Abstract
Background
The incidence of invasive fungal infections has increased globally as a result of several factors. Conventional amphotericin B (sodium deoxycholate) has been used as standard therapy for the treatment of invasive fungal infections; however, it is associated with adverse drug reactions, including acute kidney injury (AKI). New formulations of amphotericin B have aimed to improve the safety profile of the conventional formulation.
Objectives
This review aimed to assess the effects of amphotericin B deoxycholate versus liposomal amphotericin B on kidney function.
Search methods
We searched Cochrane Kidney and Transplant's Specialised Register to 10 March 2015 through contact with the Trials' Search Co‐ordinator using search terms relevant to this review.
Selection criteria
We included randomised controlled trials (RCTs) that compared amphotericin B sodium deoxycholate with liposomal amphotericin B.
Data collection and analysis
Two authors independently assessed studies for eligibility and conducted risk of bias evaluation.
Main results
We included 12 studies (2298 participants) in this review. Of these, 10 were meta‐analysed (2172 participants). Liposomal amphotericin B was found to be significantly safer than conventional amphotericin B in terms of serum creatinine increase (RR 0.49, 95% CI 0.40 to 0.59). There was significant decrease in all infusion‐related reactions in the liposomal group compared with the conventional group: fever (4 studies, 1092 participants): RR 0.39, 95% CI 0.28 to 0.55; I2 = 32%); chills and/or rigours (5 studies, 1081 participants): RR 0.27, 95% CI 0.15 to 0.48; I2 = 75%); fever and/or rigours (2 studies, 720 participants): RR 0.68, 95% CI 0.52 to 0.90; I2 = 58%); nausea (6 studies, 1187 participants): RR 0.50, 95% CI 0.35 to 0.72; I2 = 0%); and vomiting (3 studies, 1019 participants): RR 0.51, 95% CI 0.27 to 0.95; I2 = 61%). Overall, risk of bias in included studies was low or unclear for most domains. However, blinding of participants and personnel, blinding of outcome assessment and other bias (funding) tended to have a high risk of bias. The sensitivity analysis performed did not change the significance of difference in favour of the liposomal formulation. Assessment for publication bias found that review results were robust.
Authors' conclusions
Current evidence suggests that liposomal amphotericin B is less nephrotoxic than conventional amphotericin B (when the effect on kidney function is measured as an increase in serum creatinine level equal to or greater than two‐fold from the baseline level). We also found that there were fewer infusion‐related reactions associated with the liposomal formulation.
Plain language summary
Which amphotericin B formulation better preserves kidney function among people with fungal infection?
Fungal infections can cause ill health, and in some cases, death. Conventional amphotericin B has been used for many years to treat fungal infection. Although effective, this drug can cause kidney damage in about eight of every ten patients treated. People with kidney damage have longer stays in hospital, increased healthcare costs, and higher numbers of deaths.
To avoid the problems known to affect people who need treatment for fungal infection, new formulations of amphotericin B (called lipid‐associated) have been developed. Liposomal amphotericin B is a new formulation and is most often used in clinical practice to treat fungal infection.
We searched the literature published up to 10 March 2015, and identified 12 studies (2298 participants) that compared conventional amphotericin B with liposomal amphotericin B. We were able to meta‐analyse 10 studies (2172 participants). Study quality was assessed as moderate overall.
Liposomal amphotericin B was found to be associated with fewer kidney and infusion‐related reactions than conventional amphotericin B.
Summary of findings
Summary of findings for the main comparison. Amphotericin B deoxycholate versus liposomal amphotericin B: effects on kidney function.
| Increase in serum creatinine (SCr) level ≥ two‐fold from baseline level | ||||||
| Patient or population: patients in treatment with amphotericin B Settings: inpatients Intervention: liposomal amphotericin B Comparison: conventional amphotericin B | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Conventional amphotericin B | Liposomal amphotericin B | |||||
| Increase in SCr level: ≥ two‐fold from baseline level SCr level (mg/dL) | Study population | RR 0.49 (0.40 to 0.59) | 2172 (10) | ⊕⊕⊕ Moderate1 | ||
| 261 per 1000 | 128 per 1000 (105 to 154) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 The results obtained are consistent; sensitivity analysis was performed to see the influence of several factors in the effect estimate, however, the magnitude, the direction and the significance didn't change.
Background
The cause of increasing global incidence of invasive fungal infections has been attributed to a number of factors including invasive medical interventions, increased use of central venous catheters, long‐term hospital admissions and acquired or induced immunodeficiency (Pfaller 2010). The rise in incidence is notorious among immunocompromised and hospitalised patients with serious underlying diseases, although in developing countries, fungal infections also frequently affect healthy people (Brown 2012).
The most common fungal pathogens in humans are Candida spp, Cryptococcus neoformans, Pneumocystis jirovecii, Histoplasma capsulatum, Cryptoccocus gatii, Coccidoides posadasii and Aspergillus spp. (Brown 2012; Del Poeta 2012; Husain 2001; Pfaller 2010). In some European countries, annual incidence of fungaemia has been reported at between 2.2 and 11 episodes per 100,000 people (Arendrup 2008; Poikonen 2009). In population‐based studies from the United States, reported rates ranged from 6 to 8.7 per 100,000 people (Hajjeh 2004).
Among people admitted to intensive care units or who are immunocompromised, invasive fungal infections are responsible for significant morbidity and mortality. The AURORA project found that 16.5 cases of invasive fungal infections per 1000 admissions were caused by yeasts and 2.3 per 1000 admissions were caused by filamentous fungi (Montagna 2013). Although reported mortality rates for the most common pathogen ‐ Candida spp. ‐ varies, many studies report infection rates of up to 40% (Paramythiotou 2014). More than 1 million AIDS‐related deaths annually are due to invasive fungal infections and account for half of all deaths among this patient population (Armstrong‐James 2014).
The magnitude of challenges associated with management of invasive fungal infections means that identification of novel antifungal agents and approaches to improve effectiveness and safety of currently available agents has become imperative.
Description of the condition
Conventional amphotericin B (sodium deoxycholate) is a broad‐spectrum antifungal that has been used as standard therapy for treatment of many invasive fungal infections since its introduction to clinical practice in the 1950s (Bassetti 2011). Conventional amphotericin B has also been used to treat non‐fungal infections including visceral leishmaniasis and Naegleria fowleri primary amoebic meningoencephalitis.
Although other classes of antifungal drugs, such as azoles and echinocandins, have been developed in recent years, amphotericin B sodium deoxycholate continues to be widely used as the primary agent for treatment of people with serious invasive fungal infections (Laniado‐Laborín 2009; Ostrosky‐Zeichner 2003). A significant limitation of amphotericin B sodium deoxycholate is its safety profile; it is associated with adverse effects, including acute kidney injury (AKI) (Horwitz 2012; Mistro 2012) mediated by vasoconstriction and direct tubular toxicity. The reported incidence of nephrotoxicity varies widely; however, significant decrease in kidney function has been reported in over 80% of patients and is associated with higher mortality rates, length of hospital stay and costs (Bates 2001).
Description of the intervention
New lipid‐associated formulations have been developed to improve the safety profile of conventional amphotericin B including liposomal amphotericin B, amphotericin B lipid complex and amphotericin B colloidal dispersion (Graybill 1996; Kleinberg 2006). These formulations appear to offer advantages over the conventional formulation, notably a marked decrease in nephrotoxicity (Bassetti 2011; Noguchi 2013). An earlier Cochrane review reported increased nephrotoxicity associated with amphotericin B deoxycholate compared with lipid soluble formulations (Johansen 2014); however, the patient population evaluated was limited to those with cancer and neutropenia and did not account for other patient populations at high risk of nephrotoxicity.
How the intervention might work
The liposomal formulation of amphotericin B incorporates the antifungal drug into a liposomal bilayer, composed for phospholipids and cholesterol. When the liposome reaches the fungal cell, it is disrupted and the drug is released into the fungal cell membrane where it binds to the ergosterol. The liposome keeps its integrity in the presence of mammalian cells resulting in minimal toxicity (Adler‐Moore 2002).
Why it is important to do this review
The clinical relevance of nephrotoxicity is important when pharmacoeconomic analyses of lipid‐associated products are performed that attempt to assign secondary costs to amphotericin B nephrotoxicity. For a 70 kg patient, the estimated acquisition cost of liposomal amphotericin B at a dose of 5 mg/kg/day is approximately USD 1318.8/day; while the cost for amphotericin B deoxycholate at a dose 1 mg/kg/day is approximately USD 63.8/day, according to the average wholesale price (RED BOOK 2014).
Given high acquisition costs, a review of pivotal kidney health endpoints may inform resource allocation decisions for liposomal amphotericin B use, particularly in resource‐limited settings. Other adverse effects of amphotericin B products, such as infusion–related reactions, should also be considered when assessing the relative cost–effectiveness of these agents. Data limitations meant that we evaluated the effects of amphotericin B sodium deoxycholate versus liposomal amphotericin B on kidney function according to a number of kidney function parameters. Liposomal amphotericin B was selected as the comparator because it is a widely used lipid‐associated formulation.
Objectives
This review aimed to assess the effects of amphotericin B deoxycholate versus liposomal amphotericin B on kidney function.
Methods
Criteria for considering studies for this review
Types of studies
All RCTs and cluster‐RCTs comparing amphotericin B deoxycholate sodium versus liposomal amphotericin B were eligible for inclusion regardless of publication language.
Types of participants
Inclusion criteria
Patients diagnosed with proven, probable or possible invasive fungal infection were included, as well as those with documented or suspected neutropenia (absolute neutrophil count < 500 cells/mm³), those considered at high risk for developing invasive fungal infection by investigators, and those with other infectious diseases where amphotericin B is used as primary treatment.
Exclusion criteria
Patients receiving any form of parenteral amphotericin B within 10 days prior to study drug administration were excluded.
Types of interventions
Studies reporting patients receiving amphotericin B sodium deoxycholate alone or in combination with other licensed antifungal agents (control group) versus liposomal amphotericin B alone or in combination with other licensed antifungal agents (treatment group) irrespective of dose and/or duration of treatment were included. Amphotericin B formulations were administered intravenously.
Types of outcome measures
Primary outcomes
Increase in serum creatinine (SCr) level ≥ than two‐fold from baseline.
Secondary outcomes
50% increase in SCr occurring at any time during the study period
Discontinuation of amphotericin B therapy due to nephrotoxicity as determined by the investigators
Increase in SCr > 2 mg/dL at any time during the study period
Change in creatinine clearance (CrCl) from beginning to end of the study
Infusion‐related reactions as determined by the investigators.
Search methods for identification of studies
Electronic searches
We searched Cochrane Kidney and Transplant's Specialised Register to 10 March 2015 through contact with the Trials Search Co‐ordinator using search terms relevant to this review. The Specialised Register contains studies identified from the following sources.
Monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL)
Weekly searches of MEDLINE OVID SP
Handsearching of renal‐related journals and the proceedings of major renal conferences
Searching of the current year of EMBASE OVID SP
Weekly current awareness alerts for selected renal journals
Searches of the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
Studies contained in the Specialised register, are identified through search strategies for CENTRAL, MEDLINE, and EMBASE based on the scope of Cochrane Kidney and Transplant. Details of these strategies, as well as a list of handsearched journals, conference proceedings and current awareness alerts, are available in the Specialised Register section of information about the Cochrane Kidney and Transplant.
See Appendix 1 for search terms used in strategies for this review.
Searching other resources
Reference lists of review articles, relevant studies and clinical practice guidelines.
Letters seeking information about unpublished or incomplete studies to investigators known to be involved in previous studies.
Data collection and analysis
Selection of studies
The search strategy described was used to obtain titles and abstracts of studies relevant to the review. Titles and abstracts were screened independently by two authors, who discarded studies that were not applicable. However, studies and reviews thought to potentially include relevant data or information on studies were retained initially. Two authors independently assessed retrieved abstracts, and where necessary the full text of studies to determine which satisfied the inclusion criteria
Data extraction and management
Data extraction was carried out independently by two authors using a standardised data extraction form. Studies reported in languages other than English and Spanish were translated before assessment. Where more than one publication of one study existed, reports were grouped together and the publication with the most complete data was used in the analyses. Where relevant outcomes were only published in earlier versions, these data were used. Any discrepancies between published versions were to be highlighted.
Assessment of risk of bias in included studies
The following items were assessed independently by two authors using the risk of bias assessment tool (Higgins 2011) (Appendix 2).
Was there an adequate sequence generation (selection bias)?
Was allocation adequately concealed (selection bias)?
-
Was knowledge of the allocated interventions adequately prevented during the study?
Participants and personnel (performance bias)
Outcome assessors (detection bias)
Were incomplete outcome data adequately addressed (attrition bias)?
Are reports of the study free of suggestion of selective outcome reporting (reporting bias)
Was the study apparently free of other problems that could put it at a risk of bias?
Measures of treatment effect
We reported estimates for dichotomous outcomes (e.g. numbers of patients with increased SCr ≥ two‐fold from baseline level) as risk ratio (RR) with associated 95% confidence interval (CI). We calculated measures of effect by applying the random‐effects model; the Mantel‐Haenszel method (Mantel 1959) was used to pool results.
Unit of analysis issues
No cluster‐RCTs were found in the search. In studies with multiple arms (specifically those with two liposomal amphotericin B arms), data were pooled for both arms; where there were multiple arms, where one was not liposomal amphotericin B, conventional and liposomal formulation arms were included in the analysis.
Dealing with missing data
Any further information required from the original author was requested by written correspondence (e.g. e‐mailing and/or writing to corresponding author/s) and any relevant information obtained in this manner was included in the review. For all outcomes, we presented data for all randomised participants where reported; otherwise, estimates were based on complete case data.
Assessment of heterogeneity
We considered clinical heterogeneity (the degree to which RCTs varied in terms of participants, interventions, doses, duration and frequency) and statistical heterogeneity. Statistical heterogeneity was assessed using the Chi² test (P < 0.10 was considered to indicate statistically significant heterogeneity) in conjunction with the I² statistic (Higgins 2003). I² values of 25%, 50% and 75% correspond to low, medium and high levels of heterogeneity.
Assessment of reporting biases
We planned to investigate potential for small study bias by constructing and interpreting funnel plots (Higgins 2011). Our analysis found no substantial risk for small study bias.
Data synthesis
Pooled estimates of treatment effect were derived using the random‐effects model. subgroups and sensitivity analyses were conducted to prove the robustness of the model. All outcomes were dichotomous and the summary estimate as a risk ratio (RR) was presented with 95% CI.
Subgroup analysis and investigation of heterogeneity
Subgroup analyses were conducted to explore possible sources of heterogeneity. Heterogeneity in treatments could be related to prior drug(s) used, dose, duration, indication for use of amphotericin B and concomitant nephrotoxic drug use.
Sensitivity analysis
Sensitivity analyses were performed to explore the influence of the following factors on effect size:
Repeating the analysis taking risk of bias into account
Repeating the analysis excluding any very long or large studies to establish how much they dominate the results
Repeating the analysis excluding each study in turn
Excluding studies with dose or duration of liposomal amphotericin B off‐label.
Summary of findings table
Results relating to the primary outcome are presented in Table 1, which also includes an overall assessment of the evidence related to each of the main outcomes using the GRADE approach (Guyatt 2011).
Results
Description of studies
See: Characteristics of included studies and Characteristics of excluded studies
Results of the search
Our search identified 42 records of 33 studies that were assessed as full text articles. Following assessment, 26 records were excluded: 21 investigated interventions that were not relevant to this review and one study was a pharmacoeconomic. We included 12 studies that reported data for 2298 participants in the qualitative synthesis and 10 studies (2172 participants) in the meta‐analyses (Figure 1).
1.
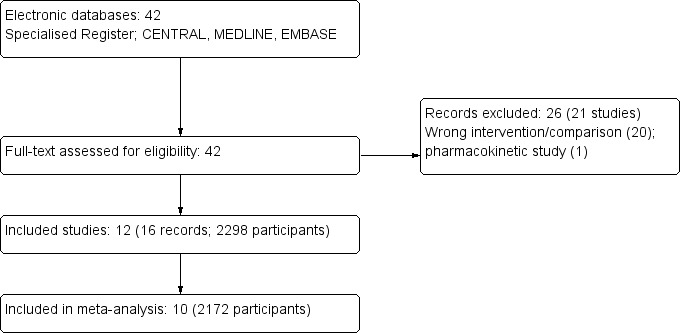
Study flow diagram
Included studies
We included 12 studies (2298 participants) in this review (Bodhe 2002; Hamill 2010; Jadhav 2012; Johnson 2002a; Leenders 1997; Leenders 1998; Prentice 1997; Shah 2005; Sundar 2010; Sundar 2004; Thakur 2001; Walsh 1999). Ten studies (2172 participants) were meta‐analysed (see Characteristics of included studies). Two studies could not be included in the meta‐analysis: Shah 2005 was the only study which assessed CrCl in mL/min and Thakur 2001 only reported rise in SCr (is not possible to know the magnitude of the change respect baseline values).
Participants
Participants included suspected or proven systemic fungal infection (Bodhe 2002); HIV or AIDS and cryptococcal meningitis (Hamill 2010; Leenders 1997); AIDS and moderate to severe histoplasmosis (Johnson 2002a); neutropenia (Leenders 1998); neutropenia and suspected invasive fungal infection (Jadhav 2012); neutropenia and fever of unknown origin (Prentice 1997; Walsh 1999); liver transplantation (Shah 2005); and signs or symptoms of visceral leishmaniasis (Sundar 2004; Sundar 2010; Thakur 2001).
Participants' ages were reported in 10 studies (two studies specified numbers of enrolled adults and children as a categorical variable (Bodhe 2002; Prentice 1997). Two studies did not report participants' sex (Jadhav 2012; Leenders 1997) and Shah 2005reported sex by episode of treatment.
Setting
Seven studies were multicentric and five were undertaken in one centre. Ten studies were undertaken in inpatients. The countries included in studies are: India, USA, Canada, Dutch, Australia, France and UK (see Characteristics of included studies).
Design
All included studies were RCTs. Eight studies included two arms and four studies were designed around three comparisons: Hamill 2010, Jadhav 2012 and Prentice 1997 investigated two doses of liposomal amphotericin B versus conventional amphotericin B and Sundar 2004 included an amphotericin B lipid complex (ABLC) arm, a liposomal amphotericin arm and the control group with conventional amphotericin.
Interventions
The comparator in all included studies was conventional amphotericin B (sodium deoxycholate salt); four studies reported the trade name of the treatment received for the control group (Fungizone®) (Bodhe 2002; Prentice 1997; Sundar 2004; Sundar 2010); in nine studies the treatment group received liposomal amphotericin B as amBisome® (Hamill 2010; Johnson 2002a; Leenders 1997; Leenders 1998; Prentice 1997; Shah 2005; Sundar 2004; Thakur 2001; Walsh 1999); and one study (Bodhe 2002) the liposomal formulation was manufactured in‐house at the Seth GS Medical College and King Edward Memorial Hospital in Mumbai (L‐AMP‐LRC‐1).
Three studies compared 1 mg/kg liposomal amphotericin B (L‐AMB) with 1 mg/kg conventional amphotericin B (C‐AMB) (Bodhe 2002; Jadhav 2012; Prentice 1997). Jadhav 2012 and Prentice 1997 also compared 3 mg/kg L‐AMB with 1 mg/kg C‐AMB
Hamill 2010 compared 3 mg/kg and 6 mg/kg L‐AMB with 0.7 mg/kg C‐AMB
Walsh 1999 compared 3 mg/kg L‐AMB with 0.6 mg/kg C‐AMB
Johnson 2002a compared 3 mg/kg L‐AMB with 0.7 mg/kg C‐AMB
Leenders 1997 compared 4 mg/kg L‐AMB with 0.7 mg/kg C‐AMB
Leenders 1998 compared 5 mg/kg L‐AMB with 1 mg/kg C‐AMB
Shah 2005 compared 50 mg/d L‐AMB with 15 mg/d C‐AMB
Sundar 2004 compared 2 mg/kg L‐AMB with 1 mg/kg C‐AMB
Sundar 2010 compared 10 mg/kg L‐AMB with 1 mg/kg C‐AMB
Thakur 2001 compared 15 mg/kg L‐AMB with 1 mg/kg C‐AMB.
Premedication to prevent infusion‐related reactions was permitted in eight studies (Jadhav 2012; Johnson 2002a; Leenders 1997; Leenders 1998; Prentice 1997; Sundar 2004; Sundar 2010; Walsh 1999), however Jadhav 2012, Johnson 2002a and Walsh 1999 allowed premedication before the first dose of the study medication. Hamill 2010 used only IV saline load and Bodhe 2002 and Thakur 2001 did not report if premedication was allowed.
Excluded studies
We excluded 26 records of 21 studies. Of these, 20 studies investigated interventions or comparators outside the scope of this review, and one was a pharmacoeconomic analysis (see Characteristics of excluded studies).
Risk of bias in included studies
Overall, study quality was suboptimal. The risk of bias in included studies was low or unclear for most domains. However, blinding of participants and personnel, blinding of outcome assessment and other bias (funding) tended to have a high risk of bias. See Figure 2 and Figure 3.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
3.
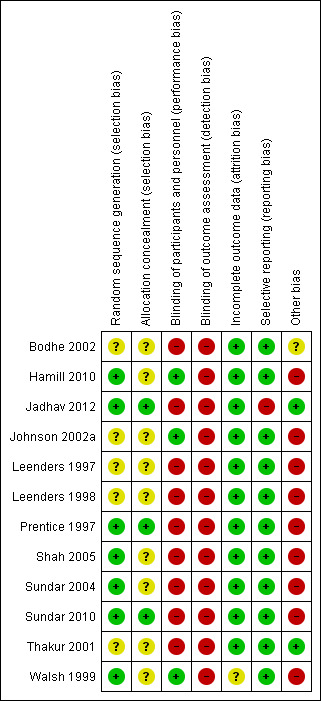
Risk of bias summary: review authors' judgements about each risk of bias item for each included study
Allocation
Seven studies clearly described random sequence generation, and were assessed at low risk of bias (Hamill 2010; Jadhav 2012; Prentice 1997; Shah 2005; Sundar 2004; Sundar 2010; Walsh 1999). Five studies did not include reporting of sequence generation methods and were deemed at unclear risk (Bodhe 2002; Johnson 2002a; Leenders 1997; Leenders 1998; Thakur 2001).
Five studies reported using sealed or closed envelopes, but did not report if envelopes were opaque and sequentially numbered, and were assessed as unclear risk of bias for this domain (Johnson 2002a; Leenders 1997; Leenders 1998; Shah 2005; Sundar 2004). Four studies did not report on the method of allocation concealment, and were also considered being at unclear risk of bias (Bodhe 2002; Hamill 2010; Thakur 2001; Walsh 1999). Three studies adequately reported concealment methods (Jadhav 2012; Prentice 1997; Sundar 2010) and were assessed at low risk of bias.
Blinding
Three studies (Hamill 2010, Johnson 2002a; Walsh 1999) were double‐blinded and assessed at low risk of bias. Nine studies were assessed at high risk of bias in relation to blinding; of these, four were described as open (Bodhe 2002; Sundar 2004; Sundar 2010; Shah 2005) and five studies did not report blinding participants or study personnel (Jadhav 2012; Leenders 1997; Leenders 1998; Prentice 1997; Thakur 2001).
None of the included studies provided statements about blinding of outcomes assessment. All were assessed at high risk of bias for this domain.
Incomplete outcome data
There was no missing outcome data in six studies (Hamill 2010; Leenders 1997; Leenders 1998; Sundar 2004; Shah 2005; Thakur 2001) and were assessed at low risk of bias. In one study, assessment was not possible due to the lack of information, so it was also assessed as at unclear risk of bias (Walsh 1999). In three studies (Bodhe 2002; Jadhav 2012; Johnson 2002a) missing outcome data were balanced in numbers across intervention groups and were assessed at low risk of bias. In Prentice 1997, missing outcome data for efficacy (an outcome different of the relevant to this review) were explained and the intention‐to‐treat analysis (ITT) was performed, and in one study no patient data were lost in follow‐up (Sundar 2010); these studies were also judged at low risk of bias of attrition bias.
Selective reporting
Most study protocols were unobtainable, so judgements were based on the agreement between the methods and the results' of the study's reports. One study was classified at high risk of bias for this domain (Jadhav 2012); the study failed to include results for a key outcome (nephrotoxicity) that would be expected to be reported, given that in methods, the authors' statement is that haematological and biochemical parameters were monitored twice weekly, including SCr levels.
Other potential sources of bias
Bodhe 2002 was assessed as unclear risk of other sources of bias due to insufficient information to permit judgement.
Pharmaceutical funding was identified in seven studies (Hamill 2010; Johnson 2002a; Leenders 1997; Leenders 1998; Prentice 1997; Shah 2005; Walsh 1999) and these studies were assessed at high risk of bias.
Sundar 2004 and Sundar 2010 were assessed at high risk of bias due to an independent sample t‐test, being used to detect differences in clinical and laboratory results (including change in creatinine) for both study groups. Tests for normal distribution were not shown or reported.
Although there were relatively few studies included in the meta‐analysis (10), a funnel plot was constructed to assess the potential existence of small study bias (Figure 4). The plot showed a lack of small studies in favour of the conventional formulation.
4.

Funnel plot of comparison: 1 Comparison between liposomal and conventional amphotericin B, outcome: 1.1 Increase in serum creatinine level ≥ two‐fold increase from baseline level
Effects of interventions
See: Table 1
Increase in serum creatinine
Ten studies (Bodhe 2002; Hamill 2010; Jadhav 2012; Johnson 2002a; Leenders 1997; Leenders 1998; Prentice 1997; Sundar 2004; Sundar 2010; Walsh 1999) compared liposomal amphotericin B with conventional amphotericin B and were included in the meta‐analysis. There was a significant increase in SCr level: ≥ two‐fold from baseline level with conventional amphotericin B compared to liposomal amphotericin B (Analysis 1.1 (10 studies, 2172 participants): RR 0.49, 95% CI 0.40 ‐ 0.59; I2 = 0%).
1.1. Analysis.
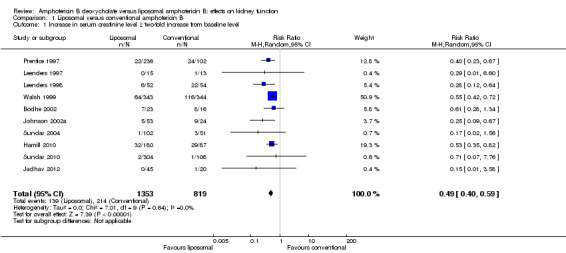
Comparison 1 Liposomal versus conventional amphotericin B, Outcome 1 Increase in serum creatinine level ≥ two‐fold increase from baseline level.
Shah 2005 and Thakur 2001 were excluded from the meta‐analysis. Shah 2005 (87 participants) reported the mean CrCl at the start and end of therapy with liposomal and conventional amphotericin B; for liposomal amphotericin B, the starting mean CrCl (mL/min) was 33.65 and at the end was 40.61 (P = 0.661). In the conventional group, the initial mean CrCl was 38.65 and at the end was 44.23 (P = 0.805). Thakur 2001 (34 participants) reported rise in SCr of 23% with conventional amphotericin B and 0% with liposomal amphotericin B. We were unsuccessful in our attempts to contact the authors for clarification.
Infusion‐related reactions
Nine studies (Hamill 2010; Jadhav 2012; Johnson 2002a; Leenders 1997; Leenders 1998; Sundar 2004; Sundar 2010; Thakur 2001; Walsh 1999) compared liposomal amphotericin B with conventional amphotericin B (sodium deoxycholate). There was significant decrease in all infusion‐related reactions in the liposomal group compared with the conventional group (Analysis 1.2): fever (4 studies, 1092 participants): RR 0.39, 95% CI 0.28 to 0.55; I2 = 32%); chills and/or rigours (5 studies, 1081 participants): RR 0.27, 95% CI 0.15 to 0.48; I2 = 75%); fever and/or rigours (2 studies, 720 participants): RR 0.68, 95% CI 0.52 to 0.90; I2 = 58%); nausea (6 studies, 1187 participants): RR 0.50, 95% CI 0.35 to 0.72; I2 = 0%); and vomiting (3 studies, 1019 participants): RR 0.51, 95% CI 0.27 to 0.95; I2 = 61%).
1.2. Analysis.

Comparison 1 Liposomal versus conventional amphotericin B, Outcome 2 Infusion‐related reactions (as determined by the investigators).
Sensitivity analyses
Sensitivity analyses were performed to explore the influence of the following factors on effect size.
Repeating the analyses taking account of risk of bias: in studies at high risk of bias the difference is not significant (Analysis 2.1; RR 0.16, 95% CI 0.03 to 1.00; I² = 0%); for studies at unclear risk of bias and low risk of bias, the difference is significant (Analysis 2.2; RR 0.39, 95% CI 0.27 to 0.55; I² = 0%) and (Analysis 2.3; RR 0.55, 95% CI 0.44 to 0.69; I² = 0%).
2.1. Analysis.

Comparison 2 Sensitivity analysis for the primary outcome, Outcome 1 Overall risk of bias: high.
2.2. Analysis.

Comparison 2 Sensitivity analysis for the primary outcome, Outcome 2 Overall risk of bias: unclear.
2.3. Analysis.

Comparison 2 Sensitivity analysis for the primary outcome, Outcome 3 Overall risk of bias: low.
Repeating the analyses excluding any very long or large studies to establish how much they dominate the results: We excluded five large studies (1857 participants): Hamill 2010; Prentice 1997; Sundar 2004; Sundar 2010; and Walsh 1999. The difference in the increase in SCr level: ≥ two‐fold from baseline level between the two treatments remained significant (Analysis 2.4; RR 0.36, 95% CI 0.22 to 0.58).
2.4. Analysis.

Comparison 2 Sensitivity analysis for the primary outcome, Outcome 4 Excluding large studies.
Repeating the meta‐analysis as many times as RCT included: a sensitivity analysis was conducted as many times as studies included, excluding one study each time. The total effect estimate was statistically significant (Analysis 2.5; RR 0.48, CI 95% 0.45 to 0.52, I² 0%).
2.5. Analysis.

Comparison 2 Sensitivity analysis for the primary outcome, Outcome 5 Sensitivity analysis excluding one study at a time.
Excluding studies with dose or duration of liposomal amphotericin B off‐label: Eight studies were compared and the total effect estimate was also statistically significant (Analysis 2.6; RR 0.49, 95% CI 0.41 to 0.59, I² = 0%).
2.6. Analysis.
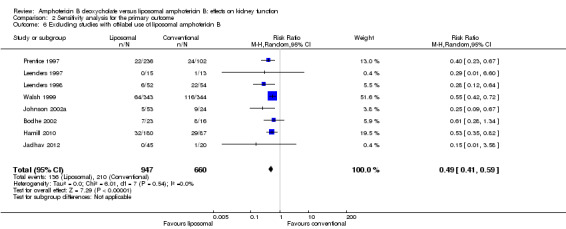
Comparison 2 Sensitivity analysis for the primary outcome, Outcome 6 Excluding studies with off‐label use of liposomal amphotericin B.
Subgroup analysis
Two subgroup analyses were also conducted. One looked at the number of days of treatment (fewer than five days and at least 11 days); the second was based in diagnostic or clinical indication of amphotericin B (IFI/neutropenia, AIDS or HIV infected + cryptococcal meningitis or histoplasmosis and visceral leishmaniasis). Only in the subgroup of fewer than five days of treatment and visceral leishmaniasis (both included the same studies), the difference was not significant (Analysis 3.1.1 (2 studies, 565 participants): RR 0.33, 95% CI 0.06 to 1.68; I2 = 0%). There is a need to clarify that these two studies (Sundar 2004; Sundar 2010) used a dose and therapy duration different from that recommended for visceral leishmaniasis. The first used a dose of 2 mg/kg/d in five consecutive infusions, and the second used a unique dose of amphotericin B of 10 mg/kg. The recommendations state 3 mg/kg/d on days one to five and 3 mg/kg/d on days 14 and 21 for immunocompetent patients, and 4 mg/kg/d on days one to five and 4 mg/kg/d on days 10, 17, 24, 31 and 38 for immunocompromised patients.
3.1. Analysis.

Comparison 3 Subgroup analysis, Outcome 1 Duration of the treatment.
Discussion
Summary of main results
We included 12 studies (2298 participants) in this review. Of these, 10 were able to be meta‐analysed (2172 participants). Bodhe 2002 evaluated patients with suspected or proven IFI; Hamill 2010 and Leenders 1997 included patients with HIV or AIDS and cryptococcal meningitis; Johnson 2002a also included patients with AIDS but with moderate to severe histoplasmosis. Four studies included patients with neutropenia; Leenders 1998 included patients with neutropenia only; participants in Jadhav 2012 had suspected IFI; Prentice 1997 and Walsh 1999 included neutropenic patients with pyrexia or fever of unknown origin; and Sundar 2004 and Sundar 2010 included patients with signs or symptoms of visceral leishmaniasis.
The primary outcome sought was SCr level increase at least two‐fold from baseline. Ten studies were included in the meta‐analysis for this outcome. For the secondary outcome (infusion‐related reactions) it was possible to obtain information about fever, chills and/or rigours, fever and/or rigours, nausea and vomiting. In all the cases, there were significantly less infusion‐related reactions with liposomal amphotericin B compared to conventional amphotericin B.
Overall, risk of bias in included studies was low or unclear for most domains. However, blinding of participants and personnel, blinding of outcome assessment and other bias (funding) tended to have a high risk of bias. Table 1 provides a concise overview and synthesis of the volume and quality of the evidence for the comparison between liposomal and conventional amphotericin B respect to the increase in SCr level ≥ two‐fold from baseline level. Publication bias was not detected and several sensitivity analyses were performed to check the robustness of the effect estimate.
Overall completeness and applicability of evidence
The studies included in this review vary in several aspects (patients (diagnosis or clinical condition), dose of amphotericin received (treatment group and control group), country, risk of bias, methods). However, the patients that would typically receive this treatment are represented. Although two eligible studies were excluded from the meta‐analysis, the included studies allowed us to obtain a clear response for both the primary and secondary outcomes.
The results reported in the included studies did not enable post hoc comparisons of renal replacement therapy or end‐stage kidney disease. Although the elevation in SCr levels is considered a clinical manifestation of AKI and it is associated with an increased risk of morbidity and mortality (Yang 2014), it is a surrogate outcome with poor specificity and sensitivity with regard to recognition of the early period of AKI (Urbschat 2011), and it is uncertain whether reductions in SCr will translate into clinically important benefits.
Quality of the evidence
The results of this review are robust; 10 studies (2172 participants) were included in the meta‐analysis for the primary outcome of which the overall risk of bias was: high for two studies (Jadhav 2012; Sundar 2004), uncertain for six studies (Bodhe 2002; Leenders 1997; Leenders 1998; Prentice 1997; Johnson 2002a; Sundar 2010 and low for the other two (Hamill 2010; Walsh 1999). The main key methodological limitation of the included studies was the blinding of participants and personnel, blinding of outcome assessment and other bias (funding). Subgroup (Analysis 3.1; Analysis 3.2) and sensitivity analyses (Analysis 2.2; Analysis 2.3; Analysis 2.4; Analysis 2.5) indicated that the magnitude and direction of the effect did not change, except in studies at high risk of bias (Analysis 2.1) and when Sundar 2004 and Sundar 2010 were analysed separately. These studies were conducted in patients with visceral leishmaniasis, with treatment doses and duration that differed from the liposomal formulation product information (off‐label).
3.2. Analysis.

Comparison 3 Subgroup analysis, Outcome 2 Diagnostic or clinical indication.
Johansen 2014 concluded that it was unclear if these formulations (all lipid‐based amphotericin B formulations) offered advantages, if conventional amphotericin B is administered under optimal circumstances; in nine of the 10 included studies in this review, loading saline or premedication with antipyretics or antihistamines were allowed for prevention of infusion‐related reactions and nephrotoxicity in the control groups and in some of the treatment groups.
Potential biases in the review process
In addition to electronic searching of bibliographic databases without language restriction, other strategies included contacting study authors and accessing registers of ongoing studies. Although searches were comprehensive, and we did not find evidence of publication bias (Figure 4), two potentially eligible studies were excluded because we were unable to extract or obtain key outcomes data. However it is unlikely that addition of unpublished data would substantially change our overall review findings.
Prentice 1997 reported nephrotoxicity incidence and accounted for use of concomitant nephrotoxic drugs with amphotericin B. This study did not find any differences in the subgroup of patients receiving concomitant nephrotoxic drugs (P = 0.11). This finding is relevant because it is pharmacologically and clinically plausible that treatment involving more than one nephrotoxic drug increases the likelihood of kidney failure, and this should be considered in treatment choices.
Agreements and disagreements with other studies or reviews
Our findings agree with Johansen 2014, who reported decreased nephrotoxicity (defined as 100% increase in SCr) associated with the liposomal formulation (RR 0.45, 95% CI 0.37 to 0.54). Mistro 2012 also suggested that liposomal amphotericin B reduced nephrotoxicity (RR 0.48; 95% CI 0.36 to 0.64). Mistro 2012 included five studies all of which were included in our review. Johansen 2014 included three studies, all of which were also included in this review.
Authors' conclusions
Implications for practice.
Liposomal amphotericin B is less nephrotoxic than conventional amphotericin B, but the high cost precludes routine use in many healthcare settings. Patients being considered to receive the liposomal formulation should be selected on the basis of diagnosis, concomitant nephrotoxic drugs, duration of therapy and dose of amphotericin B. In most included studies, premedication or saline loading was permitted to prevent infusion‐related reactions and nephrotoxicity, therefore, renal effect of each amphotericin B formulation is hard to establish.
Implications for research.
Larger, well‐designed and adequately powered studies comparing conventional amphotericin B with liposomal amphotericin B (including other lipid based formulations) and final endpoints are needed to inform clinical decision making. If this approach is considered to be unethical, new biomarkers (neutrophil gelatinase‐associated lipocalin, cystatin‐C, N‐acetyl‐β‐d‐glucosaminidase, kidney injury molecule‐1, and α1‐microglobulin, among others) with better performance in early stage AKI should be considered for incorporation in clinical practice and investigated in few studies.
Investigators should also consider adopting the recommendations of the CONSORT statement (Moher 2010) in the planning and conduct of new studies.
Analyses of cost‐effectiveness would beneficial; there are currently insufficient efficacy, safety and quality data to inform cost‐effectiveness analyses.
Acknowledgements
We wish to thank to Cochrane Kidney and Transplant for their support of this review and the referees for their comments and feedback during the preparation of this review. We also thank Dr Jadhav for providing additional study information.
Appendices
Appendix 1. Electronic search strategies
| Database | Search terms |
| CENTRAL | 1. MeSH descriptor Amphotericin B, this term only 2. deoxycholate*:ti,ab,kw 3. (#1 AND #2) in trials 4. amphotericin b deoxycholate*:ti,ab,kw in Trials 5. fungizone:ti,ab,kw or fungisone:ti,ab,kw in Trials 6. ("c‐amb":ti,ab,kw or amb:ti,ab,kw) in Trials 7. conventional next amphotericin b:ti,ab,kw in Trials 8. ambd:ti,ab,kw in Trials 9. (#3 OR #4 OR #5 OR #6 OR #7 OR #8) 10. MeSH descriptor Amphotericin B, this term only 11. liposom*:ti,ab,kw 12. (#10 AND #11) 13. "l‐amb":ti,ab,kw in Trials 14. ambisome:ti,ab,kw in Trials 15. liposomal amphotericin b:ti,ab,kw in Trials 16. (#12 OR #13 OR #14 OR #15) 17. (#9 AND #16) |
| MEDLINE | 1. Amphotericin B/ and Deoxychol$.tw. 2. Amphotericin B deoxycholate$.tw. 3. fungizone.tw. 4. ("c‐amb" or amb).tw. 5. (conventional adj2 amphotericin b).tw. 6. ambd.tw. 7. polyene antifungal$.tw. 8. or/1‐7 9. Amphotericin B/ and Liposom$.tw. 10. ("l‐amb" or ablc).tw. 11. ambisome.tw. 12. liposomal amphotericin b.tw. 13. polyene antifungal$.tw. 14. or/9‐13 15. and/8,14 |
| EMBASE | 1. Amphotericin B Deoxycholate/ 2. Amphotericin B deoxycholate$.tw. 3. fungizone.tw. 4. ("c‐amb" or amb).tw. 5. (conventional adj2 amphotericin b).tw. 6. ambd.tw. 7. polyene antifungal$.tw. 8. or/1‐7 9. Amphotericin B/ and Liposom$.tw. 10. ("l‐amb" or ablc).tw. 11. ambisome.tw. 12. liposomal amphotericin b.tw. 13. polyene antifungal$.tw. 14. or/9‐13 15. and/8,14 |
Appendix 2. Risk of bias assessment tool
| Potential source of bias | Assessment criteria |
|
Random sequence generation Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence |
Low risk of bias: Random number table; computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; minimization (minimization may be implemented without a random element, and this is considered to be equivalent to being random). |
| High risk of bias: Sequence generated by odd or even date of birth; date (or day) of admission; sequence generated by hospital or clinic record number; allocation by judgement of the clinician; by preference of the participant; based on the results of a laboratory test or a series of tests; by availability of the intervention. | |
| Unclear: Insufficient information about the sequence generation process to permit judgement. | |
|
Allocation concealment Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment |
Low risk of bias: Randomisation method described that would not allow investigator/participant to know or influence intervention group before eligible participant entered in the study (e.g. central allocation, including telephone, web‐based, and pharmacy‐controlled, randomisation; sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes). |
| High risk of bias: Using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure. | |
| Unclear: Randomisation stated but no information on method used is available. | |
|
Blinding of participants and personnel Performance bias due to knowledge of the allocated interventions by participants and personnel during the study |
Low risk of bias: No blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding; blinding of participants and key study's personnel ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding; blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Blinding of outcome assessment Detection bias due to knowledge of the allocated interventions by outcome assessors. |
Low risk of bias: No blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding; blinding of outcome assessment ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding; blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Incomplete outcome data Attrition bias due to amount, nature or handling of incomplete outcome data. |
Low risk of bias: No missing outcome data; reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias); missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size; missing data have been imputed using appropriate methods. |
| High risk of bias: Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; ‘as‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation; potentially inappropriate application of simple imputation. | |
| Unclear: Insufficient information to permit judgement | |
|
Selective reporting Reporting bias due to selective outcome reporting |
Low risk of bias: The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way; the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon). |
| High risk of bias: Not all of the study’s pre‐specified primary outcomes have been reported; one or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not pre‐specified; one or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; the study report fails to include results for a key outcome that would be expected to have been reported for such a study. | |
| Unclear: Insufficient information to permit judgement | |
|
Other bias Bias due to problems not covered elsewhere in the table |
Low risk of bias: The study appears to be free of other sources of bias. |
| High risk of bias: Had a potential source of bias related to the specific study design used; stopped early due to some data‐dependent process (including a formal‐stopping rule); had extreme baseline imbalance; has been claimed to have been fraudulent; had some other problem. | |
| Unclear: Insufficient information to assess whether an important risk of bias exists; insufficient rationale or evidence that an identified problem will introduce bias. |
Data and analyses
Comparison 1. Liposomal versus conventional amphotericin B.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Increase in serum creatinine level ≥ two‐fold increase from baseline level | 10 | 2172 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.40, 0.59] |
| 2 Infusion‐related reactions (as determined by the investigators) | 9 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Fever | 4 | 1092 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.28, 0.55] |
| 2.2 Chills and/or rigours | 5 | 1081 | Risk Ratio (M‐H, Random, 95% CI) | 0.27 [0.15, 0.48] |
| 2.3 Fever and/or rigours | 3 | 720 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.52, 0.90] |
| 2.4 Nausea | 6 | 1187 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.35, 0.72] |
| 2.5 Vomiting | 3 | 1019 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.27, 0.95] |
Comparison 2. Sensitivity analysis for the primary outcome.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall risk of bias: high | 2 | 218 | Risk Ratio (M‐H, Random, 95% CI) | 0.16 [0.03, 1.00] |
| 2 Overall risk of bias: unclear | 6 | 1000 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.27, 0.55] |
| 3 Overall risk of bias: low | 2 | 954 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.44, 0.69] |
| 4 Excluding large studies | 5 | 315 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.22, 0.58] |
| 5 Sensitivity analysis excluding one study at a time | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Excluded Leenders 1997 | 9 | 2144 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.40, 0.59] |
| 5.2 Excluded Prentice1997 | 9 | 1834 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.38, 0.61] |
| 5.3 Excluded Leenders 1998 | 9 | 2066 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.41, 0.61] |
| 5.4 Excluded Walsh 1999 | 9 | 1485 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.32, 0.55] |
| 5.5 Excluded Johnson 2002a | 9 | 2095 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.41, 0.60] |
| 5.6 Excluded Bodhe 2002 | 9 | 2133 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.36, 0.58] |
| 5.7 Excluded Sundar 2004 | 9 | 2019 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.41, 0.59] |
| 5.8 Excluded Hamill 2010 | 9 | 1915 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.34, 0.58] |
| 5.9 Excluded Sundar 2010 | 9 | 1770 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.37, 0.59] |
| 5.10 Excluded Jadhav 2012 | 9 | 2117 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.39, 0.59] |
| 6 Excluding studies with off‐label use of liposomal amphotericin B | 8 | 1607 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.41, 0.59] |
Comparison 3. Subgroup analysis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Duration of the treatment | 10 | 2172 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.40, 0.59] |
| 1.1 Fewer than 5 days of treatment | 2 | 565 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.06, 1.68] |
| 1.2 At least 11 days of treatment | 8 | 1607 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.41, 0.59] |
| 2 Diagnostic or clinical indication | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Invasive fungal infection/neutropenia | 5 | 1235 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.40, 0.62] |
| 2.2 AIDS or HIV infected + cryptococcal meningitis or histoplasmosis | 3 | 372 | Risk Ratio (M‐H, Random, 95% CI) | 0.13 [0.03, 0.50] |
| 2.3 Visceral leishmaniasis | 2 | 565 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.06, 1.68] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bodhe 2002.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | No information on method included |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of personnel and patients |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced between intervention groups (three in the intervention group and two in the control group) |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Hamill 2010.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation via centralised telephone centre |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Each infusion bag or bottle and tubing was modified to prevent distinction between liposomal amphotericin B and amphotericin B, because the drugs differ in appearance |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | Include all expected outcomes |
| Other bias | High risk | Financial support: Astellas Pharma and, in part, the Department of Veterans Affairs and the National Center for Research Resources, General Clinical Research Center Manuscript preparation. Astellas Pharma provided assistance with study design and execution, as well as statistical analyses |
Jadhav 2012.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Before administration the study drug liposomal amphotericin B (stored at 2 to 8°C) was sonicated using a bath sonicator with thermostat, at ambient temperature for 45 min to convert multilamellar vesicles into unilamellar vesicles Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated central randomisation in blocks of six |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes with a unique centre and identification number. Participants who met the inclusion criteria were assigned ID numbers and randomised as per randomisation plan |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of personnel and participants |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced among interventions |
| Selective reporting (reporting bias) | High risk | The study did not include results for a key outcome (i.e. nephrotoxicity) that would be expected, given that the methods reported recording of haematological and biochemical parameters including SCr twice weekly |
| Other bias | Low risk | The study appears to be free of other sources of bias Funding source: Department of Biotechnology, New Delhi |
Johnson 2002a.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not reported |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelope |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced between intervention groups |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported |
| Other bias | High risk | This study was supported by a research grant from the National Institute of Allergy and Infectious Diseases (NIAID) and the National Center for Research Resources, and by Gilead Sciences, Inc |
Leenders 1997.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not reported |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelope |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported |
| Other bias | High risk | The study was supported by grants from NeXstar Pharmaceuticals Inc, San Dimas, California, USA |
Leenders 1998.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Sodium supplementation prior to conventional amphotericin B infusion to prevent nephrotoxicity and medication to prevent acute reactions permitted |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of personnel and patients |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported |
| Other bias | High risk | The study was supported by grants from NeXstar Pharmaceuticals Inc., San Dimas, California, USA |
Prentice 1997.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Control group
There were no restrictions on use of premedication such as antihistamines, steroids, pethidine or paracetamol |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central randomisation; each participating centre provided with blinded, numbered envelopes which required sequential opening |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of personnel and patients |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data for efficacy were explained and ITT analysis performed |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported |
| Other bias | High risk | These studies were made possible through an educational grant and free AmBisome supplies, by Nexstar Inc, USA |
Shah 2005.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Information about the method of randomisation is described (random selection of sealed envelopes) |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of personnel and patients |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported |
| Other bias | High risk | Funding by Gilead |
Sundar 2004.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Control group
Patients in control group received antipyretic (paracetamol 500 mg) and antihistamine treatment (chlorpheniramine 50 mg) before each infusion |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence generation by computer‐based random number generator |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of personnel and patients |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported |
| Other bias | High risk | Paired‐sample t‐test was used to compare pre and post treatment values in each group, the test for normal distribution is not shown |
Sundar 2010.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Patients in the conventional therapy group who had IRR required subsequent oral pretreatment with an antipyretic (paracetamol) and an antihistamine (chlorpheniramine) with the use of standard paediatric or adult doses |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐based random number generator |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes using a computer‐based random number generator |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of personnel and patients |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up |
| Selective reporting (reporting bias) | Low risk | Protocol available and all prespecified outcomes were reported |
| Other bias | High risk | An independent sample t‐test was used to detect differences in clinical and laboratory results (included SCr) for the two study groups, test for normal distribution was not shown Supported by the Sitaram Memorial Trust and the Varanasi Physicians Research and Education Foundation |
Thakur 2001.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information on method used is described |
| Allocation concealment (selection bias) | Unclear risk | not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | not reported |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patient was lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All outcomes pre‐specified reported |
| Other bias | Low risk | The authors did not identified another possible source of bias Funding source: Balaji Utthan Sansthan |
Walsh 1999.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Before the first infusion, no premedication for the prevention of infusion‐related reactions were permitted. If a patient had an infusion‐related toxic reaction during the first infusion, it was treated. For subsequent infusions, appropriate premedication were administered at the discretion of the blinded investigator or primary physician |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computerised system randomly assigned treatment according to centre and risk stratum |
| Allocation concealment (selection bias) | Unclear risk | The pharmacist at the patient’s institution telephoned a central randomisation centre to obtain a drug assignment. No information about the concealment of the allocation is stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | To maintain the blinded conditions, the study drug concentrations were adjusted so that the volume of solution in the IV bag was the same for both study drugs. Infusion bottles were concealed by opaque bags and infusion tubing was either opaque or covered with opaque wrapping |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported |
| Other bias | High risk | Pharmaceutical funding |
CrCl ‐ creatinine clearance; ICU ‐ intensive care unit; Hb ‐ haemoglobin; HIV ‐ human immunodeficiency virus; IFI ‐ invasive fungal infection; IV ‐ intravenous; M/F ‐ male/female; RCT ‐ randomised controlled trial; SCr ‐ serum creatinine; SGOT ‐ serum glutamic oxaloacetic transaminase; SGPT ‐ serum glutamic pyruvic transaminase; WCC ‐ white cell count; ULN ‐ upper limit of normal
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Anaissie 1995 | Wrong intervention/comparison: amphotericin B lipid complex versus conventional amphotericin B |
| Cordonnier 2009 | Wrong intervention/comparison: broad spectrum betalactam and antifungal prophylaxis as needed |
| Drew 2004 | Wrong intervention/comparison: comparative safety of amphotericin B lipid complex and amphotericin B deoxycholate as aerosolized antifungal prophylaxis |
| Echevarria 2006 | Wrong intervention/comparison: oral rehydration solution to prevent nephrotoxicity of amphotericin B |
| Eriksson 2001 | Wrong intervention/comparison: comparison of effects of amphotericin B deoxycholate infused over 4 or 24 hours |
| Fleming 2001 | Wrong intervention/comparison: liposomal amphotericin B versus amphotericin B lipid complex |
| Heinemann 1994 | Study of pharmacokinetic characteristics |
| Isnard 2007 | Wrong intervention/comparison: switch from conventional to liposomal amphotericin B formulation |
| Llanos 1991 | Wrong intervention/comparison: effect of salt supplementation on amphotericin B nephrotoxicity |
| Luke 1998 | Wrong intervention/comparison: amphotericin B lipid complex |
| Moreau 1992 | Wrong intervention/comparison: amphotericin B mixed with intralipid compared with conventional amphotericin B |
| NCT00002019 | Wrong intervention/comparison: safety and efficacy of amphotericin B lipid complex |
| NCT00523965 | Wrong intervention/comparison: different combination treatment regimens of liposomal amphotericin B, paromomycin and miltefosine |
| NCT01122771 | Wrong intervention/comparison: different combination treatment regimens of liposomal amphotericin B, paromomycin and miltefosine |
| NCT01310738 | Wrong intervention/comparison: active comparator meglumine antimoniate |
| Sharkey 1996 | Wrong intervention/comparison: amphotericin B lipid complex compared with amphotericin B |
| Sorkine 1996 | Wrong intervention/comparison: administration of amphotericin B in lipid emulsion |
| Subira 2004 | Wrong intervention/comparison: amphotericin B lipid complex versus conventional amphotericin B |
| Sundar 2014 | Wrong intervention/comparison: amphotericin B emulsion versus liposomal formulation |
| Tollemar 1995 | Placebo‐controlled trial |
| Wingard 2000 | Wrong intervention/comparison: liposomal amphotericin B versus amphotericin B lipid complex |
Differences between protocol and review
This review differs from the published protocol in the following points:
Measurement of the secondary outcome was only possible to infusion‐related reactions; the others were not possible due to lack of data
A sensitivity analysis was performed investigating overall risk of bias
A sensitivity analysis was carried out excluding studies with dose or duration of liposomal amphotericin B off‐label.
Contributions of authors
Draft the protocol: JPBA
Study selection: JPBA, AMRH
Extract data from studies: JPBA, AMRH
Enter data into RevMan: JPBA
Carry out the analysis: JPBA
Interpret the analysis: JPBA, AMRH
Draft the final review: JPBA, AMRH
Disagreement resolution: JPBA, AMRH
Update the review: JPBA
Sources of support
Internal sources
Hospital Pablo Tobón Uribe, Colombia.
External sources
None, Other.
Declarations of interest
Hospital Pablo Tobón Uribe provides monetary incentives to published review authors to promote research studies.
New
References
References to studies included in this review
Bodhe 2002 {published data only}
- Bodhe PV, Kotwani RN, Kirodian BG, Kshirsagar NA, Pandya SK. Open label, randomised, comparative phase III safety and efficacy study with conventional amphotericin B and liposomal amphotericin B in patients with systemic fungal infection. Journal of the Association of Physicians of India 2002;50(5):662‐70. [MEDLINE: ] [PubMed] [Google Scholar]
Hamill 2010 {published data only}
- Hamill RJ, Sobel JD, Sadr W. Randomized double‐blind trial of AmBisome (liposomal amphotericin B) and amphotericin B in acute cryptococcal meningitis in AIDS patients [abstract no:1161]. Proceedings of the 39th Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC); 1999 Sept 26‐29; San Francisco, CA. 1999.
- Hamill RJ, Sobel JD, El‐Sadr W, Johnson PC, Graybill JR, Javaly K, et al. Comparison of 2 doses of liposomal amphotericin B and conventional amphotericin B deoxycholate for treatment of AIDS‐associated acute cryptococcal meningitis: a randomized, double‐blind clinical trial of efficacy and safety. Clinical Infectious Diseases 2010;51(2):225‐32. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Jadhav 2012 {published data only}
- Jadhav MP, Shinde VM, Chandrakala S, Jijina F, Menon H, Arora B, et al. A randomized comparative trial evaluating the safety and efficacy of liposomal amphotericin B (Fungisome) versus conventional amphotericin B in the empirical treatment of febrile neutropenia in India. Indian Journal of Cancer 2012;49(1):107‐13. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Johnson 2002a {published data only}
- Johnson PC, Wheat LJ, Cloud GA, Goldman M, Lancaster D, Bamberger DM, et al. Safety and efficacy of liposomal amphotericin B compared with conventional amphotericin B for induction therapy of histoplasmosis in patients with AIDS. Annals of Internal Medicine 2002;137(2):105‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Leenders 1997 {published data only}
- Leenders AC, Reiss P, Portegies P, Clezy K, Hop WC, Hoy J, et al. Liposomal amphotericin B (AmBisome) compared with amphotericin B both followed by oral fluconazole in the treatment of AIDS‐associated cryptococcal meningitis. AIDS 1997;11(12):1463‐71. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Leenders 1998 {published data only}
- Leenders AC, Daenen S, Jansen RL, Hop WC, Lowenberg B, Wijermans PW, et al. Liposomal amphotericin B compared with amphotericin B deoxycholate in the treatment of documented and suspected neutropenia‐associated invasive fungal infections. British Journal of Haematology 1998;103(1):205‐12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Prentice 1997 {published data only}
- Prentice HG, Hann IM, Herbrecht R, Aoun M, Kvaloy S, Catovsky D, et al. A randomized comparison of liposomal versus conventional amphotericin B for the treatment of pyrexia of unknown origin in neutropenic patients. British Journal of Haematology 1997;98(3):711‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Shah 2005 {published data only}
- Shah T, Lai WK, Gow P, Leeming J, Mutimer D. Low‐dose amphotericin for prevention of serious fungal infection following liver transplantation. Transplant Infectious Disease 2005;7(3‐4):126‐32. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sundar 2004 {published data only}
- Sundar S, Mehta H, Suresh AV, Singh SP, Rai M, Murray HW. Amphotericin B treatment for Indian visceral leishmaniasis: conventional versus lipid formulations. Clinical Infectious Diseases 2004;38(3):377‐83. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sundar 2010 {published data only}
- Sundar S, Chakravarty J, Agarwal D, Rai M, Murray HW. Single‐dose liposomal amphotericin B for visceral leishmaniasis in India. New England Journal of Medicine 2010;362(6):504‐12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Thakur 2001 {published data only}
- Thakur CP. A single high dose treatment of kala‐azar with Ambisome (amphotericin B lipid complex): a pilot study. International Journal of Antimicrobial Agents 2001;17(1):67‐70. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Walsh 1999 {published data only}
- Cagnoni PJ. Liposomal amphotericin B versus conventional amphotericin B in the empirical treatment of persistently febrile neutropenic patients. Journal of Antimicrobial Chemotherapy 2002;49 Suppl 1:81‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Cagnoni PJ, Hiemenz J, Prendergast M, Arndt C, Bodensteiner D, Greenberg R, et al. Pharmacoeconomic assessment of liposomal amphotericin B versus conventional amphotericin B deoxycholate in the empirical treatment of persistently febrile neutropenic patients [abstract no: 2268]. Journal of Clinical Oncology 1999;17:587a. [CENTRAL: CN‐00692141] [DOI] [PubMed] [Google Scholar]
- Cagnoni PJ, Walsh TJ, Prendergast MM, Bodensteiner D, Hiemenz S, Greenberg RN, et al. Pharmacoeconomic analysis of liposomal amphotericin B versus conventional amphotericin B in the empirical treatment of persistently febrile neutropenic patients.[Erratum appears in J Clin Oncol 2000 Aug;18(16):3064]. Journal of Clinical Oncology 2000;18(12):2476‐83. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Walsh TJ, Finberg RW, Arndt C, Hiemenz J, Schwartz C, Bodensteiner D, et al. Liposomal amphotericin B for empirical therapy in patients with persistent fever and neutropenia. National Institute of Allergy and Infectious Diseases Mycoses Study Group. New England Journal of Medicine 1999;340(10):764‐71. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Anaissie 1995 {published data only}
- Anaissie E, White M, Uzun O. Amphotericin B lipid complex versus amphotericin B for treatment of invasive candidiasis: a prospective, randomized, multicenter trial [abstract no: LM2]. 35th Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC);1995 Sep 17‐20; San Francisco (CA). 1995:330.
Cordonnier 2009 {published data only}
- Cordonnier C, Pautas C, Maury S, Vekhoff A, Farhat H, Suarez F, et al. Empirical versus preemptive antifungal therapy for high‐risk, febrile, neutropenic patients: a randomized, controlled trial. Clinical Infectious Diseases 2009;48(8):1042‐51. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Drew 2004 {published data only}
- Drew RH, Dodds AE, Benjamin DK Jr, Duane RD, Palmer SM, Perfect JR. Comparative safety of amphotericin B lipid complex and amphotericin B deoxycholate as aerosolized antifungal prophylaxis in lung‐transplant recipients. Transplantation 2004;77(2):232‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Echevarria 2006 {published data only}
- Echevarria J, Seas C, Cruz M, Chavez E, Campos M, Cieza J, et al. Oral rehydration solution to prevent nephrotoxicity of amphotericin B. American Journal of Tropical Medicine & Hygiene 2006;75(6):1108‐12. [MEDLINE: ] [PubMed] [Google Scholar]
Eriksson 2001 {published data only}
- Eriksson U, Seifert B, Schaffner A. Comparison of effects of amphotericin B deoxycholate infused over 4 or 24 hours: randomised controlled trial. BMJ 2001;322(7286):579‐82. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fleming 2001 {published data only}
- Fleming RV, Kantarjian HM, Husni R, Rolston K, Lim J, Raad I, et al. Comparison of amphotericin B lipid complex (ABLC) vs. ambisome in the treatment of suspected or documented fungal infections in patients with leukemia. Leukemia & Lymphoma 2001;40(5‐6):511‐20. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Heinemann 1994 {published data only}
- Heinemann V, Kahny B, Debus A, Wachholz K, Jehn U. Pharmacokinetics of liposomal amphotericin B (AmBisome) versus other lipid‐based formulations. Bone Marrow Transplantation 1994;14 Suppl 5:S8‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Isnard 2007 {published data only}
- Isnard F, Laporte JP, Chevallier P, Pigneux A, Tiffeul P, Lafuma A, et al. Benefit of early switch to liposomal amphotericin B in patients with amphotericin B deoxycholate‐induced nephrotoxicity versus late switch [abstract no: 5381]. Blood 2005;106(11 Pt 2):431. [CENTRAL: CN‐00624999] [Google Scholar]
- Isnard F, Tilleul P, Laporte JP, Chevallier P, Pigneux A, Lafuma A, et al. A benefit for renal function after early switch to liposomal amphotericin B from conventional formulation in empirical antifungal treatment. Nephrology Dialysis Transplantation 2007;22(10):3090‐1. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Isnard F, Tilleul P, Laporte JP, Chevallier P, Pigneux A, Lafuma A, et al. Impact on renal function of an early switch from conventional to liposomal amphotericin B formulation in the empirical treatment of fungal infections [Impact sur la fonction renale d'un relais precoce de la formulation conventionnelle de l'amphotericine B par sa formulation liposomale dans le cadre d'un traitement empirique antifongique (Etude Nephemat)]. Medecine et Maladies Infectieuses 2008;38(4):208‐14. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Llanos 1991 {published data only}
- Llanos A, Cieza J, Bernardo J, Echevarria J, Biaggioni I, Sabra R, et al. Effect of salt supplementation on amphotericin B nephrotoxicity. Kidney International 1991;40(2):302‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Luke 1998 {published data only}
- Luke RG, Boyle JA. Renal effects of amphotericin B lipid complex. American Journal of Kidney Diseases 1998;31(5):780‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Moreau 1992 {published data only}
- Moreau P, Milpied N, Fayette N, Ramee JF, Harousseau JL. Reduced renal toxicity and improved clinical tolerance of amphotericin B mixed with intralipid compared with conventional amphotericin B in neutropenic patients. Journal of Antimicrobial Chemotherapy 1992;30(4):535‐41. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
NCT00002019 {published data only}
- al‐Haddadin D. Safety and efficacy of amphotericin B lipid complex in the treatment of cryptococcal meningitis in patients with the acquired immunodeficiency syndrome. www.clinicaltrials.gov/ct2/show/NCT00002019 (accessed 16 November 2015).
NCT00523965 {published data only}
- Sundar S. A randomised, open‐label, parallel‐group, safety and efficacy study to evaluate different combination treatment regimens (co‐administration), of ambisome, paromomycin and miltefosine in visceral leishmaniasis (VL). www.clinicaltrials.gov/ct2/show/NCT00523965 (accessed 16 November 2015).
NCT01122771 {published data only}
- Rahman R. Phase III, open label, randomised, study of three short course combination regimens (ambisome©, miltefosine, paromomycin) compared with ambisome© alone for the treatment of visceral leishmaniasis (VL) in Bangladesh. www.clinicaltrials.gov/ct2/show/NCT01122771 (accessed 16 November 2015).
NCT01310738 {published data only}
- Romero G. Multicentric efficacy and safety study of antileishmanial drugs for treatment of visceral leishmaniasis in Brazil. www.clinicaltrials.gov/ct2/show/NCT01310738 (accessed 16 November 2015).
Sharkey 1996 {published data only}
- Sharkey PK, Graybill JR, Johnson ES, Hausrath SG, Pollard RB, Kolokathis A, et al. Amphotericin B lipid complex compared with amphotericin B in the treatment of cryptococcal meningitis in patients with AIDS. Clinical Infectious Diseases 1996;22(2):315‐21. [MEDLINE: ] [PubMed] [Google Scholar]
Sorkine 1996 {published data only}
- Sorkine P, Nagar H, Weinbroum A, Setton A, Israitel E, Scarlatt A, et al. Administration of amphotericin B in lipid emulsion decreases nephrotoxicity: results of a prospective, randomized, controlled study in critically ill patients. Critical Care Medicine 1996;24(8):1311‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Subira 2004 {published data only}
- Subira M, Martino R, Gomez L, Marti JM, Estany C, Sierra J. Low‐dose amphotericin B lipid complex vs. conventional amphotericin B for empirical antifungal therapy of neutropenic fever in patients with hematologic malignancies‐‐a randomized, controlled trial. European Journal of Haematology 2004;72(5):342‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sundar 2014 {published data only}
- Sundar S, Pandey K, Thakur CP, Jha TK, Das VN, Verma N, et al. Efficacy and safety of amphotericin B emulsion versus liposomal formulation in Indian patients with visceral leishmaniasis: a randomized, open‐label study. PLoS Neglected Tropical Diseases [electronic resource] 2014;8(9):e3169. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tollemar 1995 {published data only}
- Tollemar J, Hockerstedt K, Ericzon BG, Jalanko H, Ringden O. Liposomal amphotericin B prevents invasive fungal infections in liver transplant recipients. A randomized, placebo‐controlled study. Transplantation 1995;59(1):45‐50. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Tollemar J, Hockerstedt K, Ericzon BG, Jalanko H, Ringden O. Prophylaxis with liposomal amphotericin B (AmBisome) prevents fungal infections in liver transplant recipients: long‐term results of a randomized, placebo‐controlled trial. Transplantation Proceedings 1995;27(1):1195‐8. [MEDLINE: ] [PubMed] [Google Scholar]
- Tollemar J, Hockerstedt K, Ericzon BG, Sundberg B, Ringden O. Fungal prophylaxis with AmBisome in liver and bone marrow transplant recipients: results of two randomized double‐blind studies. Transplantation Proceedings 1994;26(3):1833. [MEDLINE: ] [PubMed] [Google Scholar]
- Tollemar J, Ringden O. Double‐blind randomized trials with AmBisome as prophylaxis in bone marrow and liver transplant patients. Bone Marrow Transplantation 1993;12(Suppl 4):S151‐2. [EMBASE: 1994059116] [PubMed] [Google Scholar]
Wingard 2000 {published data only}
- Wingard JR, White MH, Anaissie E, Raffalli J, Goodman J, Arrieta A, et al. A randomized, double‐blind comparative trial evaluating the safety of liposomal amphotericin B versus amphotericin B lipid complex in the empirical treatment of febrile neutropenia. L Amph/ABLC Collaborative Study Group. Clinical Infectious Diseases 2000;31(5):1155‐63. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Additional references
Adler‐Moore 2002
- Adler‐Moore J, Proffitt RT. AmBisome: liposomal formulation, structure, mechanism of action and pre‐clinical experience. Journal of Antimicrobial Chemotherapy 2002;49 Suppl 1:21‐30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Arendrup 2008
- Arendrup MC, Fuursted K, Gahrn‐Hansen B, Schonheyder HC, Knudsen JD, Jensen IM, et al. Semi‐national surveillance of fungaemia in Denmark 2004‐2006: increasing incidence of fungaemia and numbers of isolates with reduced azole susceptibility. Clinical Microbiology & Infection 2008;14(5):487–94. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Armstrong‐James 2014
- Armstrong‐James D, Meintjes G, Brown GD. A neglected epidemic: fungal infections in HIV/AIDS. Trends in Microbiology 2014;22(3):120‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bassetti 2011
- Bassetti M, Aversa F, Ballerini F, Benedetti F, Busca A, Cascavilla N, et al. Amphotericin B lipid complex in the management of invasive fungal infections in immunocompromised patients. Clinical Drug Investigation 2011;31(11):745‐58. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bates 2001
- Bates DW, Su L, Yu DT, Chertow GM, Seger DL, Gomes DR, et al. Mortality and costs of acute renal failure associated with amphotericin B therapy. Clinical Infectious Diseases 2001;32(5):686–93. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Brown 2012
- Brown GD, Denning DW, Levitz SM. Tackling human fungal infections. Science 2012;336(6082):647. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Del Poeta 2012
- Poeta M, Chaturvedi V. Cryptococcus and cryptococcosis in the twenty‐first century. Mycopathologia 2012;173(5‐6):283–5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Graybill 1996
- Graybill JR. Lipid formulations for amphotericin B: does the emperor need new clothes?. Annals of Internal Medicine 1996;124(10):921–3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt G, Oxman AD, Schünemann HJ, Tugwell P, Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. Journal of Clinical Epidemiology 2001;64(4):380‐2. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hajjeh 2004
- Hajjeh RA, Sofair AN, Harrison LH, Lyon GM, Arthington‐Skaggs BA, Mirza SA, et al. Incidence of bloodstream infections due to Candida species and in vitro susceptibilities of isolates collected from 1998 to 2000 in a population‐based active surveillance program. Journal of Clinical Microbiology 2004;42(4):1519‐27. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Horwitz 2012
- Horwitz E, Shavit O, Shouval R, Hoffman A, Shapiro M, Moses AE. Evaluating real‐life clinical and economical burden of amphotericin‐B deoxycholate adverse reactions. International Journal of Clinical Pharmacy 2012;34(4):611‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Husain 2001
- Husain S, Wagener MM, Singh N. Cryptococcus neoformans infection in organ transplant recipients: variables influencing clinical characteristics and outcome. Emerging Infectious Diseases 2001;7(3):375‐81. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Johansen 2014
- Johansen HK, Gøtzsche PC. Amphotericin B lipid soluble formulations versus amphotericin B in cancer patients with neutropenia. Cochrane Database of Systematic Reviews 2014, Issue 9. [DOI: 10.1002/14651858.CD000969.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kleinberg 2006
- Kleinberg M. What is the current and future status of conventional amphotericin B?. International Journal of Antimicrobial Agents 2006;27 Suppl 1:12–6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Laniado‐Laborín 2009
- Laniado‐Laborín R, Cabrales‐Vargas MN. Amphotericin B: side effects and toxicity. Revista Iberoamericana de Micología 2009;26(4):223‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mantel 1959
- Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. Journal of the National Cancer Institute 1959;22(4):719‐48. [PUBMED: 13655060] [PubMed] [Google Scholar]
Mistro 2012
- Mistro S, Maciel Ide M, Menezes RG, Maia ZP, Schooley RT, Badaro R. Does lipid emulsion reduce amphotericin B nephrotoxicity? A systematic review and meta‐analysis. Clinical Infectious Diseases 2012;54(12):1774‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Moher 2010
- Moher D, Hopewell S, Schulz KF, Montori V, Gøtzsche PC, Devereaux PJ, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:c869. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Montagna 2013
- Montagna MT, Caggiano G, Lovero G, Giglio O, Coretti C, Cuna T, et al. Epidemiology of invasive fungal infections in the intensive care unit: Results of a multicenter Italian survey (AURORA Project). Infection 2013;41(3):645–53. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Noguchi 2013
- Noguchi S, Takahashi N, Ito M, Teshima K, Yamashita T, Michishita Y, et al. Safety and efficacy of low‐dose liposomal amphotericin B as empirical antifungal therapy for patients with prolonged neutropenia. International Journal of Clinical Oncology 2013;18(6):983‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ostrosky‐Zeichner 2003
- Ostrosky‐Zeichner L, Marr K, Rex JH, Cohen SH. Amphotericin B: time for a new “gold standard”. Clinical Infectious Diseases 2003;37(3):415–25. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Paramythiotou 2014
- Paramythiotou E, Frantzeskaki F, Flevari A, Armaganidis A, Dimopoulos G. Invasive fungal infections in the ICU: how to approach, how to treat. Molecules 2014;19(1):1085‐119. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pfaller 2010
- Pfaller MA, Diekema DJ. Epidemiology of invasive mycoses in North America. Critical Reviews in Microbiology 2010;36(1):1–53. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Poikonen 2009
- Poikonen E, Lyytikäinen O, Anttila VJ, Kuusela P, Koukila‐Kähkölä P, Ollgren J, et al. Nosocomial candidaemia in a Finnish tertiary care centre during 1987‐2004. Scandinavian Journal of Infectious Diseases 2009;41(8):590‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
RED BOOK 2014
- Truven Health Analytics. Micromedex. Thomson Reuters (Healthcare) Inc, 2014. [Google Scholar]
Urbschat 2011
- Urbschat A, Obermüller N, Haferkamp A. Biomarkers of kidney injury. Biomarkers 2011;16 Suppl 1:S22‐30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Yang 2014
- Yang F, Zhang L, Wu H, Zou H, Du Y. Clinical analysis of cause, treatment and prognosis in acute kidney injury patients. PLoS One [Electronic Resource] 2014;9(2):e85214. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Botero Aguirre 2013
- Botero Aguirre JP, Restrepo Hamid AM. Amphotericin B deoxycholate versus liposomal amphotericin B: effects on kidney function. Cochrane Database of Systematic Reviews 2013, Issue 4. [DOI: 10.1002/14651858.CD010481] [DOI] [PMC free article] [PubMed] [Google Scholar]


