Abstract
Anaplastic lymphoma kinase (ALK) alterations (activating mutations, amplifications, and fusions/rearrangements) occur in ~3.3% of cancers. ALK fusions/rearrangements are discerned in >50% of inflammatory myofibroblastic tumors (IMTs) and anaplastic large cell lymphomas (ALCLs), but only in ~0.2% of other cancers outside of non-small cell lung cancer (NSCLC), a rate that may be below the viability threshold of even large-scale treatment trials. Five ALK inhibitors –alectinib, brigatinib, ceritinb, crizotinib, and lorlatinib—are FDA approved for ALK-aberrant NSCLCs, and crizotinib is also approved for ALK-aberrant IMTs and ALCL, including in children. Herein, we review the pharmacologic tractability of ALK alterations, focusing beyond NSCLC. Importantly, the hallmark of approved indications is the presence of ALK fusions/rearrangements, and response rates of ~50–85%. Moreover, there are numerous reports of ALK inhibitor activity in multiple solid and hematologic tumors (e.g., histiocytosis, leiomyosarcoma, lymphoma, myeloma, and colorectal, neuroendocrine, ovarian, pancreatic, renal, and thyroid cancer) bearing ALK fusions/rearrangements. Many reports used crizotinib or alectinib, but each of the approved ALK inhibitors have shown activity. ALK inhibitor activity is also seen in neuroblastoma, which bear ALK mutations (rather than fusions/rearrangements), but response rates are lower (~10–20%). Current data suggests that ALK inhibitors have tissue-agnostic activity in neoplasms bearing ALK fusions/rearrangements.
Subject terms: Cancer, Cancer genomics
Introduction
Anaplastic lymphoma kinase (ALK) gene alterations are gaining more attention as pan-cancer markers in both solid and hematological malignancies. Activating ALK alterations (mutations, amplifications, fusions/rearrangements) are found in various malignancies including, but not limited to, non-small lung cancer (NSCLC), anaplastic large-cell lymphoma (ALCL) (an uncommon, aggressive CD30-positive T-cell lymphoma comprising 0.5% of adult lymphomas and ~10% of non-Hodgkin lymphoma cases in children), inflammatory myofibroblastic tumors (IMT) (rare intermediate-grade neoplasms, generally found in children, which have a high recurrence rate after excision but with low metastatic potential), neuroblastomas, and inflammatory breast cancers. ALK genomic alterations are found in ~3.3% of patients with cancers, though ALK fusions/rearrangements are less common1. In large scale analyses of genomes, ALK fusions/rearrangements are detected in ~0.5–0.8% of cancers1,2. Among patients with NSCLC, the frequency of ALK fusions/rearrangements was over 3%; in contrast, the frequency in non-NSCLC tumors was just ~0.2%. Besides NSCLC, inflammatory myofibroblastic tumor (~50% have ALK fusions/rearrangements) and anaplastic large cell lymphoma (~50–80% having ALK fusions/rearrangements) are the neoplasms most frequently bearing ALK fusions. Fusion partners vary widely in non-NSCLC malignancies. Although in NSCLC, most tumors harbored an EML4-ALK fusion (83.5%), in non-NSCLC malignancies, these constituted the minority (~31%)1,2.
Normal ALK protein (cluster of differentiation (CD) 246) is a classical tyrosine kinase receptor3. It is involved in neuronal and gut development and is transcribed/translated from the normal ALK gene located on the short am of chromosome 2 (2p23)4,5. Aberrant ALK was originally found in an ALCL cell line6,7. It was described as a product of t(2;5)(p23;q35) chromosomal translocation involving a nucleophosmin partner (NPM1)---(NPM1-ALK)7,8, discerned in 75–90% of ALK-altered ALCL cases, though other ALK fusion partners also exist9,10. It is now known that ALK translocations involve different fusion partners across and within cancers. These translocations facilitate multimerization and autophosphorylation of ALK, resulting in a constitutively active tyrosine kinase enzyme that acts as an oncogenic driver (Fig. 1a)11–19.
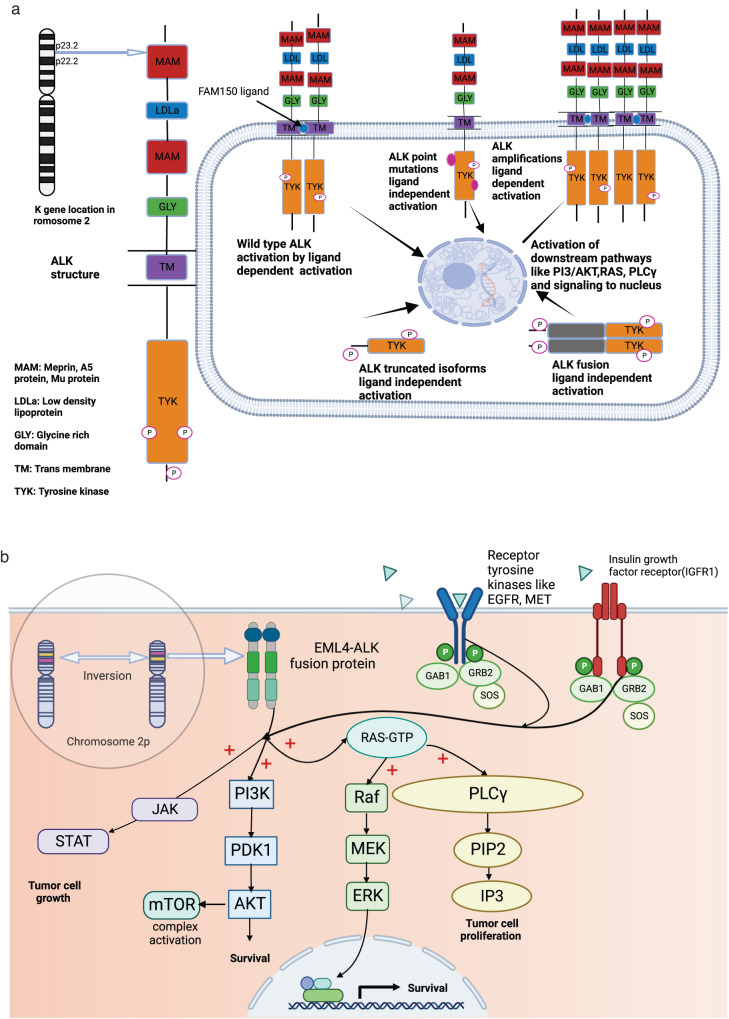
Fig. 1. Biology of ALK.
a ALK gene location, structure, and alterations. The ALK gene is located on the short arm of chromosome 2 (2p23). ALK receptor is a member of the insulin receptor superfamily and is a tyrosine kinase enzyme; its structure consists of the extracellular domain (two MAM, one LDLa), and one glycine-rich domain, a connecting transmembrane region, and an intracellular domain (tyrosine kinase domain). ALK receptors (CD246) are activated physiologically by FAM150 ligand binding followed by auto and transphosphorylation of residues, which promotes signaling to the nucleus. Intra tyrosine kinase (TYK) gain of function mutations can lead to ligand-independent downstream pathway activation. Amplified ALK gene sustains downstream signaling in a ligand-dependent manner. Similarly, truncated or isoform ALK gene that loses parts of its extracellular domain and ALK gene fusions can lead to downstream pathway activation, cell cycle progression, proliferation, migration, and angiogenesis. b Formation of EML4-ALK4 fusions and activation of downstream pathways. The figure inside the bubble depicts a small inversion within short arm of chromosome 2p, which results in the formation of a fusion gene comprising portions of (EML4) and (ALK) genes. ALK fusion protein activates downstream signaling pathways, among them, the most relevant and characterized pathways are the (MAPK/ERK), the (JAK-STAT), the (PI3K– Akt), and the (PLCγ) pathways. ALK fusion proteins have a strong oncogenic potential, and it ultimately promotes tumor cell progression. IGF1R and other receptor tyrosine kinase such as EGFR and MET also interact with ALK and lead to activation of downstream pathways. Activation of EGFR and IGF1R sometimes leads to development of bypass resistance pathways. Abbreviations: Akt protein kinase B, ALK Anaplastic lymphoma kinase, EML4 echinoderm microtubule-associated protein-like 4, ERK extracellular signal-regulated kinase, GAB1 growth factor receptor-bound 2 associated binder 1, GRB growth factor receptor-bound, GTP guanosine triphosphate, IP3 inositol triphosphates, JAK Janus kinase, MAPK mitogen-activated protein kinase, mTOR mammalian target of rapamycin, PDK1 pyruvate dehydrogenase kinase 1, PI3K phosphoinositide 3 kinase, PIP2 phosphatidylinositol 4,5-bisphosphate, PKC protein kinase C, PLC-γ phospholipase C γ, RAF rapidly accelerated fibrosarcoma, RAS rat sarcoma virus gene, SOS son of sevenless gene, STAT signal transducer and activator of transcription. Created by Biorender.com.
In NSCLC, ALK gene alterations act as oncogenic drivers, and occur in ~3–7% of cases20–22. One such molecular alteration—an activating fusion of the anaplastic lymphoma kinase (ALK) gene with echinoderm microtubule-associated protein-like 4 (EML4) gene (normally located on short am of chromosome 2 (2p21))-was first identified by Soda and colleagues20 in some NSCLCs. Both the ALK and EML4 genes are located on the same short arm of human chromosome 2, but in opposite orientations, and a small inversion involving the two loci, inv (2) (p21p23), results in gene fusion (Fig. 1b)3,20,23–25. The fusion gene EML4-ALK with its activated tyrosine kinase function induces downstream signaling pathways and promotes cell proliferation and survival. ALK gene aberrations in general are more common in the adenocarcinoma histological subtype of NSCLC, in non-smokers, and in young women. Notably, the frequency of EML4-ALK fusions commonly found in NSCLC is similar in Asians and Caucasians and the gender difference is rather small but there is a slight female preponderance26.
ALK translocations in NSCLC are often thought to be mutually exclusive with genomic alterations in the epidermal growth factor receptor (EGFR) or the Kirsten rat sarcoma viral oncogene homolog (KRAS), though they can occur together27.
There are several Food and Drug Administration (FDA) approved ALK inhibitors in the clinic: crizotinib28, ceritinib29, alectinib30, brigatinib31,32, and lorlatinib33. They are all authorized for the management of NSCLC. The FDA also approved crizotinib for pediatric patients (one year of age and older) and young adults with relapsed or refractory, systemic ALCL that is ALK-aberrant34. Of note, alectinib is approved for management of relapsed refractory ALK-aberrant ALCL in Japan35. Recently, the FDA also approved crizotinib for unresectable, recurrent, or refractory ALK-aberrant IMT36 (Table 1)28–34,36–39. The European Medicine Agency (EMA) has also approved multiple ALK inhibitors including crizotinib, ceritinib, alectinib, brigatinib and lorlatinib for treatment of ALK-aberrant NSCLC and crizotinib for unresectable ALK-aberrant IMT and ALCL in children and adolescents between the ages of 6 and 1840.
Table 1.
Examples of regulatory approval of ALK inhibitors in multiple cancers.
| Drug | FDA approved indicationa | Date of Approval | Blood brain barrier penetration (low/higher)b37,38 | ALK (IC50) c | Activity | Comment | References |
|---|---|---|---|---|---|---|---|
| Alectinib | Unresectable or metastatic ALK-positive NSCLC after progression or intolerance to crizotinib | December 2015 | Higher | 1.9 nM | ORR of 38% and 44% among 87 and 138 patients, respectively, in two single arm trials. NP28761 (NCT01871805) and NP28673 (NCT01801111) | Solid tumor approval | 30 |
| Alectinib | Unresectable or metastatic ALK-positive NSCLC | November 2017 | Higher | 1.9 nM |
Improvement in PFS: HR of 0.53 (95% CI: 0.38, 0.73; p = 0.0001) Among the 43 patients with brain lesions, the CNS ORR was 81% |
Solid tumor approval | 30 |
| Brigatinib | Unresectable or metastatic ALK-positive NSCLC after progression or intolerance to crizotinib | April 2017 | Higher | 0.37 nM | ORR, ~50% | Solid tumor approval | 31 |
| Brigatinib | Unresectable or metastatic ALK-positive NSCLC | May 2020 | Higher | 0.37 nM | ORR, 74% | Solid tumor approval | 32 |
| Ceritinib | Unresectable or metastatic ALK-positive NSCLC | May 2017 | Higher | 0.2 nM |
Improvement in PFS: HR of 0.55 (95% CI: 0.42, 0.73, p-value 0.0001) compared to platinum/pemetrexed. CNS ORR, 57% |
Solid tumor approval | 29 |
| Ceritinib | Unresectable or metastatic ALK-positive NSCLC after progression or intolerance to crizotinib | April 2014 | Higher | 0.2 nM | ORR, 44% |
Solid tumor approval Approval after Phase I trial |
|
| Crizotinib | Unresectable, recurrent, or refractory ALK-positive IMT | July 2022 | Low | 24 nM | ORR, 86% |
Solid tumor approval Includes pediatrics |
36 |
| Crizotinib | Unresectable, recurrent, or refractory ALK-positive ALCL | January 2021 | Low | 24 nM |
ORR, 88% (CR, 81%) |
Hematologic malignancy approval Includes pediatrics |
34 |
| Crizotinib | ALK-positive unresectable/metastatic NSCLC who had previously received one platinum-containing regimen |
August 2011 (accelerated) November 2013 (full approval) |
Low | 24 nM | ORR, ~50–61% | Solid tumor approval | 28 |
| Lorlatinib | Unresectable or metastatic ALK-positive NSCLC that had progressed on crizotinib and ≥1 other ALK inhibitor for metastatic disease or patients whose disease had progressed on alectinib or ceritinib as the first ALK inhibitor therapy | November 2018 | Higher | 0.07 nM |
ORR, 48% The median response duration was 12.5 months CNS ORR, 60% |
Solid tumor approval | 33 |
| Lorlatinib | Unresectable or metastatic ALK-positive NSCLC | March 2021 | Higher | 0.07 nM |
HR 0.28 (95% CI: 0.19, 0.41; p < 0.0001) (lorlatinib versus crizotinib). CNS ORR, 82% |
Solid tumor | 33 |
Abbreviations: ALCL anaplastic large cell lymphoma, CI confidence interval, CNS central nervous system, CR complete remission, HR hazard ratio, IC50 50% inhibitory concentration, IMT inflammatory myofibroblastic tumor, ORR objective response rate, NSCLC non-small cell lung cancer, PFS progression-free survival.
aALK+ infers an ALK genomic alteration.
bThere is overall consensus that crizotinib has low CNS penetration, so we labeled it “low.” In general, other TKI’s like ceritinib, alectinib, brigatinib, and lorlatinib have shown superior CNS activity compared with crizotinib, but to our knowledge, studies have not specifically compared CSF concentrations between them on an ordinal scale, and there is also a discrepancy in CSF concentrations reported in multiple studies, so we thought it would be better to label them as “higher” vs. “high”.
cAlk IC50 values were taken from Selleckchem.com. They are on cell-free assay except for crizotinib, which was only reported on cell-based assay.
ALK translocations (fusions/rearrangements) and other aberrations such as mutations and amplifications can be detected in multiple solid and hematologic malignancies. ALK translocations are particularly vulnerable to pharmacologic targeting. Several trials have therefore addressed possible pan-cancer indications for ALK inhibitors, e.g., Genentech Mypathway trial (NCT02091141) and the phase 2 TAPISTRY platform study (NCT04589845). The FDA has now authorized several tumor-agnostic gene- and immune-targeted agents. For example, agents targeting mismatch repair gene defects, high tumor mutational burden, and aberrant NTRK, RET, and BRAF are now established tumor-agnostic approvals41.
Our review summarizes the biology, diagnostic approach, therapeutic options, resistance mechanisms, and novel strategies for management of ALK-aberrant malignancies, with a particular emphasis on cancers beyond NSCLC, and the potential for viewing aberrant ALK as a tumor-agnostic target (including for both solid tumors and hematologic malignancies and for both common and uncommon cancers across the age spectrum). Tumor-agnostic indications may be especially important for rare cancer types, which represent an unmet need in oncology. We also present an illustrative case of an ultra-rare neoplasm---Erdheim Chester Disease (non-Langerhans histiocytosis) ---with an ALK fusion, and brain involvement, remarkably responsive to an ALK inhibitor.
Biology of normal and aberrant Alk
ALK (CD246) structure and function
ALK, located on the short arm of chromosome 2, is a member of the insulin receptor protein−tyrosine kinase superfamily (Fig. 1a)11–19. These transmembrane tyrosine kinase enzymes regulate cellular growth and trigger neoplastic transformation. Like other receptor tyrosine kinases, ALK undergoes a ligand-induced activation in extracellular space leading to its homo-dimerization or hetero-dimerization. FAM150A (ALKAL1 or Augmentor α) and FAM150B (ALKAL2 or Augmentor β) are group of peptides that act as ligands and activate ALK receptor tyrosine kinases17,18. The dimerization of ALK receptor tyrosine kinase results in the trans-phosphorylation of specific tyrosine residues within the cytoplasmic domain of ALK, which then leads to more tyrosine residues being phosphorylated on the same receptor tyrosine kinase. Activated ALK then phosphorylates tyrosine residues on its substrate proteins, which finally activates downstream oncogenic signaling pathways23 .
At the structural level, the human ALK gene consists of two meprin A 5 proteins, receptor protein tyrosine phosphatase μ regions (MAM), glycine-rich domains (GR), a low-density lipoprotein motif (LDLa), and an intracellular domain of tyrosine kinase (Fig. 1a)11–19.The ALK protein is a receptor tyrosine kinase that encompasses 26 exons that encode 1620 amino acid proteins of which about 180 kDa is glycosylated. ALK consists of an intracellular tyrosine kinase domain with three-tyrosine motifs (Tyr1282, Tyr1283 and Tyr1278) that act as auto-phosphorylation site for regulating kinase activity, extracellular ligand-binding domain and a transmembrane domain11,42.
ALK alteration types and biology
ALK proteins are commonly overexpressed or aberrantly expressed in tumors due to ALK gene rearrangements or fusions, copy number gains or gene amplification, and activating kinase mutations. Overall, ~3.3% of cancers across the malignancy spectrum harbor ALK alterations, with ALK fusions detected in around 0.5–0.8% of all cancers (~0.2% of cancers outside of NSCLC)1,2. Among them, the EML4-ALK fusion is the most common1. In ALCL, the most common rearrangement results in the NPM1-ALK fusion16. Other common alterations in ALK are activating kinase point mutations and ALK amplifications, observed in around 2.8% to 3.0%1,43 and 0.10% of all cancers respectively1.
The constitutive kinase activity of ALK fusions may arise from self-dimerization through N-terminal oligomerization domains, leading to auto- and transphosphorylation of ALK. Other oncogenic mechanisms leading to aberrant ALK activity include point mutations in the kinase domain (that enhance kinase enzymatic function) and gene amplification; however, not all mutations in ALK lead to ligand-independent or even kinase-active protein and some may merely represent passenger mutations. ALK rearrangements/fusions are particularly important in cancer because they are pharmacologically tractable. A variety of fusions have been described (Table 2)2,7,16,20,44–66.
Table 2.
Examples of ALK translocations, chromosomal locations, and relative frequencies in solid and hematologic malignancies.
| ALK partners | Tumor type comment | Chromosomal alterations | ALK fusion approximate frequency (%) in tumor |
References |
|---|---|---|---|---|
| Solid Tumors | ||||
| ATIC | IMT | inv (2) (p23q35) | 50–55% of all IMT | 44 |
| A2M | IMT | t (2;12) (p23; p13) | 45 | |
| CLTC | IMT | t (2;17) (p23; q23) | 16 | |
| RANBP2 | IMT | inv (2) (p23q11-13) | 16,66 | |
| TPM3 and TPM4 | IMT | t(1;2) (q21;p23) and t(2;19)(p23;p13) | 46 | |
| PPFIBP1 | IMT | t (2;12) (p23; p11) | 16 | |
| SQSTM1 | NSCLC | t (2;5) (p23.1; q35.3) | 3–7% of all NSCLC | 16 |
| CLTC | NSCLC | t (2;17) (p23; q23) | 2 | |
| HIP1 | NSCLC | t (2;7) (p23; q11.23) | 2,16 | |
| DCTN1 | NSCLC | inv (2) (p13p23) | 2 | |
| A2M | NSCLC | t (2;12) (p23; p13) | 16 | |
| TPR | NSCLC | t (1;2) (q31.1; p23) | 16 | |
| EML4 |
NSCLC Most common ALK partner in NSCLC |
inv (2) (p21p23) | 20 | |
| KIF5B | NSCLC | t (2;10) (p23; p11) | 2,16 | |
| SPTBN1 | CRC | t (2) (p16.2; p23) | <1% of all CRC | 47 |
| CAD | CRC | inv (2) (p23; p22) | 48 | |
| EML4 | CRC | inv (2) (p21p23) | 58 | |
| STRN | CRC | t (2) (p23; p22.2) | 49 | |
| VCL | RCC | t (2;10) (p23; q22) | <1% of all RCC | 16 |
| STRN | RCC | t (2) (p23; p22.2) | 50 | |
| TPM3 | RCC | t (1;2) (q21; p23) | 16 | |
| EML4 | RCC | inv (2) (p21p23) | 16 | |
| DCTN1 | PDAC | inv (2) (p13p23) | N/A | 64 |
| STRN | CCA | t (2) (p23; p22.2) | N/A | 51 |
| EML4 | BC | inv (2) (p21p23) | N/A | 52 |
| TPM4 | ESCC | t (2;19) (p23; p13) | N/A | 53 |
| FN1 | OC | inv (2) (p23q34) | N/A | 57 |
| STRN | MPM | t (2) (p23; p22.2) | N/A | 54 |
| STRN | TC | t (2) (p23; p22.2) | <1% of all TC | 55 |
| DCTN1 | TC | inv (2) (p13p23) | 16 | |
| FN1 | LMS | inv (2) (p23q34) | N/A | 56 |
| Hematological malignancies | ||||
| TFG | ALCL | t (2;3) (p23; q21) | 59 | |
| RNF213/ALO17 | ALCL | t (2;17) (p23; q25) | 16 | |
| NPM1 | ALCL | t (2;5) (p23; q35) | ~80% of pediatric and ~50% of adult ALCL harbor NPM1-ALK | 7,16 |
| CLTC | ALCL | t (2;17) (p23; q23) | 60 | |
| MSN | ALCL | t (2; X) (p32; q11-12) | 16 | |
| TPM3 | ALCL | t (1;2) (q25; p23) | 16 | |
| TPM4 | ALCL | t (2;19) (p23; p13) | 16 | |
| EML4 | DLBCL | inv (2) (p23; q21) | <1% of all DLBCL | 61 |
| SEC31A | DLBCL | t (2;4) (p24; q21) | 62 | |
| CLTC | DLBCL | t (2;17) (p23; q23) | 65 | |
| RANBP2 | AML | Inv (2) (p23; q13) | N/A | 63 |
| KIF5B | Histiocytosis | t (2;10) (p23; p11) | N/A | 2 |
Abbreviations: A2M α-2-macroglobulin, ALCL anaplastic large cell lymphoma, AML acute myeloid leukemia, ATIC 5-aminoimidazole-4-carboxamide ribonucleotide formyltransferase, BC breast cancer, CAD carbamoyl-phosphate synthetase 2, aspartate transcarbamylase, and dihydroorotase, CRC colorectal cancer, CCA cholangiocarcinoma, CLTC clathrin heavy chain, DCTN1 dynactin, DLBCL diffuse large B cell lymphoma, EML4 echinoderm microtubule-associated protein like-4, ESCC esophageal squamous cell carcinoma, FN1 fibronectin 1, HIP1 huntingtin-interacting protein 1, IMT inflammatory myofibroblastic tumor, KIF5B kinesin family member 5B, LMS leiomyosarcoma, MPM malignant peritoneal mesothelioma, MSN moesin, NA not available, NPM1 nucleophosmin1, NSCLC non-small cell lung cancer, OC ovarian carcinoma, PDAC pancreatic ductal carcinoma, PPP1CB PP1-beta-catalytic subunit, RANBP2 RAN binding protein 2, RCC renal cell carcinoma, RNF213 ring finger protein 213, SEC31A SEC31 homolog A, SPTBN1 spectrin beta non-erythrocytic 1, SQSTM1 sequestosome 1, STRN striatin, TC thyroid carcinoma, TFG TRK-fused gene, TPM3 tropomyosin 3, TPM4 tropomyosin 4, TPR translocated promoter region, VCL vinculin.
NSCLC is one of the cancers in which ALK gene fusions/rearrangements have been especially well recognized, with ~3–7% of cases affected20–22. The most common rearrangements in NSCLC result from an inter-chromosomal inversion in the short arm of chromosome 2, which creates a fusion between the 5’ portion of the EML4 gene and the 3’ portions of the ALK gene Inv (2) (p21p23) (Fig. 1b)3,20,23–25.
In vitro studies utilizing NPM1-ALK and EML4-ALK-based model systems demonstrate that dysregulation of ALK activity via fusion leads to activation of four key downstream oncogenic signaling pathways: Janus kinase - signal transducers and activators of transcription (JAK-STAT)67, mitogen-activated protein kinase/extracellular signaling regulated kinase) (MAPK/ERK)68, phospholipase C gamma (PLCγ)69 and phosphatidylinositol-3-kinase – protein kinase B) (PI3K-Akt)69. These pathways are important in cell cycle progression, proliferation, apoptosis, angiogenesis, and cell survival (Fig. 1b)3,20,23–25.
Alk alterations in solid and hematologic malignancies
The solid tumor best known for ALK alterations is NSCLC. However, ALK is also altered in a wide array of other solid tumors, as well as in hematologic cancers, with important clinical implications. ALK alterations in malignancies include fusions, mutations, amplification, and overexpression (Table 3)1,2,20–22,34,36,52,63,66,70–90.
Table 3.
Selected examples of tumors with ALK alterations and their clinical implications.
| Examples of diseases | ALK alterations and their frequency | Comments | References |
|---|---|---|---|
| All cancers |
~3.1% of cancers have ALK alterations.a ~0.2% of all cancer types have ALK fusions/rearrangements |
Alterations other than fusions may include mutations, amplifications and ALK positivity by immunohistochemistry. ALK mutations are found in about ~3% of various cancer types |
1,2 |
| Solid tumors | |||
| Breast carcinoma (including inflammatory breast cancers) |
ALK gene amplifications are found in ~13% of all breast cancers, more frequently triple negative breast cancers or inflammatory breast cancer70 75–80% of inflammatory breast cancers can have ALK copy number gains and amplifications77 EML4-ALK fusions are found in ~2.4% of breast cancers52b ALK mutations were observed in ~1.8% of cases of breast carcinomas in AACR Genie database. |
ALK protein positivity may not be present in many inflammatory breast cancers despite ALK high copy number gains and amplifications88 Data on responsiveness to ALK inhibitors in ALK-amplified breast cancer is not well described in the literature. |
1,52,70,77,88 |
| GBM |
ALK amplifications are present in around 0.2% of all of glioblastoma cases. ALK Mutations were observed in ~2.7% cases of GBM in the AACR Genie database. PPP1CB-ALK fusions were observed in ~43% (7/16) and ALK amplification was observed in 31% (5/16) of ALK-expressing pediatric glioblastoma cases89 |
ALK overexpression by IHC may be present in over 40% of cases, but fusions are uncommon. | 1,76,89 |
| IMTs |
ALK fusions are found in ~50% of IMT patients. The majority of ALK alterations seen in IMT are fusions. Multiple fusion partners to ALK occur some of them are included in (Table 2) |
ALK expression in these tumors can be associated with better prognosis. When RANBP2 is the fusion partner to ALK, it is often associated with epithelioid inflammatory myofibroblastic sarcoma, an aggressive subtype of IMT66 The FDA approved crizotinib for unresectable, recurrent, or refractory ALK-aberrant inflammatory myofibroblastic tumors. |
1,36,66,79–81 |
| Melanoma |
Spitz nevi and spitzoid melanomas are associated with ALK fusions in around 8–15% cases. ALK Mutations were observed in ~7.5% of cases of melanoma in AACR Genie database. |
1,2,75,78 | |
| NSCLC |
ALK fusions, ~3–7% of NSCLC ALK amplification were observed in~ 0.02% and ALK Mutations seen ~3.6% NSCLC cases of AACR genie data base. |
EML4-ALK is the most common ALK fusion. Multiple ALK inhibitors approved (see Table 1) |
1,20–22 |
| Neuroblastomas |
8–16% of newly diagnosed neuroblastoma have somatic ALK alterations. ALK mutations were observed in ~12.4% of cases of neuroblastoma in AACR Genie database. Around ~1–2% of neuroblastoma cases are inherited (autosomal dominant) and almost 50% of them have germline gain of function ALK mutation. ~1–3% of neuroblastoma have ALK amplification. |
ALK-mutant versus wild-type neuroblastoma have inferior survival, aggressive disease. Mutations in three positions in kinase domain—R1275, F1174, and F1245—account for around~85% of ALK mutations in neuroblastomas; R1275Q is the most common mutation, present in 45% of familial cases and a third of sporadic cases, whereas F1174 and F1245 mutants are exclusively found in sporadic disease at frequencies of around 30% and 12%, respectively. ALK can be co-amplified or co-mutated with MYCN, consistent with the proximity of these genes at 2p23-24 which is associated with aggressive prognosis. |
1,82–84 |
| Prostate |
ALK gene truncations were found in 6.4% of cases in some studies. ALK protein overexpression through IHC found in 9% cases of prostate cancer |
Rare SLC45A3-ALK fusion and ALK F1174C mutation has been observed | 1,85–87 |
| Hematologic malignancies | |||
| ALCL |
~80% of pediatric and ~50% of adult ALCL harbor NPM1-ALK ALK mutations were observed in ~2.8% cases of ALCL in the AACR Genie database. |
The FDA approved crizotinib for pediatric patients (one year of age and older) and young adults with relapsed or refractory, systemic anaplastic large cell lymphoma (ALCL) that is ALK-aberrant. | 1,34,71,72 |
| Histiocytosis | In a study of 39 ALK positive histiocytosis, 37 had ALK rearrangements, 27 with KIF5B-ALK fusion |
ALK-fusion histiocytosis is a rare subtype of histiocytic neoplasm. KIF5B is the most common ALK fusion partner. Others observed DCTN1, TPM3, EML4 and TFG fusions with ALK |
74 |
| Leukemia |
RANBP2-ALK fusion has been reported in an AML case63 ALK mutations in 2/185 (<1%) cases of leukemias) |
63,90 | |
| Multiple myeloma | TFG-ALK fusion has been reported in non-secretory multiple myeloma | TFG-ALK fusion has been reported in non-secretory multiple myeloma | 73 |
Abbreviations: ALCL anaplastic large cell lymphoma, ALL acute lymphoblastic leukemia, AML acute myelogenous leukemia, EML4 echinoderm microtubule-associated protein like-4, GBM glioblastoma multiforme, IHC immunohistochemistry, IMT inflammatory myofibroblastic tumor, KIF5B kinesin family member 5B, NPM1 nucleophosmin1, NSCLC non-small cell lung cancer, PPP1CB protein phosphatase 1 catalytic subunit beta, RANBP2 RAN binding protein 2, TFG TRK-fused gene, TPM3 tropomyosin 3.
aALK alterations in AACR genie database included rearrangements, mutations and amplification.
bThe incidence rate of EML4-ALK fusion in this study was reported by using relatively older technology (exon array profiling) and no further studies have supported this finding.
ALK gene alterations in NSCLC
ALK gene alterations are found in ~3–7% of cases20–22. ALK gene rearrangements (leading to fusions), as well as mutations and amplifications can be discerned. Desai and colleagues analyzed 11,107 tumor samples from 10,082 patients of lung adenocarcinoma from AACR Genie data base91 and found 584 (5%) samples with ALK gene alterations: 354 missense mutations (60.6%); 265 cases with fusions (45.4%); 51 with truncating mutations (8.7%); and 1 case with in-frame mutation (0.17%).
ALK gene rearrangement can manifest in the form of a translocation with another partner gene leading to formation of a fusion oncogene. These fusions often arise from fusion of the 3′ end of the ALK gene (exons 20–29) with the 5′ portion of a different gene92. For example, in some NSCLC cases, inversion rearrangement from inv(2) (p21;p23) results in EML4 replacing the extracellular and intramembranous parts of ALK and fusing with its juxtamembrane domain leading to the formation of EML4-ALK fusion oncogene20.
EML4 is the most common fusion partner in NSCLC, found most NSCLCs with ALK fusions2. Still, some studies have identified more than a dozen ALK gene rearrangements involving various EML4-ALK fusion breakpoints93.. Apart from EML4, other fusion partners seen in NSCLC include SQSTM1 (sequestosome), DCTN1(dynactin), HIP1(huntington intercating protein 1) and KIF5B (kinesin family member 5 B)16,91.
ALK gene alterations in cancers beyond NSCLC
ALK gene alterations such as gene fusions, mutations and amplification have also been described in multiple solid tumors apart from NSCLC including, but not limited to, inflammatory myelofibrotic tumor (IMT), neuroblastoma, esophageal carcinoma, renal medullary carcinoma, breast carcinoma, colorectal carcinoma, serous ovarian carcinoma, and thyroid carcinoma. STRN (striatin) and NPM1 (nucleophosmin) are the most common fusion partners of ALK in non-NSCLC tumors2.
IMT are rare mesenchymal tumors that are mostly seen in the pediatric and adolescent populations. Importantly, they are associated with ALK gene rearrangement in around 50% of cases79,80. ALK overexpressing IMT’s may have a better prognosis than ALK non-expressing tumors81. About half of IMT’s are associated with rearrangements involving the ALK gene locus on chromosome 2p23 juxtaposed to several different translocation partners: TPM3 and TPM4 (tropomyosin 3 and 4), CLTC 1 (clarithin heavy chain 1) and RANBP2 (RAN binding protein 2)16. Among them, RANBP2 is often associated with epithelioid inflammatory myofibroblastic sarcoma, which is an aggressive subset of IMT66.
As mentioned, ALK fusions are also observed in renal cell carcinoma, thyroid carcinoma, pancreatic adenocarcinoma94, spitzoid melanocytic tumors75, salivary gland cancers95, and colorectal cancers16 (Table 2)2,7,16,20,44–66. Point mutations in ALK also occur and can activate the downstream pathways in a ligand-independent manner. Gain-of-function point mutations usually occur within the kinase domain of ALK receptor tyrosine kinase enzyme. Some ALK mutations have also been identified in neuroblastomas and can either be germline or somatic12,96. Types and frequencies of different ALK alterations seen in cancer have been tabulated in (Table 3)1,2,20–22,34,36,52,63,66,70–90.
ALK alterations are present in 8–16% of newly diagnosed patients with neuroblastoma82–84. Around ~1–2% of neuroblastoma cases are inherited (autosomal dominant) and ~50% of them have germline gain-of-function ALK mutation83. The majority of ALK-altered neuroblastoma are associated with sporadically acquired somatic mutations. Among the somatic mutations, single-nucleotide variants of ALK tyrosine kinase domain at loci R1275, F1174, and F1245 are the most common and seen in ~85% of all cases with alterations82,83. ALK-mutant neuroblastomas have inferior survival compared with those with ALK wild-type (WT) tumors83,97. ALK F1174L mutated neuroblastoma is thought to be an aggressive disease phenotype, which confers resistance to crizotinib82,83. Furthermore, co-expression of ALK F1174L and MYCN amplification is associated with poor prognosis in neuroblastoma82,83.
Another mechanism of gene alteration is via ALK gene locus amplification. This process was first studied in neuroblastoma cell lines13. These amplifications often result in ligand-dependent activation of downstream ALK signaling pathway. They are found in ~4% of high-risk neuroblastomas and confer a poor prognosis14,98. ALK fusions and amplifications have also been reported in glioblastoma. ALK can be overexpressed in 43%–70% of glioblastoma cases and may have biologic relevance99–102. PPP1CB-ALK fusions were observed in~43% (7/16) and ALK amplification in 31% (5/16) of ALK-expressing pediatric glioblastoma cases89; ALK translocations are rare in adult GBM.
ALK gene amplifications are found in ~13% of breast cancers70 and ~3% of colorectal cancers103. There may be a high frequency (~75%) of ALK gene amplifications in inflammatory breast cancer104. ALK copy number gains and amplifications are also seen in some types of rhabdomyosarcomas105.
Truncated mutations are formed by elimination of the extracellular domain of ALK gene which lead to activation of downstream pathways and have been identified in neuroblastoma, squamous cell carcinoma of the skin and melanomas15,78,106. For example, In-frame deletion of exons 1 through 5 (Δ 1–5) and exons 4 through 11 (Δ 4–11) in ALK gene are seen in neuroblastoma cell lines15 whereas ALK Δ 2–17 are usually found in synovial sarcoma cell lines107. Additionally, ALK intra-kinase domain mutations have also been described; they can be acquired after exposure to certain ALK inhibitors and may perpetrate resistance.
ALK gene rearrangements/fusions are commonly found in hematological malignancies such as ALCL, which is a moderately aggressive T-cell lymphoma. ALK fusions play a vital role in ALK-altered ALCL pathogenesis. Earlier studies have shown that ALK-expressing ALCL have a much better 5-year overall survival rate (70–90% vs 15–62%) compared to non-ALK-expressing ALCL16,108. The NPM1-ALK fusion is found in >80% of pediatric ALCL and in >50% of adult ALCL cases. As described earlier, this results from the translocation of the NPM1 gene at 5q35, which encodes a nucleolar protein involved in transporting ribonucleoproteins from the cytoplasm to the nucleus, to the ALK gene at 2p23, encoding a tyrosine kinase receptor. Non-NPM1 fusion partners (e.g., TFG, TPM3, TPM4, CLTC 1, ALO17 and MSN) are also found in some ALK-altered ALCL. Among them, TPM3 is the most common in ALCL2,16.
ALK fusions are also found in DLBCL but also in differentiated B cell lymphomas, some leukemias, myelomas and histiocytosis2. ALK fusions in DLBCL are associated with poor prognosis. While most of them are associated with CLTC-ALK fusion, other ALK fusion partners such as NPM1 and EML4 are also seen16,65.
ALK alterations are rare in leukemia. RANB2-ALK fusions have been detected in three pediatric cases of atypical myeloproliferative leukemia109 and an adult patient with acute myelomonocytic leukemia63. A few cases harboring NPM1-ALK fusions have been reported in B cell acute lymphoblastic leukemia2. Oncogenic point mutations (A348D and F856S) have also been discovered in an acute myelogenous leukemia (AML) and a B-acute lymphoblastic leukemia (B-ALL) patient, respectively90. Unlike point mutations seen in neuroblastoma which are mainly found in kinase domain, the mutations found in leukemia were in the extracellular domain. Rare oncogenic deletion mutations of ALK have also been described in ALCL cells110.
Alk-targeted therapies
There are now several drugs that have achieved regulatory approval for ALK-altered tumors: alectinib, brigatinib, crizotinib, ceritinib, and lorlatinib. The majority of approvals are for NSCLC. However, the FDA has also approved ALK inhibitors such as crizotinib for IMT and for the hematologic malignancy ALCL (Table 1)28–34,36–39.
ALK inhibitors used in treatment of ALK-altered NSCLC
Alterations in the ALK gene sensitize NSCLC to ALK inhibitors, which bind to receptor tyrosine kinases and inhibit downstream signaling pathways. To date, five ALK inhibitors have received approval from the Food and Drug Administration (FDA) for ALK-altered NSCLC treatment: first-generation crizotinib, second-generation (ceritinib, alectinib, and brigatinib) and third generation ALK inhibitors (lorlatinib) based on their activity in the clinic (Supplementary Table 1)111–127.
Crizotinib was the first oral tyrosine kinase inhibitor approved for the treatment of NSCLC. Its targets include ALK, ROS1 and MET alterations. Its FDA approval in 2011 was based on landmark studies like PROFILE 1005111. These results led to the development of two other phase III studies (PROFILE 1007114 and PROFILE 1014113), which confirmed the efficacy of crizotinib and its superiority over platinum-based chemotherapy regimens in both second and first-line settings respectively. Crizotinib unfortunately has poor blood brain barrier penetrance37 and patients on crizotinib often develop resistant mutations. This led to the development of second and third generation drugs.
Ceritinib was the second oral ALK inhibitor that received FDA approval. The approval was in 2014 for patients with ALK-altered NSCLCs that progressed or were intolerant to crizotinib and as first-line in 2017. Ceritinib is 20 times as potent as crizotinib with activity against several ALK mutations such as L1196M G1 269 EA IM 1171T, and S1206Y. The initial approval of ceritinib was based on ASCEND 1119 and 2122 trials. It further received first-line treatment approval in treatment-naïve patients based on results from ASCEND 4 study118. Ceritinib inhibits autophosphorylation of ALK and targets IGF1R, and ROS1 alterations. Its ability to inhibit IGF1R may contribute to its activity in crizotinib-resistant cases24,25. Unlike crizotinib, other second and third generation ALK inhibitors have good blood-brain barrier penetration38 (Table 1)28–34,36–39.
The third ALK inhibitor to be developed was alectinib; its targets include both ALK and RET alterations. Alectinib is a 5-fold stronger ALK inhibitor compared to crizotinib and has good central nervous system activity. It does not have a substrate of P-glycoprotein, which is a key efflux transporter located at the blood-brain barrier. It has been FDA approved in ALK-altered NSCLC with or without previous treatments on crizotinib. This approval in 2015 was based on results of two single arm Phase II trials115,128. Alectinib is active in tumors harboring C1156Y, G126 9A, S1206Y and L1152R mutations but not G1202R. The superiority of alectinib over crizotinib was reported in the ALEX trial120, which compared alectinib to crizotinib in treatment-naïve patients with ALK-altered NSCLCs.
Similarly, brigatinib was another ALK inhibitor which received FDA approval based on results of ALTA trials. It is a second-generation tyrosine kinase inhibitor (TKI) that impacts the products of ALK and ROS1 fusions and interestingly also mutant EGFR L858R. It also has inhibitory activity against several acquired ALK mutations but questionable activity against G1202R. US FDA granted accelerated approval to brigatinib for treatment of individuals with advanced ALK-altered NSCLC that had progressed or were intolerant to crizotinib; approval was based on results of phase II ALTA124 and in first-line setting based on results from Phase III ALTA-1L trial121.
Finally, lorlatinib is a third generation ALK inhibitor that targets ALK and ROS1. It is specifically designed to target resistant mutations associated with first- and second-generation ALK inhibitors including the G1202R mutation. It first attained FDA approval in 2018 in clinically advanced ALK-altered NSCLC patients that had progressed on first- and second-generation ALK inhibitors such crizotinib/ceritinib/alectinib based on results of a phase II study112. Additionally, impressive results of the recently concluded CROWN study117 have led to its FDA approval in the first-line setting. Lorlatinib is also associated with excellent intracranial activity129.
ALK Inhibitors in solid tumors (beyond NSCLC) and in hematologic malignancies
ALK inhibitors have shown activity in a range of solid tumors and hematologic malignances and have achieved approval in the rare solid tumor IMT and in the hematologic malignancy ALCL, in addition to the NSCLC approval (Table 1)28–34,36–39 and (Table 4)35,48,73,74,85,94,126,130–149.
Table 4.
Examples of studies and cases of targeted therapies in ALK-altered cancers (other than NSCLC) (See Supplemental Table 1 for NSCLC studies).
| Tumor characteristics (No. of patients) Study Title | ALK alteration | Type/Phase of study | Drug | CR/PR (N/total N (%)) | Comment | References/CT.gov ID number if ongoing |
|---|---|---|---|---|---|---|
|
ALCL (N = 16) A8081013 (NCT01121588) PROFILE 1013 |
NPM1-ALK fusion | Phase IB study in non-NSCLC tumor | Crizotinib | 9/16 (56%) | 132 | |
|
ALCL (N = 26) ADVL0912 (NCT00939770) |
NPM1-ALK fusion | Phase I/II | Crizotinib |
ALCL165 group: 5/6 (83%) ALCL280 group: 18/20 (90%) |
ALCL cases treated at doses of 165 mg/m2 (ALCL165) and 280 (ALCL280) mg/m2 | 130 |
| ALCL (N = 10 in ALCL cohort) | NPM1-ALK fusion | Phase II | Alectinib | 8/10 (80%) | 35 | |
| Colon cancer (N = 1) | CAD-ALK fusion | Case report |
Entrectinib ALKA-372-001 phase I study |
1/1(100%) | DOR 4+ months | 48 |
| Colon cancer (N = 1) | EML4-ALK fusion (E21; A20) and | Case report |
Crizotinib Alectinib Lorlatinib |
1/1(100%) |
Patient was treated with crizotinb, and had a PR, Developed leptomeningeal disease switched to alectinib for few weeks and then lorlatinib; had PR with latter and DOR was 11 months |
141 |
| DLBCL (N = 2) | ALK fusion partner unknown | Case series | Crizotinib | 1/2 (50%) | 133 | |
| Erdheim-Chester disease (non-Langherhans histicocytosis) (N = 1) | ALK-KIF4B | Case report | Alectinib | 1/1(100%) | DOR 6+ months | Included in this manuscript |
| Glioma, high grade (N = 1) | SPECC1L–ALK fusion | Case report | Lorlatinib |
1/1 (100%) 3 y/o boy |
146 | |
| Histiocytosis with ALK fusions (N = 11) |
KIF5B-ALK fusion (N = 10) DCTN1-ALK fusion (N = 1) |
Retrospective study |
Crizotinib Alectinib Brigatinib Lorlatinib Ceritinib |
11/11(100%) | Median time on ALK inhibitors 16 months (range 3-43 months) | 74 |
|
Inflammatory myofibroblastic sarcoma (EIMS) (epithelioid) (N = 1) |
PRRC2B-ALK fusion | Case report |
Crizotinib Alectinib Ceritinib Lorlatinib |
1/1 (100%) | Patient was treated with four sequential ALK inhibitors and had PR to first three ALK Inhibitors | 142 |
| IMT (N = 14 pediatric patients.) (NCT00939770) | NPM-ALK fusion | Phase I/II | Crizotinib | 12/14 (86%) | Circulating tumor-derived NPM-ALK transcript decreased with response. | 130 |
| IMT (N = 2) | Usually bear ALK fusions | Case series | Brigatinib | 1/2 (50%) | 134 | |
| IMT of head and neck (N = 1 | SQSTM1-ALK fusion gene | Case report | Alectinib | 1/1 (100%) | DOR 17+ months | 135 |
| Mesothelioma (peritoneal) | STRN-ALK Fusion | Case report | Ceritinib | 1/1(100%) | DOR 3+mths | 144 |
| Multiple myeloma, non-secretory (N = 1) Myeloma | TFG-ALK fusion | Case report | Alectinib | 1/1(100%) | DOR 24+months | 73 |
| Neuroblastoma (N = 1) | ALK mutation at exon 24 F1245C | Case report | Alectinib | 1/1 (100 %) | DOR 12+ months | 136 |
|
Neuroblastoma (N = 28) A New Approaches to Neuroblastoma Consortium study |
ALK mutations/amplifications | Phase I | Lorlatinib |
In (2–17) age group, 1/18 (5%) In (15–50) age group, 4/10 (40%) |
148 | |
| Ovarian Cancer (Refractory high-grade serous) [N = 1] | EML4-ALK Fusion | Case report | Alectinib | 1/1(100%) | 147 | |
| Pancreatic adenocarcinoma (N = 1) | PPFIBP1-ALK fusion | Case report |
Alectinib/ Lorlatinib |
Minor response with alectinib lasted for 5 months.Pt. achieved stable disease for 2 months on lorlatinb | 94 | |
| Pancreatic adenocarcinoma (N = 1) | EML4-ALK fusion | Case report |
Crizotinib Alectinib |
1/1 (100%) to both crizotinib and alectinib |
PFS with crizotinib, 8 months PFS with alectinib 10 months |
137 |
| Pancreatic neuroendocrine cancer (N = 1) | KANK1-ALK Fusion | Case report | Lorlatinb | 1/1 (100%) | DOR 4+ months | 149 |
| Prostate cancer (N = 1) | ALK F1174C-activating point mutation | Case | Alectinib | Stable disease >6mths | 85 | |
| Renal cell carcinoma (N = 1) | VCL-ALK fusion | Case report |
Entrectinib ALKA-372-001 and STARTRK-1 phase I study |
1/1(100%) | 126 | |
| Thyroid cancer, medullary (N = 1) | CCDC6-ALK fusion | Case report | Crizotinib/Alectinib | 1/1(100%) | 139 | |
| Thyroid cancer, anaplastic (N = 1) | STRN-ALK fusion | Case report | Ceritinib /Brigatinib | 1/1(100%) | DOR 15+ months | 138 |
| Studies with multiple tumor types | ||||||
|
ALK-altered tumors such as ALCL [N = 1], IMT [N = 4], GBM, N = 12] and others [N = 5]) ≥1 prior systemic therapy ASCEND 10 study. (NCT02465528) |
Type of alterations not reported. But IMT and ALCL usually bear fusions..GBM generally have non-fusion ALK alterations such as overexpression) | Phase II | Ceritinib |
ALCL 1/1(100%) IMT 3/4 (75%) Glioblastoma 0/12 (0%) |
140 | |
|
Neuroblastoma (N = 11) Children’s Oncology Group consortium study of 79 pts. (including neuroblastoma, IMT, NSCLC and ALCL) |
ALK rearrangements seen in ALCL and IMT ALK mutations seen in neuroblastoma. Arg1275 Gln (patient with CR) |
Phase I/II | Crizotinib | Neuroblastoma 1/11(9%) | 131 | |
|
NCI match subgroup F Colorectal carcinoma [N = 2] Carcinoma of unknown primary [N = 1] Leiomyosarcoma [N = 1] (NCT02465060) |
EML4-ALK STRN-ALK ACTG2-ALK fusions |
Phase II | Crizotinib | 2/4 (50%) | CR in leiomyosarcoma and PR in colorectal | 145 |
| Ongoing studies | ||||||
| Plasmablastic ALK-altered large B cell lymphoma. | Phase II | Belantomab Mafodotin | NCT04676360 | |||
| ALK-positive relapsed/refractory Neuroblastoma | Phase I/II | Ceritinib+ Ribocicliib | NCT02780128 | |||
| ANBL1531: Children with Newly Diagnosed High-Risk Neuroblastoma | Phase III | 131I-Metaiodobenzylguanidine (131I-MIBG) or Crizotinib Added to Intensive Therapy | NCT03126916 | |||
| Relapsed ALK-positive lymphoma previously treated With ALK Inhibitors | Phase II | Lorlatinib | NCT03505554 | |||
| ANHL12P1: newly diagnosed Stage II-IV ALCL | Phase II | Brentuximab Vedotin or Crizotinib in Combination with Chemotherapy | Results available for the Brentuximab arb | NCT01979536 | ||
| HR-NBL2: High-Risk Neuroblastoma Study of SIOP-Europe-Neuroblastoma (SIOPEN) | Phase II | Lorlatinib | NCT04221035 | |||
| NANT 2015-02: ALK-driven Relapsed or Refractory Neuroblastoma Phase I | Phase I | Lorlatinib | NCT03107988 | |||
| Relapsing/Refractory ALK-altered Anaplastic Large Cell Lymphoma | Phase II | Nivolumab | Evaluation of response in patients with progressive disease (Cohort 1) or as consolidative immunotherapy in patients in complete remission after relapse (Cohort 2) | NCT03703050 | ||
| ALK-altered ALCL, IMT or Other Solid Tumors (Briga-PED) | Phase I/II | Brigatinib | Phase I dose escalation in ALK-altered ALCL or ALK-altered solid tumors. Phase II Cohort B1: ALK-altered IMT Cohort B2: ALK-altered ALCL | NCT04925609 | ||
| ALK Fusion-positive Solid or CNS Tumors (prior treatment has proven to be ineffective or from whom there is no curative standard treatment available) | Phase I/II | Alectinib | NCT04774718 | |||
| Solid Tumors Harboring ALK, ROS1, or NTRK1-3 Rearrangements (TRIDENT-1) | Phase I/II | Repotrectinib | NCT03093116 | |||
|
Solid tumors harboring NTRK 1/2/3 (Trk A/B/C), ROS1, or ALK gene rearrangements (Fusions) (STARTRK-2) Phase I/II |
Phase I/II | Entrectinib | NCT02568267 | |||
| Relapsed or refractory Advanced Solid Tumors, Non-Hodgkin Lymphoma, or Histiocytic Disorders with ALK or ROS1 Genomic Alterations (A Pediatric MATCH Treatment Trial) | Phase II | Ensartinib | NCT03213652 | |||
| Malignant Melanoma with ALK alterations | Phase II | Ensartinib | NCT 03420508 | |||
| Patients with Advanced NSCLC and Other Solid Tumors Harboring ALK Rearrangement or Activating ALK Mutation (ALKOVE-1) | Phase I/II | NVL-655 | NCT05384626 | |||
Abbreviations: ACTG2 Actin Gamma 2, Smooth Muscle, ALCL anaplastic large cell lymphoma, CAD carbamoyl-phosphate synthetase 2, aspartate transcarbamylase, and dihydroorotase, CCDC6 Coiled-Coil Domain Containing 6, CR complete response, DCTN1 dynactin, DLBCL diffuse large B cell lymphoma, DOR duration of response, EML4 echinoderm microtubule-associated protein like-4, GBM glioblastoma multiforme, GOPC Golgi Associated PDZ And Coiled-Coil Motif Containing, IMT inflammatory myofibroblastic tumor, KANK1 KN Motif And Ankyrin Repeat Domains 1, KIF4B/5B kinesin family member 4B/5B, NA not available, NPM1 nucleophosmin1, NSCLC non-small cell lung cancer, PFS progression free survival, PR partial response, PPFBP1 PPFIA Binding Protein 1, PRRC2B proline rich coiled-coil 2B, SD stable disease, SPECC1L sperm antigen with calponin homology and coiled-coil domains 1 like, SQSTM1 sequestosome 1, STRN striatin, TFG TRK-fused gene, VCL vinculin.
Butrynski and colleagues were one of the first to report a sustained partial response to crizotinib in a patient with ALK-rearranged IMT and mechanistically compared it with the absence of activity of crizotinib in another patient with IMT without ALK rearrangement79. Over the years, multiple basket trials have evaluated the efficacy of ALK inhibitors in tumors with ALK aberrations. The second pediatric strategy forum for ALK inhibition in pediatric malignancies provided an overview of the current status and future direction of ALK inhibitors in management of pediatric patients with ALCL, IMT and neuroblastoma98. The forum identified some key challenges of accruing patients suffering from these rare tumors in multiple clinical trials98.
A phase I/II children’s oncology group study of 26 ALK-altered ALCL and 14 IMT pediatric patients showed promising activity of crizotinib in these cancers. The overall response rate (ORR) in relapsed/refractory/unresectable IMT was around 86% and the ORR in ALK-altered ALCL was 83-90% (depending on dose). Complete response rate was 36% in IMT and over 80% in ALCL. Responses were durable130. Fukano et al reported results of a small phase II study of alectinib in 10 relapsed ALCL patients treated in Japan, which showed an impressive complete response rate of 60%- and 1-year overall survival rate of 70%35. A phase Ib study, PROFILE 1013 (NCT01121588) evaluated efficacy of crizotinib in 44 patients (≥15 years) suffering from ALK-altered ALCL, IMT and other malignancies. The ORR was 53% (95% confidence interval [CI], 28–77) for ALCL (mostly complete responses); 67% for IMTs (mostly PRs); and 12% (95% CI, 2–36) for other tumors, with two PRs in patients affected by colon carcinoma (lasting two years) and medullary thyroid cancer, respectively; an additional patient with neuroblastoma had stable disease for 19 months132. The median duration of treatment was almost three years for patients with ALCL and IMTs132. ALK-altered DLBCL is a rare subtype of large B cell lymphoma which resembles ALCL, with plasmablastic differentiation; one patient with ALK-altered DLBCL in this study had stable disease for almost four years with crizotinib132. The long-term follow up results of these studies have led to the FDA approval of crizotinib for ALK-altered ALCL and IMT34,36. Similar results have been observed in ALK-altered ALCL, IMT and some neuroblastoma cases treated ceritinib143. The ORR was ~75% (6/8) and 70% (7/10) among ALCL and IMT patients that harbor ALK rearrangements, respectively but it was only around 20% (6/30) in neuroblastoma (which usually harbors mutations in ALK). Among neuroblastoma cases, response to ceritinib were observed in cases carrying ALK p.Arg1275 mutations. Because of the trial set-up, the exact type of ALK alteration was not always reported so it is unknown if there were specific ALK point mutations in neuroblastoma that were resistant to ceritinib.
Potential reasons for the attenuated clinical activity in some groups is due to ALK alterations other than fusions/rearrangements. Some of them are reported in (Table 4)35,48,73,74,85,94,126,130–149. For instance, ALK point mutations were permitted in some studies, and mutations such as F1174L (common ALK‐activating mutations seen in neuroblastoma) have been shown to confer resistance to crizotinib in neuroblastoma and other cancers82. Investigators believed that third generation TKI’s like lorlatinib could solve this problem and be more effective in counteracting these resistant mutations in neuroblastoma98. However, results of a recently concluded phase I neuroblastoma consortium study were largely unsatisfactory. In this study 33 relapsed/refractory patients aged 2-17 (cohort A1) and aged 15-50 (cohort A2) were presented in ASCO 2020. Lorlatinib was well tolerated, in cohort A2, 10% (1/10) patients attained CR and 30% (3/10) attained PR whereas only 5.5% (1/18) patients in cohort A1 had PR148. MYCN overexpression in neuroblastoma has also been associated with resistance to ALK inhibitors82,83.
ALK aberrations have also been described, albeit uncommonly, in rare hematologic disorders such as histiocytosis. ALK-aberrant histiocytosis usually harbors a KIF5B-ALK fusion, though other fusions are also observed74. Kemps et al reported retrospective data on 39 ALK-positive histiocytosis cases with 37 confirmed ALK rearrangements. They showed that advanced stage ALK-altered histiocytosis patients including those with neurological disease can be effectively treated with ALK inhibitors (11/11 objective and sustained response were achieved with ALK inhibitors such as crizotinib, alectinib, brigatinib and lorlatinib in first, second or further line setting74).
The use of crizotinib has also led to prolonged stabilization of disease in individual cases of recurrent glioblastoma150 but its utility in management of glioblastoma’s with ALK aberrations is questionable. For example, in the ASCEND 10 study140 none of the 12 ALK positive glioblastoma patients responded to crizotinib. Unfortunately, this study was terminated early and since enrollment was only based on ALK positive immunohistochemistry, there is no data on types of ALK alterations included in the glioblastoma cohort available in public domain. One plausible reason for suboptimal treatment response to crizotinib could be the higher prevalence of ALK mutations over fusions in this cancer cohort.
Resistance to first line ALK inhibition has also been seen in some cases which are often overcome by second or third line ALK inhibition. Second and third generation ALK inhibitors like alectinib, ceritinib, brigatinib and lorlatinib have good CNS penetration and are effective in management of crizotinib resistant and relapsed/refractory patients with CNS metastasis151,152. Alectinib is being studied in pediatric patients with ALK fusion-positive solid or CNS tumors (NCT04774718). Brigatinib is also being evaluated in a clinical trial in relapsed and refractory ALCL, IMT and other pediatric cancers (NCT04925609). We have also enlisted ongoing clinical trials of ALK inhibitors with their clinical trial.gov ID’s in (Table 4)35,48,73,74,85,94,126,130–149.
In a study of nine ALK -rearranged colorectal cancers (~0.2% of colorectal malignancies), one metastatic cecal cancer patient with a STRN-ALK fusion protein was treated with the ALK inhibitor ceritinib, which resulted in a marked decrease in size of a skin metastasis, and resolution of all contrast-enhancing tumors on imaging studies. This patient-derived treatment benefit for almost 9 months49. Some other studies and case series have reported responses to second and third-generation ALK inhibitors in colorectal cancer141, renal cell carcinoma126, pancreas adenocarcinoma94 and thyroid cancers138,139, with all tumors notably bearing fusions.
Results of CTO32 study were recently presented at a national meeting. In this study, 21 patients with various tumor types harboring ALK aberrations (10 patients with ALK gene rearrangement and 11 with ALK mutation or amplifications) were treated with alectinib. Among the 10 cancer patients with ALK rearrangements, three had partial responses (PRs). Interestingly, there were no responses among the 11 patients. with ALK mutations or amplification. The median progression-free survival (PFS) was 8.2 months (95% CI, 1.7–13.6) in patients with ALK rearrangements vs 1.8 months (95% CI 1.1–5.5) for those with ALK mutation or amplifications153. Results of subgroup F (cases harboring ALK fusions) of the NCI match study145 were recently reported as well. Two patients with colorectal cancer, one with carcinoma of unknown primary and one with leiomyosarcoma were included in this cohort and treated with crizotinib. At the time of analysis, the ORR was 50% with one complete response in the leiomyosarcoma patient and partial response in one colorectal cancer patient, again showing that tumors harboring ALK fusions/rearrangement are responsive to ALK inhibitors.
It is important to note that some of the above-mentioned studies solely relied on immunohistochemistry (IHC) to identify ALK overexpressing tumors and enrolled ALK-positive cancer patients based on the IHC findings. Next generation sequencing tests were less frequently utilized to identify ALK alterations, which could have affected the final treatment outcome in some cases. We believe that response to ALK inhibitors depends on the type of ALK alterations. For example, ALK point mutations and amplifications are common in neuroblastoma, whereas ALK fusions/rearrangements are more common in IMT and ALCL, but all of these tumor types can present with ALK overexpression. Based on data collected from some international studies that included multiple disease cohorts, we can conclude that, among cancer cohorts like IMT and ALCL known to have higher frequency of ALK rearrangements, ORR were much higher, ranging from 56 to 100%35,130–132,140,143 compared to 9 to 20%131,143 observed in cancer cohorts such as neuroblastoma and other tumors where ALK mutation and amplifications are generally more prevalent). Furthermore, anecdotal responses of ALK fusion/rearranged cancers across a broad array of malignancies and with multiple different ALK inhibitors have been reported in (Table 4)35,48,73,74,85,94,126,130–149.
Poor responses in some disease groups seem attributable mostly to the ALK alterations being mutations or amplifications rather than fusions/rearrangement, the fact that ALK overexpression does not always mean that there is a fusion/rearrangement present, and conceivably due to co-existing drivers. Because of the importance of fusions/rearrangements and their potential actionability, it has been suggested that sequencing RNA rather than DNA might be more effective in detecting ALK fusions/rearrangements, given the difficulties of covering all introns from which rearrangements can arise154.
Taken together, patients with a wide variety of common and rare solid and hematologic malignancies are responsive to any one of multiple ALK inhibitors, especially if their tumors bear fusions/rearrangements, with responses occurring less frequently with other types of ALK alterations.
Tests to diagnose Alk alterations
Three main detection methods are utilized in clinical practice to detect ALK alterations. These include fluorescence in situ hybridization (FISH), immunohistochemistry (IHC) and next-generation sequencing (NGS) technologies. In a study by Bernicker et al. 155, real-world data of 41,728 patients with NSCLC diagnosed in community medical centers in the USA from January 2012 to May 2019 was evaluated to describe the ALK testing trend. The study showed that the ALK test use rates in eligible patients suffering from NSCLC dramatically rose from 59.5% in 2012 to 84.1% in the year 2019156. ALK testing rates have been higher in patients of younger age (<50 years), Asian race, non-squamous histology type, nonsmokers, and initial stage as stage IV.
FISH was the dominant method of ALK detection (81% of all testing) in the earlier part of this decade and is still considered a gold standard of ALK fusion detection, but NGS testing has quickly gained ground over the last five years. According to some estimates, by the first half of 2019, 45.99% of tests were performed by NGS compared with FISH, which was used in 37.68% of all cases155. Signal intensity variation and inter-observer variability have limited the use of FISH in a clinical setting. The cost-effectiveness and rapid turnaround time of IHC have made it an attractive choice for clinicians and pathologists. The accuracy of this technique is retained when combined with high-performance antibodies. The concordance rate between FISH and IHC was found to be around 80.6% in a large pooled metanalysis of 11,000 cases157. However, this means that substantial numbers of IHC patients do not have one of the highly actionably ALK fusions/rearrangements.
Another popular method of direct ALK fusion detection is reverse transcriptase PCR(RT-PCR) based testing. It has a rapid turnaround time and according to some estimates concordance rate between FISH and RT-PCR is around 89%158. Even so, NGS technology is quickly emerging as a preferred method for comprehensive testing in cancer. It allows broad coverage of genomic regions of interest, fusion partner characterization and improves detection of relevant genomic alterations, and most importantly, permits hundreds of genes to be interrogated with a single test. At the DNA level, it is sometimes difficult to detect gene-fusion expression, particularly if breakpoints involve long intronic regions that may not be covered by hybridization-capture probes. To address this critical issue, targeted RNA-based NGS assays have been developed which are more sensitive and effective in detecting gene fusions154,159. Overall, NGS testing has the best throughput among all these testing modalities.
Mechanisms of resistance to ALK inhibitors
Several mechanisms of ALK resistance have been proposed. These include the resistance mutations, differential sensitivity to different ALK inhibitors, type of alteration (with fusions/rearrangements being more sensitive to ALK inhibitors that mutations/amplifications and co-existent molecular drivers).
The development of secondary resistance mutations in the ALK kinase domain, can be a resistance mechanism160. For instance, L1196M mutation corresponds to a gatekeeper residue, a residue located in the ATP-binding pocket of a protein kinase that, when mutated, causes a change in the structure of the kinase that prevents ALK inhibitor binding. Certain resistance mutations affect residues adjacent to the N-terminus for example (C1156Y, L1152R, and I1151Tins) and C-terminus of the αC helix for example (F1174C/L/V)71. These mutations enhance the kinase’s ATP-binding affinity and increase its enzymatic activity. G1202R, D1203N, and S1206Y/C represent another class of solvent front ALK resistance mutations that impair drug binding likely through steric hindrance161. Earlier studies have shown that G1202R confers high-level resistance to first- and second-generation ALK inhibitors and is susceptible to third generation ALK inhibitors like lorlatinib71,160,161. Differential sensitivity to ALK mutations between different ALK inhibitors has also been reported in Fig. 225,71,115,161–170. For example, crizotinib161 can inhibit G1123S mutations which is resistant to ceritinib. Some cell lines harboring I1171N and I1171T mutations that are resistant to alectinib but are sensitive to ceritinib161,168. Conversely, L1152P, L1152R, F1174 C, F1174 L and F1174V mutations confer resistance to ceritinib161,168 but are sensitive to alectinib, brigatinib and lorlatinib. Lorlatinib is effective against mutations like F1245C, E1210K and G1202R which confer resistance to second generation ALK inhibitors. Sequential treatment with ALK inhibitors and exposure to third generation agents such as lorlatinib can also lead to development of resistant compound mutations such as ALK L1198F/C1156Y and G1269A/I1171S161. Tumors harboring these mutations can sometimes be rechallenged with first or second generation ALK inhibitors like crizotinib167 and ceritinib171 respectively. Some other highly resistant compound mutations such as G1202R/L1196M and D1203N/I1171N have also been reported that do not respond to first, second or third generation ALK inhibitors. A study by Dagogo-Jack et al. reported cooccurrence of ALK D1203N mutation in exon 23 with the I1171N exon 22 mutation in some lorlatinib-resistant cases. The allele frequencies of the two ALK-resistant mutations suggested that they were likely in cis, but the allelic configuration could not be confirmed as the two exons were separated by approximately 1600 base pairs172. Another study by Recondo et al. also reported the presence of a D1203N mutation, present in “cis” with the L1196M mutation in lorlatinib-resistant cases173. Post-treatment ALK amplifications occur less frequently than secondary mutations, but are also a recognized cause of acquired resistance to crizotinib161.
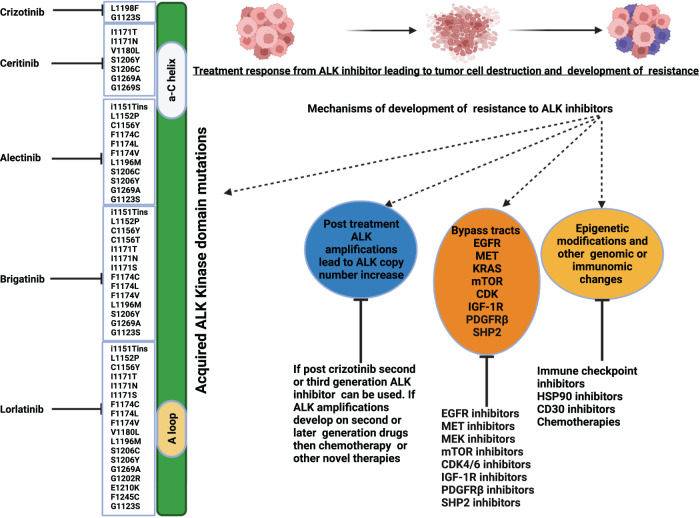
Fig. 2. Examples of Resistance Mechanisms and Treatment Strategies for ALK inhibitor resistant tumors.
This figure Illustrates mechanisms of development of resistance to ALK inhibitors and possible management strategies. For example, development of bypass pathways like EGFR, MET, PDGFR, IGF-1R, CDK, mTOR and SHP2 can lead to resistance and can be targeted by their respective inhibitors. Resistance can also develop because of epigenetic modifications and other immunomic changes in tumor, which may theoretically be targeted by heat shock protein (HSP) 90 inhibitors, Cluster differentiation (CD) 30 inhibitors and immune check point inhibitors. Similarly, formation of ALK amplifications may also be a mechanism of resistance and can be treated with chemotherapy. This figure also illustrates various acquired tyrosine kinase domain mutations which can be targeted by different generations of ALK inhibitors. The mutations listed on the left of ALK kinase domain are sensitive to respective ALK tyrosine kinase inhibitors. For example, crizotinib inhibits G1123S, L1198F resistant mutation. Ceritinib inhibits I1171T, I1171N, V1180L, S1206C, S1206Y, G1269A. Alectinib inhibits i1151Tins, G1123S, L1152P, C1156Y, F1174C, F1174L, F1174V, L1196M, S1206C, S1206Y, G1269A resistant mutations. Brigatinib inhibits i1151Tins, L1152P, C1156Y, I1171T, I1171N, I1171S, F1174C, F1174L, F1174V, V1180L, L1196M, S1206Y, G1269A and Lorlatinib inhibits i1151Tins L1152P, C1156Y, I1171T, I1171N, I1171S, F1174C, F1174L, F1174V, V1180L, L1196M, S1206C, S1206Y, E1210K, G1269A, E1210K, G1202R, E1210K, F1245C. Created by Biorender.com.
Apart from the above-mentioned ALK inhibitor-dependent resistant mechanisms, there are ALK inhibitor independent resistance pathways. As an example, epigenetic modifications and epithelial-mesenchymal transitions can lead to ALK-independent resistance174 as can aberrant activation of alternate kinases that promote ALK-independent growth through bypass pathways, including via the epidermal growth factor receptor (EGFR), human epidermal growth factor receptor type 2(HER2), and insulin-like growth factor-1(IGF-1R) receptor signals23,175 as well as platelet derived growth factor receptor beta (PDGFRβ), mammalian target of rapamycin (mTOR), Mesenchymal Epithelial Transition (MET) and others71. Activation of the MEK pathway can also contribute to ALK inhibitor resistance, through deficiency/low expression of Wiskott–Aldrich syndrome protein (WASP) protein176. Interestingly, activation of Interleukin (IL)-10RA signaling pathways also contribute to first generation ALK inhibitors resistance. IL-10 is a known immunosuppressive factor that, promotes T cell exhaustion, inhibits effector T cells and T cell activation177. Finally, high expression of P-glycoprotein (P-gp), an ATP-dependent efflux pump which is encoded by the multidrug resistance 1 (MDR1) gene can also lead to ALK independent resistance. It has been reported to be a potential cause of drug resistance and reduced CNS penetration of crizotinib and ceritinib71. Genome-wide sequencing studies have shown that protein tyrosine phosphatases such as PTPN1 and PTPN2 are involved in ALK inhibitor resistance in lymphoma. PTPN1 and PTPN2 genes regulate ALK and Src homology region 2 -containing protein tyrosine phosphatase 2 (SHP2) phosphorylation. Based on these findings, it can be postulated that combined inhibition of ALK and SHP2 is an approach that merits study in ALCL178. Prokoph et al. also reported that resistance to crizotinib in ALCL cases can be driven by upregulation of interleukin-10 receptor subunit alpha (IL10RA). The elevation of IL10RA expression rewires the signal transducer and activator of transcription 3 (STAT3) molecular signaling pathway which bypasses otherwise critical phosphorylation induced by NPM1-ALK fusion10. Figure 225,71,115,161–170 illustrates some of the common resistant mechanisms seen with ALK inhibition.
Targeting resistance with novel agents and paradigms
Resistance mutations acquired on treatment with an ALK inhibitor can sometimes be overcome by switching to a newer generation ALK inhibitor (Fig. 2)25,71,115,161–170. Earlier studies have shown that long-term ALK inhibition was also associated with enhanced IGF-1R signaling, which leads to the development of resistance pathways. IGF-1R increases the phosphorylation of ALK and its downstream effectors in ALK-aberrant ALCL cells. It further promotes the survival of these cancer cells by increasing the expression of anti-apoptotic proteins like Bcl-2 and Bcl-xl179. IGF-1R inhibitors sensitize tumor cells to the effects of ALK inhibition. Hence, co-targeting ALK and IGF-1R can improve treatment response in ALK inhibitor naïve setting and can reverse resistance in ALK inhibitor exposed setting. Ceritinib is a second-generation ALK inhibitor which is also a potent IGF-1R inhibitor25. This off-target effect of ceritinib might explain, in part, its efficacy in crizotinib-resistant tumors. Unfortunately, target kinase domain alterations and bypass signaling do not appear to be mutually exclusive and can further lead to resistance from second and third generation ALK inhibitors71.
In those unusual cases where there are co-existent ALK and EGFR alterations, which occurs in a subset of NSCLC, dual inhibition of EGFR and ALK can be a useful strategy in treating patients that develop resistance to ALK inhibitors such as crizotinib, ceritinib, and alectinib. Investigators have shown that afatinib may be a promising treatment for overcoming ceritinib resistance in ALK-positive NSCLC cells by inhibiting the EGFR162 and neuroregulatory protein (NRG1) signaling180 pathways. Interestingly brigatinib has in vitro activity against both EGFR and ALK alterations and can be used to target EGFR induced resistance to ALK inhibitors181. The mTOR pathway also acts as a bypass pathway of resistance and some investigators have shown a synergistic effect of combining ALK inhibitors with mTOR inhibitors in lymphoma cells165. There is also evolving data to show that increased expression of hepatocyte growth factor (HGF) and MET amplification can lead to resistance from some ALK inhibitors. This can be mitigated by combining metformin and second-generation ALK inhibitors such as alectinib182 or perhaps by adding a MET inhibitor183.
A preclinical study by Wood et al. showed that dual inhibition with a CDK4/6 inhibitor like ribociclib and ALK inhibitor such as ceritinib can lead to complete regression of ALK-aberrant neuroblastoma xenograft tumors164. This combination is now being investigated in a phase I Next Generation Personalized Neuroblastoma Therapy (NEPENTHE) study (NCT02780128). NPM-ALK-altered ALCL cells are associated with high expression of JUNB, JUN, PDGFRα and PDGFRβ mRNA and protein. Some preclinical studies and case reports have shown that therapeutic blockade of PDGFRβ with agents like imatinib in combination with ALK inhibitors can lead to significant reduction in size of tumor mass and alleviate relapse of ALCLs after exposure to single agent ALK inhibitors163.
A customized (N-of-1) combinatorial targeted therapy-based approach can also be utilized to tackle resistant mechanisms184–187. The efficacy and safety of NGS-informed customized combination therapy was reported in the analysis of the I-PREDICT studies in a variety of cancers. Targeting a larger fraction of known molecular alteration with a customized multidrug regimen leads to better disease control rates, higher response rates and longer PFS and survival than targeting fewer molecular alterations184–187.
Case presentation
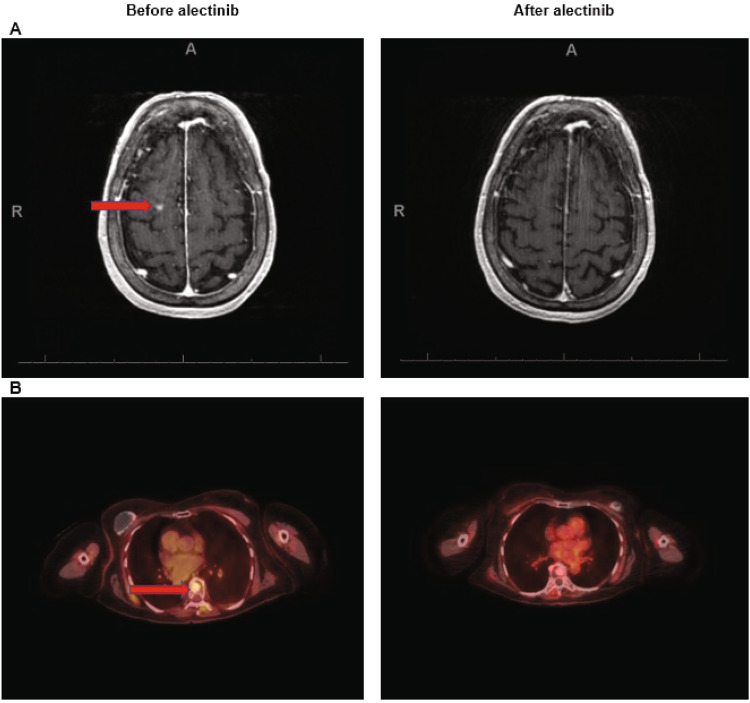
A 73-year-old woman with widespread Erdheim-Chester disease (ECD) (non-Langerhans histiocytosis) with an ALK-KIF4B fusion, who had a remarkable response to alectinib is discussed. This patient had a history of breast cancer and initially presented with right knee pain. Imaging revealed widespread osseous, extraosseous deposits and leptomeningeal disease. Biopsy of left cervical lymph node indicated histiocytic proliferation compatible ECD (negative for BRAFV600E mutation). Additional molecular testing with targeted NGS of tumor tissue and plasma derived circulating tumor DNA showed KIT M541L mutation (tissue) and TP53 mutations (C135W and C277Y) (blood biopsy). Earlier studies have shown that alterations in the MAPK pathway like MAP2K1, MAP2K2, GNAS, NF1, and RAS mutations are quite common in BRAF wildtype ECD and can be targeted by MEK inhibitors such as cobimetinib or trametinib, albeit with a need for reduced doses in patients with ECD188,189. The patient was treated with trametinib 1–1.5 mg orally daily, and imaging with PET/CT and MRI demonstrated partial response. Unfortunately, she developed cardiac complications, which precluded trametinib continuation. Because studies have demonstrated a role of proinflammatory cytokines such as interleukin-1 and -6 in the pathogenesis of ECD190 and Killu AM and colleagues had also reported a case of ECD patient with cardiac involvement, who was successfully treated with an IL-1 receptor antagonist191, she was, switched to the IL-1 receptor antagonist anakinra, which she tolerated well and had stable disease for 18 months. Patient later consented to the Institutional Review Board (IRB) approved MD Anderson Cancer Center (MDACC) protocol number RC04-567. Additional testing for gene fusions performed on archival tissue blocks revealed an ALK-KIF4B fusion. Therefore, an ALK inhibitor-- alectinib 600 mg PO daily-- was added to her treatment regimen. Follow up PET/CT and MRI after 2 months demonstrated remarkable improvement in brain lesions (Fig. 3). She continues to do well six months after starting alectinib without major toxicity.
Fig. 3. Case of 73-year-old woman with Erdheim Chester disease (non-Langerhans histiocytosis) and ALK-KIF4B fusion responds to alectinib.
Figure showing response to alectinib in follow up scans taken 2 months after treatment initiation. A MRI brain showing resolution of the lesion in the right superior frontal gyrus (arrow). B PET/CT showing decreased FDG uptake in T8 sclerotic lesion. Red arrows show lesions in the before treatment scans.
In summary
ALK is now an established therapeutic target in NSCLC and several other hematologic and solid malignancies. ALK alterations include mutations, amplifications, overexpression by IHC, and fusions/rearrangements. Multiple ALK inhibitors have entered the clinic; to date, five of them—alectinib, brigatinib, ceritinib, crizotinib, and lorlatinib-- have become standard therapies for advanced ALK-aberrant NSCLCs. Crizotinib is also FDA approved for ALK-aberrant IMT and ALCL, including in children, reflecting activity of these agents in both solid and hematologic malignancies and across the age spectrum (Table 1)28–34,36–39. Importantly, the regulatory authorizations for ALK inhibitors are for neoplasms bearing ALK fusions/rearrangements and, in those conditions, response rates are usually in the range of 50–85%. Although ALK fusions/rearrangements occur in ~3–7% of NSCLCs, ~50% of IMTs and 50–80% of ALCLs, such alterations are ultra-rare in other cancers, being found in only ~0.2% of patients, making studies in these diseases challenging, and perhaps below the feasibility threshold of prospective treatment trials, even those that are large scale such as NCI-MATCH and similar efforts192,193. Importantly, the literature is replete with reports of exceptional responses of multiple tumor types bearing ALK fusions/rearrangements, both solid and hematologic, including but not limited to colorectal cancer, histiocytosis, leiomyosarcoma, lymphoma, multiple myeloma, neuroendocrine cancer, ovarian cancer, pancreatic cancer, renal cancer, thyroid cancer (even the aggressive anaplastic variant) after the patient was given an ALK inhibitor. Most of these reports used crizotinib or alectinib, but each of the approved ALK inhibitors have demonstrated efficacy in some of the published studies (Table 4)35,48,73,74,85,94,126,130–149. Responses to ALK inhibitors can also be observed in diseases such as neuroblastoma, which bear ALK mutations (rather than fusions/rearrangements), but the response rates are generally only in the ~10–20% range. Based on current data, it seems reasonable to posit that ALK inhibitors have tissue-agnostic activity in malignancies bearing ALK fusions/rearrangements.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Author contributions
A.V.S. and R.K. conceptualized the article and made substantial contributions to conceptualization, data acquisition, review, and interpretation of data for the article. A.V.S., F.J., M.A.G. and R.K. wrote the manuscript, and all authors, including A.V.S., F.J., M.A.G., H.C., B.G., S.K. and R.K. reviewed and/or edited the manuscript before submission. In addition, all authors made a significant contribution to the discussions.
Data availability
We have not analyzed any publicly available databases for this article. We have appropriated cited all clinical data included in this review article.
Competing interests
A.V.S. MD MS: Advisory board: Taiho Oncology, Institutional research funding: Natera Pvt. Ltd. F.J. MD PHD: research support from Astex, Novartis, BioMed Valley Discoveries, Fore Biotherapeutics, Deciphera, Bristol Myers Squibb, Asana, IDEAYA Biosciences, Sanofi, Merck, F-star, JSI InnoPharm, BioXcel, Lilly, Bicara Therapeutics, PureTech Health, FUJIFILM Pharmaceuticals, SOTIO, Synlogic, NextCure, and Hutchinson MediPharma; served on the scientific advisory boards of IDEAYA Biosciences, Synlogic, SOTIO, PureTech Health, Deciphera, Crown Bioscience, Asana, Fore Biotherapeutics, Novartis, Bicara Therapeutics, and PegaOne; was a paid consultant for Mersana Therapeutics, Flame Biosciences, Cardiff Oncology, MedinCell, and ImmunoMet Therapeutics; holds a leadership position in Monte Rosa Therapeutics; has an ownership interest in Monte Rosa Oncology and Cardiff Oncology. H.-Z.C. MD PHD: 1. Consultant and speaker for Jazz Pharmaceuticals. Consultant for Biological Dynamics. B.G. MD: Consultant: Ipsen, Bristol Myers Squibb, Foundation Medicine, Taiho Oncology, BTG (Boston Scientific), Roche/Genentech, Pfizer. Research Support: Glyconex (Inst), Helix (Inst), Pfizer, Foundation Medicine (Inst), Roche/Genentech (Inst), Hoffman La-Roche (Inst), Taiho Oncology (Inst), Boehringer Ingelheim (Inst), Toray, NGM Biopharma, Hutchison Medipharma, Mirati Therapeutics, Sirnamomics (Inst), Trishula Therapeutics (Inst), Gritstone BIo (Inst). Stock Ownership: XBiotech. S.K. MD: Research contracts with ACT Genomics, Sysmex, Konica Minolta, OmniSeq, and Personalis; Speaker’s fees from Bayer and Roche; and personal fees from CureMatch, Foundation Medicine, Medpace, NeoGenomics, and Pfizer. R.K. MD: Leadership: CureMatch, CureMetrix Stock and Other Ownership Interests: CureMatch, IDbyDNA, CureMetrix. Honoraria: Roche, EUSA Pharma, NeoGenomics Laboratories, Biocom, NeoMed, LEK, AACR, Chugai Pharma USA, Wiley, Merck, Pfizer, Foundation Medicine, Turning Point Therapeutics, Bicara Therapeutics. Consulting or Advisory Role: Actuate Therapeutics, Loxo, XBiotech, NeoMed, Roche, Gaido, Soluventis, Pfizer, Merck, Turning Point Therapeutics, TD2/ Volastra, Bicara Therapeutics, AstraZeneca, Biological Dynamics, Daiichi Sankyo, Eisai, EOM Pharmaceuticals, Iylon, Prosperdtx. Speakers’ Bureau: Roche, NeoGenomics Laboratories. Research Funding: Guardant Health (Inst), Sequenom (Inst), Merck Serono (Inst), Genentech (Inst), Pfizer (Inst), Foundation Medicine (Inst), Incyte (Inst), Konica Minolta (Inst), Grifols (Inst), OmniSeq (Inst), Debiopharm Group (Inst), Boehringer Ingelheim (Inst), Top Alliance BioScience (Inst), Takeda (Inst), MedImmune (Inst), Biological Dynamics (Inst). Travel, Accommodations, Expenses: Target Cancer, NCI SWOG Other Relationship: R.K. receives research funding from Boehringer Ingelheim, Debiopharm, Foundation Medicine, Genentech, Grifols, Guardant, Incyte, Konica Minolta, Medimmune, Merck Serono, Omniseq, Pfizer, Sequenom, Takeda, and TopAlliance; as well as consultant and/or speaker fees and/or advisory board for Actuate Therapeutics, AstraZeneca, Bicara Therapeutics, Inc, Biological Dynamics, Caris, Datar Genomics, EISAI, EOM Pharmaceuticals, Iylon, Merck, NeoGenomics, Neomed, Pfizer, Prosperdtx, Regeneron, Roche, TD2/Volastra, Turning Point Therapeutics, X-Biotech; has an equity interest in CureMatch Inc and IDbyDNA; serves on the Board of CureMatch and CureMetrix, and is a co-founder of CureMatch. The other authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Aditya Shreenivas, Email: ashreenivas@mcw.edu.
Razelle Kurzrock, Email: rkurzrock@mcw.edu.
Supplementary information
The online version contains supplementary material available at 10.1038/s41698-023-00449-x.
References
- 1.Consortium APG. AACR project GENIE: powering precision medicine through an International Consortium. Cancer Discov. 2017;7:818–831. doi: 10.1158/2159-8290.CD-17-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ross JS, et al. ALK fusions in a wide variety of tumor types respond to anti-ALK targeted therapy. Oncologist. 2017;22:1444–1450. doi: 10.1634/theoncologist.2016-0488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chiarle R, Voena C, Ambrogio C, Piva R, Inghirami G. The anaplastic lymphoma kinase in the pathogenesis of cancer. Nat. Rev. Cancer. 2008;8:11–23. doi: 10.1038/nrc2291. [DOI] [PubMed] [Google Scholar]
- 4.Loren CE, et al. A crucial role for the Anaplastic lymphoma kinase receptor tyrosine kinase in gut development in Drosophila melanogaster. EMBO Rep. 2003;4:781–786. doi: 10.1038/sj.embor.embor897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moreno MM, Barrell WB, Godwin A, Guille M, Liu KJ. Anaplastic lymphoma kinase (alk), a neuroblastoma associated gene, is expressed in neural crest domains during embryonic development of Xenopus. Gene Expr. Patterns. 2021;40:119183. doi: 10.1016/j.gep.2021.119183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fischer P, et al. A Ki-1 (CD30)-positive human cell line (Karpas 299) established from a high-grade non-Hodgkin’s lymphoma, showing a 2;5 translocation and rearrangement of the T-cell receptor beta-chain gene. Blood. 1988;72:234–240. [PubMed] [Google Scholar]
- 7.Morris SW, et al. Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin’s lymphoma. Science. 1994;263:1281–1284. doi: 10.1126/science.8122112. [DOI] [PubMed] [Google Scholar]
- 8.Morris SW, et al. ALK, the chromosome 2 gene locus altered by the t(2;5) in non-Hodgkin’s lymphoma, encodes a novel neural receptor tyrosine kinase that is highly related to leukocyte tyrosine kinase (LTK) Oncogene. 1997;14:2175–2188. doi: 10.1038/sj.onc.1201062. [DOI] [PubMed] [Google Scholar]
- 9.Krumbholz M, et al. Characterization and diagnostic application of genomic NPM-ALK fusion sequences in anaplastic large-cell lymphoma. Oncotarget. 2018;9:26543–26555. doi: 10.18632/oncotarget.25489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prokoph N, et al. IL10RA modulates crizotinib sensitivity in NPM1-ALK+ anaplastic large cell lymphoma. Blood. 2020;136:1657–1669. doi: 10.1182/blood.2019003793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iwahara T, et al. Molecular characterization of ALK, a receptor tyrosine kinase expressed specifically in the nervous system. Oncogene. 1997;14:439–449. doi: 10.1038/sj.onc.1200849. [DOI] [PubMed] [Google Scholar]
- 12.Janoueix-Lerosey I, et al. Somatic and germline activating mutations of the ALK kinase receptor in neuroblastoma. Nature. 2008;455:967–970. doi: 10.1038/nature07398. [DOI] [PubMed] [Google Scholar]
- 13.Miyake I, et al. Activation of anaplastic lymphoma kinase is responsible for hyperphosphorylation of ShcC in neuroblastoma cell lines. Oncogene. 2002;21:5823–5834. doi: 10.1038/sj.onc.1205735. [DOI] [PubMed] [Google Scholar]
- 14.Bellini A, et al. Frequency and prognostic impact of ALK amplifications and mutations in the European Neuroblastoma Study Group (SIOPEN) high-risk neuroblastoma trial (HR-NBL1) J. Clin. Oncol. 2021;39:3377–3390. doi: 10.1200/JCO.21.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okubo J, et al. Aberrant activation of ALK kinase by a novel truncated form ALK protein in neuroblastoma. Oncogene. 2012;31:4667–4676. doi: 10.1038/onc.2011.616. [DOI] [PubMed] [Google Scholar]
- 16.Cao Z, et al. Anaplastic lymphoma kinase fusions: roles in cancer and therapeutic perspectives. Oncol. Lett. 2019;17:2020–2030. doi: 10.3892/ol.2018.9856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guan J, et al. FAM150A and FAM150B are activating ligands for anaplastic lymphoma kinase. Elife. 2015;4:e09811. doi: 10.7554/eLife.09811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reshetnyak AV, et al. Augmentor alpha and beta (FAM150) are ligands of the receptor tyrosine kinases ALK and LTK: Hierarchy and specificity of ligand-receptor interactions. Proc. Natl Acad. Sci. USA. 2015;112:15862–15867. doi: 10.1073/pnas.1520099112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ducray SP, Natarajan K, Garland GD, Turner SD, Egger G. The transcriptional roles of ALK fusion proteins in tumorigenesis. Cancers. 2019;11:1074. doi: 10.3390/cancers11081074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soda M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448:561–566. doi: 10.1038/nature05945. [DOI] [PubMed] [Google Scholar]
- 21.Inamura K, et al. EML4-ALK fusion is linked to histological characteristics in a subset of lung cancers. J. Thorac. Oncol. 2008;3:13–17. doi: 10.1097/JTO.0b013e31815e8b60. [DOI] [PubMed] [Google Scholar]
- 22.Chia PL, Mitchell P, Dobrovic A, John T. Prevalence and natural history of ALK positive non-small-cell lung cancer and the clinical impact of targeted therapy with ALK inhibitors. Clin. Epidemiol. 2014;6:423–432. doi: 10.2147/CLEP.S69718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang H. Anaplastic lymphoma kinase (ALK) receptor tyrosine kinase: a catalytic receptor with many faces. Int J. Mol. Sci. 2018;19:3448. doi: 10.3390/ijms19113448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilson C, Nimick M, Nehoff H, Ashton JC. ALK and IGF-1R as independent targets in crizotinib resistant lung cancer. Sci. Rep. 2017;7:13955. doi: 10.1038/s41598-017-14289-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lovly CM, et al. Rationale for co-targeting IGF-1R and ALK in ALK fusion-positive lung cancer. Nat. Med. 2014;20:1027–1034. doi: 10.1038/nm.3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou F, Zhou C. Lung cancer in never smokers-the East Asian experience. Transl. Lung Cancer Res. 2018;7:450–463. doi: 10.21037/tlcr.2018.05.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marino FRA, Accardo M, Franco R. Concomitant ALK/KRAS and ALK/EGFR mutations in non small cell lung cancer: different profile of response to target therapies. Transl. Cancer Res. 2017;6:S457–S460. [Google Scholar]
- 28.Kazandjian D, et al. FDA approval summary: crizotinib for the treatment of metastatic non-small cell lung cancer with anaplastic lymphoma kinase rearrangements. Oncologist. 2014;19:e5–e11. doi: 10.1634/theoncologist.2014-0241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.FDA. FDA broadens ceritinib indication to previously untreated ALK-positive metastatic NSCLC 2017 [05/26/2017]. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-broadens-ceritinib-indication-previously-untreated-alk-positive-metastatic-nsclc (2017).
- 30.FDA. Alectinib approved for (ALK) positive metastatic non-small cell lung cancer (NSCLC) 2017 [11/06/2017]. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/alectinib-approved-alk-positive-metastatic-non-small-cell-lung-cancer-nsclc (2017).
- 31.FDA. Brigatinib 2017. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/brigatinib (2017).
- 32.FDA. FDA approves brigatinib for ALK-positive metastatic NSCLC 2020 [05/22/2020]. Available from https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-brigatinib-alk-positive-metastatic-nsclc (2020).
- 33.FDA. FDA approves lorlatinib for metastatic ALK-positive NSCLC 2021 [03/03/2021]. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-lorlatinib-metastatic-alk-positive-nsclc (2021).
- 34.FDA. FDA approves crizotinib for children and young adults with relapsed or refractory, systemic anaplastic large cell lymphoma FDA website2022 [01/14/2022]. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-crizotinib-children-and-young-adults-relapsed-or-refractory-systemic-anaplastic-large (2022).
- 35.Fukano R, et al. Alectinib for relapsed or refractory anaplastic lymphoma kinase-positive anaplastic large cell lymphoma: An open-label phase II trial. Cancer Sci. 2020;111:4540–4547. doi: 10.1111/cas.14671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.FDA. FDA approves crizotinib for ALK-positive inflammatory myofibroblastic tumor FDA website2022 [07/14/2022]. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-crizotinib-alk-positive-inflammatory-myofibroblastic-tumor (2022).
- 37.Costa DB, et al. CSF concentration of the anaplastic lymphoma kinase inhibitor crizotinib. J. Clin. Oncol. 2011;29:e443–e445. doi: 10.1200/JCO.2010.34.1313. [DOI] [PubMed] [Google Scholar]
- 38.Thomas NJ, et al. Brain metastases in EGFR- and ALK-positive NSCLC: outcomes of central nervous system-penetrant tyrosine kinase inhibitors alone versus in combination with radiation. J. Thorac. Oncol. 2022;17:116–129. doi: 10.1016/j.jtho.2021.08.009. [DOI] [PubMed] [Google Scholar]
- 39.Chabner BA. Approval after phase I: ceritinib runs the three-minute mile. Oncologist. 2014;19:577–578. doi: 10.1634/theoncologist.2014-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.EMA. Xalkori(crizotinib) 2022 [12/02/2022]. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/xalkori (2022).
- 41.Subbiah V, et al. Accelerated approvals hit the target in precision oncology. Nat. Med. 2022;28:1976–1979. doi: 10.1038/s41591-022-01984-z. [DOI] [PubMed] [Google Scholar]
- 42.Palmer RH, Vernersson E, Grabbe C, Hallberg B. Anaplastic lymphoma kinase: signalling in development and disease. Biochem J. 2009;420:345–361. doi: 10.1042/BJ20090387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yau NK, et al. A pan-cancer review of ALK mutations: implications for carcinogenesis and therapy. Curr. Cancer Drug Targets. 2015;15:327–336. doi: 10.2174/1568009615666150225123712. [DOI] [PubMed] [Google Scholar]
- 44.Debiec-Rychter M, Marynen P, Hagemeijer A, Pauwels P. ALK-ATIC fusion in urinary bladder inflammatory myofibroblastic tumor. Genes Chromosomes Cancer. 2003;38:187–190. doi: 10.1002/gcc.10267. [DOI] [PubMed] [Google Scholar]
- 45.Tanaka M, et al. Inflammatory myofibroblastic tumors of the lung carrying a chimeric A2M-ALK gene: report of 2 infantile cases and review of the differential diagnosis of infantile pulmonary lesions. Hum. Pathol. 2017;66:177–82.. doi: 10.1016/j.humpath.2017.06.013. [DOI] [PubMed] [Google Scholar]
- 46.Lawrence B, et al. TPM3-ALK and TPM4-ALK oncogenes in inflammatory myofibroblastic tumors. Am. J. Pathol. 2000;157:377–384. doi: 10.1016/S0002-9440(10)64550-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ying J, et al. Anaplastic lymphoma kinase rearrangement in digestive tract cancer: implication for targeted therapy in Chinese population. PLoS One. 2015;10:e0144731. doi: 10.1371/journal.pone.0144731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Amatu A, et al. Novel CAD-ALK gene rearrangement is drugable by entrectinib in colorectal cancer. Br. J. Cancer. 2015;113:1730–1734. doi: 10.1038/bjc.2015.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yakirevich E, et al. Oncogenic ALK fusion in rare and aggressive subtype of colorectal adenocarcinoma as a potential therapeutic target. Clin. Cancer Res. 2016;22:3831–3840. doi: 10.1158/1078-0432.CCR-15-3000. [DOI] [PubMed] [Google Scholar]
- 50.Kusano H, et al. Two cases of renal cell carcinoma harboring a novel STRN-ALK fusion gene. Am. J. Surg. Pathol. 2016;40:761–769. doi: 10.1097/PAS.0000000000000610. [DOI] [PubMed] [Google Scholar]
- 51.Valery M, et al. Cholangiocarcinoma with STRN-ALK translocation treated with ALK inhibitors. Dig. Liver Dis. 2021;53:1664–1665. doi: 10.1016/j.dld.2021.09.001. [DOI] [PubMed] [Google Scholar]
- 52.Lin E, et al. Exon array profiling detects EML4-ALK fusion in breast, colorectal, and non-small cell lung cancers. Mol. Cancer Res. 2009;7:1466–1476. doi: 10.1158/1541-7786.MCR-08-0522. [DOI] [PubMed] [Google Scholar]
- 53.Du XL, et al. Proteomic profiling of proteins dysregulted in Chinese esophageal squamous cell carcinoma. J. Mol. Med. 2007;85:863–875. doi: 10.1007/s00109-007-0159-4. [DOI] [PubMed] [Google Scholar]
- 54.Hung YP, et al. Identification of ALK rearrangements in malignant peritoneal mesothelioma. JAMA Oncol. 2018;4:235–238. doi: 10.1001/jamaoncol.2017.2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Perot G, et al. Identification of a recurrent STRN/ALK fusion in thyroid carcinomas. PLoS One. 2014;9:e87170. doi: 10.1371/journal.pone.0087170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Testa S, Million L, Longacre T, Bui N. Uterine leiomyosarcoma with FN1-anaplastic lymphoma kinase fusion responsive to alectinib and lorlatinib. Case Rep. Oncol. 2021;14:812–819. doi: 10.1159/000516758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ren H, et al. Identification of anaplastic lymphoma kinase as a potential therapeutic target in ovarian cancer. Cancer Res. 2012;72:3312–3323. doi: 10.1158/0008-5472.CAN-11-3931. [DOI] [PubMed] [Google Scholar]
- 58.Hsiao SY, et al. Colorectal cancer with EML4-ALK fusion gene response to alectinib: a case report and review of the literature. Case Rep. Oncol. 2021;14:232–238. doi: 10.1159/000511069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hernandez L, et al. TRK-fused gene (TFG) is a new partner of ALK in anaplastic large cell lymphoma producing two structurally different TFG-ALK translocations. Blood. 1999;94:3265–3268. [PubMed] [Google Scholar]
- 60.Touriol C, et al. Further demonstration of the diversity of chromosomal changes involving 2p23 in ALK-positive lymphoma: 2 cases expressing ALK kinase fused to CLTCL (clathrin chain polypeptide-like) Blood. 2000;95:3204–3207. [PubMed] [Google Scholar]
- 61.Sakamoto K, et al. ALK-positive large B-cell lymphoma: identification of EML4-ALK and a review of the literature focusing on the ALK immunohistochemical staining pattern. Int J. Hematol. 2016;103:399–408. doi: 10.1007/s12185-016-1934-1. [DOI] [PubMed] [Google Scholar]
- 62.Bedwell C, et al. Cytogenetically complex SEC31A-ALK fusions are recurrent in ALK-positive large B-cell lymphomas. Haematologica. 2011;96:343–346. doi: 10.3324/haematol.2010.031484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Maesako Y, et al. inv(2)(p23q13)/RAN-binding protein 2 (RANBP2)-ALK fusion gene in myeloid leukemia that developed in an elderly woman. Int J. Hematol. 2014;99:202–207. doi: 10.1007/s12185-013-1482-x. [DOI] [PubMed] [Google Scholar]
- 64.Shimada Y, et al. An oncogenic ALK fusion and an RRAS mutation in KRAS mutation-negative pancreatic ductal adenocarcinoma. Oncologist. 2017;22:158–64. doi: 10.1634/theoncologist.2016-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gascoyne RD, et al. ALK-positive diffuse large B-cell lymphoma is associated with Clathrin-ALK rearrangements: report of 6 cases. Blood. 2003;102:2568–2573. doi: 10.1182/blood-2003-03-0786. [DOI] [PubMed] [Google Scholar]
- 66.Marino-Enriquez A, et al. Epithelioid inflammatory myofibroblastic sarcoma: An aggressive intra-abdominal variant of inflammatory myofibroblastic tumor with nuclear membrane or perinuclear ALK. Am. J. Surg. Pathol. 2011;35:135–144. doi: 10.1097/PAS.0b013e318200cfd5. [DOI] [PubMed] [Google Scholar]
- 67.Kasprzycka M, Marzec M, Liu X, Zhang Q, Wasik MA. Nucleophosmin/anaplastic lymphoma kinase (NPM/ALK) oncoprotein induces the T regulatory cell phenotype by activating STAT3. Proc. Natl Acad. Sci. USA. 2006;103:9964–9969. doi: 10.1073/pnas.0603507103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zou HY, et al. An orally available small-molecule inhibitor of c-Met, PF-2341066, exhibits cytoreductive antitumor efficacy through antiproliferative and antiangiogenic mechanisms. Cancer Res. 2007;67:4408–4417. doi: 10.1158/0008-5472.CAN-06-4443. [DOI] [PubMed] [Google Scholar]
- 69.Bai RY, Dieter P, Peschel C, Morris SW, Duyster J. Nucleophosmin-anaplastic lymphoma kinase of large-cell anaplastic lymphoma is a constitutively active tyrosine kinase that utilizes phospholipase C-gamma to mediate its mitogenicity. Mol. Cell Biol. 1998;18:6951–6961. doi: 10.1128/mcb.18.12.6951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Siraj AK, et al. ALK alteration is a frequent event in aggressive breast cancers. Breast Cancer Res. 2015;17:127. doi: 10.1186/s13058-015-0610-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lin JJ, Riely GJ, Shaw AT. Targeting ALK: precision medicine takes on drug resistance. Cancer Discov. 2017;7:137–55.. doi: 10.1158/2159-8290.CD-16-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Salaverria I, et al. Genomic profiling reveals different genetic aberrations in systemic ALK-positive and ALK-negative anaplastic large cell lymphomas. Br. J. Haematol. 2008;140:516–526. doi: 10.1111/j.1365-2141.2007.06924.x. [DOI] [PubMed] [Google Scholar]
- 73.Masood A, et al. Non-secretory multiple myeloma with unusual TFG-ALK fusion showed dramatic response to ALK inhibition. NPJ Genom. Med. 2021;6:23. doi: 10.1038/s41525-021-00186-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kemps PG, et al. ALK-positive histiocytosis: a new clinicopathologic spectrum highlighting neurologic involvement and responses to ALK inhibition. Blood. 2022;139:256–80. doi: 10.1182/blood.2021013338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wiesner T, et al. Kinase fusions are frequent in Spitz tumours and spitzoid melanomas. Nat. Commun. 2014;5:3116. doi: 10.1038/ncomms4116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Deuel TF. Anaplastic lymphoma kinase: “Ligand Independent Activation” mediated by the PTN/RPTPbeta/zeta signaling pathway. Biochim. Biophys. Acta. 2013;1834:2219–2223. doi: 10.1016/j.bbapap.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 77.Robertson FM, et al. Presence of anaplastic lymphoma kinase in inflammatory breast cancer. Springerplus. 2013;2:497. doi: 10.1186/2193-1801-2-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Busam KJ, et al. Primary and metastatic cutaneous melanomas express ALK through alternative transcriptional initiation. Am. J. Surg. Pathol. 2016;40:786–795. doi: 10.1097/PAS.0000000000000611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Butrynski JE, et al. Crizotinib in ALK-rearranged inflammatory myofibroblastic tumor. N. Engl. J. Med. 2010;363:1727–1733. doi: 10.1056/NEJMoa1007056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Coffin CM, et al. ALK1 and p80 expression and chromosomal rearrangements involving 2p23 in inflammatory myofibroblastic tumor. Mod. Pathol. 2001;14:569–576. doi: 10.1038/modpathol.3880352. [DOI] [PubMed] [Google Scholar]
- 81.Chun YS, Wang L, Nascimento AG, Moir CR, Rodeberg DA. Pediatric inflammatory myofibroblastic tumor: anaplastic lymphoma kinase (ALK) expression and prognosis. Pediatr. Blood Cancer. 2005;45:796–801. doi: 10.1002/pbc.20294. [DOI] [PubMed] [Google Scholar]
- 82.Bresler SC, et al. ALK mutations confer differential oncogenic activation and sensitivity to ALK inhibition therapy in neuroblastoma. Cancer Cell. 2014;26:682–694. doi: 10.1016/j.ccell.2014.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.O’Donohue T., et al. Differential Impact of ALK Mutations in Neuroblastoma. JCO Precis. Oncol.5, (2021). [DOI] [PMC free article] [PubMed]
- 84.Trigg RM, Turner SD. ALK in neuroblastoma: biological and therapeutic implications. Cancers. 2018;10:113. doi: 10.3390/cancers10040113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Carneiro BA, et al. Anaplastic lymphoma kinase mutation (ALK F1174C) in small cell carcinoma of the prostate and molecular response to alectinib. Clin. Cancer Res. 2018;24:2732–2739. doi: 10.1158/1078-0432.CCR-18-0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Song R, Ren G, Liu X, Zhang Y. [Alterations of ALK gene and protein expression in prostatic cancer and its clinical significance] Zhonghua Bing. Li Xue Za Zhi. 2015;44:382–385. [PubMed] [Google Scholar]
- 87.Patel RA, et al. Comprehensive assessment of anaplastic lymphoma kinase in localized and metastatic prostate cancer reveals targetable alterations. Cancer Res. Commun. 2022;2:277–285. doi: 10.1158/2767-9764.CRC-21-0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Colpaert C, et al. Abstract P3-06-39: Anaplastic lymphoma kinase (ALK) protein overexpression is not a feature of inflammatory breast cancer. Cancer Res. 2015;75:P3-06-39–P3-06-39. [Google Scholar]
- 89.Blandin A-F, et al. Pdtm-06. Alk amplification and rearrangements are recurrent targetable events in glioblastoma. NeuroOncol. 2018;20:vi204–vi205. doi: 10.1158/1078-0432.CCR-21-3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Maxson JE, et al. Therapeutically targetable ALK mutations in leukemia. Cancer Res. 2015;75:2146–2150. doi: 10.1158/0008-5472.CAN-14-1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Desai A, Mohammed T, Rakshit S, Krull J. 21P The landscape of ALK alterations in non-small cell lung cancer. J. Thorac. Oncol. 2021;UME 16:S707. [Google Scholar]
- 92.Marino-Enriquez A, Dal Cin P. ALK as a paradigm of oncogenic promiscuity: different mechanisms of activation and different fusion partners drive tumors of different lineages. Cancer Genet. 2013;206:357–373. doi: 10.1016/j.cancergen.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 93.Liu S, et al. The genomic characteristics of ALK fusion positive tumors in Chinese NSCLC patients. Front. Oncol. 2020;10:726. doi: 10.3389/fonc.2020.00726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gower, A., Golestany, B., Gong, J., Singhi, A. D., Hendifar, A. E. Novel ALK fusion, PPFIBP1-ALK, in pancreatic ductal adenocarcinoma responsive to alectinib and lorlatinib. JCO Precis. Oncol.4, (2020). [DOI] [PMC free article] [PubMed]
- 95.Majewska H, et al. ALK alterations in salivary gland carcinomas. Virchows Arch. 2021;478:933–941. doi: 10.1007/s00428-020-02971-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mosse YP, et al. Identification of ALK as a major familial neuroblastoma predisposition gene. Nature. 2008;455:930–935. doi: 10.1038/nature07261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang M, et al. ALK amplification and protein expression predict inferior prognosis in neuroblastomas. Exp. Mol. Pathol. 2013;95:124–130. doi: 10.1016/j.yexmp.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 98.Pearson ADJ, et al. Second Paediatric Strategy Forum for anaplastic lymphoma kinase (ALK) inhibition in paediatric malignancies: ACCELERATE in collaboration with the European Medicines Agency with the participation of the Food and Drug Administration. Eur. J. Cancer. 2021;157:198–213. doi: 10.1016/j.ejca.2021.08.022. [DOI] [PubMed] [Google Scholar]
- 99.Franceschi E, et al. The clinical and prognostic role of ALK in glioblastoma. Pathol. Res. Pr. 2021;221:153447. doi: 10.1016/j.prp.2021.153447. [DOI] [PubMed] [Google Scholar]
- 100.Karagkounis G, et al. Anaplastic lymphoma kinase expression and gene alterations in glioblastoma: correlations with clinical outcome. J. Clin. Pathol. 2017;70:593–599. doi: 10.1136/jclinpath-2016-204102. [DOI] [PubMed] [Google Scholar]
- 101.Kim JH. Prognostic and predictive markers in glioblastoma and ALK overexpression. J. Pathol. Transl. Med. 2021;55:236–237. doi: 10.4132/jptm.2021.04.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kawauchi D, et al. The ALK inhibitors, alectinib and ceritinib, induce ALK-independent and STAT3-dependent glioblastoma cell death. Cancer Sci. 2021;112:2442–2453. doi: 10.1111/cas.14885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bavi P, et al. ALK gene amplification is associated with poor prognosis in colorectal carcinoma. Br. J. Cancer. 2013;109:2735–2743. doi: 10.1038/bjc.2013.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tuma RS. ALK gene amplified in most inflammatory breast cancers. J. Natl Cancer Inst. 2012;104:87–88. doi: 10.1093/jnci/djr553. [DOI] [PubMed] [Google Scholar]
- 105.van Gaal JC, et al. Anaplastic lymphoma kinase aberrations in rhabdomyosarcoma: clinical and prognostic implications. J. Clin. Oncol. 2012;30:308–315. doi: 10.1200/JCO.2011.37.8588. [DOI] [PubMed] [Google Scholar]
- 106.Chen, J., Yu, Z., Li, X., Xu, G., Wang, Y. A novel 3’ truncated ALK cause ALK+ in a patient with minimally invasive adenocarcinoma. J. Cancer Res. Clin. Oncol.148, 1005–1006 (2021). [DOI] [PMC free article] [PubMed]
- 107.Fleuren EDG, et al. Phosphoproteomic profiling reveals ALK and MET as novel actionable targets across synovial sarcoma subtypes. Cancer Res. 2017;77:4279–4292. doi: 10.1158/0008-5472.CAN-16-2550. [DOI] [PubMed] [Google Scholar]
- 108.Hapgood G, Savage KJ. The biology and management of systemic anaplastic large cell lymphoma. Blood. 2015;126:17–25. doi: 10.1182/blood-2014-10-567461. [DOI] [PubMed] [Google Scholar]
- 109.Rottgers S, et al. ALK fusion genes in children with atypical myeloproliferative leukemia. Leukemia. 2010;24:1197–1200. doi: 10.1038/leu.2010.18. [DOI] [PubMed] [Google Scholar]
- 110.Fukuhara S, et al. Partial deletion of the ALK gene in ALK-positive anaplastic large cell lymphoma. Hematol. Oncol. 2018;36:150–158. doi: 10.1002/hon.2455. [DOI] [PubMed] [Google Scholar]
- 111.Blackhall F, et al. Final results of the large-scale multinational trial PROFILE 1005: efficacy and safety of crizotinib in previously treated patients with advanced/metastatic ALK-positive non-small-cell lung cancer. ESMO Open. 2017;2:e000219. doi: 10.1136/esmoopen-2017-000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Solomon BJ, et al. Lorlatinib in patients with ALK-positive non-small-cell lung cancer: results from a global phase 2 study. Lancet Oncol. 2018;19:1654–1667. doi: 10.1016/S1470-2045(18)30649-1. [DOI] [PubMed] [Google Scholar]
- 113.Solomon BJ, et al. Final overall survival analysis from a study comparing first-line crizotinib versus chemotherapy in ALK-mutation-positive non-small-cell lung cancer. J. Clin. Oncol. 2018;36:2251–2258. doi: 10.1200/JCO.2017.77.4794. [DOI] [PubMed] [Google Scholar]
- 114.Shaw AT, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N. Engl. J. Med. 2013;368:2385–2394. doi: 10.1056/NEJMoa1214886. [DOI] [PubMed] [Google Scholar]
- 115.Shaw AT, et al. Alectinib in ALK-positive, crizotinib-resistant, non-small-cell lung cancer: a single-group, multicentre, phase 2 trial. Lancet Oncol. 2016;17:234–242. doi: 10.1016/S1470-2045(15)00488-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Shaw AT, et al. Ceritinib versus chemotherapy in patients with ALK-rearranged non-small-cell lung cancer previously given chemotherapy and crizotinib (ASCEND-5): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2017;18:874–886. doi: 10.1016/S1470-2045(17)30339-X. [DOI] [PubMed] [Google Scholar]
- 117.Shaw AT, et al. First-line lorlatinib or crizotinib in advanced ALK-positive lung cancer. N. Engl. J. Med. 2020;383:2018–2029. doi: 10.1056/NEJMoa2027187. [DOI] [PubMed] [Google Scholar]
- 118.Soria JC, et al. First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ASCEND-4): a randomised, open-label, phase 3 study. Lancet. 2017;389:917–929. doi: 10.1016/S0140-6736(17)30123-X. [DOI] [PubMed] [Google Scholar]
- 119.Kim DW, et al. Activity and safety of ceritinib in patients with ALK-rearranged non-small-cell lung cancer (ASCEND-1): updated results from the multicentre, open-label, phase 1 trial. Lancet Oncol. 2016;17:452–463. doi: 10.1016/S1470-2045(15)00614-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Camidge DR, et al. Updated efficacy and safety data and impact of the EML4-ALK fusion variant on the efficacy of alectinib in untreated ALK-positive advanced non-small cell lung cancer in the global phase III ALEX study. J. Thorac. Oncol. 2019;14:1233–1243. doi: 10.1016/j.jtho.2019.03.007. [DOI] [PubMed] [Google Scholar]
- 121.Camidge DR, et al. Brigatinib versus crizotinib in advanced ALK inhibitor-naive ALK-positive non-small cell lung cancer: second interim analysis of the phase III ALTA-1L trial. J. Clin. Oncol. 2020;38:3592–3603. doi: 10.1200/JCO.20.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Crino L, et al. Multicenter phase II study of whole-body and intracranial activity with ceritinib in patients with ALK-rearranged non-small-cell lung cancer previously treated with chemotherapy and crizotinib: results from ASCEND-2. J. Clin. Oncol. 2016;34:2866–2873. doi: 10.1200/JCO.2015.65.5936. [DOI] [PubMed] [Google Scholar]
- 123.Horn L, et al. Ensartinib vs crizotinib for patients with anaplastic lymphoma kinase-positive non-small cell lung cancer: a randomized clinical trial. JAMA Oncol. 2021;7:1617–1625. doi: 10.1001/jamaoncol.2021.3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Huber RM, et al. Brigatinib in crizotinib-refractory ALK+ NSCLC: 2-year follow-up on systemic and intracranial outcomes in the phase 2 ALTA trial. J. Thorac. Oncol. 2020;15:404–415. doi: 10.1016/j.jtho.2019.11.004. [DOI] [PubMed] [Google Scholar]
- 125.El Darsa H, Abdel-Rahman O, Sangha R. Pharmacological and clinical properties of lorlatinib in the treatment of ALK-rearranged advanced non-small cell lung cancer. Expert Opin. Pharmacother. 2020;21:1547–1554. doi: 10.1080/14656566.2020.1774552. [DOI] [PubMed] [Google Scholar]
- 126.Drilon A, et al. Safety and antitumor activity of the multitargeted Pan-TRK, ROS1, and ALK inhibitor entrectinib: combined results from two phase I Trials (ALKA-372-001 STARTRK-1) Cancer Discov. 2017;7:400–409. doi: 10.1158/2159-8290.CD-16-1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Novello S, et al. Alectinib versus chemotherapy in crizotinib-pretreated anaplastic lymphoma kinase (ALK)-positive non-small-cell lung cancer: results from the phase III ALUR study. Ann. Oncol. 2018;29:1409–1416. doi: 10.1093/annonc/mdy121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ou SH, et al. Alectinib in crizotinib-refractory ALK-rearranged non-small-cell lung cancer: A Phase II Global Study. J. Clin. Oncol. 2016;34:661–668. doi: 10.1200/jco.2015.63.9443. [DOI] [PubMed] [Google Scholar]
- 129.Bauer TM, et al. Brain penetration of lorlatinib: cumulative incidences of CNS and Non-CNS progression with lorlatinib in patients with previously treated ALK-positive non-small-cell lung cancer. Target Oncol. 2020;15:55–65. doi: 10.1007/s11523-020-00702-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mosse YP, et al. Targeting ALK With crizotinib in pediatric anaplastic large cell lymphoma and inflammatory myofibroblastic tumor: A Children’s Oncology Group Study. J. Clin. Oncol. 2017;35:3215–3221. doi: 10.1200/JCO.2017.73.4830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mosse YP, et al. Safety and activity of crizotinib for paediatric patients with refractory solid tumours or anaplastic large-cell lymphoma: a Children’s Oncology Group phase 1 consortium study. Lancet Oncol. 2013;14:472–480. doi: 10.1016/S1470-2045(13)70095-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gambacorti-Passerini C, et al. Long-term effects of crizotinib in ALK-positive tumors (excluding NSCLC): A phase 1b open-label study. Am. J. Hematol. 2018;93:607–614. doi: 10.1002/ajh.25043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gambacorti Passerini C, et al. Crizotinib in advanced, chemoresistant anaplastic lymphoma kinase-positive lymphoma patients. J. Natl Cancer Inst. 2014;106:djt378. doi: 10.1093/jnci/djt378. [DOI] [PubMed] [Google Scholar]
- 134.Gettinger SN, et al. Activity and safety of brigatinib in ALK-rearranged non-small-cell lung cancer and other malignancies: a single-arm, open-label, phase 1/2 trial. Lancet Oncol. 2016;17:1683–1696. doi: 10.1016/S1470-2045(16)30392-8. [DOI] [PubMed] [Google Scholar]
- 135.Honda K, et al. Durable response to the ALK inhibitor alectinib in inflammatory myofibroblastic tumor of the head and neck with a novel SQSTM1-ALK fusion: a case report. Invest. N. Drugs. 2019;37:791–795. doi: 10.1007/s10637-019-00742-2. [DOI] [PubMed] [Google Scholar]
- 136.Heath JA, Campbell MA, Thomas A, Solomon B. Good clinical response to alectinib, a second generation ALK inhibitor, in refractory neuroblastoma. Pediatr. Blood Cancer. 2018;65:e27055. doi: 10.1002/pbc.27055. [DOI] [PubMed] [Google Scholar]
- 137.Ou K, et al. ALK rearrangement-positive pancreatic cancer with brain metastasis has remarkable response to ALK inhibitors: a case report. Front. Oncol. 2021;11:724815. doi: 10.3389/fonc.2021.724815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Leroy L, et al. Remarkable response to ceritinib and brigatinib in an anaplastic lymphoma kinase-rearranged anaplastic thyroid carcinoma previously treated with crizotinib. Thyroid. 2020;30:343–344. doi: 10.1089/thy.2019.0202. [DOI] [PubMed] [Google Scholar]
- 139.Hillier K, et al. A novel ALK fusion in pediatric medullary thyroid carcinoma. Thyroid. 2019;29:1704–1707. doi: 10.1089/thy.2019.0041. [DOI] [PubMed] [Google Scholar]
- 140.Moreno V, et al. An open-label, multicenter, phase II study of ceritinib in patients with advanced ALK+ non-lung solid tumors and hematological malignancies (ASCEND-10) J. Clin. Oncol. 2020;38:3520. [Google Scholar]
- 141.He, X. et al. Clinical responses to crizotinib, alectinib, and lorlatinib in a metastatic colorectal carcinoma patient with ALK gene rearrangement: a case report. JCO Precis. Oncol.5, (2021). [DOI] [PMC free article] [PubMed]
- 142.Wang Z, et al. Durable clinical response to ALK tyrosine kinase inhibitors in epithelioid inflammatory myofibroblastic sarcoma harboring PRRC2B-ALK rearrangement: a case report. Front. Oncol. 2022;12:761558. doi: 10.3389/fonc.2022.761558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Fischer M, et al. Ceritinib in paediatric patients with anaplastic lymphoma kinase-positive malignancies: an open-label, multicentre, phase 1, dose-escalation and dose-expansion study. Lancet Oncol. 2021;22:1764–1776. doi: 10.1016/S1470-2045(21)00536-2. [DOI] [PubMed] [Google Scholar]
- 144.Ruschoff JH, et al. STRN -ALK rearranged malignant peritoneal mesothelioma with dramatic response following ceritinib treatment. JCO Precis. Oncol. 2019;3:1–6. doi: 10.1200/PO.19.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mansfield AS, et al. Crizotinib in patients with tumors harboring ALK or ROS1 rearrangements in the NCI-MATCH trial. NPJ Precis Oncol. 2022;6:13. doi: 10.1038/s41698-022-00256-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bagchi A, et al. Lorlatinib in a child with ALK-fusion-positive high-grade glioma. N. Engl. J. Med. 2021;385:761–763. doi: 10.1056/NEJMc2101264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hui B, et al. EML4-ALK, a potential therapeutic target that responds to alectinib in ovarian cancer. Jpn J. Clin. Oncol. 2020;50:1470–1474. doi: 10.1093/jjco/hyaa156. [DOI] [PubMed] [Google Scholar]
- 148.Goldsmith KC, et al. Phase I trial of lorlatinib in patients with ALK-driven refractory or relapsed neuroblastoma: A New Approaches to Neuroblastoma Consortium study. J. Clin. Oncol. 2020;38:10504. [Google Scholar]
- 149.Dayyani F, Lee W, Houshyar R, Fontaine P. Rapid and Deep Response to Lorlatinib in Pancreatic High-Grade Neuroendocrine Carcinoma With a Treatment Emergent Novel KANK1-ALK Fusion. JCO Precis Oncol. 2023;7:e2200230. doi: 10.1200/PO.22.00230. [DOI] [PubMed] [Google Scholar]
- 150.Le Rhun E, et al. Patterns of response to crizotinib in recurrent glioblastoma according to ALK and MET molecular profile in two patients. CNS Oncol. 2015;4:381–386. doi: 10.2217/cns.15.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Parker BM, Parker JV, Lymperopoulos A, Konda V. A case report: pharmacology and resistance patterns of three generations of ALK inhibitors in metastatic inflammatory myofibroblastic sarcoma. J. Oncol. Pharm. Pr. 2019;25:1226–1230. doi: 10.1177/1078155218781944. [DOI] [PubMed] [Google Scholar]
- 152.Verran J, Mathavan V. Alectinib monotherapy in isolated central nervous system relapse of ALK-positive anaplastic large cell lymphoma. Case Rep. Hematol. 2022;2022:4749452. doi: 10.1155/2022/4749452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Swanton C, et al. Abstract CT032: activity and safety of alectinib for ALK-altered solid tumors from MyPathway. Cancer Res. 2022;82:CT032–CT032. [Google Scholar]
- 154.Benayed R, et al. High yield of RNA sequencing for targetable kinase fusions in lung adenocarcinomas with no mitogenic driver alteration detected by DNA sequencing and low tumor mutation burden. Clin. Cancer Res. 2019;25:4712–4722. doi: 10.1158/1078-0432.CCR-19-0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Bernicker EH, et al. Adherence to National Comprehensive Cancer Network ALK testing guidelines for patients with advanced non-small cell lung cancer in U.S. Community Medical Centers. Oncologist. 2021;26:e1050–e1057. doi: 10.1002/onco.13779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.O’Donnell P, et al. Analytic performance studies and clinical reproducibility of a real-time PCR assay for the detection of epidermal growth factor receptor gene mutations in formalin-fixed paraffin-embedded tissue specimens of non-small cell lung cancer. BMC Cancer. 2013;13:210. doi: 10.1186/1471-2407-13-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Pyo JS, Kang G, Sohn JH. ALK immunohistochemistry for ALK gene rearrangement screening in non-small cell lung cancer: a systematic review and meta-analysis. Int J. Biol. Markers. 2016;31:e413–e421. doi: 10.5301/jbm.5000218. [DOI] [PubMed] [Google Scholar]
- 158.Wang J, et al. Clinical characteristics and outcomes of patients with primary lung adenocarcinoma harboring ALK rearrangements detected by FISH, IHC, and RT-PCR. PLoS One. 2014;9:e101551. doi: 10.1371/journal.pone.0101551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Haynes BC, et al. An integrated next-generation sequencing system for analyzing DNA mutations, gene fusions, and RNA expression in lung cancer. Transl. Oncol. 2019;12:836–845. doi: 10.1016/j.tranon.2019.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Katayama R, et al. Mechanisms of acquired crizotinib resistance in ALK-rearranged lung Cancers. Sci. Transl. Med. 2012;4:120ra17. doi: 10.1126/scitranslmed.3003316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Pan Y, Deng C, Qiu Z, Cao C, Wu F. The resistance mechanisms and treatment strategies for ALK-rearranged non-small cell lung cancer. Front. Oncol. 2021;11:713530. doi: 10.3389/fonc.2021.713530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Miyawaki M, et al. Overcoming EGFR bypass signal-induced acquired resistance to ALK tyrosine kinase inhibitors in ALK-translocated lung cancer. Mol. Cancer Res. 2017;15:106–114. doi: 10.1158/1541-7786.MCR-16-0211. [DOI] [PubMed] [Google Scholar]
- 163.Laimer D, et al. PDGFR blockade is a rational and effective therapy for NPM-ALK-driven lymphomas. Nat. Med. 2012;18:1699–1704. doi: 10.1038/nm.2966. [DOI] [PubMed] [Google Scholar]
- 164.Wood AC, et al. Dual ALK and CDK4/6 inhibition demonstrates synergy against neuroblastoma. Clin. Cancer Res. 2017;23:2856–2868. doi: 10.1158/1078-0432.CCR-16-1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Redaelli S, et al. Synergistic activity of ALK and mTOR inhibitors for the treatment of NPM-ALK positive lymphoma. Oncotarget. 2016;7:72886–72897. doi: 10.18632/oncotarget.12128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Rigaud, C., et al. Efficacy of nivolumab in a patient with systemic refractory ALK+ anaplastic large cell lymphoma. Pediatr. Blood Cancer65, (2018). [DOI] [PubMed]
- 167.Shaw AT, et al. Resensitization to crizotinib by the lorlatinib ALK resistance mutation L1198F. N. Engl. J. Med. 2016;374:54–61. doi: 10.1056/NEJMoa1508887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Gainor JF, et al. Molecular mechanisms of resistance to first- and second-generation ALK inhibitors in ALK-rearranged lung cancer. Cancer Discov. 2016;6:1118–1133. doi: 10.1158/2159-8290.CD-16-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Friboulet L, et al. The ALK inhibitor ceritinib overcomes crizotinib resistance in non-small cell lung cancer. Cancer Discov. 2014;4:662–673. doi: 10.1158/2159-8290.CD-13-0846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Shaw AT, et al. ALK resistance mutations and efficacy of lorlatinib in advanced anaplastic lymphoma kinase-positive non-small-cell lung cancer. J. Clin. Oncol. 2019;37:1370–1379. doi: 10.1200/JCO.18.02236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Okada K, et al. Prediction of ALK mutations mediating ALK-TKIs resistance and drug re-purposing to overcome the resistance. EBioMedicine. 2019;41:105–119. doi: 10.1016/j.ebiom.2019.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Dagogo-Jack I, et al. Treatment with next-generation ALK inhibitors fuels plasma ALK mutation diversity. Clin. Cancer Res. 2019;25:6662–6670. doi: 10.1158/1078-0432.CCR-19-1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Recondo G, et al. Diverse resistance mechanisms to the third-generation ALK inhibitor lorlatinib in ALK-rearranged lung cancer. Clin. Cancer Res. 2020;26:242–255. doi: 10.1158/1078-0432.CCR-19-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Camidge DR, Pao W, Sequist LV. Acquired resistance to TKIs in solid tumours: learning from lung cancer. Nat. Rev. Clin. Oncol. 2014;11:473–481. doi: 10.1038/nrclinonc.2014.104. [DOI] [PubMed] [Google Scholar]
- 175.Yamaguchi N, et al. Dual ALK and EGFR inhibition targets a mechanism of acquired resistance to the tyrosine kinase inhibitor crizotinib in ALK rearranged lung cancer. Lung Cancer. 2014;83:37–43. doi: 10.1016/j.lungcan.2013.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Menotti M, et al. Wiskott-Aldrich syndrome protein (WASP) is a tumor suppressor in T cell lymphoma. Nat. Med. 2019;25:130–140. doi: 10.1038/s41591-018-0262-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Sawant DV, et al. Adaptive plasticity of IL-10(+) and IL-35(+) Treg cells cooperatively promotes tumor T cell exhaustion. Nat. Immunol. 2019;20:724–735. doi: 10.1038/s41590-019-0346-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Karaca Atabay E, et al. Tyrosine phosphatases regulate resistance to ALK inhibitors in ALK+ anaplastic large cell lymphoma. Blood. 2022;139:717–731. doi: 10.1182/blood.2020008136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Shi P, et al. IGF-IR tyrosine kinase interacts with NPM-ALK oncogene to induce survival of T-cell ALK+ anaplastic large-cell lymphoma cells. Blood. 2009;114:360–370. doi: 10.1182/blood-2007-11-125658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Chen H, et al. Afatinib reverses ceritinib resistance (CR) in ALK/ROS1-positive non-small-cell lung cancer cell (NSCLC) via suppression of NRG1 pathway. Onco Targets Ther. 2018;11:8201–8209. doi: 10.2147/OTT.S173008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Mezquita L, Planchard D. The role of brigatinib in crizotinib-resistant non-small cell lung cancer. Cancer Manag. Res. 2018;10:123–130. doi: 10.2147/CMAR.S129963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Chen H, et al. Metformin reduces HGF-induced resistance to alectinib via the inhibition of Gab1. Cell Death Dis. 2020;11:111. doi: 10.1038/s41419-020-2307-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Dagogo-Jack I, et al. MET alterations are a recurring and actionable resistance mechanism in ALK-positive lung cancer. Clin. Cancer Res. 2020;26:2535–2545. doi: 10.1158/1078-0432.CCR-19-3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Sicklick JK, et al. Molecular profiling of cancer patients enables personalized combination therapy: the I-PREDICT study. Nat. Med. 2019;25:744–750. doi: 10.1038/s41591-019-0407-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Kato S, et al. Real-world data from a molecular tumor board demonstrates improved outcomes with a precision N-of-One strategy. Nat. Commun. 2020;11:4965. doi: 10.1038/s41467-020-18613-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Rodon J, et al. Genomic and transcriptomic profiling expands precision cancer medicine: the WINTHER trial. Nat. Med. 2019;25:751–758. doi: 10.1038/s41591-019-0424-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Sicklick JK, et al. Molecular profiling of advanced malignancies guides first-line N-of-1 treatments in the I-PREDICT treatment-naive study. Genome Med. 2021;13:155. doi: 10.1186/s13073-021-00969-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Janku F, et al. Molecular profiling of tumor tissue and plasma cell-free DNA from patients with non-langerhans cell histiocytosis. Mol. Cancer Ther. 2019;18:1149–1157. doi: 10.1158/1535-7163.MCT-18-1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Saunders IM, Goodman AM, Kurzrock R. Real-world toxicity experience with BRAF/MEK inhibitors in patients with erdheim-chester disease. Oncologist. 2020;25:e386–e390. doi: 10.1634/theoncologist.2019-0606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Aouba A, et al. Rationale and efficacy of interleukin-1 targeting in Erdheim-Chester disease. Blood. 2010;116:4070–4076. doi: 10.1182/blood-2010-04-279240. [DOI] [PubMed] [Google Scholar]
- 191.Killu AM, Liang JJ, Jaffe AS. Erdheim-Chester disease with cardiac involvement successfully treated with anakinra. Int J. Cardiol. 2013;167:e115–e117. doi: 10.1016/j.ijcard.2013.04.057. [DOI] [PubMed] [Google Scholar]
- 192.Kurzrock R, et al. Alpha-T: An innovative decentralized (home-based) phase 2 trial of alectinib in ALK-positive (ALK+) solid tumors in a histology-agnostic setting. J. Clin. Oncol. 2021;39:TPS3155–TPS3155. [Google Scholar]
- 193.Salgia SK, Govindarajan A, Salgia R, Pal SK. ALK-directed therapy in Non-NSCLC malignancies: are we ready? JCO Precis. Oncol. 2021;5:767–770. doi: 10.1200/PO.21.00078. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
We have not analyzed any publicly available databases for this article. We have appropriated cited all clinical data included in this review article.