Abstract
The safety, tolerability, and pharmacokinetics of an oral solution of itraconazole and its active metabolite hydroxyitraconazole were investigated in an open multicenter study of 26 infants and children aged 6 months to 12 years with documented mucosal fungal infections or at risk for the development of invasive fungal disease. The most frequent underlying illness was acute lymphoblastic leukemia, except in the patients aged 6 months to 2 years, of whom six were liver transplant recipients. The patients were treated with itraconazole at a dosage of 5 mg/kg of body weight once daily for 2 weeks. Blood samples were taken after the first dose, during treatment, and up to 8 days after the last itraconazole dose. On day 1, the mean peak concentrations in plasma after the first and last doses (Cmax) and areas under the concentration-time curve from 0 to 24 h (AUC0–24) for itraconazole and hydroxyitraconazole were lower in the children aged 6 months to 2 years than in children aged 2 to 12 years but were comparable on day 14. The mean AUC0–24-based accumulation factors of itraconazole and hydroxyitraconazole from day 1 to 14 ranged from 3.3 to 8.6 and 2.3 to 11.4, respectively. After 14 days of treatment, Cmax, AUC0–24, and the half-life, respectively, were (mean ± standard deviation) 571 ± 416 ng/ml, 6,930 ± 5,830 ng · h/ml, and 47 ± 55 h in the children aged 6 months to 2 years; 534 ± 431 ng/ml, 7,330 ± 5,420 ng · h/ml, and 30.6 ± 25.3 h in the children aged 2 to 5 years; and 631 ± 358 ng/ml, 8,770 ± 5,050 ng · h/ml, and 28.3 ± 9.6 h in the children aged 5 to 12 years. There was a tendency to have more frequent low minimum concentrations of the drugs in plasma for both itraconazole and hydroxyitraconazole for the children aged 6 months to 2 years. The oral bioavailability of the solubilizer hydroxypropyl-β-cyclodextrin was less than 1% in the majority of the patients. In conclusion, an itraconazole oral solution given at 5 mg/kg/day provides potentially therapeutic concentrations in plasma, which are, however, substantially lower than those attained in adult cancer patients, and is well tolerated and safe in infants and children.
Invasive fungal infections are a growing cause of morbidity and mortality in infants and children with hematological malignancies or undergoing liver transplantation (12, 19). These infections are most frequently caused by Candida or Aspergillus species (12, 19). Unfortunately, treatment of these invasive mycoses is complicated by problems in diagnosis (4, 19) and the limited efficacy and toxicities of available systemic antifungal agents (19). Itraconazole is an orally active triazole antifungal agent with a wide spectrum of activity and pronounced lipophilic properties (17, 18). The pharmacokinetics of itraconazole in healthy adult volunteers are characterized by good oral absorption, an extensive tissue distribution with concentrations in tissue considerably higher than in plasma, a relatively long elimination half-life (t1/2) (about 1 day), and biotransformation into a large number of metabolites (7, 8, 16). Itraconazole is thus potentially useful in the prophylaxis or treatment of invasive candidiasis or aspergillosis in immunocompromised children. However, the available capsule formulation of itraconazole may be difficult to administer to infants and children. Recently, a 10-mg/ml oral solution of itraconazole was developed, with hydroxypropyl-β-cyclodextrin as a solubilizer. With a dosage of 5 mg/kg of body weight once daily, maximum concentrations in serum of itraconazole exceeded 250 ng/ml in adult patients during autologous bone marrow transplantation (13) or during remission induction for acute myeloblastic leukemia (14). These concentrations of itraconazole in serum were judged to be suitable for antifungal prophylaxis because of data suggesting an excess of episodes of invasive pulmonary aspergillosis in patients whose maximum concentrations of the drug in serum were less than 250 ng/ml (1). However, pharmacokinetic data on itraconazole in children are limited, and questions with regard to dosage schedules in prevention and treatment of systemic fungal infections often arise. The objective of the present study was to assess the pharmacokinetics of itraconazole and its active metabolite hydroxyitraconazole during and after repeated dosing of itraconazole in oral solution, 5 mg/kg per day for 2 weeks, in infants and children requiring systemic antifungal prophylaxis or treatment.
MATERIALS AND METHODS
Patients.
Male and female children aged 6 months to 12 years with a documented mucosal fungal infection or at risk for the development of invasive fungal disease were eligible for this study. Their hospitalization was planned for at least 14 days after the first itraconazole dose. The protocol was approved by the ethics committee of each participating center, and written informed consent was obtained from the children’s legal representatives.
Patients were excluded if any of the following applied: concomitant use of other systemically absorbed antifungal drugs; treatment with rifampin, phenytoin, phenobarbital, carbamazepine, terfenadine, astemizole, warfarin, rifabutin, cisapride, or loratadine concomitantly or in the 2 weeks prior to start of the itraconazole treatment; a known sensitivity to the azole groups of antifungals; signs of hepatic dysfunction defined by liver function test results greater than three times the laboratory’s normal ranges, unless etiologically well documented in cases of liver transplantation; and participation in an investigational drug trial, except for chemotherapy and growth factor trials, within 30 days prior to the start of the trial.
Drug administration.
Patients were treated with itraconazole at dosages of 5 mg/kg once daily for 14 days, provided in an oral solution containing 10 mg of itraconazole per ml and 400 mg of hydroxypropyl-β-cyclodextrin per ml. The required volume was either pipetted directly into the mouth or given to the child to drink with water. Whenever possible, the dose was given after an overnight fast and at least 30 min before breakfast.
Pharmacokinetic and safety assessments.
Venous blood samples (2 ml) for the measurement of itraconazole and its active metabolite hydroxyitraconazole were taken through a central catheter immediately before the first dose; 2, 4, 8, and 24 h after the first dose; immediately before the dose on days 5, 8, and 11; immediately before the last dose; 2, 4, 8, and 24 h after the last dose; and 2, 3, 5, and 8 days after the last dose. Blood was collected into heparinized tubes and centrifuged for 10 min at 1,000 × g within 2 h. Plasma was stored at −20°C until required for assay. Itraconazole and hydroxyitraconazole were measured by high-performance liquid chromatography with UV detection (20). The limit of quantification was 5 ng/ml for itraconazole and 10 ng/ml for hydroxyitraconazole. The mean coefficients of variation were 3.4, 1.3, and 1.9% for itraconazole at concentrations in plasma of 15, 95, and 600 ng/ml, respectively. For hydroxyitraconazole, the coefficients of variation were 6.1, 2.8, and 3.4% at the same concentrations in plasma.
Whenever possible, a predose urine sample and the complete urinary output during the intervals 0 to 8 h and 8 to 24 h after the first and last dose of itraconazole were collected for measurement of hydroxypropyl-β-cyclodextrin. Urine volume and pH were recorded, and a 20-ml sample was stored at −20°C before analysis. Hydroxypropyl-β-cyclodextrin was measured by size exclusion chromatography with postcolumn complexation (15). The limit of detection was 1 μg/ml, and the coefficients of variation were 6.3, 4.5, 2.9, and 10.8% at concentrations of 3, 30, 150, and 300 μg/ml, respectively.
Data on adverse events were collected throughout the study. In addition, blood was collected for hematological and biochemical tests within 1 week before the first dose of itraconazole and 24 h after the last dose.
Data analyses.
Based on the plasma concentration-time curves of individual patients, the following pharmacokinetic parameters were determined for itraconazole and hydroxyitraconazole: minimal (predose) concentration in plasma (Cmin); peak concentration in plasma after the first and last dose (Cmax); time to attain Cmax (Tmax); area under the plasma concentration-time curve of a dosing interval after the first and last dose (AUC0–24), determined by trapezoidal summation (6); metabolic ratio, calculated as the AUC0–24 for hydroxyitraconazole divided by the AUC0–24 for itraconazole (ratiomet); accumulation factor, calculated as the AUC0–24 for the last dose divided by the AUC0–24 for the first dose (R); elimination rate constant after the last dose (6); and terminal t1/2 after the last dose (t1/2term). The amount of hydroxypropyl-β-cyclodextrin excreted in the urine was calculated by multiplying the concentration in urine by the volume of urine.
RESULTS
Twenty-six patients were recruited, of whom eight were between 6 months and 2 years old, seven were 2 to 5 years old, and 11 were 5 to 12 years old. During the open treatment period, three patients in the group of those aged 5 to 12 years dropped out. Two patients with acute lymphoblastic leukemia and neuroblastoma, respectively, withdrew on days 10 and 7 because of fever, which was considered a prophylactic endpoint, and were given intravenous amphotericin B. A third patient, with acute lymphoblastic leukemia, dropped out on day 6 because of lack of cooperation in taking the medication. In addition, one patient with acute lymphoblastic leukemia in the same age group discontinued taking the medication because of vomiting on day 11 only but continued to have assessments. During the run-out period, one patient in the group of those aged 6 months to 2 years dropped out due to an intercurrent event (the patient moved). The demographic characteristics and diagnoses of the patients are summarized in Table 1. The most frequent intercurrent illness was acute lymphoblastic leukemia, except in the patients aged between 6 months and 2 years, of whom six were liver transplant recipients. All patients were neutropenic, except those who underwent a liver transplantation.
TABLE 1.
Demographic characteristics, diagnoses, and intercurrent diseases
| Characteristics | Age (yr)
|
Total | ||
|---|---|---|---|---|
| 0.5–2 | 2–5 | 5–12 | ||
| Patients | 8 | 7 | 11 | 26 |
| Male, n (%) | 4 (50) | 5 (71.4) | 9 (81.8) | 18 (69.2) |
| Female, n (%) | 4 (50) | 2 (28.6) | 2 (18.2) | 8 (30.8) |
| Age, mo (mean ± SD) | 14.6 ± 2.2 | 44.1 ± 3.9 | 89.9 ± 8.2 | 54.4 ± 7.4 |
| Ht, cm (mean ± SD) | 73.8 ± 3.4 | 100.2 ± 2.7 | 119.5 ± 4.2 | 100.2 ± 4.4 |
| Wt, kg (mean ± SD) | 8.7 ± 1.0 | 15.3 ± 1.1 | 23.9 ± 2.4 | 16.9 ± 1.7 |
| Diagnosis, n (%) | ||||
| Mucosal yeast infection | 3 (37.5) | 0 (0) | 0 (0) | 3 (11.5) |
| Prophylaxis | 5 (62.5) | 7 (100) | 11 (100) | 23 (88.5) |
| Intercurrent disease, n (%) | ||||
| Acute lymphoblastic leukemia | 2 (25) | 4 (57.1) | 6 (54.5) | 12 (46.2) |
| Orthotopic liver transplantation | 6 (75) | 6 (23.1) | ||
| Acute myeloblastic leukemia | 2 (28.6) | 2 (7.7) | ||
| Acute monocytic leukemia | 1 (14.3) | 1 (3.8) | ||
| Burkitt’s lymphoma | 1 (9.1) | 1 (3.8) | ||
| Neuroblastoma | 2 (18.2) | 2 (7.7) | ||
| Relapse of lymphoma | 1 (9.1) | 1 (3.8) | ||
| Myelodysplastic syndrome | 1 (9.1) | 1 (3.8) | ||
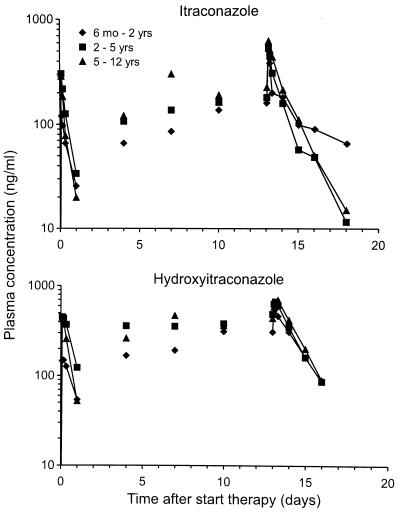
The time courses of the mean concentrations of itraconazole and hydroxyitraconazole in plasma are shown in Fig. 1. The pharmacokinetic parameters of itraconazole and hydroxyitraconazole are summarized in Table 2. On day 1, the mean Cmaxs and AUC0–24s of itraconazole and hydroxyitraconazole were lower for the group of those aged 6 months to 2 years than for the other two groups but were comparable on day 14. The same pattern was observed for the mean ratiomet. The Tmaxs were comparable in the three age groups and did not change significantly during repeated doses. The mean AUC0–24-based accumulation factor of itraconazole and hydroxyitraconazole from day 1 to 14 ranged from 3.3 to 8.6 and 2.3 to 11.4, respectively. There was a tendency to have more frequent low Cmins for both itraconazole and hydroxyitraconazole in the group of those aged 6 months to 2 years. The t1/2term values measured at steady state were comparable in the three age groups for both itraconazole and hydroxyitraconazole.
FIG. 1.
Semilogarithmic plot of the mean concentrations in plasma of itraconazole and hydroxyitraconazole as a function of time after oral administration of itraconazole in oral solution, 5 mg/kg/day, for 2 weeks to children.
TABLE 2.
Pharmacokinetic parameters of itraconazole and hydroxyitraconazolea
| Age (yr) | Itraconazole
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Day 1
|
Day 14
|
|||||||||
| Tmax (h) | Cmax (ng/ml) | AUC (ng · h/ml) | Predose concn (ng/ml) | Tmax (h) | Cmax (ng/ml) | AUC (ng · h/ml) | R | t1/2term (h) | ||
| 0.5–2 | 2.9 ± 1.1 | 138 ± 91 | 1,340 ± 780 | 159 ± 218 | 1.9 ± 0.1 | 571 ± 416 | 6,930 ± 5,830 | 6.2 ± 5.0 | 47.4 ± 55.0 | |
| 2–5 | 2.4 ± 0.8 | 314 ± 105 | 2,740 ± 1,080 | 179 ± 101 | 2.9 ± 2.5 | 534 ± 431 | 7,330 ± 5,420 | 3.3 ± 3.0 | 30.6 ± 25.3 | |
| 5–12 | 2.8 ± 1.8 | 298 ± 292 | 2,010 ± 1,580 | 223 ± 145 | 3.1 ± 2.1 | 631 ± 358 | 8,770 ± 5,050 | 8.6 ± 7.4 | 28.3 ± 9.6 | |
| 3.9 ± 2.7 | 179 ± 101 | 2,340 ± 1,490 | 1.6 ± 0.6 | 308 ± 436 | 4.4 ± 2.3 | 690 ± 445 | 13,200 ± 11,400 | 11.4 ± 16.0 | 1.7 ± 0.7 | 18.0 ± 18.1 |
| 4.1 ± 2.7 | 493 ± 106 | 6,730 ± 1,950 | 2.6 ± 0.6 | 487 ± 314 | 4.8 ± 2.7 | 687 ± 419 | 13,400 ± 9,110 | 2.3 ± 1.9 | 2.1 ± 0.7 | 17.1 ± 14.5 |
| 3.1 ± 1.8 | 447 ± 365 | 4,920 ± 4,390 | 2.4 ± 0.6 | 437 ± 246 | 10.8 ± 14.3 | 699 ± 234 | 13,450 ± 7,190 | 6.4 ± 5.6 | 1.7 ± 0.5 | 17.9 ± 8.7 |
Data are means ± standard deviations.
In the majority of patients (11 of 14), less than 1% of the hydroxypropyl-β-cyclodextrin dose was excreted in the urine on days 1 and/or 14. In the other three patients (one in the group of those aged 2 to 5 years and two in the group of those aged 5 to 12 years), the percentage of the hydroxypropyl-β-cyclodextrin dose excreted in the urine did not exceed 11.8%. Given that after intravenous administration 80 to 90% of the dose is excreted unchanged in the urine (9), the mean oral bioavailability of hydroxypropyl-β-cyclodextrin in the majority of the patients can be estimated to be less than 1%.
During the open treatment period, adverse events—mainly gastrointestinal system and general disorders—were reported for all patients, except for two in the group of those aged 6 months to 2 years. The most frequent adverse event was vomiting, which is also reported for adult cancer patients treated with itraconazole in oral solution (13, 14). During the run-out period, adverse events occurred in two patients in the group of those aged 6 months to 2 years, five occurred in the group of those aged 2 to 5 years, and six occurred in the group of those aged 5 to 12 years. Adverse events occurring in at least three patients in any group are shown in Table 3. The incidence of adverse events was higher in patients aged 5 to 12 years. No treatment-related severe or serious adverse events occurred. All patients showed abnormal laboratory data at some time during treatment but no consistent, clinically relevant changes were observed. In the group of those aged 6 months to 2 years, high values for alanine aminotransferase, aspartate aminotransferase, and gamma-glutamyl transferase were observed in three liver transplant patients but were considered normal at that stage after transplantation. Increases in these parameters were also noted in two patients with acute lymphoblastic leukemia in the group of those aged 2 to 5 years but were attributed to antineoplastic chemotherapy. Finally, a borderline increase in alanine aminotransferase in a patient with acute lymphoblastic leukemia in the group of those aged 5 to 12 years was considered to be possibly related to itraconazole.
TABLE 3.
Adverse events occurring in more than three patients in any group
| Adverse event | No of events
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Age (yr)
|
Total
|
|||||||
| 0.5–2
|
2–5
|
5–12
|
||||||
| Oa | Rb | O | R | O | R | O | R | |
| Abdominal pain | 3 | 5 | 4 | 8 | 4 | |||
| Diarrhea | 1 | 3 | 2c | 4 | 8 | 2 | ||
| Epistaxis | 1 | 3 | 1 | 3 | 2 | |||
| Fever | 1 | 3 | 1 | 4 | 2 | 7 | 4 | |
| Headache | 1 | 1 | 5 | 6 | 1 | |||
| Hypertension | 3 | 3 | 0 | |||||
| Leg pain | 1 | 3 | 4 | 0 | ||||
| Nausea | 2 | 7 | 2 | 9 | 2 | |||
| Pain | 1 | 5 | 2 | 6 | 2 | |||
| Pharyngitis | 1 | 2 | 3 | 1 | 6 | 1 | ||
| Rigors | 3 | 2 | 3 | 2 | ||||
| Vomiting | 1 | 5 | 2 | 7 | 2 | 13 | 4 | |
O, open treatment.
R, runout period.
Including Clostridium difficile and bloody diarrhea.
DISCUSSION
Itraconazole has a broader spectrum of activity than the other azoles and is the only commercially available antifungal in this class with in vitro activity against Aspergillus. Clinical efficacy of itraconazole has been shown in adult patients with candidal thrush or esophagitis (5) and inferred in patients with invasive aspergillosis (3). It is thus potentially useful in the prophylaxis and treatment of mucosal and invasive fungal infections in children. This first pharmacokinetic study of itraconazole oral solution in children was prompted by potential differences between children and adults, as illustrated by the shorter t1/2 of fluconazole in serum in children with neoplastic diseases than in adults (10).
The pharmacokinetics of the itraconazole capsule preparation have been well defined in healthy adult volunteers and immunocompromised patients. In healthy volunteers, intake of the capsules after a meal enhanced bioavailability (8). In different studies, oral dosing of 100 mg once daily for 2 weeks produced mean Cmaxs of 378 and 672 ng/ml, AUC0–24s of 5,330 and 9,416 ng · h/ml, a t1/2 of 34 h, and a Tmax of 3 h (7, 8, 16), but wide intersubject variations were observed. Steady state was attained in 10 to 14 days, and oral bioavailability was disproportionately augmented by increasing the dose. Much lower itraconazole concentrations were attained in patients with acute leukemia or autologous bone marrow transplantation than in healthy volunteers (2, 11). In one study (2), repeated doses of 200 mg of the itraconazole capsule preparation once daily for 2 weeks produced a mean Cmax of 412 ng/ml and an AUC0–24 of 6,040 ng · h/ml, compared to 1,028 ng/ml and 15,400 ng · h/ml in healthy adult volunteers (7). Capsules are thus poorly absorbed in neutropenic cancer patients at greatest risk of fungal disease. Accordingly, an oral solution of 10 mg of itraconazole per ml and 400 mg of hydroxypropyl-β-cyclodextrin per ml which improves bioavailability by as much as 30% when administered to healthy volunteers (data on file; Janssen Research Foundation) was developed. In contrast to the capsule formulation, itraconazole in oral solution does not need to be administered with food (data on file; Janssen Research Foundation), and Tmax is attained more rapidly (8). Repeated-dose pharmacokinetics of itraconazole in oral solution, 5 mg/kg daily, were essentially identical in adult autologous bone marrow transplant recipients (13) and patients receiving chemotherapy for acute myeloid leukemia (14). After 2 weeks, mean Cmaxs were 1,464 and 1,486 ng/ml, and mean AUC0–24s were 24,476 and 22,710 ng · h/ml, respectively, for these two groups of patients. Achievable concentrations in serum were thus considerably improved in adult neutropenic cancer patients receiving itraconazole in oral solution compared to capsules. In fact, itraconazole in oral solution restored the Cmaxs and AUC0–24s to values obtained with an equivalent dose (200 mg) of the capsule preparation in healthy volunteers (7) and thus corrected for reduced absorption from capsules in adult neutropenic cancer patients.
Unlike the case for adults, there is a paucity of pharmacokinetic, tolerability, and safety data on the effects of itraconazole in children. In a small pilot study, repeated dosing with itraconazole capsules, 50 mg once daily, in seven neutropenic children aged 3 to 15 years achieved a mean Cmax of 120 ng/ml after 2 weeks (8). Data from this study were difficult to interpret because itraconazole was not administered on the basis of body weight. We thus conducted the first systematic repeated-dose pharmacokinetic study of itraconazole in oral solution in infants and children. Immunocompromised patients at high risk for fungal disease were selected because they represent a substantial proportion of children who may benefit from receiving itraconazole, and the pharmacokinetics for this group of patients may differ from those for healthy children or those with other underlying diseases, as well as those for healthy or immunocompromised adult patients. In addition, itraconazole in oral solution is potentially useful in children because of difficulties in administration and adjusting dosages on a milligram-per-kilogram basis with the capsule preparation.
The results of the present study demonstrate that the pharmacokinetics of itraconazole in oral solution administered to children with neoplastic disease differ substantially from those in adults with cancer. In the children aged 2 to 12 years, all of whom had neoplastic disease, Cmaxs, Cmins, and AUC0–24s for itraconazole were only about a third of those attained for adult cancer patients (13, 14) treated with an identical dosage of 5 mg/kg daily for 14 days. However, the t1/2 of about 30 h in children was similar to that reported in adult volunteers (7) and was not shortened in children, as it is with fluconazole (10). Lower concentrations of itraconazole in plasma in children with neoplastic disease than in adults most likely resulted from decreased absorption from the gastrointestinal tract, because of either mucositis or vomiting. The relative contributions of age and underlying disease to the observed lower levels in the patients aged 2 to 12 years than in adults cannot be determined from the present study and will require pharmacokinetic data in healthy volunteers from this same age group. However, children with neoplastic disease may require higher doses on a milligram-per-kilogram basis than adults for equivalent prophylactic and therapeutic benefits. Because the pharmacokinetics of itraconazole are related nonlinearly to dose, it would be worthwhile to investigate the pharmacokinetics at higher doses in defined populations of immunocompromised children. It would be difficult to unequivocally state that the itraconazole levels obtained at 5 mg/kg daily in this study are therapeutic, since correlations between levels and outcome are sparse (1). However, since lower levels are achieved in children than in adults, severe infections would probably require higher doses. In addition, the wide interpatient variations in Cmaxs and AUC0–24s indicate that plasma itraconazole assays would be required in the treatment of invasive fungal infections. Steady state was reached by about day 11, which is similar to the result reported for adults.
In conclusion, itraconazole in oral solution at 5 mg/kg daily for 2 weeks provides potentially therapeutic levels in plasma, which, however, are substantially lower than those obtained at a similar dosage in adult cancer patients. Itraconazole in oral solution has potential in prophylaxis and treatment of mucosal and invasive fungal infections in children.
ACKNOWLEDGMENTS
This study was supported by a grant from the Janssen Research Foundation.
We gratefully acknowledge the participation of M. Schroeder, K. Groen, P. Wallemacq, P. Stoffels, R. Wiels, R. Woestenborghs, A. Daems, A. Van Peer, J. Heykants, M. Peeters, H. Joosen, and B. Banks. We also thank S. Tassé for expert secretarial assistance.
REFERENCES
- 1.Boogaerts, M. A., G. E. Verhoef, P. Zachee, H. Demuynck, L. Verbist, and K. De Beule. 1989. Antifungal prophylaxis with itraconazole in prolonged neutropenia: correlation with plasma levels. Mycoses 32(Suppl. 1):103–108. [DOI] [PubMed]
- 2.Bradford C R, Prentice A G, Warnock D W, Copplestone J A. Comparison of the multiple dose pharmacokinetics of two formulations of itraconazole during remission induction for acute myeloblastic leukemia. J Antimicrob Chemother. 1991;28:555–560. doi: 10.1093/jac/28.4.555. [DOI] [PubMed] [Google Scholar]
- 3.Denning D W, Lee J Y, Hostetler J S, Pappas P, Kauffman C A, Dewsnup D H, Galgiani J N, Graybill J R, Sugar A M, Catanzaro A, Gallis H, Perfect J R, Dockery B, Dismukes W E, Stevens D A. NIAID mycoses study group multicenter trial of oral itraconazole therapy for invasive aspergillosis. Am J Med. 1994;97:135–144. doi: 10.1016/0002-9343(94)90023-x. [DOI] [PubMed] [Google Scholar]
- 4.de Repentigny, L. 1992. Serodiagnosis of candidiasis, aspergillosis and cryptococcosis. Clin. Infect. Dis. 14(Suppl. 1):S11–S22. [DOI] [PubMed]
- 5.de Repentigny L, Ratelle J. Comparison of itraconazole and ketoconazole in HIV-positive patients with oropharyngeal or esophageal candidiasis. Chemotherapy (Basel) 1996;42:374–383. doi: 10.1159/000239469. [DOI] [PubMed] [Google Scholar]
- 6.Gibaldi M, Perrier D. Pharmacokinetics. 2nd ed. New York, N.Y: Marcel Dekker, Inc.; 1982. [Google Scholar]
- 7.Hardin T C, Graybill J R, Fetchick R, Woestenborghs R, Rinaldi M G, Kuhn J G. Pharmacokinetics of itraconazole following oral administration to normal volunteers. Antimicrob Agents Chemother. 1988;32:1310–1313. doi: 10.1128/aac.32.9.1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heykants J, Michiels M, Meuldermans W, Monbaliu J, Lavrijsen K, Van Peer A, Levron J C, Woestenborghs R, Cauwenbergh G. The pharmacokinetics of itraconazole in animals and man: an overview. In: Fromtling R A, editor. Recent trends in the discovery, development and evaluation of antifungal agents. J. R. Barcelona, Spain: Prous Science Publishers; 1987. pp. 57–83. [Google Scholar]
- 9.Jacqmin P, Van Peer A, De Beule K, Stoffels P, Heykants J. Clinical pharmacokinetics summary. Beerse, Belgium: Janssen Research Foundation; 1995. Hydroxypropyl-β-cyclodextrin pharmacokinetics and safety in man: a review of the data available until February 1995. [Google Scholar]
- 10.Lee J W, Seibel N L, Amantea M, Whitcomb P, Pizzo P A, Walsh T J. Safety and pharmacokinetics of fluconazole in children with neoplastic diseases. J Pediatr. 1992;120:987–993. doi: 10.1016/s0022-3476(05)81975-4. [DOI] [PubMed] [Google Scholar]
- 11.Persat F, Marzullo C, Guyotat D, Rochet M-J, Piens M A. Plasma itraconazole concentrations in neutropenic patients after repeated high-dose treatment. Eur J Cancer. 1992;28:838–841. doi: 10.1016/0959-8049(92)90127-n. [DOI] [PubMed] [Google Scholar]
- 12.Pizzo P A, Rubin M, Freifeld A, Walsh T J. The child with cancer and infection. II. Nonbacterial infections. J Pediatr. 1991;119:845–857. doi: 10.1016/s0022-3476(05)83032-x. [DOI] [PubMed] [Google Scholar]
- 13.Prentice A G, Warnock D W, Johnson S A N, Phillips M J, Oliver D A. Multiple dose pharmacokinetics of an oral solution of itraconazole in autologous bone marrow transplant recipients. J Antimicrob Chemother. 1994;34:247–252. doi: 10.1093/jac/34.2.247. [DOI] [PubMed] [Google Scholar]
- 14.Prentice A G, Warnock D W, Johnson S A N, Taylor P C, Oliver D A. Multiple dose pharmacokinetics of an oral solution of itraconazole in patients receiving chemotherapy for acute myeloid leukemia. J Antimicrob Chemother. 1995;36:657–663. doi: 10.1093/jac/36.4.657. [DOI] [PubMed] [Google Scholar]
- 15.Szathmary S C. Determination of hydroxypropyl-β-cyclodextrin in plasma and urine by size-exclusion chromatography with post-column complexation. J Chromatogr. 1989;487:99–105. doi: 10.1016/s0378-4347(00)83011-x. [DOI] [PubMed] [Google Scholar]
- 16.Van Cauteren, H., J. Heykants, R. De Coster, and G. Cauwenbergh. 1987. Itraconazole: pharmacologic studies in animals and humans. Rev. Infect. Dis. 9(Suppl. 1):S43–S46. [DOI] [PubMed]
- 17.Van Cutsem J, Van Gerven F, Van Der Ven M-A, Borgers M, Janssen P A J. Itraconazole, a new triazole that is orally active in aspergillosis. Antimicrob Agents Chemother. 1984;26:527–534. doi: 10.1128/aac.26.4.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Cutsem J, Van Gerven F, Janssen P A J. The in vitro and in vivo antifungal activity of itraconazole. In: Fromtling R A, editor. Recent trends in the discovery, development and evaluation of antifungal agents. J. R. Barcelona, Spain: Prous Science Publishers; 1987. pp. 5–20. [Google Scholar]
- 19.Walsh T J, Gonzalez C, Lyman C A, Chanock S J, Pizzo P A. Invasive fungal infections in children: recent advances in diagnosis and treatment. Adv Pediatr Infect Dis. 1996;11:187–290. [PubMed] [Google Scholar]
- 20.Woestenborghs R, Lorreyne W, Heykants J. Determination of itraconazole in plasma and animal tissues by high-performance liquid chromatography. J Chromatogr. 1987;413:332–337. doi: 10.1016/0378-4347(87)80249-9. [DOI] [PubMed] [Google Scholar]