Abstract
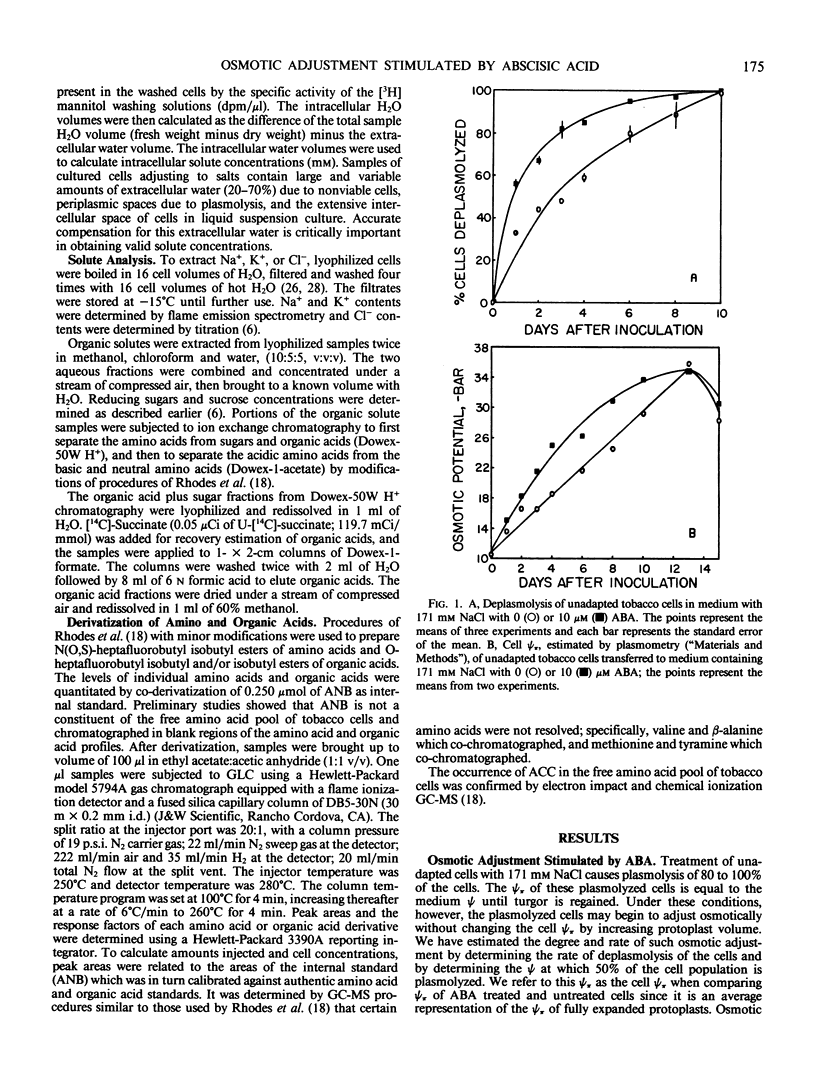
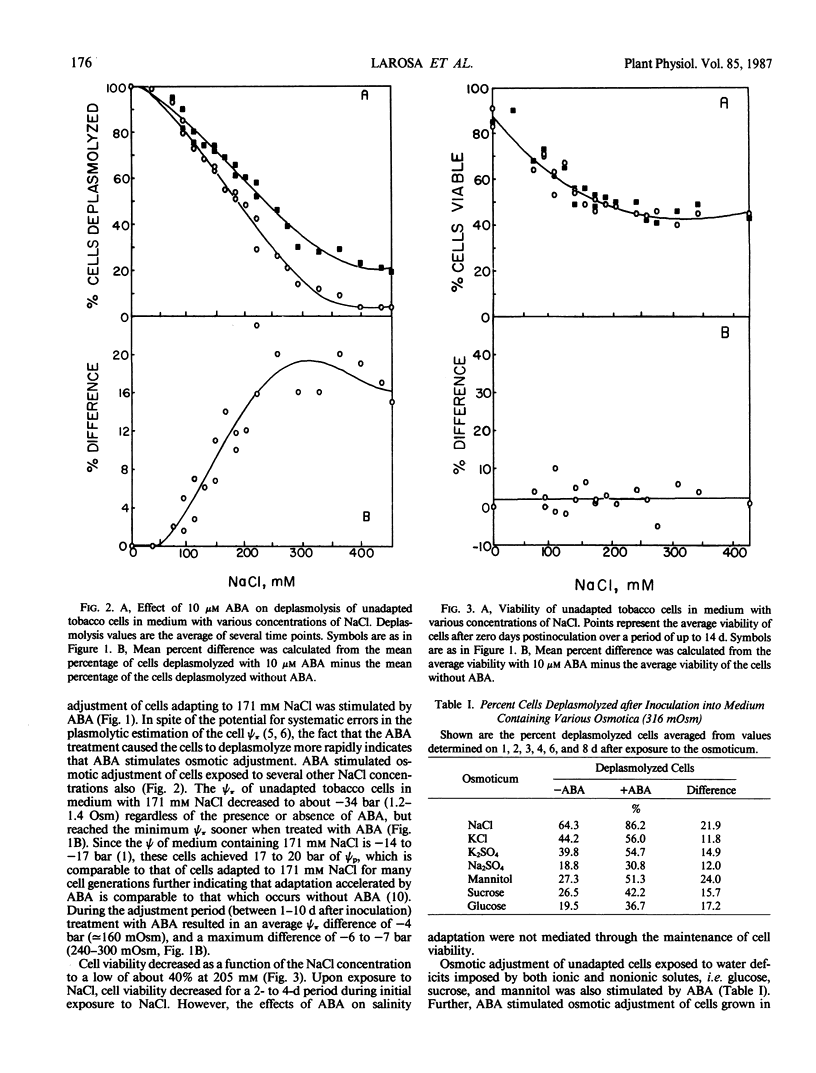
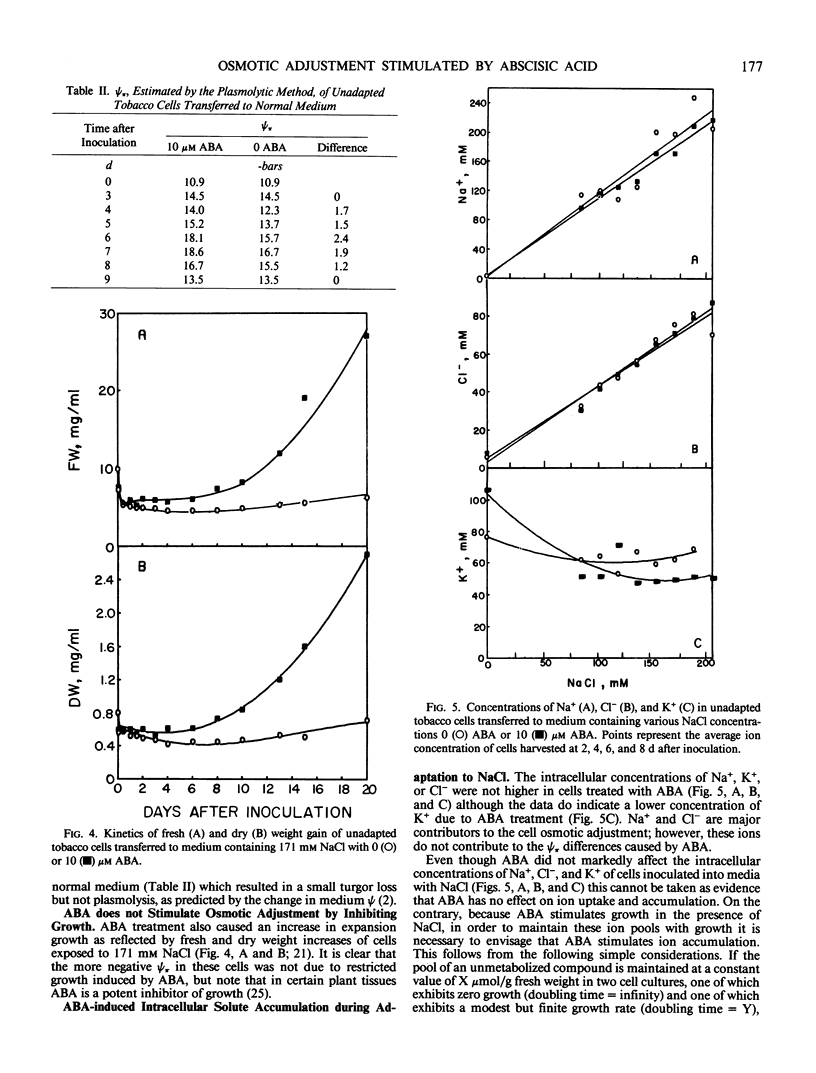
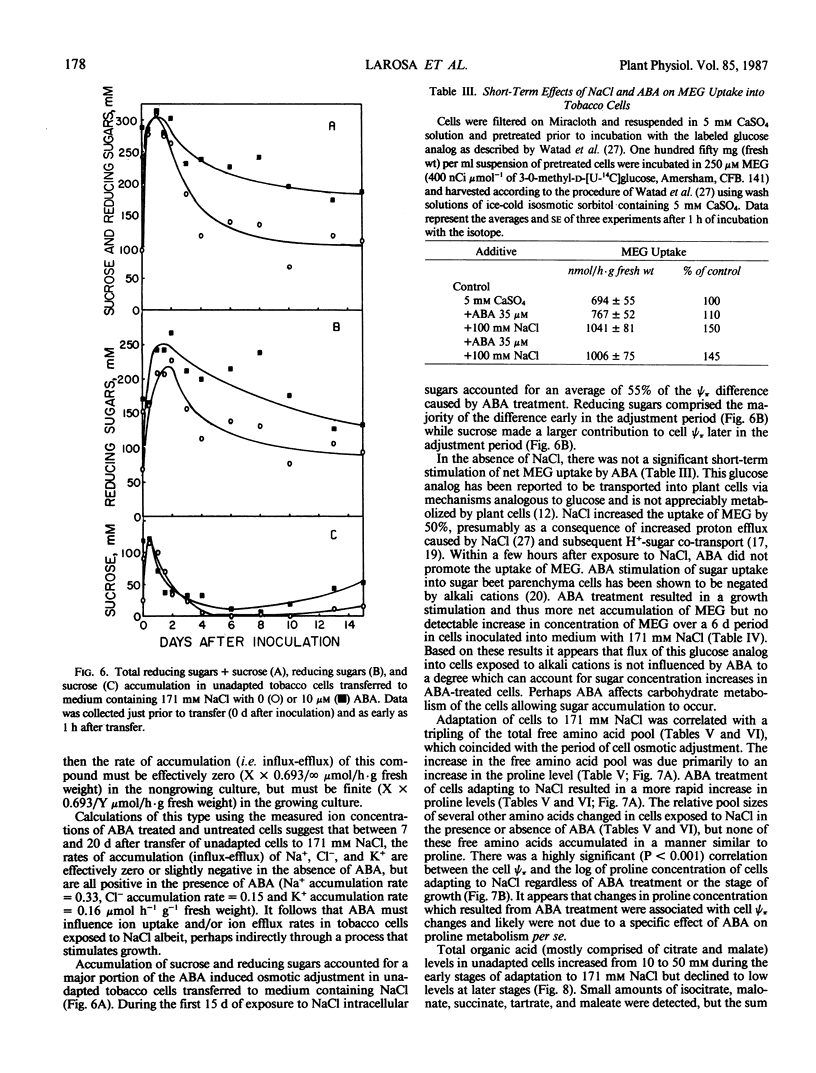
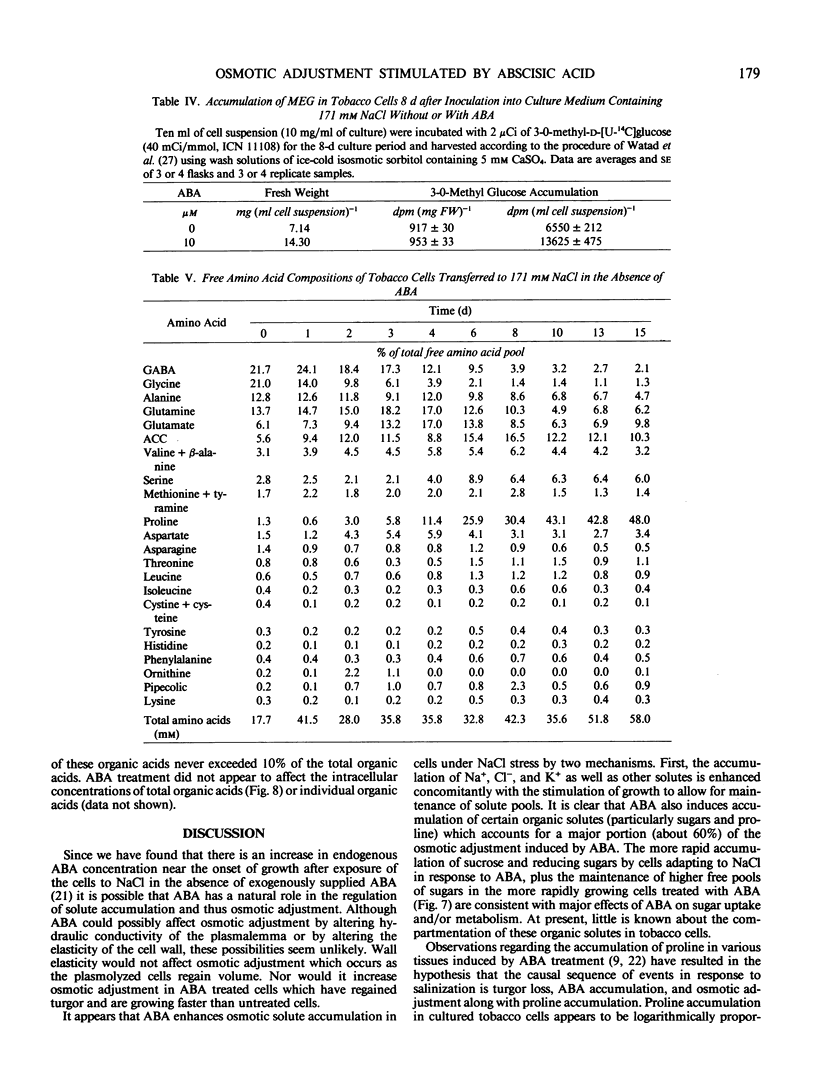
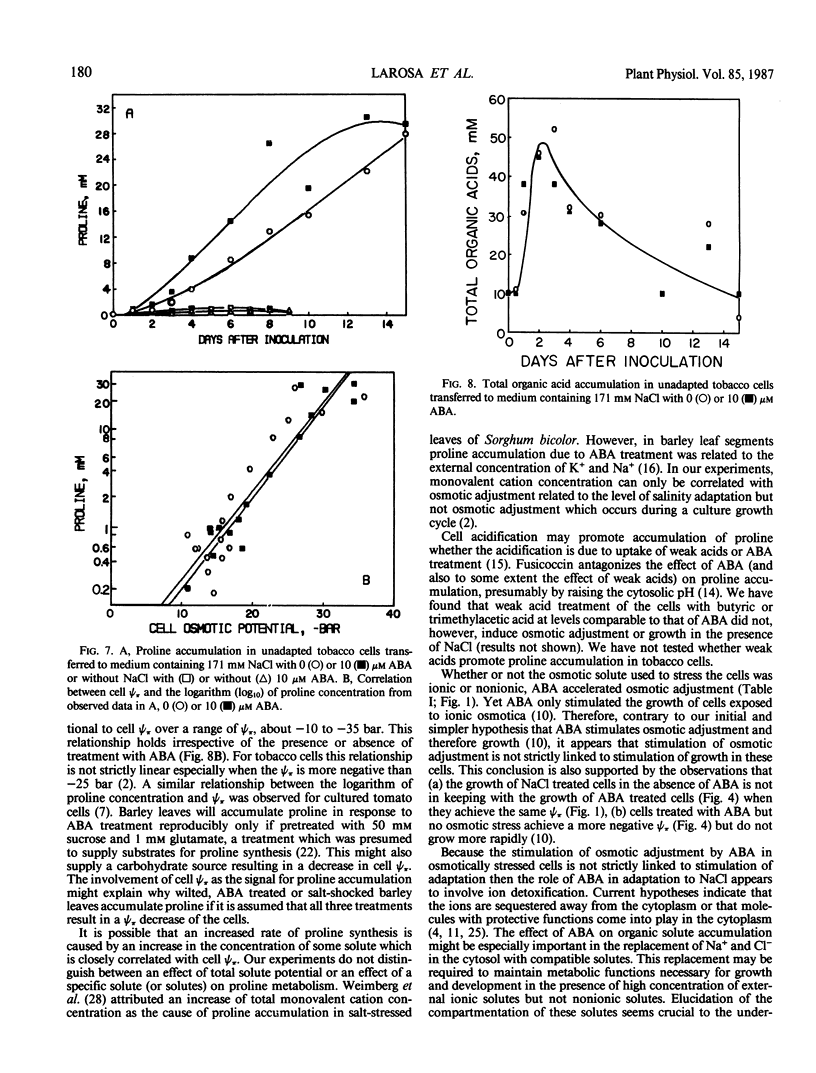
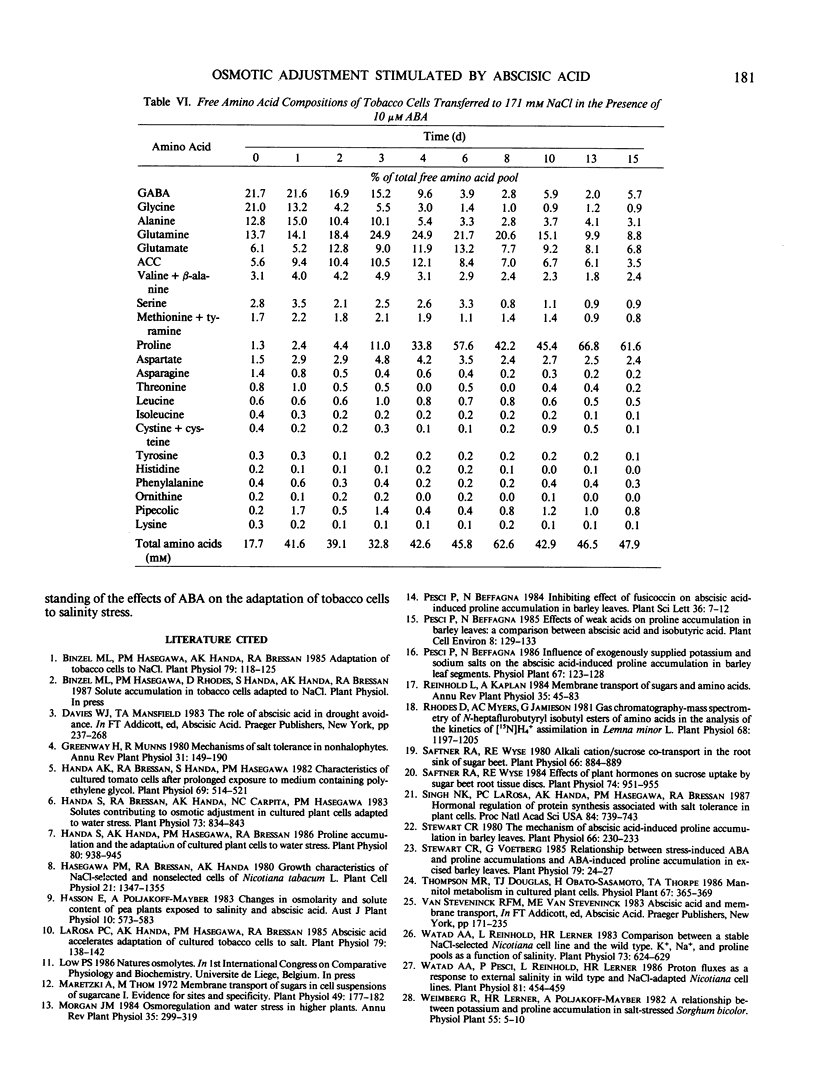
Osmotic adjustment of cultured tobacco (Nicotiana tabacum L. var Wisconsin 38) cells was stimulated by 10 micromolar (±) abscisic acid (ABA) during adaptation to water deficit imposed by various solutes including NaCl, KCl, K2SO4, Na2SO4, sucrose, mannitol, or glucose. The maximum difference in cell osmotic potential (Ψπ) caused by ABA treatment during adaptation to 171 millimolar NaCl was about 6 to 7 bar. The cell Ψπ differences elicited by ABA were not due to growth inhibition since ABA stimulated growth of cells in the presence of 171 millimolar NaCl. ABA caused a cell Ψπ difference of about 1 to 2 bar in medium without added NaCl. Intracellular concentrations of Na+, K+, Cl−, free amino acids, or organic acids could not account for the Ψπ differences induced by ABA in NaCl treated cells. However, since growth of NaCl treated cells is more rapid in the presence of ABA than in its absence, greater accumulation of Na+, K+, and Cl− was necessary for ion pool maintenance. Higher intracellular sucrose and reducing sugar concentrations could account for the majority of the greater osmotic adjustment of ABA treated cells. More rapid accumulation of proline associated with ABA treatment was highly correlated with the effects of ABA on cell Ψπ. These and other data indicate that the role of ABA in accelerating salt adaptation is not mediated by simply stimulating osmotic adjustment.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Handa S., Handa A. K., Hasegawa P. M., Bressan R. A. Proline accumulation and the adaptation of cultured plant cells to water stress. Plant Physiol. 1986 Apr;80(4):938–945. doi: 10.1104/pp.80.4.938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larosa P. C., Handa A. K., Hasegawa P. M., Bressan R. A. Abscisic Acid accelerates adaptation of cultured tobacco cells to salt. Plant Physiol. 1985 Sep;79(1):138–142. doi: 10.1104/pp.79.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes D., Myers A. C., Jamieson G. Gas Chromatography-Mass Spectrometry of N- Heptafluorobutyryl Isobutyl Esters of Amino Acids in the Analysis of the Kinetics of [N]H(4) Assimilation in Lemna minor L. Plant Physiol. 1981 Nov;68(5):1197–1205. doi: 10.1104/pp.68.5.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saftner R. A., Wyse R. E. Alkali Cation/Sucrose Co-transport in the Root Sink of Sugar Beet. Plant Physiol. 1980 Nov;66(5):884–889. doi: 10.1104/pp.66.5.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N. K., Larosa P. C., Handa A. K., Hasegawa P. M., Bressan R. A. Hormonal regulation of protein synthesis associated with salt tolerance in plant cells. Proc Natl Acad Sci U S A. 1987 Feb;84(3):739–743. doi: 10.1073/pnas.84.3.739. [DOI] [PMC free article] [PubMed] [Google Scholar]


