Abstract
The D-amino acid content of Ishizuchi-kurocha, a post-fermented tea produced in Ehime, Japan, was measured. Ishizuchi-kurocha mainly contains D-glutamic acid and D-alanine, but it also contains a small amount of D-aspartic acid. Two types of lactic acid bacteria, Lactiplantibacillus plantarum and Levilactobacillus brevis, are the main species involved in lactic acid fermentation during the tea fermentation process. Therefore, the D-amino acid-producing abilities of strains of these two species isolated from Ishizuchi-kurocha were examined. Specifically, the production of D-aspartic acid, D-alanine, and D-glutamic acid by L. brevis and L. plantarum strains was observed. The amount of D-aspartic acid produced by L. plantarum was low. D-glutamine was detected in culture supernatant but not in bacterial cells. D-arginine was detected in bacterial cells of the L. plantarum strains but not in the culture supernatant. Both the L. brevis and L. plantarum strains possessed at least three kinds of putative racemase genes: alanine racemase, glutamate racemase, and aspartate racemase. However, their expression and enzyme activity remain unknown. L. plantarum and L. brevis could play an important role in the production of D-amino acids in Ishizuchi-kurocha. In fact, Ishizuchi-kurocha is expected to possess the effective physiological activities of D-amino acids.
Keywords: D-amino acid, amino acid racemase, Lactiplantibacillus plantarum, Levilactobacillus brevis, Ishizuchi-kurocha
INTRODUCTION
Twenty α-amino acids are important biological molecules consisting of proteins. Nineteen of them, excluding glycine, have an optical isomer. Bacteria can synthesize a variety of D-amino acids via several racemases. In particular, D-alanine (D-Ala) and D-glutamic acid (D-Glu) are essential constituents of bacterial cell wall peptidoglycans. Although humans can only synthesize some D-amino acids, they can absorb the D-amino acids produced by intestinal bacteria [1]. D-Amino acids, first observed in the mammalian brain, have multiple physiological functions [2]. D-Serine (D-Ser) plays a key role in the physiology of the brain. Free D-Ser acts as a co-agonist against the N-methyl-D-aspartate receptor associated with mammalian brain functions such as memory and learning [3]. D-Ser has also been found to influence the clinical status of patients with Alzheimer’s disease and schizophrenia [4,5,6]. Ser racemase has been found in the rat and mouse brain, suggesting its involvement in D-Ser synthesis. Moreover, D-Ser enhances the growth of kidney cells and improves kidney function [7]. D-Ser, produced by intestinal bacteria, also has a protective effect on the kidney [8]. The physiological functions of free D-aspartic acid (D-Asp) include a melatonin antisecretory effect, prolactin secretion activation, and testosterone synthesis promotion [9,10,11]. D-Amino acids, particularly D-Ala, D-Ser, and D-arginine (D-Arg), suppress lipid accumulation in hepatocytes [12]. As mentioned above, D-amino acids have various physiological functions that differ from those of L-amino acids. Therefore, D-amino acids are important for maintaining human physical functions and improving quality of life. However, because humans cannot synthesize a variety of D-amino acids, synthesis by intestinal bacteria or intake from foods is important for D-amino acid supply. Because lactic acid bacteria can synthesize D-amino acids, foods produced by lactate fermentation contain D-amino acids [13,14,15]. Ishizuchi-kurocha is a post-fermented tea produced in Ehime, Japan. The fermentation processes of post-fermented teas, such as Ishizuchi-kurocha, Goishi-cha (Kochi, Japan), Awa-bancha (Tokushima, Japan), and Miang (Northern Thailand), include lactate fermentation. Ishizuchi-kurocha is produced by a two-step fermentation process [16]. First, harvested tea leaves are steamed, and primary fermentation is performed aerobically by fungi, mainly Aspergillus niger and Aspergillus luchuensis. The secondary fermentation for Ishizuchi-kurocha is lactate fermentation, in which the tea leaves are fermented anaerobically by lactic acid bacteria. The dominant bacterial flora species in secondary fermentation are Lactiplantibacillus plantarum and Levilactobacillus brevis. The composition of lactic acid bacteria in Ishizuchi-kurocha is 80–100% L. plantarum and 20–0% L. brevis during secondary fermentation. Sometimes, L. brevis is not detected during secondary fermentation. L. plantarum is frequently isolated from plant-derived fermented foods. The genome sequence of L. plantarum strain IYO1511, which was isolated from Ishizuchi-kurocha in 2015, has been determined [17]. According to the genome sequence, L. plantarum IYO1511 possesses a racemase gene. However, the D-amino acid concentration of Ishizuchi-kurocha has yet to be determined. In fact, it remains unclear whether D-amino acids are present in Ishizuchi-kurocha. The D-amino acid producibility of L. brevis is also unknown. Therefore, in this study, the D-amino acid content of Ishizuchi-kurocha was determined, and the contributions of L. plantarum and L. brevis were examined.
MATERIALS AND METHODS
Tea samples and lactic acid bacteria
Samples of Ishizuchi-kurocha tea were provided by the Ishizuchi-kurocha producer Visee (Saijo, Ehime, Japan). The Ishizuchi-kurocha used in the present study was produced in 2020 and 2021 in Saijo, Ehime, Japan. One hundred milliliters of boiling distilled water were added to 2 g of sun-dried tea leaves and left to stand for 5 min. Then, the elution was passed through a 0.22 μm filter. This solution was filtered through an Amicon Ultra-0.5 Centrifugal Filter Unit (3 kDa NMWCO pore size; MilliporeSigma, Burlington, MA, USA) with centrifugation (14,000 × g, for 15 min at 4°C). Sample solutions were stored at −80°C until use and analyzed with an ultra-performance liquid chromatography (UPLC) system (Waters, Milford, MA, USA). Lactic acid bacteria (L. brevis IYO2091, L. plantarum IYO2092, and L. plantarum IYO2179) were isolated from the tea leaves after secondary fermentation (production lots 2020-3 and 2021-1). Isolation and identification of the lactic acid bacteria were performed with the method reported by Horie et al. [18]. The isolated species were identified by the homology of the 16S rRNA gene sequence. Each sequence of the 16S rRNA gene has been submitted to the DNA Data Bank of Japan (DDBJ). Details of isolates and accession numbers of 16S rRNA gene sequences are shown in Table 1.
Table 1. Properties of lactic acid bacteria used in this study.
| Species | Strain | Source | DDBJ Accession No. | |||
|---|---|---|---|---|---|---|
| 16S rRNA gene | Glutamate | Aspartate | Alanine | |||
| racemase | racemase | racemase | ||||
| Levilactobacillus brevis | IYO2091 | Ishizuchi-kurocha Lot 2020 | LC687616 | LC742524 | LC742523 | LC742522 |
| Lactiplantibacillus plantarum | IYO2092 | Ishizuchi-kurocha Lot 2020 | LC687615 | LC742518 | LC742517 | LC742516 |
| Lactiplantibacillus plantarum | IYO2179 | Ishizuchi-kurocha Lot 2021 | LC742525 | LC742521 | LC742520 | LC742519 |
DDBJ: DNA Data Bank of Japan.
Preparation of culture media and cell-free extracts of lactic acid bacteria for amino acid analysis
The three isolates were cultured under static conditions for 24 hr at 37°C in de Man, Rogosa and Sharpe (MRS) medium (100 mL). The culture supernatant and cells were separated by centrifugation at 10,000 × g for 15 min at 4°C. For amino acid analysis of culture supernatant, aliquots (1 mL) of medium were filtered with an Amicon Ultra-0.5 Centrifugal Filter Unit (3 kDa NMWCO pore size). The prepared samples were stored at −80°C until use and analyzed with a UPLC system. The collected cells were suspended in 20 mM potassium phosphate buffer (pH 7.2) and broken using glass beads in a Multi-beads Shocker homogenizer (Yasui Kikai, Osaka, Japan). The resultant homogenate was centrifuged at 12,000 × g for 10 min at 4°C. Then, the supernatant was filtered with an Amicon Ultra-0.5 Centrifugal Filter Unit (3 kDa NMWCO pore size). The filtrate was used as a cell-free extract for amino acid analysis with a UPLC system.
Derivatization of amino acids for UPLC analysis
Derivatization of amino acids was carried out using o-phthaldialdehyde (OPA; Nova Biochemical, Waltham, MA, USA) plus N-acetyl-l-cysteine (NAC; FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan) or N-tert-butyloxycarbonyl-l-cysteine (NBC; Nacalai Tesque, Kyoto, Japan). For the amino acid derivatives, a methanolic solution was prepared by dissolving 8 mg of OPA and 10 mg of NAC (OPA-NAC) or 10 mg OPA and 10 mg NBC (OPA-NBC) in 1 mL of methanol. The reaction mixture (250 µL) for derivatization consisted of 25 µL of sample, 50 µL of methanolic solution, and 175 µL of 0.4 M borate-NaOH buffer (pH 10.4). After incubation for 2 min at room temperature in the dark for derivatization, aliquots (1 µL) of the reaction mixture were introduced into a UPLC system.
Analysis and quantification of amino acids using UPLC
The derivatives formed with OPA-NAC or OPA-NBC were applied to a 2.1 × 100 mm AccQ-Tag Ultra column (Waters) in an ACQUITY UPLC TUV system consisting of a Waters Binary Solvent Manager, Waters Sample Manager, and Waters FLR Detector. The excitation and emission wavelengths for fluorescent detection of the derivatized amino acids were 350 nm and 450 nm, respectively. The system was operated at a flow rate of 0.25 mL/min at 30°C. For the analysis of OPA-NAC derivatives (A=50 mM sodium acetate, pH 5.9, and B=methanol), the UPLC gradient system was 10–20% B over 3.2 min, 20% B for 1.2 min, 20–40% B over 3.6 min, 40% B for 1.2 min, 40–60% B over 3.8 min, 60% B for 1 min, and 60–10% B over 0.01 min. The gradient system used for analysis of OPA-NBC derivatives (A=50 mM sodium acetate, pH 5.9, and B=acetonitrile) was 15–21% B over 7 min, 21–27.5% B over 1.5 min, 27.5% B for 2 min, 27.5–30% B over 1 min, 30–40% B over 2 min, 40% B for 0.5 min, and 40–15% B over 0.01 min.
Peak heights were used for quantification of amino acids. The standard solution consisted of 32 kinds of amino acids: D- and L- forms of Ala, Arg, Asn, Asp, Gln, Glu, His, Leu, Met, Phe, Ser, Trp, Tyr, and Val plus L-Ile, D-allo-Ile, L-Thr, and D-allo-Thr. To construct the standard calibration curves, the standard solution was diluted with 0.05 M HCl to eight concentrations (5, 10, 25, 50, 100, 250, 500 and 1,000 μM), and the eight standard solutions were analyzed on a UPLC system. Peak heights were plotted against amino acid concentrations to create a calibration curve. For all amino acid types tested, the relation was linear, with regression coefficients above 0.999. If the concentration of amino acid in a sample was over 1,000 μM, an appropriately diluted sample was derivatized, and aliquots (1 µL) of the reaction mixture were applied to the UPLC system.
Determination of racemase gene sequences of isolates
Racemase genes were amplified by polymerase chain reaction (PCR). The racemase gene primers were designed based on the genome of L. plantarum IYO1511 isolated from Ishizuchi-kurocha [17] and L. plantarum JCM 1149T. The primer sequences are listed in Table 2. Each lactic acid bacterial strain was cultured in MRS broth for 18 hr. Then, bacterial cells were harvested by centrifugation at 10,000 × g for 10 min. DNA was extracted from pellets with a DNeasy Tissue & Blood Kit (Qiagen, Hilden, Germany). PCR amplification was performed in a TaKaRa PCR Thermal Cycler (Takara Bio Inc., Kusatsu, Japan). The PCRs were conducted in a total reaction volume of 50 µL containing 1 µL of template DNA, 0.5 μmol/L of each primer, Ex Taq polymerase, 5 µL of PCR buffer, and 0.2 mmol/L of deoxynucleotide triphosphate. All primers were synthesized by Eurofins Genomics K.K. (Tokyo, Japan). Thermal cycling of the Taq polymerase was conducted under the following conditions: 2 min at 93°C for initial denaturation; 30 cycles of 30 sec at 93°C, 30 sec at 55°C, and 30 sec at 72°C; and then 1 min at 72°C. PCR products were analyzed by electrophoresis in 2.0% agarose gel followed by GelRedTM (Biotium, Inc., Fremont, CA, USA) staining. Amplicons were purified by NucleoSpin® Gel and PCR Clean-up (Macherey-Nagel GmbH & Co. KG., Düren, Germany). DNA sequencing was performed by Macrogen Japan Corp. (Tokyo, Japan). Each primer used for DNA sequencing was a forward primer. Alignment of the primary structures of the translated amino acid racemases and calculation of the phylogenetic trees were performed by ClustalW. Phylogenetic analysis was performed based on maximum likelihood with the bootstrap method using PhyML [19]. Each sequence of the racemase genes has been submitted to DDBJ. Accession numbers of the racemase gene sequences are shown in Table 1.
Table 2. Sequence of primers for amino acid racemase.
| Target | Species | Primer name | Sequence (5′-3′) |
|---|---|---|---|
| Glutamate racemase | L. plantarum | PlaGluRasF | CGTTTCCACTAATGTGTCGG |
| PlaGluRasR1 | GATACCGTTC TCCTTAATTT CAGG | ||
| L. brevis | BreGluRac-3F | CGCGAAGTTCTTAATGACAGAA | |
| BreGluRac-3R | CACTGCTGTCGCCTTTAAAG | ||
| Aspartate racemase | L. plantarum | PlaAspRac-F | GCTTGTTAGGAGGTGCATGTTAG |
| PlaAspRac-R | CAACGATTTGAGCGCTTACAA | ||
| L. brevis | BreAmiRasF1 | GTTCCAAGTGCAAGACGTG | |
| BreAmiRasR1 | TTGATTGCCG CTGGCAAGTA TG | ||
| Alanine racemase | L. plantarum | PlaAlaRac_2-F | CCAGAATTTCTCCGGCACTCA |
| PlaAlaRac_2-R | AGACAACGCCCAGGGTAAAC | ||
| PlaAlaRac_2b-F | GGCGCAAACCTATCGTCTGA | ||
| PlaAlaRac_2b-R | TATCCAAGTTGCTCAGGCCG | ||
| PlaAlaRac_2c-F | TACGTGGCACCATAGCTGAC | ||
| PlaAlaRac_2c-R | ATCGAGTTTATGGCAGGGCG | ||
| L. brevis | BreAlaRac-F | GACATTGAGATTCGGGATAATG | |
| BreAlaRac-R | GCTGCTTGGTACGAGTCATG | ||
| BreAlaRac_2-F | CGGTCGTGATGACACAGGTT | ||
| BreAlaRac_2-R | GCCGCAACGTTACTGGTTTC |
These primers were designed based on genome sequence of L. plantarum JCM1149T (CP039121.1) and L. brevis UCCLBBS124 (CP031169.1).
RESULTS
D- and L-Amino acid contents of Ishizuchi-kurocha
The D- and L-amino acid contents of Ishizuchi-kurocha produced in 2020 and 2021 were determined (Table 3). Three types of D-amino acids (D-Asp, D-Glu, and D-Ala) were identified. However, the composition of these D-amino acids differed according to the year of production. The Ishizuchi-kurocha produced in 2020 contained D-Asp, D-Glu, and D-Ala, while the tea produced in 2021 contained only D-Glu and D-Ala. The D-Ala content was higher in production lot 2021 than in production lot 2020. D-Amino acids were not detected in Batabata-cha (another post-fermented tea), which was produced without lactic fermentation (data not shown).
Table 3. D- and L-Amino acid content of Ishizuchi-kurocha (μM).
| 2020 | 2021 | |||
|---|---|---|---|---|
| NAC | NBC | NAC | NBC | |
| L-Asp | 122.6 | 121.6 | 161.6 | 166.0 |
| D-Asp | 4.9 | 6.7 | 0.0 | 0.0 |
| d/d+l ratio | 0.04 | 0.05 | 0.00 | 0.00 |
| L-Glu | 51.2 | 186.0 | ||
| D-Glu | 5.7 | 6.4 | ||
| d/d+l ratio | 0.10 | 0.03 | ||
| L-Ser | 25.6 | 34.0 | ||
| L-Gln | 3.3 | 1.8 | 0.0 | 0.0 |
| L-His | 72.6 | 31.1 | 72.3 | 34.2 |
| L-Arg | 12.5 | 83.3 | ||
| L-Ala | 85.2 | 83.6 | 123.6 | 123.1 |
| D-Ala | 39.3 | 28.1 | 67.9 | 35.4 |
| d/d+l ratio | 0.32 | 0.25 | 0.35 | 0.22 |
| L-Tyr | 11.1 | 43.5 | ||
| L-Val | 76.1 | 74.2 | 85.5 | 86.3 |
| L-Met | 0.0 | 0.0 | 14.0 | 14.8 |
| L-Trp | 33.9 | 12.0 | 28.5 | 15.5 |
| L-Phe | 57.5 | 57.7 | 65.6 | 67.9 |
| L-Ile | 42.3 | 44.0 | 47.8 | 51.2 |
| L-Leu | 128.9 | 124.1 | 152.3 | 150.4 |
NAC: N-acetyl-L-cysteine; NBC: N-tert-butyloxycarbonyl-L-cysteine.
Production of D-amino acids by lactic acid bacteria isolated from Ishizuchi-kurocha
The D-amino acid producibility of the L. plantarum and L. brevis strains isolated from Ishizuchi-kurocha was examined. The strains were isolated from the same lots of Ishizuchi-kurocha for which the D- and L-amino acids were measured in this study. They included L. plantarum IYO2092 and L. brevis IYO2091 isolated from production lot 2020 and L. plantarum IYO2179 isolated from product lot 2021. L. brevis was not detected in production lot 2021. The D- and L-amino acid contents in culture supernatants and bacterial cells after 24 hr of cultivation were determined (Tables 4 and 5). Four types of D-amino acids (D-Asp, D-Ser, D-Ala, and D-Glu) were detected in the culture supernatants of these strains. In addition, four types of D-amino acids (D-Asp, D-Glu, D-Ser, and D-Ala) were detected in the bacterial cells of L. brevis IYO2091. The D-amino acids detected in the culture supernatants were not detected in the MRS medium. The D-amino acid to total amino acid (D/D+L) ratios for D-Asp, D-Glu, D-Ser, and D-Ala were approximately 43–50% (Table 3). On the other hand, D-Asp was not detected in the bacterial cells of L. plantarum IYO2092. The intracellular D-Asp concentration of L. plantarum IYO2179 was lower than that of L. brevis IYO2091. Compared with the contents in the culture supernatants, the D/D+L ratios of amino acids in the bacterial cells were high. The production of D-Asp and D-Glu was significantly higher in L. brevis IYO2091 than in L. plantarum strains. Although L. brevis IYO2091 produced D-Ala and D-Ser, the production of D-Ala and D-Ser by L. plantarum strains was low. On the other hand, although D-Arg was not detected in the culture supernatants, the bacterial cells of L. plantarum strains contained D-Arg. D-Arg was not detected in either the culture supernatant or bacterial cells of L. brevis IYO2091. It was only detected in the cells of the L. plantarum strains.
Table 4. D- and L-Amino acid contents in the culture supernatant and the bacteria cells of lactic acid bacteria (OPA-NAC derivatives).
| L.brevis IYO2091 | L. plantarum IYO2092 | L. plantarum IYO2179 | ||||
|---|---|---|---|---|---|---|
| Culture supernatant | Bacterial cell | Culture supernatant | Bacterial cell | Culture supernatant | Bacterial cell | |
| μM | nmol/g1) | μM | nmol/g1) | μM | nmol/g1) | |
| L-Asp | 1,553 | 6,061 | 2,542 | 3,241 | 588 | 596 |
| D-Asp | 217 | 5,063 | 79 | 0 | 41 | 36 |
| d/d+l ratio | 0 | 0 | 0 | 0 | 0 | 0 |
| L-Ser | 2,358 | 700 | 76 | 0 | 17 | 49 |
| D-Ser | 28 | 644 | 29 | 0 | 0 | 0 |
| d/d+l ratio | 0 | 0 | 0 | 0 | 0 | 0 |
| L-Gln | 48 | 119 | 43 | 93 | 66 | 0 |
| D-Gln | 0 | 0 | 0 | 0 | 0 | 0 |
| L-His | 689 | 585 | 1,119 | 129 | 546 | 211 |
| D-His | 0 | 0 | 0 | 0 | 0 | 0 |
| L-Arg | 0 | 164 | 3,835 | 270 | 1,931 | 429 |
| D-Arg | 0 | 0 | 0 | 220 | 0 | 223 |
| d/d+l ratio | 0 | 0 | 0 | 0 | 0 | 0 |
| L-Ala | 2,787 | 2,808 | 3,937 | 807 | 1,834 | 213 |
| D-Ala | 1,065 | 2,884 | 1,273 | 843 | 596 | 362 |
| d/d+l ratio | 0 | 1 | 0 | 1 | 0 | 1 |
| D-allo-Thr | 0 | 0 | 152 | 0 | 0 | 97 |
| L-Tyr | 682 | 89 | 950 | 0 | 439 | 0 |
| D-Tyr | 0 | 0 | 0 | 0 | 0 | 0 |
| L-Val | 3,078 | 799 | 5,446 | 174 | 2,834 | 196 |
| D-Val | 0 | 0 | 0 | 0 | 0 | 0 |
| L-Met | 1,130 | 0 | 1,810 | 0 | 861 | 0 |
| D-Met | 0 | 0 | 0 | 0 | 0 | 0 |
| L-Trp | 0 | 202 | 1,379 | 0 | 663 | 0 |
| D-Trp | 0 | 0 | 0 | 0 | 0 | 0 |
| L-Phe | 2,446 | 365 | 4,085 | 94 | 2,054 | 74 |
| D-Phe | 0 | 0 | 0 | 0 | 0 | 0 |
| L-Ile | 2,460 | 494 | 4,072 | 101 | 2,091 | 129 |
| D-allo-Ile | 0 | 0 | 0 | 0 | 0 | 0 |
| L-Leu | 5,673 | 1,050 | 9,684 | 308 | 5,332 | 319 |
| D-Leu | 0 | 0 | 0 | 0 | 0 | 0 |
1)Amount of substance (nmol) per 1 g of wet weight of cells calculated from concentration (μM).
OPA: o-phthaldialdehyde; NBC: N-tert-butyloxycarbonyl-L-cysteine.
Table 5. D- and L-Amino acid contents in the culture supernatant and the bacteria cells of lactic acid bacteria (OPA-NBC derivatives).
| L.brevis IYO2091 | L. plantarum IYO2092 | L. plantarum IYO2179 | ||||
|---|---|---|---|---|---|---|
| Culture supernatant | Bacterial cell | Culture supernatant | Bacterial cell | Culture supernatant | Bacterial cell | |
| μM | nmol/g1) | μM | nmol/g1) | μM | nmol/g1) | |
| L-Asp | 1,419 | 5,227 | 1,069 | 2,855 | 553 | 526 |
| D-Asp | 162 | 3,985 | 0 | 0 | 21 | 31 |
| d/d+l ratio | 0 | 0 | 0 | 0 | 0 | 0 |
| L-Glu | 2,447 | 12,958 | 3,357 | 2,805 | 3,206 | 265 |
| D-Glu | 270 | 12,589 | 131 | 2,658 | 79 | 1,715 |
| d/d+l ratio | 0 | 0 | 0 | 0 | 0 | 1 |
| L-Asn | 1,002 | 0 | 689 | 1,425 | 661 | 1,329 |
| D-Asn | 0 | 0 | 0 | 0 | 0 | 0 |
| L-Ser | 2,517 | 652 | 0 | 111 | 0 | 75 |
| D-Ser | 0 | 610 | 0 | 0 | 0 | 0 |
| d/d+l ratio | 0 | 0 | 0 | 0 | 0 | 0 |
| L-Gln | 0 | 241 | 0 | 51 | 56 | 0 |
| D-Gln | 220 | 0 | 192 | 0 | 216 | 0 |
| d/d+l ratio | 1 | 0 | 1 | 0 | 1 | 0 |
| L-His | 613 | 454 | 470 | 111 | 445 | 200 |
| D-Arg | 0 | 0 | 0 | 211 | 0 | 218 |
| L-Ala | 2,891 | 2,744 | 2,070 | 840 | 1,838 | 228 |
| D-Ala | 1,004 | 2,636 | 573 | 748 | 540 | 317 |
| d/d+l ratio | 0 | 0 | 0 | 0 | 0 | 1 |
| D-Tyr | 0 | 0 | 0 | 0 | 0 | 0 |
| L-Val | 3,445 | 839 | 3,161 | 183 | 3,054 | 210 |
| D-Val | 0 | 0 | 0 | 0 | 0 | 0 |
| L-Met | 1,085 | 0 | 947 | 0 | 874 | 0 |
| D-Met | 0 | 0 | 0 | 0 | 0 | 0 |
| L-Ile | 2,719 | 487 | 2,277 | 104 | 2,198 | 136 |
| L-Trp | 673 | 0 | 621 | 0 | 597 | 0 |
| L-Phe | 2,505 | 341 | 2,142 | 83 | 2,032 | 67 |
| D-Phe | 0 | 0 | 0 | 0 | 0 | 0 |
| L-Leu | 5,132 | 951 | 4,843 | 293 | 4,708 | 297 |
| D-Leu | 0 | 0 | 0 | 0 | 0 | 0 |
1) Amount of substance (nmol) per 1 g of wet weight of cells calculated from concentration (μM).
OPA: o-phthaldialdehyde; NBC: N-tert-butyloxycarbonyl-L-cysteine.
Detection of amino acid racemase gene sequences in the L. plantarum and L. brevis strains
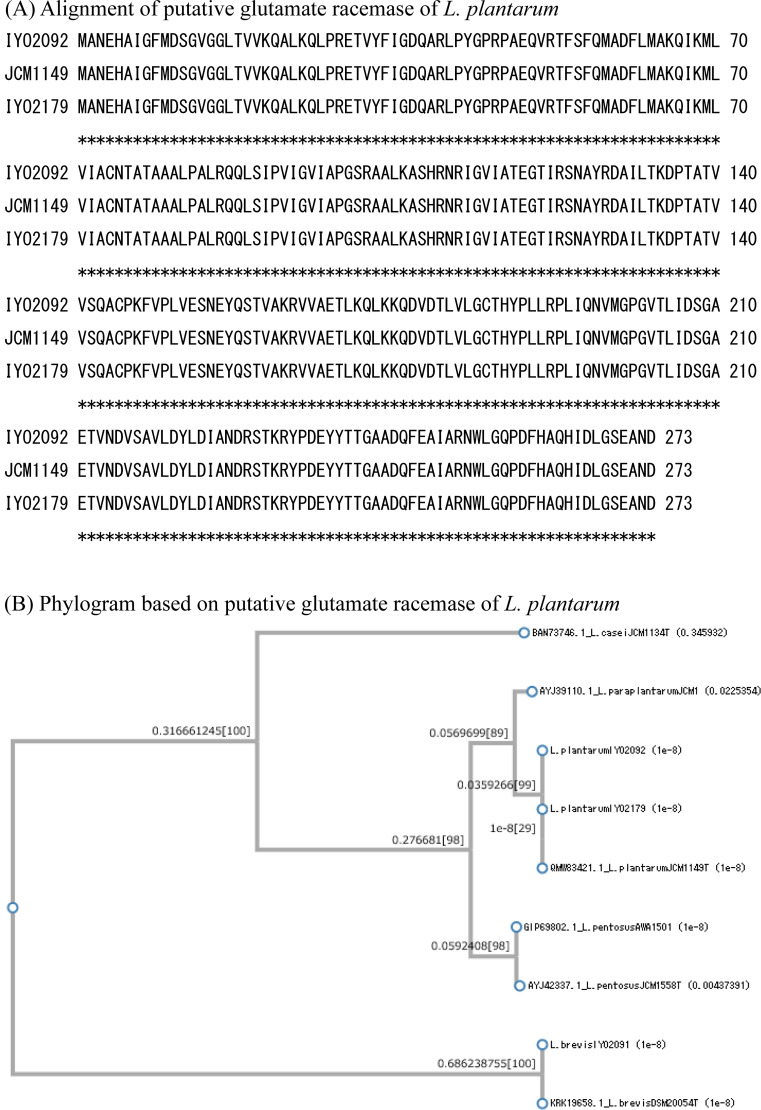
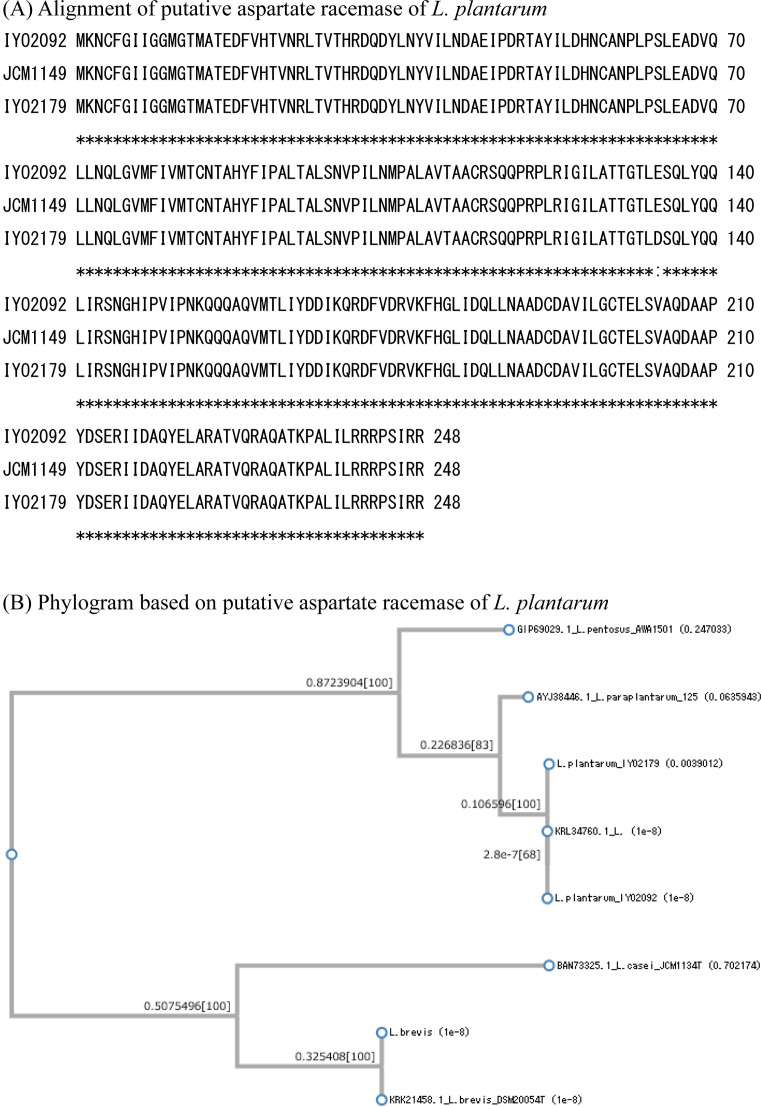
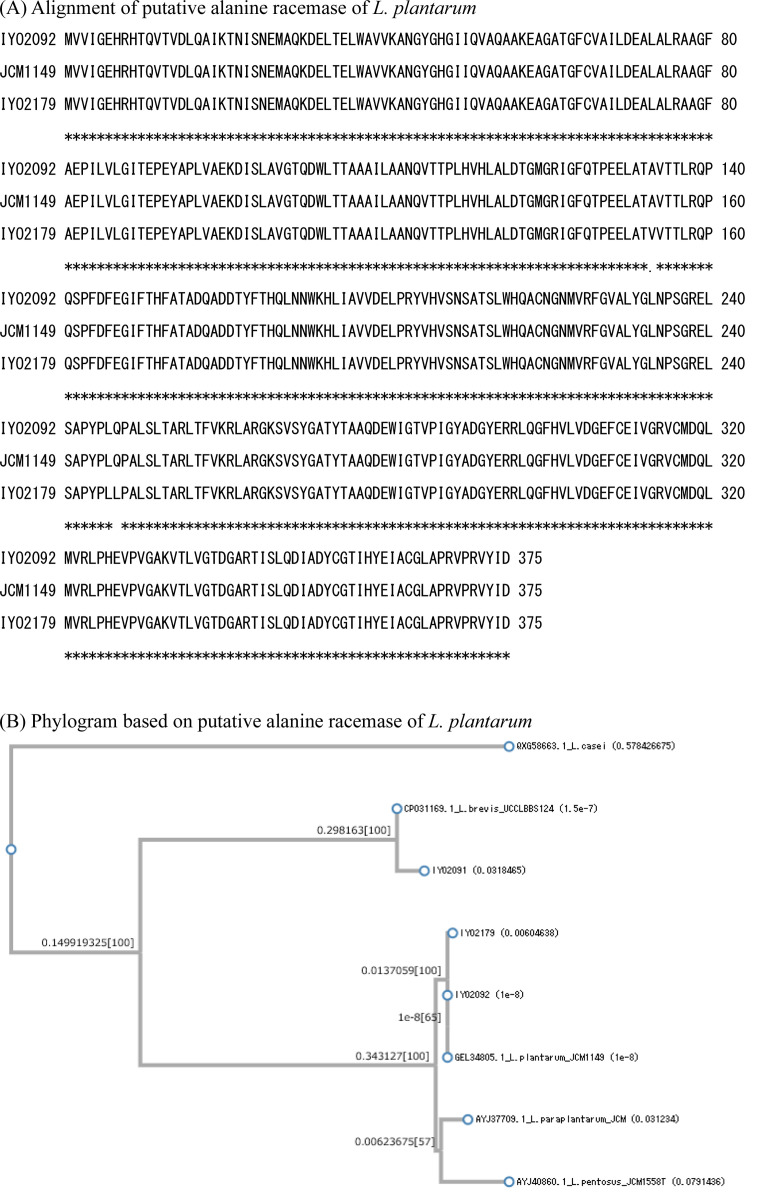
The gene sequences of the three types of amino acid racemases (glutamate racemase, alanine racemase, and aspartate racemase) were determined. The PCR primers for these racemase genes were designed based on the whole genome sequences of L. plantarum JCM1149T, L. plantarum IYO1511, and L. brevis ATCC 14869T. All the known racemase genes in L. plantarum IYO2092, L. plantarum IYO2179, and L. brevis IYO2091 were amplified by PCR. These strains contained at least three types of amino acid racemases: alanine racemase, aspartate racemase, and glutamate racemase. The primary structure of each racemase was determined according to its gene sequence. For glutamate racemase, the homology of the primary structures of IYO2092, IYO2179, and JCM1149T was 100% (Fig. 1). The primary structure of L. brevis IYO2091 glutamate racemase was the same as that of L. brevis DSM20054T (KRK19658). For aspartate racemase, the homology of the primary structures of IYO2092 and JCM1149T was 100% (Fig. 2). However, the primary structure of IYO2179 aspartate racemase differed from that of IYO2092 and JCM1149T by one amino acid (Glu134Asp). The primary structure of L. brevis IYO2091 aspartate racemase was the same as that of L. brevis DSM20054T (KRK21458). For alanine racemase, the homology of the primary structures of IYO2092 and JCM1149T was 100% (Fig. 3). However, the primary structure of IYO2179 alanine racemase differed from that of IYO2012 and JCM1149T by two amino acids (Ala153Val and Gln247Leu). The primary structure of L. brevis IYO2091 alanine racemase was the same as that of L. brevis JCM1059T (=DSM20054T) (OQ957164). The primary structures of amino acid racemases were well conserved in L. plantarum species. On the other hand, the primary structure of the L. plantarum strains differed from that of other species in the L. plantarum group, i.e., L. pentosus and L. paraplantarum. The primary structures of the amino acid racemases significantly differed between L. plantarum group species and L. brevis. According to alignment by BLAST, the identities of the glutamate, aspartate, and alanine racemases between L. plantarum JCM1149T and L. brevis DSM20054T were 49%, 44%, and 67%, respectively.
Fig. 1.
Comparison of the primary structures of glutamate racemases in the Lactiplantibacillus plantarum group and Levilactobacillus brevis.
(A) Alignment of the primary structures of the glutamate racemases of L. plantarum IYO2092, IYO2179, and JCM1149T. (B) Glutamate racemase phylogenic tree. Sequences of different types of strains were employed as a reference. The reference sequences of L. plantarum, L. paraplantarum, L. pentosus, and L. brevis were obtained from GenBank. L. casei was employed as the outgroup species. The accession numbers of the reference species are indicated in the figure.
Fig. 2.
Comparison of the primary structures of aspartate racemases in the Lactiplantibacillus plantarum group and Levilactobacillus brevis.
(A) Alignment of the primary structures of the amino acid racemases of L. plantarum IYO2092, IYO2179, and JCM1149T. (B) Aspartate racemase phylogenic tree. Sequences of different types of strains were employed as a reference. The reference sequences of L. plantarum, L. paraplantarum, L. pentosus, and L. brevis were obtained from GenBank. L. casei was employed as the outgroup species. The accession numbers of the reference species are indicated in the figure.
Fig. 3.
Comparison of the primary structures of alanine racemases in the Lactiplantibacillus plantarum group and Levilactobacillus brevis.
(A) Alignment of the primary structures of the amino acid racemases of L. plantarum IYO2092, IYO2179, and JCM1149T. (B) Alanine racemase phylogenic tree. Sequences of different types of strains were employed as a reference. The reference sequences of L. plantarum, L. paraplantarum, L. pentosus, and L. brevis were obtained from GenBank. L. casei was employed as the outgroup species. The accession numbers of the reference species are indicated in the figure.
DISCUSSION
In the present study, the D-amino acid content of Ishizuchi-kurocha was determined. Ishizuchi-kurocha from production lot 2020 contained three types of D-amino acids: D-Asp, D-Glu, and D-Ala. However, D-Asp was not detected in Ishizuchi-kurocha from production lot 2021; it contained only D-Glu and D-Ala.
All three lactic acid bacterial strains used in this study (L. brevis IYO2091, L. plantarum IYO2092, and L. plantarum IYO2179) possessed putative aspartate racemases. Compared with L. brevis IYO 2091, the D-Asp production by L. plantarum IYO2092 and IYO2179 was low. In particular, D-Asp was not detected in the bacterial cells of L. plantarum IYO2092. The intracellular D-Asp concentration of L. brevis IYO2091 was higher than the extracellular concentration. The homology of the primary structure of the putative aspartate racemase between L. plantarum and L. brevis was low. According to BLAST alignment, the identity between these aspartate racemases was 44%. Therefore, it is necessary to investigate the activity of these two enzymes.
The dominant bacterial species in Ishizuchi-kurocha during secondary fermentation is L. plantarum, followed by L. brevis [20]. These two species are present in the tea leaves of Ishizuchi-kurocha after secondary fermentation each year. However, L. brevis is not always detected in individual production lots of Ishizuchi-kurocha [20]. L. brevis was not detected in production lot 2021-1, which was analyzed in this study. In contrast, both L. plantarum and L. brevis were isolated from production lot 2020-3. The reason why some production lots did not contain L. brevis is unknown. The composition of D-amino acids in Ishizuchi-kurocha is dependent on the composition of lactic acid bacteria during fermentation. During secondary fermentation, the composition of lactic acid bacteria in Ishizuchi-kurocha is 80–100% L. plantarum and 20–0% L. brevis. When L. brevis is involved in the fermentation of Ishizuchi-kurocha, D-Asp may be produced. Compared with L. brevis IYO2091, the production of D-Asp by L. plantarum IYO2092 and IYO2179 was very low. If L. brevis is absent during the fermentation of Ishizuchi-kurocha, little to no D-Asp may be detected in the Ishizuchi-kurocha lot. L. brevis is an important species for D-Asp production in Ishizuchi-kurocha. Although D-Arg was not detected outside cells, it was detected in the cells of L. plantarum IYO2092 and IYO2179. The D/D+L ratios of Arg in L. plantarum IYO2092 and IYO2179 were 44.9 and 34.1, respectively. To date, arginine racemase has only been reported in Pseudomonas sp. [21]. The arginine racemase activity of Pseudomonas taetrolens was almost exclusively present in the periplasm [22]. MalY from Latilactobacillus sakei [23] and Lys racemase from Oenococcus oeni are broad-spectrum racemases that may be present in L. plantarum IYO2092 and IYO217. D-Ser was detected in the bacterial cells of L. brevis IYO 2091 but not in the culture supernatant. L. plantarum did not produce D-Ser in either bacterial cells or culture supernatant.
In contrast, D-Arg was detected in the bacterial cells of L. plantarum IYO 2092 and IYO2179 but not in their culture supernatants. L. brevis did not produce D-Arg in either bacterial cells or culture supernatant. Although D-Ser, D-Arg, and D-Glu were detected in the culture supernatants or bacterial cells of isolated lactic acid bacteria, they were not found in the tea leaves of Ishizuchi-kurocha. These D-amino acids were detected when lactic acid bacteria were cultured in MRS broth. MRS broth is suitable for the growth of lactic acid bacillus, whereas fermented tea leaves provide a more severe environment because they contain antimicrobial molecules, such as catechins [24]. The nutritional conditions for bacterial growth are significantly different between MRS medium and tea leaves. The amount of substrate amino acids in tea leaves would be much lower than in MRS medium. These differences may be major factors involved in the difference in the production of D-amino acids. Furthermore, other microorganisms and their metabolites may affect the growth of lactic acid bacteria. For example, the L. plantarum strain isolated from Ishizuchi-kurocha exhibited stronger antibiotic resistance than L. plantarum strains isolated from Awa-bancha, which does not undergo aerobic fermentation by molds [18]. Thus, the metabolism and gene expression of L. plantarum and L. brevis in MRS broth and in fermented tea leaves could be significantly different. The interaction between the gene expression of amino acid racemases and D-amino acid production and secretion by L. plantarum and L. brevis during the fermentation of Ishizuchi-kurocha is an interesting process.
In conclusion, Ishizuchi-kurocha contains at least three kinds of D-amino acids (D-Asp, D-Glu, and D-Ala) with various physiological benefits, such as improved brain function and protection against kidney injury. Drinking Ishizuchi-kurocha is expected to affect the physiological activities of D-amino acids. L. plantarum and L. brevis isolated from Ishizuchi-kurocha have practical food industry applications. The co-culture of L. plantarum and L. brevis could improve the production of various D-amino acids.
CONFLICTS OF INTEREST
There is no potential conflict of interest to declare.
REFERENCES
- 1.Matsumoto M, Kunisawa A, Hattori T, Kawana S, Kitada Y, Tamada H, Kawano S, Hayakawa Y, Iida J, Fukusaki E. 2018. Free D-amino acids produced by commensal bacteria in the colonic lumen. Sci Rep 8: 17915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hashimoto A, Nishikawa T, Hayashi T, Fujii N, Harada K, Oka T, Takahashi K. 1992. The presence of free D-serine in rat brain. FEBS Lett 296: 33–36. [DOI] [PubMed] [Google Scholar]
- 3.Shleper M, Kartvelishvily E, Wolosker H. 2005. D-serine is the dominant endogenous coagonist for NMDA receptor neurotoxicity in organotypic hippocampal slices. J Neurosci 25: 9413–9417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Piubelli L, Pollegioni L, Rabattoni V, Mauri M, Princiotta Cariddi L, Versino M, Sacchi S. 2021. Serum D-serine levels are altered in early phases of Alzheimer’s disease: towards a precocious biomarker. Transl Psychiatry 11: 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng YJ, Lin CH, Lane HY. 2021. D-Amino acids and pLG72 in Alzheimer’s disease and schizophrenia. Int J Mol Sci 22: 10917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calcia MA, Madeira C, Alheira FV, Silva TC, Tannos FM, Vargas-Lopes C, Goldenstein N, Brasil MA, Ferreira ST, Panizzutti R. 2012. Plasma levels of D-serine in Brazilian individuals with schizophrenia. Schizophr Res 142: 83–87. [DOI] [PubMed] [Google Scholar]
- 7.Hesaka A, Tsukamoto Y, Nada S, Kawamura M, Ichimaru N, Sakai S, Nakane M, Mita M, Okuzaki D, Okada M, Isaka Y, Kimura T. 2021. D-Serine mediates cellular proliferation for kidney remodeling. Kidney360 2: 1611–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakade Y, Iwata Y, Furuichi K, Mita M, Hamase K, Konno R, Miyake T, Sakai N, Kitajima S, Toyama T, Shinozaki Y, Sagara A, Miyagawa T, Hara A, Shimizu M, Kamikawa Y, Sato K, Oshima M, Yoneda-Nakagawa S, Yamamura Y, Kaneko S, Miyamoto T, Katane M, Homma H, Morita H, Suda W, Hattori M, Wada T. 2018. Gut microbiota-derived D-serine protects against acute kidney injury. JCI Insight 3: e97957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ishio S, Yamada H, Hayashi M, Yatsushiro S, Noumi T, Yamaguchi A, Moriyama Y. 1998. D-aspartate modulates melatonin synthesis in rat pinealocytes. Neurosci Lett 249: 143–146. [DOI] [PubMed] [Google Scholar]
- 10.D’Aniello G, Tolino A, D’Aniello A, Errico F, Fisher GH, Di Fiore MM. 2000. The role of D-aspartic acid and N-methyl-D-aspartic acid in the regulation of prolactin release. Endocrinology 141: 3862–3870. [DOI] [PubMed] [Google Scholar]
- 11.Furuchi T, Homma H. 2005. Free D-aspartate in mammals. Biol Pharm Bull 28: 1566–1570. [DOI] [PubMed] [Google Scholar]
- 12.Sato T, Umekawa Y, Shindo S. 2022. Suppressive effects of D-amino acids on the lipid accumulation in human hepatocyte. J Brew Soc Japan 117: 131–138. [Google Scholar]
- 13.Mutaguchi Y, Ohmori T, Akano H, Doi K, Ohshima T. 2013. Distribution of D-amino acids in vinegars and involvement of lactic acid bacteria in the production of D-amino acids. Springerplus 2: 691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kobayashi J. 2019. D-Amino acids and lactic acid bacteria. Microorganisms 7: 690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oikawa T. 2015. “D-amino acid”, a new ingredient effecting on Sake taste. J Brew Soc Japan 110: 189–197. [Google Scholar]
- 16.Horie M, Nara K, Sugino S, Umeno A, Yoshida Y. 2016. Comparison of antioxidant activities among four kinds of Japanese traditional fermented tea. Food Sci Nutr 5: 639–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Niwa R, Syaputri Y, Horie M, Iwahashi H. 2020. Draft genome sequence of Lactobacillus plantarum IYO1511, isolated from Ishizuchi-Kurocha. Microbiol Resour Announc 9: e00143–e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horie M, Sato H, Tada A, Nakamura S, Sugino S, Tabei Y, Katoh M, Toyotome T. 2019. Regional characteristics of Lactobacillus plantarum group strains isolated from two kinds of Japanese post-fermented teas, Ishizuchi-kurocha and Awa-bancha. Biosci Microbiota Food Health 38: 11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, Gascuel O. 2010. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol 59: 307–321. [DOI] [PubMed] [Google Scholar]
- 20.Horie M, Tada A, Kanamoto N, Tamai T, Fukuda N, Sugino S, Toyotome T, Tabei Y. 2019. Evaluation of lactic acid bacteria and component change during fermentation of Ishizuchi‐kurocha. J Food Process Preserv 43: e14186. [Google Scholar]
- 21.Yorifuji T, Ogata K. 1971. Arginine racemase of Pseudomonas graveolens. I. Purification, crystallization, and properties. J Biol Chem 246: 5085–5092. [PubMed] [Google Scholar]
- 22.Matsui D, Oikawa T, Arakawa N, Osumi S, Lausberg F, Stäbler N, Freudl R, Eggeling L. 2009. A periplasmic, pyridoxal-5′-phosphate-dependent amino acid racemase in Pseudomonas taetrolens. Appl Microbiol Biotechnol 83: 1045–1054. [DOI] [PubMed] [Google Scholar]
- 23.Kato S, Oikawa T. 2018. A novel bifunctional amino acid racemase with multiple substrate specificity, MalY from Lactobacillus sakei LT-13: genome-based identification and enzymological characterization. Front Microbiol 9: 403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Toda M, Okubo S, Ikigai H, Shimamura T. 1990. [Antibacterial and anti-hemolysin activities of tea catechins and their structural relatives]. Jpn J Bacteriol 45: 561–566 (in Japanese). [DOI] [PubMed] [Google Scholar]