Abstract
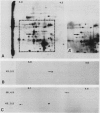
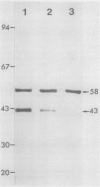
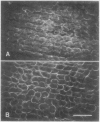
Internodal cells of Chara corallina Klein ex. Wild have been studied to determine the number of actin isoforms they contain and whether actin occurs at locations in the cortical cytoplasm outside the filament bundles. A monoclonal antibody to chicken actin is specific for actin in numerous animal cells but binds to two Chara proteins after their separation by two-dimensional polyacrylamide gel electrophoresis. One protein resembles known actins in relative molecular mass (43,000-Mr) and isoelectric point (5.5) while the other is distinctly different (58,000-Mr, isoelectric point = 4.8). Because it is indetectable in cells whose actin bundles have been extracted, the 43,000-Mr protein is assigned to the bundles and concluded to be rare or absent in the remaining cortical cytoplasm. The 58,000-Mr protein, in contrast, does not extract with the actin bundles. It was localized within the chloroplasts by immunofluorescence and by the dependence of proteolysis on the permeabilization of the chloroplast envelope.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blikstad I., Markey F., Carlsson L., Persson T., Lindberg U. Selective assay of monomeric and filamentous actin in cell extracts, using inhibition of deoxyribonuclease I. Cell. 1978 Nov;15(3):935–943. doi: 10.1016/0092-8674(78)90277-5. [DOI] [PubMed] [Google Scholar]
- Bullard B., Bell J., Craig R., Leonard K. Arthrin: a new actin-like protein in insect flight muscle. J Mol Biol. 1985 Apr 5;182(3):443–454. doi: 10.1016/0022-2836(85)90203-7. [DOI] [PubMed] [Google Scholar]
- Cline K., Werner-Washburne M., Andrews J., Keegstra K. Thermolysin is a suitable protease for probing the surface of intact pea chloroplasts. Plant Physiol. 1984 Jul;75(3):675–678. doi: 10.1104/pp.75.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrels J. I., Gibson W. Identification and characterization of multiple forms of actin. Cell. 1976 Dec;9(4 Pt 2):793–805. doi: 10.1016/0092-8674(76)90142-2. [DOI] [PubMed] [Google Scholar]
- Hightower R. C., Meagher R. B. Divergence and differential expression of soybean actin genes. EMBO J. 1985 Jan;4(1):1–8. doi: 10.1002/j.1460-2075.1985.tb02309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A., Raziuddin, Finlay T. H., Thomas J. O., Szer W. Isolation of a minor species of actin from the nuclei of Acanthamoeba castellanii. Biochemistry. 1984 Dec 18;23(26):6753–6757. doi: 10.1021/bi00321a072. [DOI] [PubMed] [Google Scholar]
- Lin J. J. Mapping structural proteins of cultured cells by monoclonal antibodies. Cold Spring Harb Symp Quant Biol. 1982;46(Pt 2):769–783. doi: 10.1101/sqb.1982.046.01.073. [DOI] [PubMed] [Google Scholar]
- Marano F., Galleron C., Minty A. J., Montarras D., Bornens M. An actin-like protein and gene in the unicellular green alga Dunaliella. Cell Biol Int Rep. 1982 Dec;6(12):1085–1092. doi: 10.1016/0309-1651(82)90025-x. [DOI] [PubMed] [Google Scholar]
- McCurdy D. W., Williamson R. E. An actin-related protein inside pea chloroplasts. J Cell Sci. 1987 Apr;87(Pt 3):449–456. doi: 10.1242/jcs.87.3.449. [DOI] [PubMed] [Google Scholar]
- Nagao R. T., Shah D. M., Eckenrode V. K., Meagher R. B. Multigene family of actin-related sequences isolated from a soybean genomic library. DNA. 1981;1(1):1–9. doi: 10.1089/dna.1.1981.1.1. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Palevitz B. A., Hepler P. K. Identification of actin in situ at the ectoplasm-endoplasm interface of Nitella. Microfilament-chloroplast association. J Cell Biol. 1975 Apr;65(1):29–38. doi: 10.1083/jcb.65.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardee J. D., Spudich J. A. Purification of muscle actin. Methods Cell Biol. 1982;24:271–289. doi: 10.1016/s0091-679x(08)60661-5. [DOI] [PubMed] [Google Scholar]
- Piperno G., Luck D. J. An actin-like protein is a component of axonemes from Chlamydomonas flagella. J Biol Chem. 1979 Apr 10;254(7):2187–2190. [PubMed] [Google Scholar]
- Schwartz R. J., Stone E. M. Cloning of contractile protein genes. Cell Muscle Motil. 1983;3:195–257. doi: 10.1007/978-1-4615-9296-9_7. [DOI] [PubMed] [Google Scholar]
- Shah D. M., Hightower R. C., Meagher R. B. Complete nucleotide sequence of a soybean actin gene. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1022–1026. doi: 10.1073/pnas.79.4.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J. H., Boustead C. M., Witzemann V., Shaw G., Weber K., Osborn M. Cytoskeletal proteins at the cholinergic synapse: distribution of desmin, actin, fodrin, neurofilaments, and tubulin in Torpedo electric organ. Eur J Cell Biol. 1985 Jul;38(1):123–133. [PubMed] [Google Scholar]
- Williamson R. E. Cytochalasin B stabilises the sub-cortical actin bundles of Chara against a solution of low ionic strength. Cytobiologie. 1978 Oct;18(1):107–113. [PubMed] [Google Scholar]
- Williamson R. E. Cytoplasmic streaming in Chara: a cell model activated by ATP and inhibited by cytochalasin B. J Cell Sci. 1975 May;17(3):655–668. doi: 10.1242/jcs.17.3.655. [DOI] [PubMed] [Google Scholar]
- Williamson R. E., Hurley U. A. Growth and regrowth of actin bundles in Chara: bundle assembly by mechanisms differing in sensitivity to cytochalasin. J Cell Sci. 1986 Sep;85:21–32. doi: 10.1242/jcs.85.1.21. [DOI] [PubMed] [Google Scholar]
- Williamson R. E., Perkin J. L., Hurley U. A. Selective extraction of Chara actin bundles: identification of actin and two coextracting proteins. Cell Biol Int Rep. 1985 Jun;9(6):547–554. doi: 10.1016/0309-1651(85)90019-0. [DOI] [PubMed] [Google Scholar]