, Bharti K, Chakrabarti D. Evaluation of efficacy of low dose recombinant human erythropoietin in combination with androgen therapy in anaemia of chronic renal failure. Journal, Indian Academy of Clinical Medicine 2005;6(3):208‐15. [EMBASE: 2005526349] [Google Scholar]
Aslanhan 2001 {published data only}
- Aslanhan I, Ersoy A, Sarandol E, Demiray M, Usta M, Dalkilic E, et al. The comparison of the effects of rHuEPO and testosterone ester compounds on lipid peroxidation in male hemodialysis patients [abstract]. 38th Congress. European Renal Association. European Dialysis and Transplantation Association; 2001 Jun 24‐27; Vienna, Austria. 2001:212. [CENTRAL: CN‐00636137]
- Aslanhan I, Ersoy A, Usta M, Ersoy C, Yavuz M, Gullulu M, et al. The comparison of the effects of erythropoietin and testosterone ester compounds on lipid and lipoprotein profile in the male hemodialysis patients [abstract]. Nephrology Dialysis Transplantation 2001;16(6):A138. [CENTRAL: CN‐00444248] [Google Scholar]
Ballal 1991 {published data only}
- Ballal SH, Domoto DT, Polack DC, Marciulonis P, Martin KJ. Androgens potentiate the effects of erythropoietin in the treatment of anemia of end‐stage renal disease. American Journal of Kidney Diseases 1991;17(1):29‐33. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Berns 1992 {published data only}
- Berns JS, Rudnick MR, Cohen RM. A controlled trial of recombinant human erythropoietin and nandrolone decanoate in the treatment of anemia in patients on chronic hemodialysis. Clinical Nephrology 1992;37(5):264‐7. [MEDLINE: ] [PubMed] [Google Scholar]
Brockenbrough 2006 {published data only}
- Brockenbrough AT, Dittrich MO, Page ST, Smith T, Stivelman JC, Bremner WJ. Transdermal androgen therapy to augment EPO in the treatment of anemia of chronic renal disease. American Journal of Kidney Diseases 2006;47(2):251‐62. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Buchwald 1977 {published data only}
- Buchwald D, Argyres S, Easterling RE, Oelshlegel FJ, Brewer GJ, Schoomaker EB, et al. Effect of nandrolone decanoate on the anemia of chronic hemodialysis patients. Nephron 1977;18(4):232‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Diez 1997 {published data only}
- Diez JJ, Iglesias P, Bajo MA, Alvaro FD, Selgas R. Effects of erythropoietin on gonadotropin responses to gonadotropin‐releasing hormone in uremic patients. Nephron 1997;77(2):169‐75. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Eiam‐Ong 2007 {published data only}
- Eiam‐Ong S, Buranaosot S, Eiam‐Ong S, Wathanavaha A, Pansin P. Nutritional effect of nandrolone decanoate in predialysis patients with chronic kidney disease. Journal of Renal Nutrition 2007;17(3):173‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gascon 1999 {published data only}
- Gascon A, Belvis JJ, Berisa F, Iglesias E, Estopinan V, Teruel JL. Nandrolone decanoate is a good alternative for the treatment of anemia in elderly male patients on hemodialysis. Geriatric Nephrology & Urology 1999;9(2):67‐72. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hendler 1974 {published data only}
- Hendler ED, Goffinet JA, Ross S, Longnecker RE, Bakovic V. Controlled study of androgen therapy in anemia of patients on maintenance hemodialysis. New England Journal of Medicine 1974;291(20):1046‐51. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lee 2002 {published data only}
- Lee MS, Ahn SH, Song JH. Effects of adjuvant androgen on anemia and nutritional parameters in chronic hemodialysis patients using low‐dose recombinant human erythropoietin. Korean Journal of Internal Medicine 2002;17(3):167‐73. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Logan 2005 {published data only}
- Logan JL, Lien YH, Silva S, DeLong M, Yong K. Six months of testosterone replacement improves musculoskeletal health but not mental health or anemia management in men on hemodialysis [abstract no: F‐PO690]. Journal of the American Society of Nephrology 2005;16:485A. [Google Scholar]
Mirahmadi 1979 {published data only}
- Mirahmadi MK, Vaziri ND, Gorman JT. Controlled evaluation of hemodialysis patients on nandrolone decanoate (ND) vs testosterone enanthate (TE) (Androgens and dialysis patient). Transactions ‐ American Society for Artificial Internal Organs 1979;25:449‐54. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mora 2001 {published data only}
- Mora C, Macía ML, García J, Navarro JF. Effect of nandrolone decanoate on the lipid profile of male peritoneal dialysis patients. Peritoneal Dialysis International 2001;21(6):611‐14. [MEDLINE: ] [PubMed] [Google Scholar]
Naik 1978 {published data only}
- Naik RB, Gibbons AR, Gyde OH, Harris BR, Robinson BH. Androgen trial in renal anaemia. Proceedings of the European Dialysis & Transplant Association 1978;15:136‐43. [MEDLINE: ] [PubMed] [Google Scholar]
Neff 1981 {published data only}
- Neff MS, Goldberg J, Slifkin RF, Eiser AR, Calamia V, Kaplan M, et al. A comparison of androgens for anemia in patients on hemodialysis. New England Journal of Medicine 1981;304(15):871‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Saxena 1997 {published data only}
- Saxena S, Dash SC, Tiwari SC, Agarwal SK, Jain PK, Aslam J. Effect of nandrolone decanoate on response to low dose erythropoietin (EPO) in anemia of end stage renal disease (ESRD) patients on maintenance hemodialysis (MHD) [abstract]. Nephrology 1997;3(Suppl 1):S310. [CENTRAL: CN‐00461666] [Google Scholar]
Solomon 1987 {published data only}
- Solomon LR, Hendler ED. Androgen therapy in haemodialysis patients. II. Effects on red cell metabolism. British Journal of Haematology 1987;65(2):223‐30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Solomon LR, Hendler ED. Androgen therapy in haemodialysis patients: further observations on erythropoiesis and ferrokinetics. British Journal of Haematology 1988;69(3):426‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Solomon LR, Hendler ED. Androgen therapy in hemodialysis patients: effects on red cell metabolism and exercise tolerance [abstract: 10]. Blood 1986;68(5 Suppl 1):58a. [CENTRAL: CN‐00485953] [Google Scholar]
von Hartitzsch 1976 {published data only}
- Hartitzsch B, Kerr DN. Response to parenteral iron with and without androgen therapy in patients undergoing regular haemodialysis. Nephron 1976;17(6):430‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Williams 1974 {published data only}
- Williams JS, Stein JH, Ferris TF. Nandrolone decanoate therapy for patients receiving hemodialysis. A controlled study. Archives of Internal Medicine 1974;134(2):289‐92. [MEDLINE: ] [PubMed] [Google Scholar]
References to studies awaiting assessment
Ganguli 2003 {published data only}
- Ganguli A, Singh NP, Ahuja N. A comparative study of nandrolone decanoate and erythropoietin on albumin levels, quality of life, and progression of renal disease in Indian predialysis CKD patients [abstract no: W455]. Nephrology Dialysis Transplantation 2003;18(Suppl 4):692. [CENTRAL: CN‐00653811] [Google Scholar]
- Ganguli A, Singh NP, Singh T, Agarwal SK, Neeraj A. Nandrolone decanoate is equiefacious to erythropoietin in correcting anemia and quality of life in predialysis chronic kidney disease patients [abstract no: 137]. Journal of the Association of Physicians of India 2003;51(Dec):1188. [CENTRAL: CN‐00765715] [Google Scholar]
- Singh NP, Anirban G, Singh T, Agarwal SK, Neera A. Long term effects of anemia correction on progression of renal disease and cognitive function using erythropoietin and androgenic steroids [abstract no: 136]. Journal of the Association of Physicians of India 2003;51(Dec):1188. [CENTRAL: CN‐00783567] [Google Scholar]
- Singh NP, Ganguli A, Singh T. A comparative study of nandrolone decanoate versus recombinant human erythropoietin on anemia in Indian predialysis chronic kidney disease patients [abstract no: W456]. Nephrology Dialysis Transplantation 2003;18(Suppl 4):692‐3. [CENTRAL: CN‐00447760] [Google Scholar]
Ota 1986 {published data only}
- Ota K, Koshikawa S, Mimura N, Mizoguchi H, Katataoka K, Kon T, et al. Effects of Org DD‐100 (Nandrolone decanoate) on renal anemia: a multi‐center double‐blind study. Rinsho Hyoka 1986;14:827‐57. [CENTRAL: CN‐00320731] [Google Scholar]
- Ota K, Mimura N, Koshikawa S, Mizoguchi H. High dose nandrolone decanoate therapy on the anemia of haemodialysis patients [abstract]. Kidney International 1985;28(2):349. [Google Scholar]
Suzuki 1986 {published data only}
- Suzuki T. Therapeutic efficacy of nandrolone decanoate for anemia in hemodialysis patients: a multicenter randomized dose‐finding study comparing 3 dose regimens. Kiso to Rinsho 1986;20(4):2473‐87. [CENTRAL: CN‐00636146] [Google Scholar]
Additional references
Adamu 2012
- Adamu B, Ma'aji SM, Erwin PJ, Tleyjeh IM. Meta‐analysis of randomized controlled trials on androgens versus erythropoietin for anaemia of chronic kidney disease: Implications for developing countries. International Journal of Nephrology, 2012. http://www.hindawi.com/journals/ijn/2012/580437/ (accessed 23 August 2014). [DOI: 10.1155/2012/580437] [DOI] [PMC free article] [PubMed]
BOE 2007
- Britannica Online Encyclopaedia. Androgens. http://www.britannica.com/eb/article‐292/androgen (accessed 23 August 2014).
Brian 2005
- Leyland‐Jones B, Semiglazov V, Pawlicki M, Pienkowski T, Tjulandin S, Manikhas G, et al. Maintaining normal hemoglobin levels with epoetin alfa in mainly nonanemic patients with metastatic breast cancer receiving first‐line chemotherapy: a survival study. Journal of Clinical Oncology 2005;23(25):5960‐72. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cody 2005
- Cody J, Daly C, Campbell M, Donaldson C, Khan I, Rabindranath K, et al. Recombinant human erythropoietin for chronic renal failure in pre‐dialysis patients. Cochrane Database of Systematic Reviews 2005, Issue 3. [DOI: 10.1002/14651858.CD003266.pub2] [DOI] [PubMed] [Google Scholar]
Combe 2001
- Combe C, Pisoni RL, Port FK, Young EW, Canaud B, Mapes DL, et al. Dialysis Outcomes and Practice Patterns Study: data on the use of central venous catheters in chronic hemodialysis [Dialysis Outcomes and Practice Patterns Study: donnees sur l'utilisation des catheters veineux centraux en hemodialyse chronique]. Nephrologie 2001;22(8):379‐84. [MEDLINE: ] [PubMed] [Google Scholar]
Efficace 2003
- Efficace F, Bottomley A, Osoba D, Gotay C, Flechtner H, D'haese S, et al. Beyond the development of health‐related quality‐of‐life (HRQOL) measures: a checklist for evaluating HRQOL outcomes in cancer clinical trials‐‐does HRQOL evaluation in prostate cancer research inform clinical decision making?. Journal of Clinical Oncology 2003;21(18):3502‐11. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Efficace 2006
- Efficace F, Horneber M, Lejeune S, Dam F, Leering S, Rottmann M, et al. Methodological quality of patient‐reported outcome research was low in complementary and alternative medicine in oncology. Journal of Clinical Epidemiology 2006;59(12):1257‐65. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Efficace 2007
- Efficace F, Osoba D, Gotay C, Sprangers M, Coens C, Bottomley A. Has the quality of health‐related quality of life reporting in cancer clinical trials improved over time? Towards bridging the gap with clinical decision making. Annals of Oncology 2007;18(4):775‐81. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Eschbach 1989
- Eschbach JW. The anemia of chronic renal failure: pathophysiology and the effects of recombinant erythropoietin. Kidney International 1989;35(1):134‐48. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Geddes 2006
- Geddes CC, Woo YM. The European Best Practice Guidelines (EBPG) for peritoneal dialysis recommendation for minimum Kt/Vurea is not supported by current evidence. Nephrology Dialysis Transplantation 2006;21(9):2674. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Henke 2003
- Henke M, Laszig R, Rube C, Schafer U, Haase KD, Schilcher B, et al. Erythropoietin to treat head and neck cancer patients with anaemia undergoing radiotherapy: randomised, double‐blind, placebo‐controlled trial. Lancet 2003;362(9392):1255‐60. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analysis. BMJ 2003;327(7174):227‐60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hsu 2002
- Hsu CY, McCulloch CE, Curhan GC. Epidemiology of anemia associated with chronic renal insufficiency among adults in the United States: results from the Third National Health and Nutrition Examination Survey. Journal of the American Society of Nephrology 2002;13(2):504‐10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lin 1985
- Lin FK, Suggs S, Lin CH, Browne JK, Smalling R, Egrie JC, et al. Cloning and expression of the human erythropoietin gene. Proceedings of the National Academy of Sciences of the United States of America 1985;82(22):7580‐4. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Locatelli 2004a
- Locatelli F, Aljama P, Barany P, Canaud B, Carrera F, Eckardt KU, et al. Revised European best practice guidelines for the management of anaemia in patients with chronic renal failure. Nephrology Dialysis Transplantation 2004;19 Suppl 2:ii1‐47. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Locatelli 2004b
- Locatelli F, Pisoni RL, Combe C, Bommer J, Andreucci VE, Piera L, et al. Anaemia in haemodialysis patients of five European countries: association with morbidity and mortality in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Nephrology Dialysis Transplantation 2004;19(1):121‐32. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
McClellan 2004
- McClellan W, Aronoff SL, Bolton WK, Hood S, Lorber DL, Tang KL, et al. The prevalence of anemia in patients with chronic kidney disease. Current Medical Research & Opinion 2004;20(9):1501‐10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Navarro 2001a
- Navarro JF, Mora C. Androgen therapy for anemia in elderly uremic patients. International Urology & Nephrology 2001;32(4):549‐57. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Navarro 2001b
- Navarro JF, Mora C. Effect of androgens on anemia and malnutrition in renal failure: implications for patients on peritoneal dialysis. Peritoneal Dialysis International 2001;21(1):14‐24. [MEDLINE: ] [PubMed] [Google Scholar]
Navarro 2003
- Navarro JF. In the erythropoietin era, can we forget alternative or adjunctive therapies for renal anaemia management? The androgen example. Nephrology Dialysis Transplantation 2003;18(11):2222‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
NKF 2002
- National Kidney Foundation. K/DOQI Clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. American Journal of Kidney Diseases 2002;39(2 Suppl 1):S1‐266. [MEDLINE: ] [PubMed] [Google Scholar]
NKF 2006
- National Kidney Foundation. Clinical practice guidelines and clinical practice recommendations for anemia in chronic kidney disease. http://www.kidney.org/Professionals/kdoqi/guidelines_anemia/index.htm (accessed 23 August 2014).
NKF 2007
- National Kidney Foundation. About chronic kidney disease. http://www.kidney.org/kidneydisease/aboutckd.cfm (accessed 23 August 2014).
Omwancha 2006
- Omwancha J, Brown TR. Selective androgen receptor modulators: in pursuit of tissue‐selective androgens. Current Opinion in Investigational Drugs 2006;7(10):873‐81. [MEDLINE: ] [PubMed] [Google Scholar]
Pielecka 2006
- Pielecka J, Quaynor SD, Moenter SM. Androgens increase gonadotropin‐releasing hormone neuron firing activity in females and interfere with progesterone negative feedback. Endocrinology 2006;147(3):1474‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sanchez 2006
- Sanchez de la Nieta MD, Caparros G. Rivera F. Epoetin‐induced pure red cell aplasia successfully treated with androgens. Journal of Nephrology 2006;19(2):220‐1. [MEDLINE: ] [PubMed] [Google Scholar]
Shahani 2009
- Shahani S, Braga‐Basaria M, Maggio M, Basaria S. Androgens and erythropoiesis: past and present. Journal of Endocrinological Investigation 2009;32(8):704‐16. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Teruel 1996a
- Teruel JL, Marcen R, Navarro‐Antolin J, Aguilera A, Fernandez‐Juarez G, Ortuno J. Androgen versuserythropoietin for the treatment of anemia in hemodialyzed patients: a prospective study. Journal of the American Society of Nephrology 1996;7(1):140‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Teruel 1996b
- Teruel JL, Aguilera A, Marcen R, Antolin JN, Otero GG, Ortuno J. Androgen therapy for anaemia of chronic renal failure. Scandinavian Journal of Urology & Nephrology 1996;30(5):403‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
USRDS 2006
- United States Renal Data System. Atlas of end‐stage renal disease in the United States. hthttp://www.usrds.org/atlas06.aspx (accessed 23 August 2014).
Ward 1990
- Ward HJ. Implications of recombinant erythropoietin therapy for renal transplantation. American Journal of Nephrology 1990;10 Suppl 2:44‐52. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Winearls 1986
- Winearls CG, Oliver DO, Pippard MJ, Reid C, Downing MR, Cotes PM. Effect of human erythropoietin derived from recombinant DNA on the anaemia of patients maintained by chronic haemodialysis. Lancet 1986;2(8517):1175‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Yared 1997
- Yared K, Gagnon RF, Brox AG. Mechanisms of action of androgen treatment on the anemia of experimental chronic renal failure [abstract]. Journal of the American Society of Nephrology 1997;Program & Abstracts(8):633A. [Google Scholar]
References to other published versions of this review
Tang 2008
- Tang X, Gu R, Xie XS, Wu T. Androgens for the anaemia of chronic kidney disease in adults. Cochrane Database of Systematic Reviews 2008, Issue 1. [DOI: 10.1002/14651858.CD006881] [DOI] [PMC free article] [PubMed] [Google Scholar]


