Abstract
The objective of this systematic review and meta-analysis is to examine the effects of resistance exercise training on muscle stem cells in older adults. A database search was performed (PubMed, Scopus, Web of Science and Google Scholar) to identify controlled clinical trials in English language. The mean difference (MD) with 95% confidence intervals (CIs) and overall effect size were calculated for all comparisons. The PEDro scale was used to assess the methodological quality. Nineteen studies were included in the review. The meta-analysis found a significant effect of resistance training (RT) on muscle stem cells in the elderly (difference in means=-0.008, Z=-3.415, P=0.001). Also, muscle stem cells changes were similar in men and women (difference in means=-0.004, Z=-1.558, P=0.119) and significant changes occur in type II muscle fibers (difference in means=-0.017, Z=-7.048, P=0.000). Resistance-type exercise training significantly increased muscle stem cells content in intervention group that this result is similar in men and womenthis increase occurred more in type II muscle fibers.
Keywords: Elderly, Muscle Stem Cells, Older Adult, Resistance Training, Satellite Cells
Introduction
Ageing leads to a decrease in the ability to regenerate body tissues in all obstetric species. This is mainly due to a functional decrease in the tissue-resident stem cell population. The population of skeletal muscle resident stem or satellite cells are numerically and functionally compromised, and the microenvironment (the niche) of the satellite cell becomes less supportive (1). About 40 to 50% of the adult human body weight is composed of muscle. Approximately 600 of these muscles comprise the largest tissue in the body. Mononuclear stem cells of skeletal muscles were first identified half a century ago by Mauro (2). The unique self-renewal and multiple differentiation capabilities of these cells have led to tremendous interest in investigating their applications in muscle regeneration and repair (3). Satellite cells play an important role in postnatal growth and regeneration of skeletal muscles in mammals. Muscle fibre hypertrophy is caused by the addition of satellite cell nuclei to existing myofibres. Also, the satellite cell population has the nuclear reserve to enable muscle regeneration (2).
Resistance exercise is usually recommended to prevent or reverse age-related loss of skeletal muscle capacity and size, which can compromise independence in activities of daily living and quality of life (4). Unfortunately, the hypertrophic response of skeletal muscle to resistance exercise is less observed or delayed in older adults (5). To date, the full mechanism of this delayed response remains unknown; however, impaired ribosome biogenesis, translational efficiency defects in anabolic hormones, low-grade inflammation, and low fibre capillarization with ageing may play a role (6).
Evidence has shown that resistance exercise increases the number of satellite cells and increases muscle fibre size in the elderly (7). However, the response of satellite cells to a single bout of exercise may decrease or be delayed with ageing (8). The slow and delayed response of satellite cells with ageing may be a limiting factor for muscle fibre hypertrophy, as the response to progressive resistance training appears to be related to satellite cell content or mode of response (9). As a source of new myonucleus, satellite cells play a key role during hypertrophy when myonucleus addition is needed (10).
Resistance and endurance exercise via endo-, para-, or autocrine mechanisms activate satellite cells from a quiescent state where they undergo proliferation, commitment, and differentiation to add myonuclei to pre-existing myofibrils or self-renew, and return to the quiescent state. During early stages of myogenic Lineage Pax7, CD56, and Myf5 derive the activation and proliferation process; however, at later stages MRF4 and myogenin have a master regulatory role by controlling terminal differentiation (11).
Pax7 transcription factor plays a crucial role in the formation and differentiation of skeletal muscle precursor cells during embryonic development. It controls the expression of other myogenic regulators and also acts as an anti-apoptotic factor. MyoD is a 3D genome structure organiser for muscle cell identity. CD56 is the archetypal phenotypic marker of natural killer cells but can actually be expressed by numerous immune cells, including alpha beta T cells, gamma delta T cells, dendritic cells, and monocytes. Myogenin is a muscle-specific transcription factor that can induce myogenesis in a variety of cell types in tissue cultures and is essential for the development of functional skeletal muscle. Although myogenic regulatory factor MRF4 is highly expressed in adult skeletal muscle, its function is unknown (2).
Our literature searches and research thus far indicate that no meta-analysis study has been done regarding the effect of resistance training on muscle stem cells in the elderly. Therefore, it is innovative and necessary to carefully study the effects of resistance exercise training on muscle stem cells in older adults. The aim of our study is to investigate these effects according to our three hypotheses: i. The effects of resistance exercise training on muscle stem cells in the older adult trained group are higher than the control group; ii. The effect in an older male group is higher than in an older female group; and iii. There are more type 2 muscle fibres than type 1 in the elderly who partake in resistance training.
Materials and Methods
Protocol and registration
The present systematic review study protocol was registered prospectively in the PROSPERO database with the registration code CRD42022345876 and can be found at: http://crd.york.ac.uk/PROSPERO/display_record. asp?ID=CRD42022345876.
Literature search strategy
We applied the PRISMA strategies to report this meta-analytical review (12). Independent searches were conducted by two researchers of the PubMed, Web of Science, Scopus, and Google Scholar databases to extract eligible health and sports-related studies. The search was conducted from June 1, 2022, until August 7, 2022. The search conditions included three steps combined with Boolean sentences (AND/OR). The first search step used the research phrases "strength exercise OR exercise training OR resistance exercise OR resistance training OR strength training OR physical activity,"; the second step used "AND (satellite cell OR pax7 OR MyoD OR CD56 OR Myogenin OR Mrf4 OR Muscle stem cell)"; and the third step used "AND (old OR elderly OR older adults OR ageing)". The screening process included the title, abstract, and full text. First, all articles identified in the search were screened manually by the two researchers and discussed for competency. All of the chosen titles were then moved to a reference management software (EndNote, version 20). Both researchers screened the abstracts separately and then discussed them. Irrelevant studies were excluded and the full texts were screened for the final inclusion/exclusion of the articles.
Study inclusion and exclusion criteria
We used the PICOS approach [population (P), intervention (I), comparators (C), main outcome (O) and study design consider (S)] to define the inclusion and exclusion criteria. Only studies with a full text published in the English language and target group of individuals 60 years and older (P) were included in the meta-analysis. The inclusion criterion was that the study had to be an intervention study (I) with resistance training (at least two weeks) as a controlled trial. There should be at least one control group in each article (C). Muscle stem cells that consisted of satellite cells, Pax7, MyoD, CD56, Myogenin or Mrf4 (O) should be reported in the article as the pre-test and post-test (S). Exclusion criteria were the use of dietary supplements or changes in the diet as part of the intervention.
Study selection
Two reviewers separately screened the titles and abstracts, and read the full texts of the eligible studies. Differences of opinion were resolved by discussion between the two authors.
Methodological quality assessment
The The methodological quality of the eligible randomised controlled trials was rated using the PEDro scale. The PEDro score includes 11 criteria comprised of concealed allocation; random allocation; blind subjects; baseline comparability; blind assessor; blind therapists; intention-to-treat-analysis; adequate follow-up; point estimates and variability; between groups comparisons. Eligible trials received either a "yes", or "no" rating. The maximum PEDro concession is 10 points (13).
Data extraction
The results of the muscle stem cell data were extracted and transferred to a separate Excel sheet that contained all of the information for the relevant calculations described in the statistical analysis section. Other pertinent article information [year, author, number of participants, body mass index (BMI), participants’ characteristics (gender), intervention groups, and details about the intervention] were extracted and are shown in Tables 1-3.
Statistical analysis
Statistical analyses were conducted using the Comprehensive Meta-Analysis Software version 2.0. We performed a meta-analysis to specify the change in pretest and post-test muscle stem cell data by calculating the standardized mean difference (SMD) between the intervention and control groups with a 95% confidence interval (CI). We also use this software to analyse the heterogeneity of the studies, followed by Begg and Egger, s tests to determine publication bias.
Results
We identified 689 studies according to the search strategy; after screening, there were 57 related studies. We excluded 27 studies after reviewing their abstracts and the full text because they did not meet the study criteria. Hence, 19 articles met the inclusion criteria and were included in the study (Tables1-3). All of the included studies had a PEDro score of 5 or higher, with an average score of 6.21 ± 0.91.
Table 1.
Continued
|
| |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Study | Exercise + Control = Total sample size (baseline) | Sex | Participants’ characteristics | Baseline BMI (kg/m2) (mean ± SD) | Frequency (days/week) | Intervention duration (weeks) | Exercise intervention | Results | PEDro score |
|
| |||||||||
| Bermon et al. (14) | 16+16=32 | Female/Male | Healthy older adults | Exercise: 24.8 ± 0.5Control: 26.0 ± 0.6 | 3 | 8 | Strength training program, three sets of eight repetitions, 80% of 1RM | Eight weeks of strength training appeared to be a very short period to adjust the number of lymphocyte subsets at rest in sedentary older adults. | 5 |
| Blocquiaux et al. (15) | 30+10=40 | Male | Healthy older adults | Exercise: No dataControl: No data | 3 | 12 | Upper and lower body resistance exercises with sets and repetitions from 2 to 3 and from 15 to 8, respectively | Muscle strength and changes in muscle fibres were largely maintained after 12 weeks of detraining. | 6 |
| Flynn et al. (16) | 15+14=29 | Female | Healthy older adults | Exercise: 25.7 ± 4.5Control: 26.5 ± 2.0 | 3 | 10 | Progressive resistance exercise | 10 weeks of resistance exercise did not influence resting immune measures in elderly women. | 5 |
| Table 1: Continued | |||||||||
| Study | Exercise + Control = Total sample size (baseline) | Sex | Participants’ characteristics | Baseline BMI (kg/m2) (mean ± SD) | Frequency(days/week) | Intervention duration (weeks) | Exercise intervention | Results | PEDro score |
| Karlsen et al. (17) | 24+13=37 | Female/Male | Healthy older adults | Exercise: No dataControl: No data | 3 | 12 | Heavy resistance training in two intervention groups | Type II muscle fibre size, satellite cell content, and myonuclear domain were significantly smaller in elderly men compared to young men. Heavy resistance training could not improve these factors in the elderly. | 7 |
| McFarlin et al. (18) | 19+6=25 | Female | Healthy older adults | Exercise: 25.2 ± 5.6Control: 27.6 ± 3.4 | 3 | 10 | Resistance trainingwith training intensity at 70% of 1RM for the first week.The training intensity was 80% of 1RM for weeks 2-10. | Resistance exercise increased natural killer cell activity at rest and in response to an acute exercise period. | 6 |
| Mero et al. (19) | 10+18=18 | Male | Healthy older adults | Exercise: No dataControl: No data | 2 | 21 | Progressive hypertrophicresistance training | After resistance training, muscle fibre size increased less in older men than in younger men. | 7 |
| Raso et al. (20) | 22+20=42 | Female | Healthy older adults | Exercise: 24.0 ± 7.0Control: 27.0 ± 5.0 | 3 | 48 | Moderate resistance exercise with 60% of 1RM | This resistance exercise program increased muscle strength but did not change the phenotypic and functional immune parameters in sedentary elderly women. | 5 |
|
| |||||||||
BMI; Body mass index and 1RM; One-repetition maximum.
Table 3.
Specifications of analyzed trials to evaluate type I and II muscle fibers
|
| |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Study | Total sample size (Baseline) | Sex | Participants characteristics | Body mass index (kg/m2) (Baseline) | Frequency (days/week) | Intervention duration (weeks) | Exercise intervention | Results | PEDro score |
|
| |||||||||
| Karlsen et al. (26) | 24 | Male | Healthy older adults | 25.9 ± 3.6 | 3 | 13 | Resistance training (RT) for both legs | Boosting SC availability in healthy elderly men does not enhance the subsequent muscle hypertrophy response to RT. | 7 |
| Moro et al. (27) | 19 | Female/Male | Healthy older adults | 29.7 ± 3.1 | 3 | 12 | progressive Resistance exercise training (RET) program | This study provides intriguing evidence for a fibre type-specific response to RET in older adults and suggests flexibility in the myonuclear domain of type II fibres during a hypertrophic stimulus. | 8 |
| Snijders et al. (28) | 22 | Male | Healthy older adults | 27.4 ± 0.6 | 3 | 24 | Progressive resistance type exercise training | Type II muscle fibre capillarization at baseline may be a critical factor for allowing muscle fibre hypertrophy to occur during prolonged resistance exercise training in older men. | 6 |
| Snijders et al. (29) | 14 | Male | Healthy older adults | 28.0 ± 4.0 | 2 | 12 | A resistance exercise session consisted of a 5 min warm-up on a cycle ergometer, followed by three sets of four separate exercises in the following order: leg press, chest press or lateral pull-down, horizontal row or shoulder press, and leg extension | The acute muscle satellite cell response following exercise can be improved by prolonged exercise training in older men. | 7 |
| Verdijk et al. (29) | 13 | Male | Healthy older adults | 27.4 ± 1.1 | 3 | 12 | Training consisted of 5 minutes of warming-up on a cycle ergometer, followed by four sets on both the leg press (LP) and leg extension (LE) machines, and a 5-minute cooling-down period on the cycle ergometer | No changes were observed in the Type I muscle fibers. In older adults, skeletal muscle tissue is still capable of inducing SC proliferation and differentiation, resulting in Type II muscle fiber hypertrophy. | 5 |
| Verdijk et al. (31) | 53 | Male | Healthy older adults | No data | 3 | 12 | Training consisted of a 5-minutes warm-up on a cycle ergometer, followed by four sets on both the leg press and leg-extension machines, followed by a 5- minutes cooling down period on the cycle ergometer | Twelve weeks of resistance-type exercise training significantly increased type II muscle fiber size and satellite cell content. | 6 |
| Verney et al. (32) | 10 | Male | Healthy older adults | 27.5 ± 3.0 | 3 | 14 | Resistance training bouts included three exercises with three sets per exercise that targeted muscles of the arms and trunk | This study provide a practical reference for the determination of optimal exercise protocols for improved muscle function and regeneration in elderly individuals. | 6 |
|
| |||||||||
Resistance Training and Muscle Stem Cells in Older Adult
Comparison of control and intervention groups
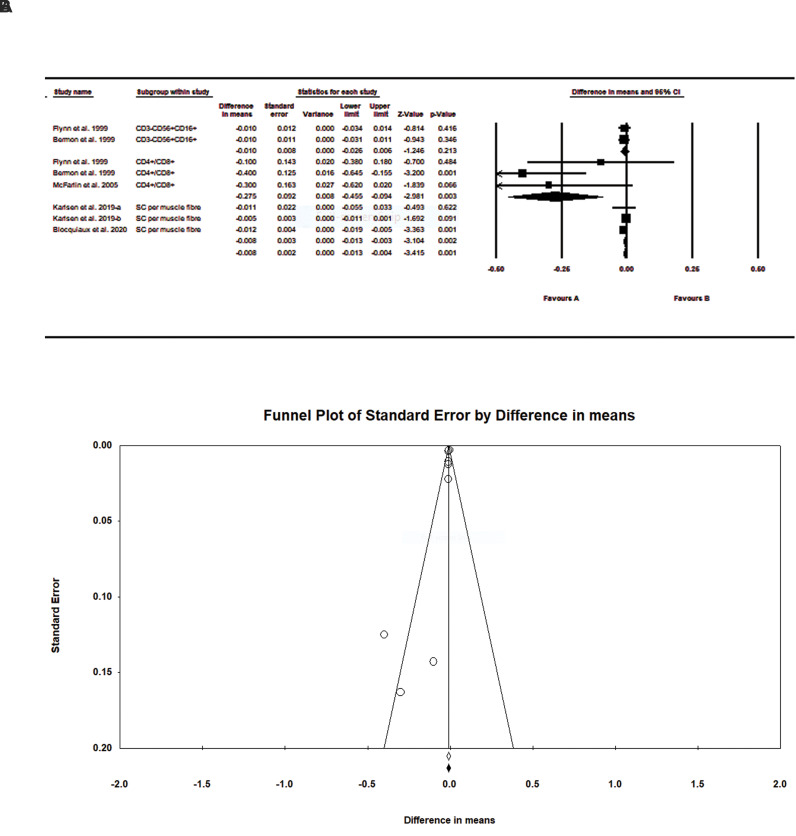
The our findings showed a significant effect of resistance exercise on muscle stem cells in older adults [difference in means: -0.008, standard error (SE)=0.002, P=0.001, Fig.1A]. A subgroup analysis of CD3- CD56+ CD16+ , CD4+ /CD8+ and satellite cell resistance training indicated a significant effect between the control and intervention groups on muscle stem cells in the elderly; however, CD3- CD56+CD16+ was not significant. Our findings showed that resistance training significantly increased satellite cell content in the intervention group. The funnel plot in Figure 1B does not show any significant publication bias. In other words, these findings are largely trustworthy. Begg’s test (P=0.26) and Egger’s test (P=0.09) results show no significant publication bias in this study.
Fig 1.
Meta-analysis of the effects of resistance exercise on muscle stem cells in older adults in control and intervention groups. A. Forest plot indicates pooled mean differences (MD) with 95% confidence intervals (CIs) for nine effect sizes obtained from five trials. B. Funnel plot of muscle stem cells for each trial. Duval and Tweedie’s trim and fill method does not show any significant bias.
In this meta-analysis, forest plots that report the I2 statistic [total (95% CI)] due to the heterogeneity of the continuous data were obtained. Although the analysis provided high evidence of between-group heterogeneity (I2 = 56%, P= 0.02), intra-group analysis of the subgroups indicated no significant heterogeneity
An average value of 0.5 was used to determine the correlation between pre-test and post-test articles. The risk of bias was performed by the one study removed method. Excluding any of the studies, indicating a low risk of bias, the meta-analysis results did not change significantly, indicating a low risk of bias.
Comparison of men and women groups
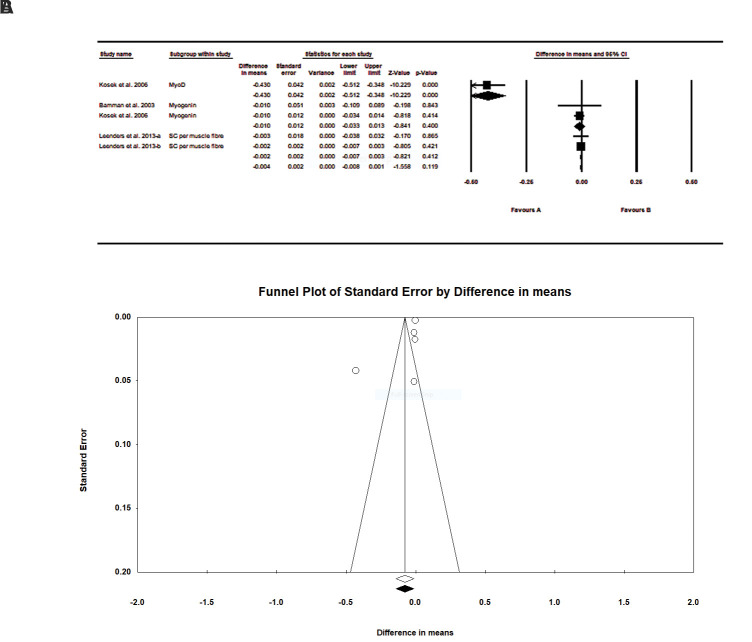
The Our findings did not find a significant effect of resistance exercise on muscle stem cells between older males and females (difference in means:-0.004, SE=0.002, P=0.119, Fig .2A). A subgroup analysis with MyoD, myogenin and satellite cells showed no significant effect on muscle stem cells between older men and women. Our findings indicated similar results between men and women. Figure 2B shows a funnel plot to check for publication bias; the results indicated no significant bias. In other words, these findings are largely trustworthy. The results of Begg’s test (P=0.46) and Egger’s test (P=0.31) show that there is no significant publication bias in this study.
Fig 2.
Meta-analysis of the effects of resistance training on muscle stem cells in the elderly in men and women groups. A. forest plot showings pooled mean differences with 95% confidence intervals (CIs) for 5 effect sizes obtained from 3 trials. B. Funnel plot of muscle stem cells for each trial. Duval and Tweedie’s trim and fill method does not show significant bias
We generated forest plots in this meta-analysis that report the I2 statistic [total (95% CI)] due to the heterogeneity of the continuous data. The analysis provided strong evidence of between-group heterogeneity (I2 =96%, P=0.00). However, intra-group analysis of the subgroups indicated no significant heterogeneity.
An average value of 0.5 was used to determine the correlation between pre-test and post-test articles. The risk for bias was performed by the one study removed method. Excluding any of the studies, the meta-analysis results did not change significantly, indicating a low risk of bias.
Table 2.
Specifications of the analyzed randomized between men and women groups
|
| ||||||||
|---|---|---|---|---|---|---|---|---|
| Study | Male +Female= Total sample size (Baseline) | Participants characteristics | Body mass index (kg/m2) (Baseline) | Frequency (days/week) | Intervention duration (weeks) | Exercise intervention | Results | PEDro score |
|
| ||||||||
| Bamman et al. (21) | 9+5=14 | Healthy older adults | Male: 24.60 ± 4.3Female: 25.51 ± 5.7 | 3 | 26 | Knee extensor training at 65-80% of one-repetition maximum | Gender differences in load-induced myofibre hypertrophy among older adults cannot be explained by levels of circulating IGF-1, dehydroepiandrosterone sulphate, or by expression of the myogenic transcripts examined | 5 |
| Bamman et al. (22) | 11+9=20 | Healthy older adults | Male: 27.73 ± 2.9Female: 24.94 ± 4.2 | 2-3 | 26 | Three sets of 80% one-repetition maximum for squat, leg press, and knee extension | Myogenin levels increased in the elderly | 7 |
| Kosek et al. (23) | 13+12=25 | Healthy older adults | Male: 27.81 ± 3.0Female: 25.28 ± 3.0 | 3 | 16 | The resistance training program focused on knee extensors | Muscle hypertrophy was more in men | 6 |
| Leenders et al. (24) | 29+24=53 | Healthy older adults | Male: 27.00 ± 0.5Female: 24.60 ± 0.4 | 3 | 24 | Progressive resistance training | The results were similar in men and women | 7 |
| Mackey et al. (25) | 13+16=29 | Healthy older adults | Male: 25.00 ± 1.3Female: 25.28 ± 1.0 | 3 | 12 | The exercise protocol included three dynamic strength exercises | The results were similar in men and women | 7 |
|
| ||||||||
Comparison of type I and II muscle fibers
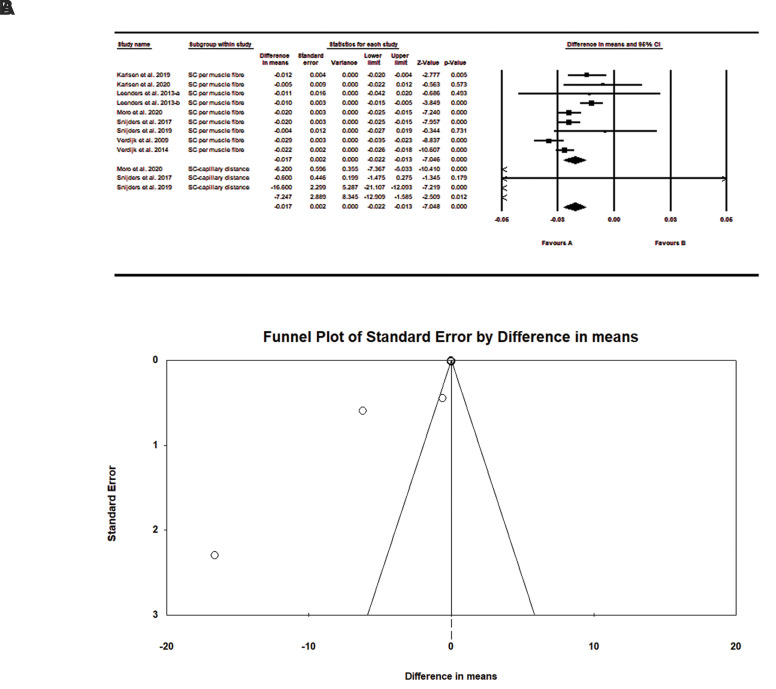
The Our findings showed a significant effect of resistance exercise on muscle stem cells between type I and II muscle fibres in older adults (difference in means:-0.017, SE=0.002, P=0.000, Fig .3A). A subgroup analysis of satellite cell and satellite cell-capillary distance showed a significant effect between type I and II muscle fibres on muscle stem cells in the elderly. Therefore, our findings showed that resistance exercise training significantly increased type II muscle fibre size and satellite cell content. Figure 3B shows a funnel plot assessment of publication bias. The findings indicated no significant bias. In other words, these findings are largely trustworthy. Begg’s test (P=0.24) and Egger’s test (P=0.08) results also indicated no significant publication bias in this study.
Fig 3.
Meta analysis of the effects of resistance training on muscle stem cells in the elderly in type I and II muscle fibers. A. Forest plot showings pooled mean differences with 95% confidence intervals (CIs) for 12 effect sizes obtained from 8 trials. B. Funnel plot of muscle stem cells for each trial. Duval and Tweedie’s trim and fill method does not show significant bias.
In this meta-analysis, forest plots that report the I2 statistic [total (95% CI)] due to the heterogeneity of the continuous data were obtained. The analysis provided strong evidence of between-group heterogeneity (I2 =94%, P=0.00). Intra-group analysis of the subgroups showed no significant heterogeneity.
An average value of 0.5 was used to determine the correlation between pre-test and post-test articles. The risk of bias was assessed by the one study removed method. The results did not change significantly and this indicated a low risk of bias.
Discussion
According to our findings, resistance exercise can increase muscle stem cells in the elderly, and this increase is almost the same in men and women. On the other hand, in this research, we showed that regular resistance training for 12 weeks and three sessions per week can increase the number of satellite cells in the elderly. We also found that this increase was significantly greater in type II muscle fibres than in type I.
Resistance training intensity is said to be one of the most detrimental functional indicators that alter the response of satellite cells to resistance training. There are sample results that support the effectiveness of moderate to high-intensity resistance training, either alone (33, 34) or combined with sports supplements (35) on satellite cell activation. The minimum intensity of resistance training to activate satellite cells is still unknown. On the other hand, the results of several studies have shown that even low-intensity resistance training can stimulate activation of satellite cells (36, 37).
A complete satellite cell response to resistance training has been observed 72-96 hours after the athletic competition where the number of satellite cells peaks (38). Although more studies analysed satellite cell responses in young volunteers after eccentric resistance training sessions, they have not thoroughly investigated other indicators such as age, gender and exercise attributes (frequency, intensity and duration). Additional researches in the future can provide promising results (39). Scientists believe that regular, long-term exercise can positively impact muscle structure and function (40).
Ageing causes atrophy and a gradual decrease in muscle mass and quality, which is determined at the myocellular level by a decrease in type II muscle fibre frequency and cross-sectional area. In addition to obvious muscle atrophy, older people have more grouped type I muscle fibres (41), which is probably associated with the gradual death of nerves related to type II muscle fibres in this population. In order to prevent progressive neuronal death in type II muscle fibres, they reinnervate axons close to type I muscle fibres and enhance the transition from type II to type I phenotype (42, 43). Therefore, the number of type I muscle fibres increases and type I muscle fibres appear "grouped" with less penetration of type II muscle fibres. Resistance training has been proven to increase type II muscle fibre cross-sectional area and frequency in both the young (44) and elderly (45).
Tremendous progress has been made in exploring the importance of satellite cells in the prevention and treatment of sarcopenia (46, 47). It is well-established that the function and quantity of muscle satellite cells decrease with age, and this impairs the regenerative response and muscle fibre development. Whether muscle satellite cells are causally relevant to sarcopenia is arguable. Therefore, the theory that the function and amount of satellite cells can be effective as an important factor in the treatment of sarcopenia is debatable (48). Our findings may be important because resistance training can prevent sarcopenia in the elderly.
It is well-known that resistance training can be beneficial as a therapeutic program to deal with the loss of muscle mass and strength in older adults (49, 50). Significant improvements in muscle mass, strength, and functional performance have been demonstrated following longterm resistance training, even in very elderly adults (51). It has been observed that both older men (52) and women (53) retain the capacity to increase muscle fibre size and function at the muscle fibre level. Despite the overwhelming evidence that shows the efficacy of resistance training for increasing muscle mass and strength in the older adult population, there are many differences in the proposed benefits of resistance training in older females compared to older males. Previous contradictory evidence suggests that muscle mass and strength gains following long-term resistance training are equal (54), less (55), or even greater (56) in older females compared to older males. The findings of the present meta-analysis show that the positive benefits of resistance training to prevent sarcopenia are similar in older men and women.
Conclusion
This review shows that older adults can improve their muscle cells by participating in resistance exercise programs. Resistance exercise training intervention significantly increased muscle fibre size and satellite cell content in a similar manner in both men and women. This increase occurred more in type II muscle fibres. However, more studies are needed to assess the effects of different types of resistance exercise training on muscle stem cells in the elderly.
Acknowledgments
The authors declare that they have no financial support and conflict of interests.
Author’s Contributions
D.H., B.B.; Conceptualization, Methodology, and Software of the study. D.H.; Conducted the formal analysis and Wrote the original draft. H.Sh., B.B.; Performed validation and Wrote, Reviewed and Edited the manuscript. H.Sh., A.Sh.; Supervised the study, Reviewed and Edited the manuscript. All authors read and approved the final manuscript.
References
- 1.Hwang AB, Brack AS. Muscle stem cells and aging. Curr Top Dev Biol. 2018;126:299–322. doi: 10.1016/bs.ctdb.2017.08.008. [DOI] [PubMed] [Google Scholar]
- 2.Bazgir B, Fathi R, Rezazadeh Valojerdi M, Mozdziak P, Asgari A. Satellite cells contribution to exercise mediated muscle hypertrophy and repair. Cell J. 2017;18(4):473–484. doi: 10.22074/cellj.2016.4714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morgan JE, Zammit PS. Direct effects of the pathogenic mutation on satellite cell function in muscular dystrophy. Exp Cell Res. 2010;316(18):3100–3108. doi: 10.1016/j.yexcr.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 4.Hodson N, West DWD, Philp A, Burd NA, Moore DR. Molecular regulation of human skeletal muscle protein synthesis in response to exercise and nutrients: a compass for overcoming age-related anabolic resistance. Am J Physiol Cell Physiol. 2019;317(6):C1061–C1078. doi: 10.1152/ajpcell.00209.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilkinson DJ, Piasecki M, Atherton PJ. The age-related loss of skeletal muscle mass and function: Measurement and physiology of muscle fibre atrophy and muscle fibre loss in humans. Ageing Res Rev. 2018;47:123–132. doi: 10.1016/j.arr.2018.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alturki M, Beyer I, Mets T, Bautmans I. Impact of drugs with antiinflammatory effects on skeletal muscle and inflammation: a systematic literature review. Exp Gerontol. 2018;114:33–49. doi: 10.1016/j.exger.2018.10.011. [DOI] [PubMed] [Google Scholar]
- 7.Dirks ML, Tieland M, Verdijk LB, Losen M, Nilwik R, Mensink M, et al. Protein supplementation augments muscle fiber hypertrophy but does not modulate satellite cell content during prolonged resistance- type exercise training in frail elderly. J Am Med Dir Assoc. 2017;18(7):608–615. doi: 10.1016/j.jamda.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 8.Brook MS, Din U, Tarum J, Selby A, Quinlan J, Bass JJ, et al. Omega-3 supplementation during unilateral resistance exercise training in older women: A within subject and double-blind placebocontrolled trial. Clin Nutr ESPEN. 2021;46:394–404. doi: 10.1016/j.clnesp.2021.09.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bellamy LM, Joanisse S, Grubb A, Mitchell CJ, McKay BR, Phillips SM, et al. The acute satellite cell response and skeletal muscle hypertrophy following resistance training. PLoS One. 2014;9(10):e109739–e109739. doi: 10.1371/journal.pone.0109739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petrella JK, Kim JS, Mayhew DL, Cross JM, Bamman MM. Potent myofiber hypertrophy during resistance training in humans is associated with satellite cell-mediated myonuclear addition: a cluster analysis. J Appl Physiol (1985) 2008;104(6):1736–1742. doi: 10.1152/japplphysiol.01215.2007. [DOI] [PubMed] [Google Scholar]
- 11.McCarthy JJ, Mula J, Miyazaki M, Erfani R, Garrison K, Farooqui AB, et al. Effective fiber hypertrophy in satellite cell-depleted skeletal muscle. Development. 2011;138(17):3657–3666. doi: 10.1242/dev.068858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162(11):777–784. doi: 10.7326/M14-2385. [DOI] [PubMed] [Google Scholar]
- 13.Tayebi M, Heidary D, Mehdipour A. Effects of resistance training on performance and physiological indices in patients with ischemic stroke: a systematic review and meta-analysis. J Mazandaran Univ Med Sci. 2022;32(208):164–178. [Google Scholar]
- 14.Bermon S, Philip P, Ferrari P, Candito M, Dolisi C. Effects of a shortterm strength training programme on lymphocyte subsets at rest in elderly humans. Eur J Appl Physiol Occup Physiol. 1999;79(4):336–340. doi: 10.1007/s004210050517. [DOI] [PubMed] [Google Scholar]
- 15.Blocquiaux S, Gorski T, Van Roie E, Ramaekers M, Van Thienen R, Nielens H, et al. The effect of resistance training, detraining and retraining on muscle strength and power, myofibre size, satellite cells and myonuclei in older men. Exp Gerontol. 2020;133:110860–110860. doi: 10.1016/j.exger.2020.110860. [DOI] [PubMed] [Google Scholar]
- 16.Flynn MG, Fahlman M, Braun WA, Lambert CP, Bouillon LE, Brolinson PG, et al. Effects of resistance training on selected indexes of immune function in elderly women. J Appl Physiol (1985) 1999;86(6):1905–1913. doi: 10.1152/jappl.1999.86.6.1905. [DOI] [PubMed] [Google Scholar]
- 17.Karlsen A, Bechshøft RL, Malmgaard-Clausen NM, Andersen JL, Schjerling P, Kjaer M, et al. Lack of muscle fibre hypertrophy, myonuclear addition, and satellite cell pool expansion with resistance training in 83-94-year-old men and women. Acta Physiol (Oxf) 2019;227(1):e13271–e13271. doi: 10.1111/apha.13271. [DOI] [PubMed] [Google Scholar]
- 18.McFarlin BK, Flynn MG, Phillips MD, Stewart LK, Timmerman KL. Chronic resistance exercise training improves natural killer cell activity in older women. J Gerontol A Biol Sci Med Sci. 2005;60(10):1315–1318. doi: 10.1093/gerona/60.10.1315. [DOI] [PubMed] [Google Scholar]
- 19.Mero AA, Hulmi JJ, Salmijärvi H, Katajavuori M, Haverinen M, Holviala J, et al. Resistance training induced increase in muscle fibre size in young and older men. Eur J Appl Physiol. 2013;113(3):641–650. doi: 10.1007/s00421-012-2466-x. [DOI] [PubMed] [Google Scholar]
- 20.Raso V, Benard G, DA Silva Duarte AJ, Natale VM. Effect of resistance training on immunological parameters of healthy elderly women. Med Sci Sports Exerc. 2007;39(12):2152–2159. doi: 10.1249/mss.0b013e318156e9fa. [DOI] [PubMed] [Google Scholar]
- 21.Bamman MM, Hill VJ, Adams GR, Haddad F, Wetzstein CJ, Gower BA, et al. Gender differences in resistance-training-induced myofiber hypertrophy among older adults. J Gerontol A Biol Sci Med Sci. 2003;58(2):108–116. doi: 10.1093/gerona/58.2.b108. [DOI] [PubMed] [Google Scholar]
- 22.Bamman MM, Ragan RC, Kim JS, Cross JM, Hill VJ, Tuggle SC, et al. Myogenic protein expression before and after resistance loading in 26- and 64-yr-old men and women. J Appl Physiol (1985) 2004;97(4):1329–1337. doi: 10.1152/japplphysiol.01387.2003. [DOI] [PubMed] [Google Scholar]
- 23.Kosek DJ, Kim JS, Petrella JK, Cross JM, Bamman MM. Efficacy of 3 days/wk resistance training on myofiber hypertrophy and myogenic mechanisms in young vs.older adults. J Appl Physiol (1985) 2006;101(2):531–544. doi: 10.1152/japplphysiol.01474.2005. [DOI] [PubMed] [Google Scholar]
- 24.Leenders M, Verdijk LB, van der Hoeven L, van Kranenburg J, Nilwik R, van Loon LJ. Elderly men and women benefit equally from prolonged resistance-type exercise training. J Gerontol A Biol Sci Med Sci. 2013;68(7):769–779. doi: 10.1093/gerona/gls241. [DOI] [PubMed] [Google Scholar]
- 25.Mackey AL, Esmarck B, Kadi F, Koskinen SO, Kongsgaard M, Sylvestersen A, et al. Enhanced satellite cell proliferation with resistance training in elderly men and women. Scand J Med Sci Sports. 2007;17(1):34–42. doi: 10.1111/j.1600-0838.2006.00534.x. [DOI] [PubMed] [Google Scholar]
- 26.Karlsen A, Soendenbroe C, Malmgaard-Clausen NM, Wagener F, Moeller CE, et al. Preserved capacity for satellite cell proliferation, regeneration, and hypertrophy in the skeletal muscle of healthy elderly men. FASEB J. 2020;34(5):6418–6436. doi: 10.1096/fj.202000196R. [DOI] [PubMed] [Google Scholar]
- 27.Moro T, Brightwell CR, Volpi E, Rasmussen BB, Fry CS. Resistance exercise training promotes fiber type-specific myonuclear adaptations in older adults. J Appl Physiol (1985) 2020;128(4):795–804. doi: 10.1152/japplphysiol.00723.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Snijders T, Nederveen JP, Joanisse S, Leenders M, Verdijk LB, van Loon LJ, et al. Muscle fibre capillarization is a critical factor in muscle fibre hypertrophy during resistance exercise training in older men. J Cachexia Sarcopenia Muscle. 2017;8(2):267–276. doi: 10.1002/jcsm.12137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Snijders T, Nederveen JP, Bell KE, Lau SW, Mazara N, Kumbhare DA, et al. Prolonged exercise training improves the acute type II muscle fibre satellite cell response in healthy older men. J Physiol. 2019;597(1):105–119. doi: 10.1113/JP276260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Verdijk LB, Gleeson BG, Jonkers RA, Meijer K, Savelberg HH, Dendale P, et al. Skeletal muscle hypertrophy following resistance training is accompanied by a fiber type-specific increase in satellite cell content in elderly men. J Gerontol A Biol Sci Med Sci. 2009;64(3):332–339. doi: 10.1093/gerona/gln050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Verdijk LB, Snijders T, Drost M, Delhaas T, Kadi F, van Loon LJ. Satellite cells in human skeletal muscle; from birth to old age. Age (Dordr) 2014;36(2):545–547. doi: 10.1007/s11357-013-9583-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Verney J, Kadi F, Charifi N, Féasson L, Saafi MA, Castells J, et al. Effects of combined lower body endurance and upper body resistance training on the satellite cell pool in elderly subjects. Muscle Nerve. 2008;38(3):1147–1154. doi: 10.1002/mus.21054. [DOI] [PubMed] [Google Scholar]
- 33.Hyldahl RD, Olson T, Welling T, Groscost L, Parcell AC. Satellite cell activity is differentially affected by contraction mode in human muscle following a work-matched bout of exercise. Front Physiol. 2014;5:485–485. doi: 10.3389/fphys.2014.00485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burd NA, West DW, Staples AW, Atherton PJ, Baker JM, Moore DR, et al. Low-load high volume resistance exercise stimulates muscle protein synthesis more than high-load low volume resistance exercise in young men. PLoS One. 2010;5(8):e12033–e12033. doi: 10.1371/journal.pone.0012033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Farup J, Rahbek SK, Riis S, Vendelbo MH, Paoli Fd, Vissing K. Influence of exercise contraction mode and protein supplementation on human skeletal muscle satellite cell content and muscle fiber growth. J Appl Physiol (1985) 2014;117(8):898–909. doi: 10.1152/japplphysiol.00261.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kowsari E, Asgari A, Shirvani H, Bazgir B, Rahimi M. Determining the combined effects of three weeks of consumption of HMB supplement and one session of extreme eccentric resistance exercise on the expression rates of MyoG and CD56 genes in satellite cells. Rev Bras de Medicina do Esporte. 2019;13(77):94–105. [Google Scholar]
- 37.Heiat F, Ghanbarzadeh M, Ranjbar R, Shojaeifard M. Continuous swimming training arises a remarkable effect on some longevity biomarkers in rat skeletal muscles. Ann Appl Sport Sci. 2020;8(2):41–47. [Google Scholar]
- 38.Paulsen G, Egner IM, Drange M, Langberg H, Benestad HB, Fjeld JG, et al. A COX-2 inhibitor reduces muscle soreness, but does not influence recovery and adaptation after eccentric exercise. Scand J Med Sci Sports. 2010;20(1):e195–207. doi: 10.1111/j.1600-0838.2009.00947.x. [DOI] [PubMed] [Google Scholar]
- 39.Mangan G, Bombardier E, Mitchell AS, Quadrilatero J, Tiidus PM. Oestrogen-dependent satellite cell activation and proliferation following a running exercise occurs via the PI3K signalling pathway and not IGF-1. Acta Physiol (Oxf) 2014;212(1):75–85. doi: 10.1111/apha.12317. [DOI] [PubMed] [Google Scholar]
- 40.Bazgir B, Salesi M, Koushki M, Amirghofran Z. Effects of eccentric and concentric emphasized resistance exercise on IL-15 serum levels and its relation to inflammatory markers in athletes and nonathletes. Asian J Sports Med. 2015;6(3):e27980–e27980. doi: 10.5812/asjsm.27980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kelly NA, Hammond KG, Stec MJ, Bickel CS, Windham ST, Tuggle SC, et al. Quantification and characterization of grouped type I myofibers in human aging. Muscle Nerve. 2018;57(1):E52–E59. doi: 10.1002/mus.25711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tayebi SM, Siahkouhian M, Keshavarz M, Mahdian R, Shamsi MM, Shahbazi S. The effects of high intensity interval training on Mir-23a expression and related factors involved in muscular atrophy of aged rats. Int J Appl Exerc Physiol. 2019;8(1):170–176. [Google Scholar]
- 43.Tayebi SM, Siahkouhian M, Keshavarz M, Yousefi M. The effects of high-intensity interval training on skeletal muscle morphological changes and denervation gene expression of aged rats. Monten J Sports Sci Med. 2019;8(2):39–45. [Google Scholar]
- 44.Olsen S, Aagaard P, Kadi F, Tufekovic G, Verney J, Olesen JL, et al. Creatine supplementation augments the increase in satellite cell and myonuclei number in human skeletal muscle induced by strength training. J Physiol. 2006;573(Pt 2):525–534. doi: 10.1113/jphysiol.2006.107359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bazgir B, Rezazadeh Valojerdi M, Rajabi H, Fathi R, Ojaghi SM, Emami Meybodi MK, et al. Acute cardiovascular and hemodynamic responses to low intensity eccentric resistance exercise with blood flow restriction. Asian J Sports Med. 2016;7(4):e38458–e38458. doi: 10.5812/asjsm.38458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Snijders T, Parise G. Role of muscle stem cells in sarcopenia. Curr Opin Clin Nutr Metab Care. 2017;20(3):186–190. doi: 10.1097/MCO.0000000000000360. [DOI] [PubMed] [Google Scholar]
- 47.The effects of plyometric and resistance training on selected fitness variables among university soccer-playing adults. Ann Appl Sport Sci. 2020;8(8):e817–e817. [Google Scholar]
- 48.Fujita R. Role of muscle stem cells in sarcopenia. Sarcopenia: Elsevier; 2021. pp. 109–138. [Google Scholar]
- 49.Saeidi A, Seifi-Ski-Shahr F, Soltani M, Daraei A, Shirvani H, Laher I, et al. Resistance training, gremlin 1 and macrophage migration inhibitory factor in obese men: a randomised trial. Arch Physiol Biochem. 2020;129(3):640–648. doi: 10.1080/13813455.2020.1856142. [DOI] [PubMed] [Google Scholar]
- 50.Hassabi M, Abedi Yekta AH, Poursaeid Esfahani M, Salehi S, Labibzadeh N. The effects of aerobic and resistance exercise therapy with and without weight bearing on the outcomes of stem cell therapy for knee osteoarthritis: a randomised clinical trial. Ann Appl Sport Sci. 2022;10(8):e1067–e1067. [Google Scholar]
- 51.Fiatarone MA, O’Neill EF, Ryan ND, Clements KM, Solares GR, Nelson ME, et al. Exercise training and nutritional supplementation for physical frailty in very elderly people. N Engl J Med. 1994;330(25):1769–1775. doi: 10.1056/NEJM199406233302501. [DOI] [PubMed] [Google Scholar]
- 52.Trappe S, Williamson D, Godard M, Porter D, Rowden G, Costill D. Effect of resistance training on single muscle fiber contractile function in older men. J Appl Physiol (1985) 2000;89(1):143–152. doi: 10.1152/jappl.2000.89.1.143. [DOI] [PubMed] [Google Scholar]
- 53.Trappe S, Godard M, Gallagher P, Carroll C, Rowden G, Porter D. Resistance training improves single muscle fiber contractile function in older women. Am J Physiol Cell Physiol. 2001;281(2):C398–C406. doi: 10.1152/ajpcell.2001.281.2.C398. [DOI] [PubMed] [Google Scholar]
- 54.Ivey FM, Tracy BL, Lemmer JT, NessAiver M, Metter EJ, Fozard JL, et al. Effects of strength training and detraining on muscle quality: age and gender comparisons. J Gerontol A Biol Sci Med Sci. 2000;55(3):B152–B157. doi: 10.1093/gerona/55.3.b152. [DOI] [PubMed] [Google Scholar]
- 55.Tracy BL, Ivey FM, Hurlbut D, Martel GF, Lemmer JT, Siegel EL, et al. Muscle quality.II.Effects Of strength training in 65- to 75-yrold men and women. J Appl Physiol (1985) 1999;86(1):195–201. doi: 10.1152/jappl.1999.86.1.195. [DOI] [PubMed] [Google Scholar]
- 56.Häkkinen K, Kallinen M, Izquierdo M, Jokelainen K, Lassila H, Mälkiä E, et al. Changes in agonist-antagonist EMG, muscle CSA, and force during strength training in middle-aged and older people. J Appl Physiol (1985) 1998;84(4):1341s–1341s. doi: 10.1152/jappl.1998.84.4.1341. [DOI] [PubMed] [Google Scholar]