Abstract
The quality characteristics of pineapple slices coated with emulsions enriched with pomegranate seed oil (PSO) and grape seed oil (GSO) by electrospray coating (ESC) and dip-coating (DC) methods were investigated. The ESC method was evaluated as an alternative to conventional DC. Pineapple slices were stored in clear polystyrene cups for seven days at 5 °C and 80% RH. The weight loss (%), pH, titratable acidity, color, firmness, total antioxidant activity (TAA), total phenolic content (TPC), microbiological, and sensory qualities of fresh-cut pineapple slices were evaluated. Coated samples had significantly lower weight loss values than the non-coated samples after 7 days of storage. The usage of GSO-enriched emulsion with the ESC method was found to be more successful in preserving the titratable acidity. Although all the samples exhibited a significant decrease in yellowness (b*), the electrospray-coated pineapple slices had the highest. Incorporating GSO into the emulsions helped protect the tissue of the fresh-cut pineapples, regardless of the coating method used. The TPC and TAA values of the samples coated by the ESC method with emulsions enriched with PSO showed a lower decrease compared to other treatments. It was determined that the ESC method was more successful in preserving the sensory qualities of fresh-cut pineapples. These findings suggested that using ESC as a coating method with EO-enriched emulsions has positive effects on the quality features of fresh-cut pineapples.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13197-023-05839-4.
Keywords: Electrospraying, Fresh-cut fruit, Coating, Water-in-oil (w/o) emulsion
Introduction
Pineapple, a non-climacteric fruit, has a significant amount of minerals, fibers, and antioxidants (Treviño-Garza et al. 2017; Hossain and Rahman 2011). It is a popular fruit because of its unique taste and flavor. Fresh-cut pineapple is preferred by consumers as they are easier to eat than whole pineapple (Prakash et al. 2020). However, texture loss, enzymatic browning, water loss, microbial spoilage, and decreased nutritional quality occur during the storage of fresh-cut (minimally processed) fruits (Kumar et al. 2021a). Since fresh-cut fruits do not have the same shelf life as whole fruits (Mendonça et al. 2020), numerous studies on technologies like controlled and modified atmosphere packaging, chemical treatments, and edible coatings (Gardesh et al. 2016) have been investigated.
Edible coatings help to create sufficient barriers that protect fruits from their surroundings, resulting in a slowdown of water loss, which helps to protect textural properties, flavor, and appearance as well as a decrease in gas exchange between the fruits and their environments, and inhibits the loss of natural volatile flavor compounds. They can also carry functional ingredients that may decrease microbial loads, and improve characteristics (Pirozzi et al. 2021a). Furthermore, edible coatings are less invasive, help to limit the use of plastic packaging, and allow for secondary packaging simplicity (Pirozzi et al. 2021b). Thus, edible coatings appear to be worth considering to increase the shelf life of these products. Edible coating techniques mainly include spraying, dipping, drum coating, spray-fluidization, pan coating, and falling-film (Debeaufort and Voilley 2009; Andrade et al. 2012). One of the most common methods to coat fruits, vegetables, and meat products is by immersion of food samples in a coating-forming dispersion, which involves three steps: immersion and dwelling, deposition, and evaporation of solvents (Tavassoli-Kafrani et al. 2016). This process creates a thin, film-like membrane over the surface of the product. In this process, the thickness of the created film-like membrane depends on the properties such as density, viscosity, and surface tension of the coating solution (Skurtys et al. 2010). Electrospraying is a non-residue, energy-saving, and coating material-saving non-thermal technology that is suitable for industrial production in the liquid coating of foods and prevents material waste by providing fine droplets and uniform distribution on the surface of the coated materials (Dhiman et al. 2021). During electrospraying, the coating material becomes charged as it passes through a nozzle connected to a high-voltage supply. These charges overcome the surface tension of the liquid, leading to the formation of micro-droplets (Jaworek 2007). The charged micro-droplets repel each other and create a cloud of liquid in the air, with coalescence eliminated by the charging of the droplets to ensure uniform sizes. These charged droplets are then attracted toward a grounded surface and release their charge after deposition (Wilhelm et al. 2003). The efficiency of coating and film thickness in electrospraying is influenced by the size of the droplets and how they are deposited on the surface where various factors, such as the applied voltage, flow rate, distance between the needle and the grounded collector, as well as the properties of the coating material, including surface tension, density, viscosity, electrical conductivity, and contact angle, influence the size of the droplets and how the coating material is deposited (Khan et al. 2017).
Proteins, polysaccharides, lipids, or composites are structural materials for edible coatings. In emulsion edible coatings, lipids have a moisture barrier function, while polysaccharides and proteins have mechanical properties improving and oxygen permeability reducing functions (Prakash et al. 2020). Different lipids (fats and oils), bioactive compounds, and stabilizers are used in the film-forming solutions to create emulsion-based structures and stabilize emulsions (Galus and Kadzińska 2015). Maltodextrin and whey proteins are used as stabilizers in emulsions (Nurhadi et al. 2016).
Essential oils (EOs) are widely used in edible coatings as a bioactive component. EOs are defined as oily aromatic liquids extracted from different parts of plants (Van de Braak and Leijten 1999; Burt 2004). The presence of tocopherols, carotenoids, polyunsaturated fatty acids, flavonoids, and polyphenolic compounds in seed oils (Al-Sabahi et al. 2017); led to the evaluation of the seed oils like pomegranate and grape seed, which are by-products (Passos et al. 2010; Liu et al. 2012). Grape seed is a by-product of juice and wine production and it contains 10–16% of oil (Tangolar et al. 2009). Grape seed oil (GSO) contains bioactive compounds such as phytosterols, flavonoids, and phenolic acids, as well as natural antioxidants namely tocopherol and tocotrienol, which are a rich source of vitamin E in high quantities (Fernandes et al. 2013; Maier et al. 2009; Bail et al. 2008). The presence of these bioactive compounds has been linked to a decrease in the occurrence of various diseases including cardiovascular disease, cancer, hypertension, autoimmune disorders, and arteriosclerosis (Matthaus 2008; Shinagawa et al. 2015). Because of its potential health benefits, it is favored for consumption (Dabetic et al. 2020).
The pomegranate seed oil (PSO) is extracted from the pomegranate seeds, which are the primary by-product of juice production (Fazaeli et al. 2013). This oil is rich in unsaturated fatty acids, especially oleic acid, linoleic acid, and conjugated linolenic acid which is known as punicic acid (Al-Juhaimi et al. 2017) along with phenolic compounds, flavonoids, anthocyanins, and tannins (de Melo et al. 2016). PSO has become a focus of interest as a functional ingredient because of its compounds (Siraj et al. 2019; Pamisetty et al. 2020) which were associated with several potential health benefits (Paul et al. 2020).
Previous studies have used EOs or their components, such as cinnamon essential oil (Basaglia et al. 2021), cinnamaldehyde (Mantilla et al. 2013), citral (Prakash et al. 2020), lemongrass essential oil (Azarakhsh et al. 2014) in the edible coatings of fresh-cut pineapples and examined their quality characteristics. However, to the best of our knowledge, there has been no previous study on electrospray-coated pineapples with seed oil-enriched emulsions. The study aimed to evaluate the effectiveness of ESC as a coating method for fresh-cut pineapples, using water-in-oil emulsions with GSO/PSO seed oils as enrichment bioactive constituents. The study aimed to investigate the effects of various factors, including the coating method, essential oil, water phase, and storage time, on the physicochemical, textural, microbiological, and sensory characteristics of fresh-cut pineapple slices.
Materials and methods
Materials
Refined olive oil (TARİŞ, Izmir), polyglycerol polyrycinolate (PGPR; Esterchem, UK; Elvan Gıda San. ve Tic. A.Ş., Istanbul), PSO and GSO (Zade Vital, Helvacızade Gıda İlaç Kimya San. Tic. A.Ş., Konya), whey protein isolates (WPIs; Hardline Nutrition, Hipro Isowhey, İstanbul), and DE 6–8 MD (Paselli™ MD6, Avebe Nişasta Ltd. Şti., Izmir) were used in the production of the coating emulsions.
Golden sweet pineapples (Ananas comosus (L.) Merr.) (imported crop from Costa Rica) were purchased from a market in Izmir and were stored at 5–6 °C for a maximum of 24 h until the application of the slicing and coating process.
Gallic acid, Folin–Ciocalteau reagent, and methanol (Merck KGaA, Germany); sodium hydroxide, 1,1-diphenyl-2-picrylhydrazyl, and 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox) standard (Sigma-Aldrich, Germany); and sodium bicarbonate (Horasan Kimya, Turkey) were obtained from local suppliers.
Preparation of edible coating formulations
The emulsions, the formulations of which are provided in Table 1, were homogenized (IKA, T25 Ultra-Turrax, Germany). The oil and water phases, PGPR ratios, and emulsification parameters were determined based on preliminary studies. The emulsions were prepared using olive oil, distilled water, PGPR, MD, WPI, PSO, and GSO. All the emulsions contained an 80% oil phase (78% olive oil and 2% PSO/GSO), a 20% water phase, and a PGPR of 5% (w/w) of the total oil and water phase. The water phase consisted of MD, WPI, or MD and WPI (50:50 w/w), and they accounted for 16% (w/w) of the water phase, except for the control GSO/PSO. Dry materials were added to the distilled water, and the constituents of the water phase were mixed with a magnetic stirrer. Thereafter, olive oil, PGPR, PSO, and GSO were weighed into 50 mL centrifuge tubes. The oil and water phases and PGPR were mixed using the Ultra-Turrax at 8,000 rpm for 5 min.
Table 1.
Voltages applied in electrospraying and emulsion compositionsa of coating methods
| Emulsion | Voltage (kV)b | Olive oil (%, w/w) | EO (%, w/w) | EO type | MD (% of water, w/w) | WPI (% of water, w/w) | MD + WPI (% of water, w/w) |
|---|---|---|---|---|---|---|---|
| Control-0 | 11 | 80 | 0 | – | – | – | – |
| Control-MD | 12 | 80 | 0 | – | 16 | – | – |
| Control-WPI | 12 | 80 | 0 | – | – | 16 | – |
| Control-MW | 13 | 80 | 0 | – | – | – | 16 |
| GSO-0 | 10 | 78 | 2 | GSO | – | – | – |
| GSO-MD | 11 | 78 | 2 | GSO | 16 | – | – |
| GSO-WPI | 13 | 78 | 2 | GSO | – | 16 | – |
| GSO-MW | 12 | 78 | 2 | GSO | – | – | 16 |
| PSO-0 | 11 | 78 | 2 | PSO | – | – | – |
| PSO-MD | 12 | 78 | 2 | PSO | 16 | – | – |
| PSO-WPI | 12 | 78 | 2 | PSO | – | 16 | – |
| PSO-MW | 12 | 78 | 2 | PSO | – | – | 16 |
aWeight percentages (w/w) in emulsion formulations are calculated over 30 g. The total weight of the emulsions with 5% PGPR is 31.5 g
b3.5 mL/h flow rate, 14 cm tip to collector distance, 25 s operation time
EO Essential oil, GSO and PSO Grape and pomegranate seed oil, MD Maltodextrin, WPI Whey protein isolate, MW Mixture of maltodextrin and whey protein isolate (50:50)
Coating of fresh-cut pineapples
Pineapples having green leaves and outer shells of green-yellow color distribution were chosen for this study. The pineapples were washed with tap water, then kept in a 100 ppm sodium hypochlorite (NaOCl) solution for 5 min to minimize the microbial load on the fruits. Water was taken with a paper towel, and then pineapples were sliced into one-quarter of the ring with a thickness of 8.6 ± 0.5 cm.
In this study, the same ESC vertical equipment design consisting of a single nozzle and ESC process steps described in Cakmak et al. (2018) was used with minor modifications. The electrospraying was applied on each pineapple slice for 25 s with a 3.5 mL/h feeding flow and at a 14 cm collector–needle tip distance. The voltages applied according to the Taylor cone-jet principle in the coating process are shown in Table 1.
The DC process was conducted in sterile transparent PET trays for 20 s. The pineapples were dipped into the emulsion in the PET trays, and the surplus emulsion was let to drip off. For storage evaluation, the coated and control samples were placed in sterile transparent PET trays and stored in a humidity chamber (HPP 750, Memmert, Germany) at 5 °C and 80% RH for 7 days.
Weight loss, pH, titratable acidity, color, and texture analyses were examined on days 0, 3, 5, and 7 of storage. TAA, TPC, microbiological, and sensory analyses were conducted on days 0 and 5 of storage.
Weight loss
The weight loss of the fruits was determined using a weighing balance with an accuracy of ± 1 mg (ATX224; Shimadzu, Japan). Supplementary Material S1 file is provided for more information.
Titratable acidity and pH
The pH of the fresh-cut pineapples was measured using a pH meter (WTW GmbH, model: 7110 Germany). Titratable acidity and pH were determined based on the study of Montero-Calderón et al. (2008). The samples were diluted at a ratio of 1:10 (pineapple slices/distilled water) and then homogenized. The results were expressed as g citric acid/100 g of sample.
Color analysis
Color changes throughout storage were monitored with a Konica Minolta spectrophotometer (CM-700D, Konica Minolta Sensing, Japan) using the CIE L*, a*, and b* scale. Each treatment had four replicates, and the slices were scanned at three different regions. The color changes were expressed as L*, and b*.
Firmness analysis
A texture analyzer (TA.XT Express, Stable Microsystems Ltd., UK) was used to evaluate the firmness of the pineapple slices during storage. Penetration tests were conducted using a 2 mm diameter needle probe at a pretest speed of 1.5 mm s−1, a test speed of 1 mm s−1, and a post-test speed of 10 mm s−1. The slices were penetrated to a depth of 4 mm. At least 18 measurements were taken from three different points of the pineapple slices, and the results were expressed in Newtons (N).
Sample extraction for determining TAA and TPC
A modified extraction method described by Diamanti (2010) was used to determine the TAA and TPC in the pineapples. Supplementary Material S1 file is provided for the extraction procedure.
Total Antioxidant Activity and Total Phenolic Content
The TAA was determined using a DPPH free radical scavenging capacity assay according to the method of Hossain and Rahman (2011) with minor modifications. The TPC was determined using a modified method described by Lutterodt et al. (2011). The exact protocols of analyses are given in Supplementary Material S1.
Microbiological analysis
Total viable aerobic mesophilic bacteria (TAMB) and yeast and mold (YM) counts were determined by manufacturer’s instructions of TEMPO® AC and TEMPO® YM. The procedure steps are described in detail in Supplementary Material S1.
Sensory analysis
A 9-point hedonic scale (Mantilla et al. 2013) was used to conduct a consumer taste test for the pineapple slices on the first, third, and fifth days after coating. A group of 25 partially trained panelists in the age group of 20–50 years old (16 female and 9 male), evaluated the sensory parameters of pineapple slices. The panelists evaluated the color, texture (hardness), juiciness (freshness), flavor, and general acceptability of the pineapple slices.
Statistical analysis
The significance of the relationship between the results of the analysis and each factor/factor interaction was examined with the Univariate General Linear Model, and the effect size was given as partial eta squared () (Supplementary Table S1.1, S1.2, S1.3, and S1.4). Additional information on the interpretation of the relationship between analysis results and factor/factor interactions is given in Supplementary Material S1. The grouping scheme of the samples is given in Supplementary Material S1, Figure S1.
The comparison of the averages of the analysis results was conducted using the ANOVA-Duncan Multiple Comparison test. The statistical differences in the results of TAA, TPC, TAMB, and YM between the first and fifth days were analyzed using the paired samples t-test. All statistical analyzes were carried out using SPSS software (SPSS Inc., Ver 20.0) with 95% confidence intervals (significant level p = 0.05). Three fruit slices made up the experimental unit, and there were at least three repetitions of each treatment.
Results and discussion
Weight loss
Cutting or peeling fruits exposes their internal tissues and weight loss occurs with the acceleration of metabolic processes (Kumar et al. 2021b).
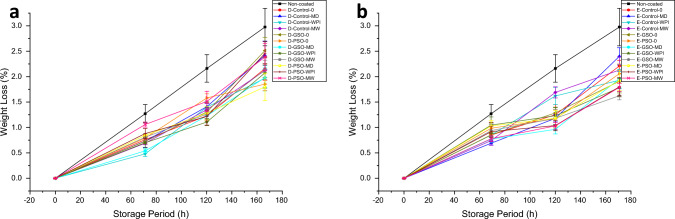
The weight loss of the fresh-cut pineapple slices throughout the storage is displayed in Fig. 1. Although no other factors exhibited a significant impact with a large effect size on weight loss, except for storage time, the essential oil, storage time, and water phase triple interaction demonstrated a substantial effect on weight loss (Supplementary Material S1.1). The constituents of water phases did not cause a significant difference in weight loss during storage (p > 0.05). Along the storage, the groups coated with the emulsion that contain the EOs had lower weight loss than the non-coated sample (Supplementary Material S2) (p < 0.05) regardless of the water phases of the emulsion. W/O emulsions with and without EOs provide effective barrier-forming properties through the coating process due to the strong moisture barrier caused by the hydrophobic characteristics of lipids (Suhag et al. 2020; Debeaufort and Voilley 2009).
Fig. 1.
Weight loss of the fresh-cut pineapple slices during storage. a Dip-coated fresh-cut pineapple slices, b Electrospray-coated fresh-cut pineapple slices
Parallel results were observed in kiwis (Manzoor et al. 2021) and strawberries (Mendonça et al. 2020) that were coated with emulsion. In a study conducted by Manzoor et al. (2021), the samples coated with emulsions had less weight loss than the control sample at the end of storage because the emulsion coatings acted as a barrier against water loss from the surface of the fruit. Mendonça et al. (2020) discovered that when strawberries were coated with w/o emulsions made with citronella essential oil and avocado oil, the control sample had a higher weight loss than the coated samples, but there were no significant changes between them (p > 0.05).
Titratable acidity and pH
The titratable acidity and pH of the samples (Table 2) were significantly influenced by the factors of essential oil and storage time with a large effect size. Although the method and the water phase did not have a large effect size, the other independent variables EO and storage time along with triple interaction between EO, storage time, and method had a significant effect on pH and TA values with a large effect size (Supplementary Material S1.1). At the end of the storage, the pH values of all slices decreased (p < 0.05) significantly. TA values increased in most of the samples after the third day of storage.
Table 2.
Titratable acidity and pH values of the fresh-cut pineapple samples during storage
| Samples | pH | TA (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| Storage time (day) | Storage time (day) | |||||||
| 0 | 3 | 5 | 7 | 0 | 3 | 5 | 7 | |
| Non-coated | 3.428 ± 0.030b,A | 3.324 ± 0.096d,e,f,A | 3.260 ± 0.016a,b,c,d,A | 3.254 ± 0.023e,f,g,A | 0.823 ± 0.017d,A | 0.859 ± 0.019f,g,A | 0.850 ± 0.008i,A | 1.055 ± 0.041 g,B |
| D-Control-0 | 3.438 ± 0.055b,C | 3.246 ± 0.013a,b,A,B | 3.237 ± 0.020a,b,c,A | 3.307 ± 0.034h,B | 0.752 ± 0.043b,c,A | 0.814 ± 0.048e,f,B | 0.727 ± 0.028d,e,A | 0.750 ± 0.016a,A |
| D-Control-MD | 3.437 ± 0.054b,C | 3.258 ± 0.003a,b,c,A | 3.332 ± 0.019c,d,e,f,B | 3.294 ± 0.029g,h,C | 0.708 ± 0.023a,b,B | 0.869 ± 0.013g,D | 0.599 ± 0.025b,A | 0.761 ± 0.016a,b,C |
| D-Control-WPI | 3.413 ± 0.076b,C | 3.238 ± 0.009a,A | 3.365 ± 0.006e,f,B,C | 3.293 ± 0.005g,h,A,B | 0.720 ± 0.038a,b,c,B | 0.880 ± 0.012g,D | 0.596 ± 0.022b,A | 0.765 ± 0.032a,C |
| D-Control-MW | 3.397 ± 0.072b,C | 3.210 ± 0.017a,A | 3.328 ± 0.023b,c,d,e,f,B,C | 3.281 ± 0.029g,h,A,B | 0.730 ± 0.029a,b,c,B | 0.845 ± 0.036e,f,g,C | 0.677 ± 0.032c,A | 0.724 ± 0.029a,B |
| D-GSO-0 | 3.308 ± 0.020a,B | 3.307 ± 0.008c,d,B | 3.219 ± 0.013a,A | 3.192 ± 0.018c,d,A | 0.824 ± 0.031d,B | 0.753 ± 0.017d,A | 0.923 ± 0.020j,C | 0.842 ± 0.021d,e,f,B |
| D-GSO-MD | 3.319 ± 0.004a,B | 3.300 ± 0.020b,c,d,B | 3.191 ± 0.011a,A | 3.213 ± 0.011c,d,e,A | 0.867 ± 0.010d,B | 0.709 ± 0.013c,d,A | 0.956 ± 0.01j,D | 0.850 ± 0.013d,e,f,B |
| D-GSO-WPI | 3.346 ± 0.035a,B | 3.335 ± 0.009d,e,f,g,B | 3.271 ± 0.005a,b,c,d,e,A | 3.250 ± 0.030e,f,g,A | 0.843 ± 0.012d,B | 0.739 ± 0.016d,A | 0.855 ± 0.016i,B | 0.831 ± 0.027c,d,e,B |
| D-GSO-MW | 3.426 ± 0.040b,C | 3.318 ± 0.010d,e,B | 3.279 ± 0.008a,b,c,d,e,B | 3.224 ± 0.011c,d,e,f,A | 0.723 ± 0.013a,b,c,A | 0.722 ± 0.018c,d,A | 0.774 ± 0.009f,g,B | 0.901 ± 0.013f,C |
| D-PSO-0 | 3.419 ± 0.009b,C | 3.459 ± 0.021i,j,D | 3.281 ± 0.008a,b,c,d,e,B | 3.233 ± 0.010d,e,f,A | 0.695 ± 0.010a,A | 0.803 ± 0.020e,C | 0.765 ± 0.001e,f,B | 0.841 ± 0.013d,e,f,D |
| D-PSO-MD | 3.409 ± 0.003b,B | 3.462 ± 0.022i,j,C | 3.218 ± 0.008a,A | 3.232 ± 0.009d,e,f,A | 0.763 ± 0.026c,A | 0.753 ± 0.018d,A | 0.840 ± 0.012 h,i,B | 0.879 ± 0.012d,e,f,C |
| D-PSO-WPI | 3.429 ± 0.002b,C | 3.485 ± 0.020j,D | 3.194 ± 0.012a,A | 3.267 ± 0.020f,g,h,B | 0.709 ± 0.024a,b,A | 0.745 ± 0.004d,B | 0.827 ± 0.005 h,i,C | 0.827 ± 0.010c,d,e,C |
| D-PSO-MW | 3.419 ± 0.012b,C | 3.481 ± 0.015j,D | 3.209 ± 0.010a,A | 3.292 ± 0.008 g,h,B | 0.730 ± 0.012a,b,c,A | 0.758 ± 0.010d,B | 0.798 ± 0.005f,g,h,C | 0.762 ± 0.020a,b,B |
| E-Control-0 | 3.428 ± 0.030b,C | 3.203 ± 0.018a,A | 3.232 ± 0.013a,b,c,A | 3.303 ± 0.014h,B | 0.823 ± 0.022d,A | 0.871 ± 0.040g,B | 0.806 ± 0.039f,g,h,i,A | 0.885 ± 0.002e,f,B |
| E-Control-MD | 3.428 ± 0.030b,C | 3.247 ± 0.014a,b,A | 3.227 ± 0.010a,b,A | 3.310 ± 0.011h,B | 0.823 ± 0.022d,A | 0.813 ± 0.019e,f,A | 0.835 ± 0.044h,i,A | 0.847 ± 0.020d,e,f,A |
| E-Control-WPI | 3.428 ± 0.030b,C | 3.239 ± 0.030a,A | 3.213 ± 0.010a,A | 3.355 ± 0.012i,B | 0.823 ± 0.022d,A | 0.816 ± 0.028e,f,A | 0.857 ± 0.037i,A | 0.818 ± 0.032b,c,d,A |
| E-Control-MW | 3.428 ± 0.030b,C | 3.234 ± 0.031a,A | 3.242 ± 0.033a,b,c,A | 3.309 ± 0.057h,B | 0.823 ± 0.022d,A,B | 0.813 ± 0.011e,f,A | 0.841 ± 0.017h,i,B | 0.869 ± 0.021d,e,f,C |
| E-GSO-0 | 3.428 ± 0.030b,D | 3.349 ± 0.010d,e,f,g,C | 3.277 ± 0.003a,b,c,d,e,B | 3.189 ± 0.012c,d,A | 0.823 ± 0.022d,B | 0.632 ± 0.027b,A | 0.834 ± 0.015 h,i,B | 1.028 ± 0.027 g,C |
| E-GSO-MD | 3.428 ± 0.030b,C | 3.373 ± 0.011e,f,g,h,B | 3.346 ± 0.008d,e,f,B | 3.182 ± 0.009b,c,A | 0.823 ± 0.022d,B | 0.566 ± 0.020a,A | 0.828 ± 0.015 h,i,B | 1.053 ± 0.032 g,C |
| E-GSO-WPI | 3.428 ± 0.030b,C | 3.372 ± 0.008e,f,g,h,B | 3.366 ± 0.008e,f,B | 3.193 ± 0.019c,d,A | 0.823 ± 0.022d,B | 0.581 ± 0.022a,A | 0.791 ± 0.035f,g,h,B | 1.153 ± 0.007 h,C |
| E-GSO-MW | 3.428 ± 0.030b,C | 3.366 ± 0.016e,f,g,h,B | 3.362 ± 0.019d,e,f,B | 3.218 ± 0.022c,d,e,A | 0.823 ± 0.022d,B | 0.572 ± 0.020a,A | 0.820 ± 0.029 g,h,I,B | 1.133 ± 0.042 h,C |
| E-PSO-0 | 3.428 ± 0.030b,C | 3.386 ± 0.010 g,h,B | 3.359 ± 0.008d,e,f,B | 3.117 ± 0.021a,A | 0.823 ± 0.022d,C | 0.740 ± 0.010d,B | 0.681 ± 0.024c,A | 0.846 ± 0.017d,e,f,C |
| E-PSO-MD | 3.428 ± 0.030b,C | 3.442 ± 0.018i,j,C | 3.388 ± 0.006f,g,B | 3.139 ± 0.009a,b,A | 0.823 ± 0.022d,B | 0.677 ± 0.026c,A | 0.690 ± 0.012c,d,A | 0.865 ± 0.036d,e,f,C |
| E-PSO-WPI | 3.428 ± 0.030b,B | 3.381 ± 0.011f,g,h,B | 3.567 ± 0.185 h,B | 3.134 ± 0.006a,A | 0.823 ± 0.022d,D | 0.730 ± 0.020d,B | 0.634 ± 0.010b,A | 0.775 ± 0.030a,b,c,C |
| E-PSO-MW | 3.428 ± 0.030b,B,C | 3.420 ± 0.001 h,I,B | 3.468 ± 0.011 g,C | 3.144 ± 0.035a,b,A | 0.823 ± 0.022d,C | 0.752 ± 0.021d,B | 0.520 ± 0.022a,A | 0.745 ± 0.031a,B |
a–lDifferent letters in the same column are statistically different (p < 0.05)
A–DDifferent letters in the same row are statistically different (p < 0.05)
Non-coated: Untreated sample, D: Dip-coating, E: Electrospray coating, Control: Essential oil-free, GSO and PSO: Grape and Pomegranate Seed Oil, 0: MD/WPI/MW-free emulsion in water phase, MD: Maltodextrin, WPI: Whey Protein Isolate, MW: Mixture of Maltodextrin and Whey Protein Isolate
Medeiros Teodosio et al. (2021) who worked with Spondias tuberosa fruit determined a similar ascending trend to the present study for titratable acidity results. In the aforementioned study, Chlorella sp. and PSO were used as edible coating material, and the increase in TA values was associated with the accumulation of galacturonic acid, which is released during the hydrolysis of cell wall components.
The titratable acidity values of the fresh-cut pineapple slices slightly increased along the storage. The samples with no coating showed the highest titratable acidity value while those coated with the DC method without EOs showed the lowest value. The samples treated with the DC method had lower titratable acidity values than those treated with ESC, which indicates that the DC method was more effective in slowing down the respiratory activity of the fresh-cut pineapples, thereby preserving the organic acids in the fruit (de Morais et al. 2010). This efficiency may have been due to the creation of a thicker film-like membrane on the surface during the dip-coating process compared to the ESC. The use of EO enrichment had an impact when combined with the ESC method. Using the ESC method with PSO-enriched emulsion was found to be more effective in maintaining the titratable acidity values than using the ESC method with GSO-enriched emulsions.
Color
Color, one of the coating optical properties, is an important quality parameter for consumer acceptance of coated food products and antioxidants that delay oxidation and discoloration (Pirozzi et al. 2021b).
Table 3 shows the L*and b* values of all the coated and non-coated fresh-cut pineapples during 7 days of storage. It was found that the essential oil, method interaction showed a large effect size on the L* values (Supplementary Material S1.1) where the samples containing the same essential oil showed different L* values when coated with DC and ESC methods (p < 0.05). At the end of storage, DC samples without EO and with GSO showed the lowest L* values, while ESC samples with GSO showed the highest L* values (p < 0.05). Although the change in the L* values of the coated and control samples was significant (p < 0.05), no distinctive trend was observed in the samples. The maturation mechanism of pineapples varies from the fleshy tissue of the fruit to the crown. Such a complex fruit anatomy and maturation structure makes it difficult to detect a uniform distribution in the color and texture values (Paull and Chen 2014; Montero-Calderón et al. 2008).
Table 3.
Color results of the fresh-cut pineapple samples during storage
| Samples | L* | b* | ||||||
|---|---|---|---|---|---|---|---|---|
| Storage time (day) | Storage time (day) | |||||||
| 0 | 3 | 5 | 7 | 0 | 3 | 5 | 7 | |
| Control | 73.51 ± 2.06a,b,c,A | 75.04 ± 2.88j,k,B | 75.13 ± 1.66 m,B | 73.52 ± 2.03d,ef,g,h,I,A | 31.35 ± 1.60d,C | 27.11 ± 4.03d,e,A | 29.21 ± 2.01g,h,i,B | 28.49 ± 2.55m,B |
| D-Control-0 | 73.26 ± 2.57a,b,C | 71.83 ± 4.79c,d,e,B | 70.21 ± 2.67b,c,A | 73.82 ± 1.91e,f,g,h,i,C | 32.81 ± 2.91e,C | 21.41 ± 1.57a,A | 25.94 ± 2.27c,d,B | 26.22 ± 1.17h,i,B |
| D-Control-MD | 73.92 ± 1.92a,b,c,d,C | 73.44 ± 4.13f,g,h,C | 70.40 ± 4.78b,c,d,A | 72.26 ± 2.69b,B | 27.65 ± 1.57a,D | 21.87 ± 2.07a,A | 23.80 ± 2.90a,B | 25.53 ± 1.74h,C |
| D-Control-WPI | 74.91 ± 2.41e,f,C | 73.71 ± 2.95g,h,i,B | 72.86 ± 4.27h,i,j,B | 70.52 ± 2.16a,A | 29.94 ± 3.07c,D | 22.92 ± 2.69b,A | 24.78 ± 2.71b,B | 26.80 ± 1.65i,j,k,C |
| D-Control-MW | 74.15 ± 2.00b,c,d,e,B | 74.66 ± 2.07i,j,B | 71.46 ± 3.72d,e,f,g,A | 74.09 ± 3.82e,f,g,h,i,B | 28.93 ± 0.95b,D | 21.56 ± 2.81a,A | 23.47 ± 2.71a,B | 26.26 ± 1.44h,i,C |
| D-GSO-0 | 72.99 ± 1.86a,B | 70.37 ± 1.43b,A | 70.62 ± 1.96c,d,e,A | 70.99 ± 3.47a,A | 31.20 ± 1.49d,C | 29.49 ± 1.44i,j,B | 30.35 ± 1.63j,k,B | 22.50 ± 2.74e,A |
| D-GSO-MD | 73.48 ± 1.21a,b,c,B | 71.33 ± 1.92b,c,d,A | 72.05 ± 2.63f,g,h,A | 73.59 ± 2.43e,f,g,h,i,B | 30.77 ± 1.62c,d,C | 29.91 ± 1.79i,j,B,C | 29.30 ± 2.48h,i,B | 17.89 ± 1.32a,A |
| D-GSO-WPI | 73.27 ± 0.93a,b,B | 72.08 ± 1.61d,e,A | 71.51 ± 1.75e,f,g,A | 74.38 ± 2.36 g,h,i,C | 31.78 ± 1.84d,D | 27.45 ± 1.26e,f,B | 28.93 ± 1.71g,h,C | 20.11 ± 1.34c,A |
| D-GSO-MW | 74.43 ± 1.50c,d,e,C | 70.87 ± 1.34b,c,A | 71.99 ± 1.81f,g,h,B | 74.11 ± 3.43e,f,g,h,C | 31.20 ± 1.87d,C | 28.40 ± 1.89g,h,B | 28.90 ± 1.81g,h,B | 18.82 ± 1.25b,A |
| D-PSO-0 | 75.33 ± 1.61f,B | 77.21 ± 1.64 m,C | 73.59 ± 2.92j,k,A | 73.45 ± 1.54d,e,f,g,h,A | 28.94 ± 1.48b,C | 24.04 ± 1.22c,A | 26.48 ± 1.72d,e,B | 23.83 ± 1.30f,g,A |
| D-PSO-MD | 74.57 ± 1.25d,e,f,B | 78.47 ± 1.07n,C | 73.17 ± 2.85i,j,k,A | 74.02 ± 1.38e,f,g,h,i,A,B | 27.93 ± 1.08a,b,C | 21.10 ± 0.92a,A | 25.74 ± 0.92c,B | 21.12 ± 1.20d,A |
| D-PSO-WPI | 74.13 ± 0.88b,c,d,e,A | 76.33 ± 1.58 l,m,C | 74.95 ± 2.33 l,m,B | 75.85 ± 0.58 k,C | 27.66 ± 1.11a,D | 21.41 ± 1.72a,B | 26.76 ± 0.27e,f,C | 19.74 ± 1.15c,A |
| D-PSO-MW | 73.53 ± 1.27a,b,c,A | 75.66 ± 1.14 k,l,C | 75.06 ± 1.75 l,m,B,C | 74.60 ± 0.82i,j,B | 28.35 ± 1.71a,b,C | 21.97 ± 1.26a,A | 27.42 ± 0.23f,B | 21.65 ± 1.02d,A |
| E-Control-0 | 73.51 ± 2.06a,b,c,C | 70.37 ± 5.11b,A | 72.22 ± 2.39g,h,i,B | 74.01 ± 2.07e,f,g,h,i,C | 31.35 ± 1.60d,C | 31.78 ± 2.44k,C | 30.40 ± 1.64j,k,B | 27.62 ± 1.72k,l,A |
| E-Control-MD | 73.51 ± 2.06a,b,c,B | 69.15 ± 3.01a,A | 73.66 ± 2.68j,k,B | 74.29 ± 2.32f,g,h,i,B | 31.35 ± 1.60d,C | 33.65 ± 3.10l,D | 29.90 ± 2.57i,j,B | 27.19 ± 2.31j,k,A |
| E-Control-WPI | 73.51 ± 2.06a,b,c,B | 68.99 ± 2.78a,A | 74.08 ± 2.68k,l,B | 73.22 ± 2.81b,c,d,e,f,B | 31.35 ± 1.60d,C | 31.63 ± 4.64k,C | 29.49 ± 1.81h,i,B | 27.58 ± 2.12k,l,A |
| E-Control-MW | 73.51 ± 2.06a,b,c,C | 68.62 ± 3.44a,A | 70.31 ± 3.39b,c,B | 73.10 ± 1.73b,c,d,e,C | 31.35 ± 1.60d,C | 32.18 ± 2.16k,C | 31.59 ± 1.42l,B,C | 28.18 ± 1.98l,m,A |
| E-GSO-0 | 73.51 ± 2.06a,b,c,A | 73.57 ± 1.13 g,h,A | 73.81 ± 1.16j,k,A | 75.87 ± 0.67 k,B | 31.35 ± 1.60d,D | 26.76 ± 1.22d,e,B | 29.19 ± 0.83g,h,i,C | 23.31 ± 0.95f,A |
| E-GSO-MD | 73.51 ± 2.06a,b,c,A | 73.38 ± 1.39f,g,h,A | 73.48 ± 2.08j,k,A | 75.79 ± 1.74 k,B | 31.35 ± 1.60d,C | 27.53 ± 1.53e,f,g,A | 28.44 ± 1.15g,B | 27.44 ± 1.94j,k,l,A |
| E-GSO-WPI | 73.51 ± 2.06a,b,c,B | 73.54 ± 1.46 g,h,B | 72.16 ± 1.80 g,h,I,A | 75.51 ± 0.48j,k,C | 31.35 ± 1.60d,D | 26.27 ± 1.31d,A | 28.50 ± 1.87g,C | 27.44 ± 1.9j,k,l,B |
| E-GSO-MW | 73.51 ± 2.06a,b,c,C | 72.05 ± 1.26d,B | 70.98 ± 2.08c,d,e,A | 75.80 ± 0.41 k,D | 31.35 ± 1.60d,C | 29.06 ± 1.64h,i,B | 30.78 ± 1.58k,B | 24.28 ± 1.66g,A |
| E-PSO-0 | 73.51 ± 2.06a,b,c,B | 73.86 ± 1.47 h,I,B | 70.92 ± 0.22c,d,e,A | 73.33 ± 2.33c,d,e,f,g,B | 31.35 ± 1.60d,B | 28.13 ± 1.27f,g,h,A | 30.75 ± 1.06,k,B | 27.54 ± 1.72k,l,A |
| E-PSO-MD | 73.51 ± 2.06a,b,c,C | 72.49 ± 1.30e,f,B | 70.07 ± 1.89b,c,A | 72.38 ± 1.13b,c,B | 31.35 ± 1.60d,C | 30.05 ± 1.02j,B | 30.85 ± 1.44k,C | 26.58 ± 2.31i,j,A |
| E-PSO-WPI | 73.51 ± 2.06a,b,c,B | 72.70 ± 1.33e,f,g,B | 69.46 ± 0.61a,b,A | 72.48 ± 2.94b,c,d,B | 31.35 ± 1.60d,C | 29.05 ± 2.01h,i,B | 30.77 ± 1.48k,C | 27.42 ± 1.92j,k,l,A |
| E-PSO-MW | 73.51 ± 2.06a,b,c,B | 75.64 ± 0.90 k,l,D | 69.03 ± 2.68a,A | 74.48 ± 0.96 h,I,C | 31.35 ± 1.60d,C | 26.46 ± 1.02d,A | 31.11 ± 0.86k,l,C | 27.42 ± 2.01j,k,l,B |
a–lDifferent letters in the same column are statistically different (p < 0.05)
A–DDifferent letters in the same row are statistically different (p < 0.05)
Non-coated: Uncoated sample, D: Dip-coating, E: Electrospray coating, Control: Essential oil-free, GSO and PSO: Grape and Pomegranate Seed Oil, 0: MD/WPI/MW-free emulsion in water phase, MD: Maltodextrin, WPI: Whey Protein Isolate, MW: Mixture of Maltodextrin and Whey Protein Isolate
When the b* values of all the samples were examined, the samples were found to exhibit a decrease in b* values during storage. It was thought that one of the reasons for the decrease in b* values was the translucent mechanism of the pineapple fruit flesh (Xu et al. 2022). Similar results were found by Treviño-Garza et al. (2017) and Prakash et al. (2020), who applied edible coatings to fresh-cut pineapples, and emphasized that b* values decrease during storage.
The essential oil and water phase did not show a significant impact with a large effect size while the method and storage time showed a significant impact large effect size on b* values (p < 0.05). It was also determined that the triple interaction of essential oil, storage time, and method showed a large effect on b* values (Supplementary Material S1.1). At the end of storage, there were significant differences between the b* values of DC and ESC samples containing the same EO. Considering the same essential oil groups, the b* values of ESC-treated samples were higher than DC-treated samples. This delayed change in the b* values was thought to be caused by the smaller droplet sizes ensuring homogeneous coating (Oh et al. 2017). In our study, ESC was more homogeneous than DC and had no uncoated area deficiency. Thus, ESC can be claimed to improve the color properties of fresh-cut pineapple slices by providing small particle sizes and ensuring a homogeneous coating.
Firmness
The firmness of the samples showed an increase until the third day of storage, then the firmness of all slices, except D-GSO, decreased (Table 4). All factors, except for the water phase, had a significant impact with the large effect sizes (p < 0.05). Additionally, the triple interaction of essential oil, storage time, and method also had a substantial effect on firmness values (Supplementary Material S1.1). The formation of a hard layer-like structure on the fruit surfaces after drying upon the first weight loss caused an increase in firmness values on the third day. The degradation of the components that ensure the structural hardness and integrity of fruits (particularly insoluble pectin and protopectin) and the decrease in turgor pressure might explain the tissue softening and firmness diminution of the pineapple slices detected after the third day of storage (Kumar et al. 2018; Basaglia et al. 2021). Tissue softening of pineapple slices during storage was detected by Basaglia et al. (2021), who used chitosan−based edible coating with the incorporation of cinnamon essential oil, as well as Treviño-Garza et al. (2017), who coated pineapples with an edible coating based on chitosan, pullulan, linseed, nopal cactus, and aloe mucilage.
Table 4.
Firmness values of the fresh-cut pineapple samples during storage
| Samples | Firmness (N) | |||
|---|---|---|---|---|
| Storage time (day) | ||||
| 0 | 3 | 5 | 7 | |
| Non-coated | 1.806 ± 0.438b,A | 2.149 ± 0.387d,B | 1.852 ± 0.072d,e,f,A | 1.895 ± 0.323d,e,A |
| D-Control-0 | 1.773 ± 0.403b,A | 2.560 ± 0.567 g,h,i,C | 2.009 ± 0.356 g,h,B | 1.950 ± 0.333e,B |
| D-Control-MD | 1.785 ± 0.388b,A | 2.518 ± 0.412f,g,h,B | 1.912 ± 0.334f,A | 1.885 ± 0.452c,d,e,A |
| D-Control-WPI | 1.712 ± 0.391b,A,B | 2.442 ± 0.424e,f,C | 1.806 ± 0.365c,d,B | 1.602 ± 0.305b,A |
| D-Control-MW | 1.802 ± 0.384b,A | 2.411 ± 0.510e,B | 1.827 ± 0.369d,e,A | 1.896 ± 0.364d,e,A |
| D-GSO-0 | 1.787 ± 0.175b,A | 2.047 ± 0.244c,B | 2.256 ± 0.170j,C | 2.401 ± 0.317 g,D |
| D-GSO-MD | 1.548 ± 0.164a,A | 1.960 ± 0.206c,B | 2.205 ± 0.090j,C | 2.739 ± 0.360 k,D |
| D-GSO-WPI | 1.504 ± 0.103a,A | 2.161 ± 0.212d,B | 2.366 ± 0.156 k,C | 3.154 ± 0.344 l,D |
| D-GSO-MW | 1.424 ± 0.107a,A | 2.049 ± 0.203c,B | 2.416 ± 0.069 k,l,C | 3.106 ± 0.405 l,D |
| D-PSO-0 | 1.711 ± 0.194b,A | 3.125 ± 0.172 l,m,B | 2.099 ± 0.251i,B | 1.802 ± 0.222c,A |
| D-PSO-MD | 1.843 ± 0.225b,A | 3.756 ± 0.302n,D | 2.053 ± 0.081 h,i,B | 2.599 ± 0.308i,j,C |
| D-PSO-WPI | 1.993 ± 0.198c,A | 3.148 ± 0.346 m,C | 1.980 ± 0.047 g,A | 2.660 ± 0.246j,k,B |
| D-PSO-MW | 1.752 ± 0.209b,A | 3.047 ± 0.323 l,C | 2.443 ± 0.168 l,B | 2.421 ± 0.234 g,B |
| E-Control-0 | 1.806 ± 0.438b,C | 1.422 ± 0.29a,A | 1.657 ± 0.361b,B,C | 1.604 ± 0.376b,B |
| E-Control-MD | 1.806 ± 0.438b,B | 1.567 ± 0.394b,A | 1.756 ± 0.323c,B | 1.506 ± 0.263a,A |
| E-Control-WPI | 1.806 ± 0.438b,B | 1.604 ± 0.376b,A | 1.644 ± 0.309b,A,B | 1.672 ± 0.266b,A,B |
| E-Control-MW | 1.806 ± 0.438b,B,C | 1.441 ± 0.318a,A | 1.855 ± 0.323d,e,f,C | 1.687 ± 0.270b,B |
| E-GSO-0 | 1.806 ± 0.438b,A | 2.672 ± 0.354j,C | 2.226 ± 0.098j,B | 2.275 ± 0.252f,B |
| E-GSO-MD | 1.806 ± 0.438b,A | 2.820 ± 0.338 k,C | 1.909 ± 0.267f,A | 2.614 ± 0.134i,j,B |
| E-GSO-WPI | 1.806 ± 0.438b,A | 2.640 ± 0.204i,j,B | 1.850 ± 0.204d,e,f,A | 2.537 ± 0.130 h,i,B |
| E-GSO-MW | 1.806 ± 0.438b,B | 2.607 ± 0.284 h,i,j,C | 1.542 ± 0.128a,A | 2.473 ± 0.163 g,h,C |
| E-PSO-0 | 1.806 ± 0.438b,A | 2.490 ± 0.273e,f,g,B | 1.883 ± 0.195e,f,A | 1.840 ± 0.146c,A |
| E-PSO-MD | 1.806 ± 0.438b,A | 2.690 ± 0.201j,B | 1.676 ± 0.115b,A | 1.654 ± 0.133b,A |
| E-PSO-WPI | 1.806 ± 0.438b,A | 2.625 ± 0.338i,j,B | 1.883 ± 0.245e,f,A | 1.795 ± 0.154c,A |
| E-PSO-MW | 1.806 ± 0.438b,B | 2.873 ± 0.236 k,C | 1.976 ± 0.239 g,B | 1.662 ± 0.159b,A |
a–jDifferent letters in the same column are statistically different (p < 0.05)
A–DDifferent letters in the same row are statistically different (p < 0.05)
Non-coated: Untreated sample, D: Dip-coating, E:Electrospray coating, Control: Essential oil-free, GSO and PSO: Grape and Pomegranate Seed Oil, 0: MD/WPI/MW-free emulsion in water phase, MD: Maltodextrin, WPI: Whey Protein Isolate, MW: Mixture of Maltodextrin and Whey Protein Isolate
The pineapple slices that were coated with GSO emulsions displayed less tissue deterioration during storage than the samples coated using Control and PSO emulsions regardless of the method. It was thought that the GSO might cause to increase in the elasticity of the samples and a decrease in their brittleness, which led to an increase in the firmness (Jokar et al. 2021). The ESC method has been shown to confer comparable protective effects on firmness using significantly less coating material.
Total antioxidant activity and total phenolic content
Phenolic components are antioxidants with a high free radical scavenging activity, and pineapple fruits are a prominent source of these components (Kumar et al. 2021a; Mohd Ali et al. 2020).
TAA results of the pineapple slices are shown in Table 5. It was observed that the use of different EOs did not have a significant impact with a large effect size on TAA and TPC results. Among the factors, the method and storage time showed large effects size on TAA and TPC values (Supplementary Material S1.3). TPC values of all samples decreased at the end of storage (Table 5). Although there were no significant differences between the TPC in the non-coated and electrosprayed pineapple slices at the beginning of storage, the non-coated slices had a lower TPC than the electrosprayed pineapple slices at the end of storage (p < 0.05). TPC values of D-Control-GSO and D-Control-PSO samples were higher than non-coated and ESC pineapple slices. Since the coating material amount used in the DC method is more than in the ESC method, higher TPC values of the dip-coated samples were obtained. The storage time did not affect the TPC of the samples with electrosprayed GSO and PSO (p > 0.05). In other words, the TPC in the pineapple slices coated by the ESC method was maintained throughout storage. Kumar et al. (2021b) indicated that the phenolic content of coated pineapple samples containing carboxymethyl cellulose and ascorbic acid was better preserved than that of the uncoated samples.
Table 5.
Changes in the total antioxidant activity and total phenolic content of the fresh cut pineapple samples during storage
| Storage time (days) | Samples | Total antioxidant activity* (µmol TEAC/g sample) | Total phenolic content** (mg GAE/kg sample) |
|---|---|---|---|
| Non-coated | 5.66 ± 0.17a,A | 513.61 ± 3.14a,B | |
| 0 | D-GSO-0 | 6.76 ± 0.31b,B | 781.61 ± 1.89c,B |
| D-PSO-0 | 5.80 ± 0.13a,B | 678.76 ± 34.74b,B | |
| E-GSO-0 | 6.76 ± 0.35b,B | 510.58 ± 40.29a,A | |
| E-PSO-0 | 6.37 ± 0.12b,A | 534.06 ± 28.66a,A |
| Non-coated | 5.88 ± 0.11b,A | 402.92 ± 9.44a,A | |
| 5 | D-GSO-0 | 4.54 ± 0.17a,A | 598.24 ± 22.74c,A |
| D-PSO-0 | 4.80 ± 0.25a,A | 619.51 ± 36.91c,A | |
| E-GSO-0 | 4.60 ± 0.05a,A | 534.96 ± 31.11b,A | |
| E-PSO-0 | 6.01 ± 0.22b,A | 525.11 ± 40.09b,A |
*Total antioxidant activity was determined using the DPPH method and expressed as trolox equivalent antioxidant capacity (TEAC) equivalent
**Total phenolic content was expressed as gallic acid equivalent (GAE)
a–cDifferent letters indicate a statistical difference between samples on the same storage day (p < 0.05) (Duncan)
A–BDifferent letters indicate a statistical difference between storage days of the same sample (p < 0.05) (paired samples t-test)
Non-coated: Untreated sample, D: Dip-coating, E:Electrospray coating, Control: Essential oil-free, GSO and PSO: Grape and Pomegranate Seed Oil, 0: MD/WPI/MW-free emulsion in water phase, MD: Maltodextrin, WPI: Whey Protein Isolate, MW: Mixture of Maltodextrin and Whey Protein Isolate
At the end of storage, the non-coated sample and the sample coated by the ESC method with PSO had higher TAAs than the other samples (p < 0.05). But only the PSO sample coated by the ESC method retained both TPC and TAA values during storage. Manzoor et al. (2021), who coated kiwi slices with emulsions based on alginate, carboxymethylcellulose, and ascorbic acid, discovered that the coated slices exhibited a slower decrease in TAA and better TAA protection than the uncoated slices at the end of storage.
Microbiological analysis
Fruits have unique protective skin that plays a significant role in their shelf life by protecting them against most pathogens and spoilage microorganisms. This protective skin cannot retain the properties of fruits when they are subjected to cutting, and the fresh-cut sliced fruits become exposed to risks of damage and contamination from the environment (Liao et al. 2023).
The results of the TAMB and YM showing the control and coated samples based on the zeroth and fifth days of storage were given in Table 6. The values of TAMB counts and YM counts correspond to the acceptable limit value (6 log CFU/g) of the Institute of Food Science and Technology (IFST) in studies on the shelf life of fruits (Manzoor et al. 2021). The values of the TAMB in most of the samples remained below 2 log CFU/g on the fifth day of storage (Table 6). There was no large effect size of the method on TAMB values. The interaction of essential oil and method showed a significant impact on YM values with a large effect size (p < 0.05). (Supplementary Material S1.4). The YM values at the end of storage were found to range from 3.23 to 3.86 for DC samples containing GSO and 3.56 to 3.92 for ESC samples containing GSO. Similarly, the YM values ranged from 3.91 to 4.96 for DC samples containing PSO and 3.68 to 4.43 for ESC samples containing PSO. Notably, the YM values were similar for samples coated using both methods, indicating that the ESC method, which uses less coating material, is equally effective as the DC method.
Table 6.
Microbiological analysis results of the fresh-cut pineapple samples during storage
| Samples | Total aerobic mesophilic bacteria (log CFU/g) | Mold and yeast (log CFU/g) | ||
|---|---|---|---|---|
| Storage time (day) | Storage time (day) | |||
| 0 | 5 | 0 | 5 | |
| Non-coated | < 1 | < 2 | 3.89 | 4.57 |
| D-Control-0 | 1 | 2 | 3.08 | 3.70 |
| D-Control-MD | < 1 | 2.32 | 2.97 | 3.08 |
| D-Control-WPI | 1.52 | 2 | 3.18 | 3.76 |
| D-Control-MW | 1 | < 2 | 2.97 | 2.97 |
| D-GSO-0 | 1 | < 2 | 3.32 | 3.85 |
| D-GSO-MD | < 1 | < 2 | 3.60 | 3.86 |
| D-GSO-WPI | 1.32 | < 2 | 2.91 | 3.23 |
| D-GSO-MW | < 1 | < 2 | 2.98 | 3.45 |
| D-PSO-0 | < 1 | < 2 | 3.41 | 3.97 |
| D-PSO-MD | < 1 | < 2 | > 4.69 | 4.96 |
| D-PSO-WPI | 1.32 | < 2 | 3.04 | 3.97 |
| D-PSO-MW | 1.86 | < 2 | 4.23 | 3.91 |
| E-Control-0 | 1 | 2.30 | 2.85 | 3.38 |
| E-Control-MD | < 1 | < 2 | 2.91 | 3.75 |
| E-Control-WPI | < 1 | < 2 | 2.88 | 4.46 |
| E-Control-MW | 1.76 | < 2 | 3.08 | 3.79 |
| E-GSO-0 | 1 | < 2 | 3.40 | 3.76 |
| E-GSO-MD | 1 | < 2 | 2.91 | 3.56 |
| E-GSO-WPI | < 1 | < 2 | 3.08 | 3.92 |
| E-GSO-MW | 1 | < 2 | 3.04 | 3.85 |
| E-PSO-0 | 1.32 | < 2 | 3.15 | 3.84 |
| E-PSO-MD | < 1 | < 2 | 2.45 | 4.04 |
| E-PSO-WPI | 1.49 | < 2 | 2.75 | 4.43 |
| E-PSO-MW | 1 | < 2 | 3.23 | 3.68 |
Non-treated sample: Control: Essential oil-free, D: Dip-coating, E:Electrospray coating, GSO and PSO: Grape and Pomegranate Seed Oil, 0: MD/WPI/MW-free emulsion in water phase, MD: Maltodextrin, WPI: Whey Protein Isolate, MW: Mixture of Maltodextrin and Whey Protein Isolate
Sensory analysis
Table 7 shows the sensory analysis results for the color, texture, juiciness, flavor, and general acceptability of the fresh-cut pineapple slices on the first, third, and fifth days of storage. Images representing uncoated, dip-coated, and electrospray-coated fresh-cut pineapple samples during storage are given in Supplementary Material S2. None of the factors (essential oil, water phase, method, and storage time) had a significant impact with a large effect size on the color and texture of the sensory analysis parameters. However, the method showed a large effect on juiciness, flavor, and general acceptability (Supplementary Material S1.2). Samples coated with ESC had higher juiciness, flavor, and general acceptability values than samples coated with DC (p < 0.05). Samples coated with the ESC method have higher juiciness, flavor, and general acceptability values than samples coated with the DC method. In particular, it was observed that DC samples containing PSO received lower scores than ESC-PSO of samples.
Table 7.
Sensory analysis results of the fresh-cut pineapple samples during storage
| Storage time (day) | Storage time (day) | Storage time (day) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 3 | 5 | |||||||||||||
| Samples | Color | Texture | Juiciness | Flavor | General acceptability | Color | Texture | Juiciness | Flavor | General acceptability | Color | Texture | Juiciness | Flavor | General acceptability |
| Non-coated | 7.6a | 7.8a | 7.8a,b | 7.6b | 7.6c,d | 7.1a,b,c,d,e,f,g | 7.6j | 7.5f,g | 7.5g,h,i,j | 7.3g,h,i,j | 6.9 c,d,e,f | 6.8a,b,c,d,e | 6.8a,b | 6.9d,e,f,g,h | 6.9d,e,f,g,h |
| D-Control-0 | 7.7a | 7.3a | 7.3a | 6.5a | 6.8a,b | 7.4d,e,f,g,h | 6.2a,b | 6.1d | 3.2a | 4.1a | 6.0a | 6.3a | 6.2a | 5.0a | 5.7a |
| D-Control-MD | 7.7a | 7.3a | 7.3a | 6.5a | 6.8a,b | 6.9a,b,c,d,e | 6.7a,b,c,d,e,f | 7.0e,f,g | 4.3b | 5.2b,c,d | 6.5a,b,c | 6.5a,b,c | 6.3a | 5.6b | 5.9a,b |
| D-Control-WPI | 7.7a | 7.3a | 7.3a | 6.5a | 6.8a,b | 7.3c,d,e,f,g,h | 6.9c,d,e,f,g,h | 4.5c | 7.4f,g,h,i,j | 6.5e,f | 7.3d,e,f,g,h | 7.1d,e | 7.6c,d,e,f | 7.3f,g,h,i | 7.3g,h,i |
| D-Control-MW | 7.7a | 7.3a | 7.3a | 6.5a | 6.8a,b | 7.1b,c,d,e,f,g,h | 6.2a | 4.2a,b,c | 6.6c,d | 4.7a,b | 7.3d,e,f,g,h | 7.3d,e | 7.5c,d,e,f | 6.2b,c,d | 6.7c,d,e,f |
| D-GSO-0 | 8.0a | 7.3a | 7.6a,b | 7.5b | 7.1a,b,c | 7.2c,d,e,f,g,h | 6.8b,c,d,e,f,g | 3.6a | 7.3e,f,g,h,i,j | 4.7a,b | 7.5f,g,h | 7.0 b,c,d,e | 7.3b,c,d,e | 6.7c,d,e,f | 7.1e,f,g,h,i |
| D-GSO-MD | 8.0a | 7.3a | 7.6a,b | 7.5b | 7.1a,b,c | 6.7a,b,c | 7.0c,d,e,f,g,h,i | 7.2e,f,g | 6.5c | 6.4e | 7.5f,g,h | 7.0 b,c,d,e | 7.2b,c,d | 7.0e,f,g,h,i | 7.0e,f,g,h,i |
| D-GSO-WPI | 8.0a | 7.3a | 7.6a,b | 7.5b | 7.1a,b,c | 7.0a,b,c,d,e,f | 6.6a,b,c,d,e | 4.2a,b,c | 7.2d,e,f,g,h,i | 5.8d | 7.7h | 7.3d,e | 7.4c,d,e,f | 7.1e,f,g,h,i | 7.4h,i |
| D-GSO-MW | 8.0a | 7.3a | 7.6a,b | 7.5b | 7.1a,b,c | 6.9a,b,c,d,e | 6.5a,b,c | 4.3b,c | 6.9c,d,e,f,g | 5.1b,c | 7.0c,d,e,f | 7.2d,e | 7.8d,e,f | 6.3b,c,d | 7.0e,f,g,h |
| D-PSO-0 | 7.6a | 7.2a | 7.6a,b | 6.8a | 6.6a | 6.6a,b | 6.5a,b,c,d | 4.0a,b,c | 6.6c,d | 4.7a,b | 7.3e,f,g,h | 7.2d,e | 7.2b,c,d | 6.1b,c | 6.6c,d,e,f |
| D-PSO-MD | 7.6a | 7.2a | 7.6a,b | 6.8a | 6.6a | 6.6a,b | 7.1c,d,ef,g,h,i,j | 7.4e,f,g | 6.7c,d,e,f | 6.5e,f | 7.2d,e,f,g,h | 7.2d,e | 7.4c,d,e,f | 6.0b,c | 6.5c,d,e,f |
| D-PSO-WPI | 7.6a | 7.2a | 7.6a,b | 6.8a | 6.6a | 7.0a,b,c,d,e,f | 6.6a,b,c,d,e | 6.1d | 7.1d,e,f,g,h | 6.5e,f | 7.3d,e,f,g,h | 7.0b,c,d,e | 7.2b,c,d | 6.0b,c | 6.5b,c,d,e |
| D-PSO-MW | 7.6a | 7.2a | 7.6a,b | 6.8a | 6.6a | 6.5a | 6.4a,b,c | 3.8a,b | 6.8c,d,e,f | 5.5c,d | 7.3d,e,f,g,h | 7.2d,e | 7.4b,c,d,e,f | 6.0b,c | 6.3a,b,c,d |
| E-Control-0 | 7.6a | 7.7a | 8.0b | 8.0b | 7.9d | 7.7g,h | 7.1e,f,g,h,i,j | 7.5f,g | 7.3e,f,g,h,i,j | 7.0e,f,g,h | 7.3f,g,h | 7.2d,e | 7.9e,f | 7.6h,i | 7.5h,i |
| E-Control-MD | 7.6a | 7.7a | 8.0b | 8.0b | 7.9d | 7.3c,d,e,f,g,h | 7.2f,g,h,i,j | 6.9e,f | 7.0c,d,e,f,g,h | 6.9e,f,g | 6.7b,c,d | 6.4a,b | 6.3a | 6.3b,c,d | 6.2a,b,c |
| E-Control-WPI | 7.6a | 7.7a | 8.0b | 8.0b | 7.9d | 6.8a,b,c,d | 7.1e,f,g,h,i,j | 6.8e | 7.2d,e,f,g,h,i | 6.5e,f | 6.3a,b | 6.6a,b,c,d | 7.0b,c | 6.8d,e,f,g | 6.7c,d,e,f,g |
| E-Control-MW | 7.6a | 7.7a | 8.0b | 8.0b | 7.9d | 7.3c,d,e,f,g,h | 7.3f,g,h,i,j | 7.2e,f,g | 7.7h,i,j | 7.2g,h,i,j | 7.0c,d,e,f,g | 7.3d,e | 7.3b,c,d,e,f | 7.5g,h,i | 7.1e,f,g,h,i |
| E-GSO-0 | 7.6a | 7.5a | 7.6a,b | 7.6b | 7.4b,c,d | 7.4e,f,g,h | 7.4g,h,i,j | 7.6g | 7.9j | 7.8j | 6.5a,b,c | 7.3e | 7.2b,c,d | 7.3f,g,h,i,j | 7.2f,g,h,i |
| E-GSO-MD | 7.6a | 7.5a | 7.6a,b | 7.6b | 7.4b,c,d | 7.4d,e,f,g,h | 6.7a,b,c,d,e,f | 7.1e,f,g | 6.7c,d,e | 7.1f,g,h,i | 7.7h | 7.2d,e | 7.8d,e,f | 7.7i | 7.2f,g,h,i |
| E-GSO-WPI | 7.6a | 7.5a | 7.6a,b | 7.6b | 7.4b,c,d | 7.3c,d,e,f,g,h | 7.3g,h,i,j | 7.2e,f,g | 7.3f,g,h,i,j | 7.3g,h,i,j | 6.7b,c,d,e | 7.2d,e | 7.4c,d,e,f | 7.2e,f,g,h,i | 7.2f,g,h,i |
| E-GSO-MW | 7.6a | 7.5a | 7.6a,b | 7.6b | 7.4b,c,d | 7.7h | 7.2f,g,h,i,j | 7.5f,g | 7.7h,i,j | 7.6h,i,j | 7.4f,g,h | 7.2d,e | 7.4c,d,e,f | 6.6c,d,e,f | 7.1e,f,g,h,i |
| E-PSO-0 | 7.6a | 7.6a | 7.7a,b | 7.7b | 7.8c,d | 7.5e,f,g,h | 7.6i,j | 7.6g | 7.8i,j | 7.8i,j | 7.0c,d,e,f,g | 7.1c,d,e | 7.7d,e,f | 6.6c,d,e | 7.1e,f,g,h,i |
| E-PSO-MD | 7.6a | 7.6a | 7.7a,b | 7.7b | 7.8c,d | 7.5f,g,h | 7.4h,i,j | 7.5f,g | 7.4g,h,i,j | 7.6h,i,j | 7.3d,e,f,g,h | 6.8a,b,c,d,e | 7.4c,d,e,f | 7.2e,f,g,h,i | 7.2f,g,h,i |
| E-PSO-WPI | 7.6a | 7.6a | 7.7a,b | 7.7b | 7.8c,d | 7.1b,c,d,e,f,g,h | 7.3g,h,i,j | 7.4e,f,g | 7.3f,g,h,i,j | 7.4g,h,i,j | 7.6g,h | 7.0b,c,d,e | 7.3b,c,d,e | 7.0e,f,g,h,i | 7.0e,f,g,h,i |
| E-PSO-MW | 7.6a | 7.6a | 7.7a,b | 7.7b | 7.8c,d | 7.7g,h | 7.3f,g,h,i,j | 7.2e,f,g | 7.8h,i,j | 7.2g,h,i,j | 7.1d,e,f,g,h | 7.4e | 8.0f | 7.7i | 7.7i |
a–j Different letters in the same column are statistically different (p < 0.05)
A–D Different letters in the same row are statistically different (p < 0.05)
Non-coated: Untreated sample, D: Dip-coating, E:Electrospray coating, Control: Essential oil-free emulsion, GSO and PSO: Grape and Pomegranate Seed Oil based emulsion, 0: MD/WPI/MW-free emulsion in water phase, MD: Maltodextrin, WPI: Whey Protein Isolate, MW: Mixture of Maltodextrin and Whey Protein Isolate
The dip-coated pineapples were observed to have lower juiciness and general acceptability on the third day of storage than on the first and fifth days. The low juiciness values of the dip-coated samples on the third day were associated with the increased firmness values (Table 4) caused by the weight loss experienced during the first three days of storage (Fig. 1). Although flavor, juiciness, and general acceptability values decreased on the third day, the juiciness and general acceptability values increased on the fifth day (Table 7).
Aroma and flavor characteristics during the ripening of pineapple fruit caused an increase in the panelists' juiciness and overall acceptability scores on the fifth day of storage. Oh et al. (2017) also obtained similar results in the sensory analysis results of grape samples coated with lemongrass oil-based emulsions. On day 0, the panelists assigned the uncoated samples higher flavor and taste scores than the coated samples (Oh et al. 2017). However, they found that this difference did not change significantly after the seventh day of storage, and this was because the flavor formed by grapes during ripening masked the flavor of the lemongrass essential oil.
Conclusions
In this study, the usage of the ESC method with EO-enriched emulsions on fresh-cut pineapples was investigated. The results showed that, in electrospray coating, the distribution of emulsions on the surface of the pineapple slices in the form of homogeneous particles with small diameters increased the edible coating efficiency and contributed to the preservation of titratable acidity, color, texture, TPC, and TAA. In addition, the use of EO-enriched emulsions with ESC exhibited preserving characteristics that positively affected the quality of the fresh-cut pineapples. The ESC method has demonstrated efficacy comparable to that of the DC method. Nonetheless, the ESC has been shown to confer comparable protective effects using significantly less coating material. These findings suggest that the ESC method, when combined with emulsions enriched with EO as a coating agent, may offer a practicable and promising alternative for preserving fresh-cut pineapple.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We are grateful to Ege University Planning and Monitoring Coordination of Organizational Development and Directorate of Library and Documentation for their support in editing and proofreading service of this study.
Abbreviations
- PSO
Pomegranate seed oil
- GSO
Grape seed oil
- ESC
Electrospray coating
- DC
Dip-coating
- TAA
Total antioxidant activity
- TPC
Total phenolic content
- MD
Maltodextrin
- EO
Essential oil
- w/o
Water-in-oil emulsion
- PGPR
Polyglycerol polyrycinolate
- WPI
Whey protein isolate
- TAMB
Total viable aerobic mesophilic bacteria
- YM
Yeast and mold
Author contributions
GEA: Formal analysis, Investigation, Data curation, Writing—Original draft, Writing—Review & Editing, Visualization. NB: Investigation, Writing—Review & Editing, Supervision. ST: Conceptualization, Writing—Review & Editing, Resources, Project administration, Supervision. SK: Conceptualization, Writing—Review & Editing, Preparation, Resources, Project administration, Supervision.
Funding
This work was financially supported by the Scientific and Technological Council of Turkey (TUBITAK) with project number 214-O-405 and the Ege University Scientific Research Fund with project number 15-BIL-024.
Data availability
All data supporting the findings of the study are included in the article and can be submitted to researchers who request it.
Code availability
Not applicable.
Declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical approval
Not applicable.
Consent to participate
All participants were informed about the content (ingredients) of the samples and their consent was obtained before they participated in the sensory analysis.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Gozde Ela Albayrak, Email: gozdeelaalbayrak@gmail.com.
Neslihan Bozdogan, Email: neslihanbozdogan5@gmail.com.
Sebnem Tavman, Email: sebnem.tavman@ege.edu.tr.
Seher Kumcuoglu, Email: seher.kumcuoglu@ege.edu.tr.
References
- Al-Juhaimi F, Özcan MM, Ghafoor K. Characterization of pomegranate (Punica granatum L.) seed and oils. Eur J Lipid Sci Technol. 2017;119(10):1700074. doi: 10.1002/ejlt.201700074. [DOI] [Google Scholar]
- Al-Sabahi BN, Fatope MO, Essa MM, Subash S, Al-Busafi SN, Al-Kusaibi FSM, Manivasagam T. Pomegranate seed oil: Effect on 3-nitro propionic acid-induced neurotoxicity in PC12 cells and elucidation of unsaturated fatty acids composition. Nutr Neurosci. 2017;20:40–48. doi: 10.1179/1476830514Y.0000000155. [DOI] [PubMed] [Google Scholar]
- Andrade RD, Skurtys O, Osorio FA. Atomizing spray systems for application of edible coatings. Compr Rev Food Sci Food Saf. 2012;11:323–337. doi: 10.1111/j.1541-4337.2012.00186.x. [DOI] [Google Scholar]
- Azarakhsh N, Osman A, Ghazali HM, Tan CP, Mohd Adzahan N. Lemongrass essential oil incorporated into alginate-based edible coating for shelf-life extension and quality retention of fresh-cut pineapple. Postharvest Biol Technol. 2014;88:1–7. doi: 10.1016/j.postharvbio.2013.09.004. [DOI] [Google Scholar]
- Bail S, Stuebiger G, Krist S, Unterweger H, Buchbauer G. Characterisation of various grape seed oils by volatile compounds, triacylglycerol composition, total phenols and antioxidant capacity. Food Chem. 2008;108:1122–1132. doi: 10.1016/j.foodchem.2007.11.063. [DOI] [PubMed] [Google Scholar]
- Basaglia RR, Pizato S, Santiago NG, Maciel de Almeida MM, Pinedo RA, Cortez-Vega WR. Effect of edible chitosan and cinnamon essential oil coatings on the shelf life of minimally processed pineapple (Smooth cayenne) Food Biosci. 2021;41:100966. doi: 10.1016/j.fbio.2021.100966. [DOI] [Google Scholar]
- Benítez S, Soro L, Achaerandio I, Sepulcre F, Pujolá M. Combined effect of a low permeable film and edible coatings or calcium dips on the quality of fresh-cut pineapple: edible coatings or calcium dips on fresh-cut pineapple. J Food Process Eng. 2014;37:91–99. doi: 10.1111/jfpe.12063. [DOI] [Google Scholar]
- Burt S. Essential oils: their antibacterial properties and potential applications in foods—a review. Int J Food Microbiol. 2004;94(3):223–253. doi: 10.1016/j.ijfoodmicro.2004.03.022. [DOI] [PubMed] [Google Scholar]
- Cakmak H, Kumcuoglu S, Tavman S. Production of edible coatings with twin-nozzle electrospraying equipment and the effects on shelf-life stability of fresh-cut apple slices. J Food Process Eng. 2018;41:e12627. doi: 10.1111/jfpe.12627. [DOI] [Google Scholar]
- Dabetic NM, Todorovic VM, Djuricic ID, Antic Stankovic JA, Basic ZN, Vujovic DS, Sobajic SS. Grape seed oil characterization: a novel approach for oil quality assessment. Eur J Lipid Sci Technol. 2020;122(6):1900447. doi: 10.1002/ejlt.201900447. [DOI] [Google Scholar]
- de Melo IL, Carvalho E, Yoshime L, Sattler J, Pavan R, Mancini-Filho J. Characterization of constituents, quality and stability of pomegranate seed oil (Punica granatum L.) Food Sci Technol. 2016;36:132–139. doi: 10.1590/1678-457X.0069. [DOI] [Google Scholar]
- de Morais FA, de Araújo FM, Machado AV, Ricarte FD, Junior RS. Influência da atmosfera modificada sob a vida útil pós-colheita do mamão ‘formosa’. Revista Verde Agroecologia e Desenvolvimento Sustentável. 2010;5(4):5. [Google Scholar]
- Debeaufort F, Voilley A. Lipid-based edible films and coatings. In: Huber KC, Embuscado ME, editors. Edible films and coatings for food applications. New York: Springer; 2009. pp. 135–168. [Google Scholar]
- Dhiman A, Suhag R, Singh A, Prabhakar PK. Mechanistic understanding and potential application of electrospraying in food processing: a review. Crit Rev Food Sci Nutr. 2021 doi: 10.1080/10408398.2021.1926907. [DOI] [PubMed] [Google Scholar]
- Diamanti J (2010) Quality, nutritional quality and nutraceutical value as a1 new task for strawberry breeding, (PhD Thesis), Universita Politecnica Delle Marche, Facolta di Agraria, Ancona, Italy
- Fazaeli M, Yousefi S, Emam-Djomeh Z. Investigation on the effects of microwave and conventional heating methods on the phytochemicals of pomegranate (Punica granatum L.) and black mulberry juices. Food Res Int. 2013;50(2):568–573. doi: 10.1016/j.foodres.2011.03.043. [DOI] [Google Scholar]
- Fernandes L, Casal S, Cruz R, Pereira JA, Ramalhosa E. Seed oils of ten traditional Portuguese grape varieties with interesting chemical and antioxidant properties. Int Food Res J. 2013;50:161–166. doi: 10.1016/j.foodres.2012.09.039. [DOI] [Google Scholar]
- Galus S, Kadzińska J. Food applications of emulsion-based edible films and coatings. Trends Food Sci Technol. 2015;45:273–283. doi: 10.1016/j.tifs.2015.07.011. [DOI] [Google Scholar]
- Gardesh ASK, Badii F, Hashemi M, Ardakani AY, Maftoonazad N, Gorji AM. Effect of nanochitosan based coating on climacteric behavior and postharvest shelf-life extension of apple cv. Golab Kohanz LWT. 2016;70:33–40. doi: 10.1016/j.lwt.2016.02.002. [DOI] [Google Scholar]
- Graça A, Esteves E, Nunes C, Abadias M, Quintas C. Microbiological quality and safety of minimally processed fruits in the marketplace of southern Portugal. Food Control. 2017;73:775–783. doi: 10.1016/j.foodcont.2016.09.046. [DOI] [Google Scholar]
- Hossain MA, Rahman SMM. Total phenolics, flavonoids and antioxidant activity of tropical fruit pineapple. Food Res Int. 2011;44:672–676. doi: 10.1016/j.foodres.2010.11.036. [DOI] [Google Scholar]
- Jaworek A. Electrospray droplet sources for thin film deposition. J Mater Sci. 2007;42:266–297. doi: 10.1007/s10853-006-0842-9. [DOI] [Google Scholar]
- Jokar A, Barzegar H, Maftoon Azad N, Shahamirian M. Effects of cinnamon essential oil and Persian gum on preservation of pomegranate arils. Food Sci Nutr. 2021;9(5):2585–2596. doi: 10.1002/fsn3.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan MKI, Nazir A, Maan AA. Electrospraying: a Novel Technique for Efficient Coating of Foods. Food Eng Rev. 2017;9:112–119. doi: 10.1007/s12393-016-9150-6. [DOI] [Google Scholar]
- Kumar P, Sethi S, Sharma RR, Singh S, Varghese E. Improving the shelf life of fresh-cut ‘Royal Delicious’ apple with edible coatings and anti-browning agents. J Food Sci Technol. 2018;55:3767–3778. doi: 10.1007/s13197-018-3308-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P, Sethi S, Varghese E. Impact of carboxymethyl cellulose coating functionalized with browning inhibitors for maintaining quality attributes of fresh-cut pineapple cubes. J Food Process Preserv. 2021;45(12):e16053. doi: 10.1111/jfpp.16053. [DOI] [Google Scholar]
- Kumar P, Tanwar R, Gupta V, Upadhyay A, Kumar A, Gaikwad KK. Pineapple peel extract incorporated poly (vinyl alcohol)-corn starch film for active food packaging: preparation, characterization and antioxidant activity. Int J Biol Macromol. 2021;187:223–231. doi: 10.1016/j.ijbiomac.2021.07.136. [DOI] [PubMed] [Google Scholar]
- Liao X, Xing Y, Fan X, Qiu Y, Xu Q, Liu X. Effect of composite edible coatings combined with modified atmosphere packaging on the storage quality and microbiological properties of fresh-cut pineapple. Foods. 2023;12(6):1344. doi: 10.3390/foods12061344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Xu X, Gong Y, He L, Gao Y. Effects of supercritical CO2 extraction parameters on chemical composition and free radical-scavenging activity of pomegranate (Punica granatum L.) seed oil. Food Bioprod Process. 2012;90:573–578. doi: 10.1016/j.fbp.2011.11.004. [DOI] [Google Scholar]
- Lutterodt H, Slavin M, Whent M, Turner E, Yu (Lucy) L. Fatty acid composition, oxidative stability, antioxidant and antiproliferative properties of selected cold-pressed grape seed oils and flours. Food Chem. 2011;128:391–399. doi: 10.1016/j.foodchem.2011.03.040. [DOI] [PubMed] [Google Scholar]
- Maier T, Schieber A, Kammerer DR, Carle R. Residues of grape (Vitis vinifera L.) seed oil production as a valuable source of phenolic antioxidants. Food Chem. 2009;112:551–559. doi: 10.1016/j.foodchem.2008.06.005. [DOI] [Google Scholar]
- Mantilla N, Castell-Perez ME, Gomes C, Moreira RG. Multilayered antimicrobial edible coating and its effect on quality and shelf-life of fresh-cut pineapple (Ananas comosus) LWT Food Sci Technol. 2013;51:37–43. doi: 10.1016/j.lwt.2012.10.010. [DOI] [Google Scholar]
- Manzoor S, Gull A, Wani SM, Ganaie TA, Masoodi FA, Bashir K, Malik AR, Dar BN. Improving the shelf life of fresh cut kiwi using nanoemulsion coatings with antioxidant and antimicrobial agents. Food Biosci. 2021;41:101015. doi: 10.1016/j.fbio.2021.101015. [DOI] [Google Scholar]
- Matthäus B. Virgin grape seed oil: Is it really a nutritional highlight? Eur J Lipid Sci Technol. 2008;110(7):645–650. doi: 10.1002/ejlt.200700276. [DOI] [Google Scholar]
- Medeiros Teodosio AEM, Carlos Rocha Araújo RH, Figueiredo Lima Santos BG, Linné JA, da Silva Medeiros ML, Alves Onias E, Alves de Morais F, de Melo SS, de Lima JF. Effects of edible coatings of Chlorella sp. containing pomegranate seed oil on quality of Spondias tuberosa fruit during cold storage. Food Chem. 2021;338:127916. doi: 10.1016/j.foodchem.2020.127916. [DOI] [PubMed] [Google Scholar]
- Mendonça CRB, Borges CD, Kringel AL, da Silveira RP, da Silva FA, Schulz GAS. Application of microemulsions as coating in fresh cut strawberries. J Food Sci Technol. 2020;57:2764–2770. doi: 10.1007/s13197-020-04515-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero-Calderón M, Rojas-Graü MA, Martín-Belloso O. Effect of packaging conditions on quality and shelf-life of fresh-cut pineapple (Ananas comosus) Postharvest Biol Technol. 2008;50:182–189. doi: 10.1016/j.postharvbio.2008.03.014. [DOI] [Google Scholar]
- Nurhadi B, Roos YH, Maidannyk V. Physical properties of maltodextrin DE 10: water sorption, water plasticization and enthalpy relaxation. J Food Eng. 2016;174:68–74. doi: 10.1016/j.jfoodeng.2015.11.018. [DOI] [Google Scholar]
- Oh YA, Oh YJ, Song AY, Won JS, Song KB, Min SC. Comparison of effectiveness of edible coatings using emulsions containing lemongrass oil of different size droplets on grape berry safety and preservation. LWT Food Sci Technol. 2017;75:742–750. doi: 10.1016/j.lwt.2016.10.033. [DOI] [Google Scholar]
- Pamisetty A, Juvvi P, Ramakrishna C, Singh RP. Development of ω-5 and antioxidant enriched bar with pomegranate seed powder. J Food Process Preserv. 2020;44(3):e14359. doi: 10.1111/jfpp.14359. [DOI] [Google Scholar]
- Passos CP, Silva RM, Da Silva FA, Coimbra MA, Silva CM. Supercritical fluid extraction of grape seed (Vitis vinifera L.) oil. Effect of the operating conditions upon oil composition and antioxidant capacity. J Chem Eng. 2010;160:634–640. doi: 10.1016/j.cej.2010.03.087. [DOI] [Google Scholar]
- Paul A, Radhakrishnan M. Pomegranate seed oil in food industry: Extraction, characterization, and applications. Trends Food Sci Technol. 2020;105:273–283. doi: 10.1016/j.tifs.2020.09.014. [DOI] [Google Scholar]
- Paull RE, Chen CC. Pineapple: Postharvest quality-maintenance guidelines, fruit, nut, and beverage crops. Fruit Nut Beverage Crops. 2014;2:1–6. [Google Scholar]
- Pirozzi A, Del Grosso V, Ferrari G, Pataro G, Donsi F. Combination of edible coatings containing oregano essential oil nanoemulsion and pulsed light treatments for improving the shelf life of tomatoes. Chem Eng Trans. 2021;87:61–66. doi: 10.3303/CET2187011. [DOI] [Google Scholar]
- Pirozzi A, Ferrari G, Donsì F. The use of nanocellulose in edible coatings for the preservation of perishable fruits and vegetables. Coatings. 2021;11:990. doi: 10.3390/coatings11080990. [DOI] [Google Scholar]
- Prakash A, Baskaran R, Vadivel V. Citral nanoemulsion incorporated edible coating to extend the shelf life of fresh cut pineapples. LWT. 2020;118:108851. doi: 10.1016/j.lwt.2019.108851. [DOI] [Google Scholar]
- Riaz A, Aadil RM, Amoussa AMO, Bashari M, Abid M, Hashim MM. Application of chitosan-based apple peel polyphenols edible coating on the preservation of strawberry (Fragaria ananassa cv Hongyan) fruit. J Food Process. 2021;45(1):e15018. doi: 10.1111/jfpp.15018. [DOI] [Google Scholar]
- Shinagawa FB, Santana FC, Torres LR, Mancini-Filho J. Grape seed oil: a potential functional food? Food Sci Technol. 2015;35:399–406. doi: 10.1590/1678-457X.6826. [DOI] [Google Scholar]
- Siraj N, Shabbir MA, Khan MR, Rehman KU. Preventing oxidation of canola and sunflower oils by addition of pomegranate seed oil. Acta Aliment. 2019;48(1):18–27. doi: 10.1556/066.2018.0005. [DOI] [Google Scholar]
- Skurtys O, Acevedo C, Pedreschi F, Enrione J, Osorio F, Aguilera JM. Food hydrocolloids edible films and coatings. In: Hollingworth CS, editor. Food Hydrocolloids: Characteristics. New York: Properties and Structures, Nova Science Publishers; 2010. pp. 41–80. [Google Scholar]
- Suhag R, Kumar N, Petkoska AT, Upadhyay A. Film formation and deposition methods of edible coating on food products: a review. Food Res Int. 2020;136:109582. doi: 10.1016/j.foodres.2020.109582. [DOI] [PubMed] [Google Scholar]
- Tangolar SG, Özoğul Y, Tangolar S, Torun A. Evaluation of fatty acid profiles and mineral content of grape seed oil of some grape genotypes. Int J Food Sci Nutr. 2009;60(1):32–39. doi: 10.1080/09637480701581551. [DOI] [PubMed] [Google Scholar]
- Tavassoli-Kafrani E, Shekarchizadeh H, Masoudpour-Behabadi M. Development of edible films and coatings from alginates and carrageenans. Carbohydr Polym. 2016;137:360–374. doi: 10.1016/j.carbpol.2015.10.074. [DOI] [PubMed] [Google Scholar]
- Treviño-Garza MZ. Layer-by-layer edible coatings based on mucilages, pullulan, and chitosan and its effect on quality and preservation of fresh-cut pineapple (Ananas comosus) Postharvest Biol Technol. 2017;128:63–75. doi: 10.1016/j.postharvbio.2017.01.007. [DOI] [Google Scholar]
- Van de Braak SAAJ, Leijten GCJJ (1999) Essential oils and oleoresins: a survey in the Netherlands and other major markets in the European Union. CBI centre for the promotion of imports from developing countries Rotterdam, 116
- Wilhelm O, Mädler L, Pratsinis SE. Electrospray evaporation and deposition. J Aerosol Sci. 2003;34(7):815–836. doi: 10.1016/S0021-8502(03)00034-X. [DOI] [Google Scholar]
- Xu S, Ren J, Lu H, Wang X, Sun X, Liang X. Nondestructive detection and grading of flesh translucency in pineapples with visible and near-infrared spectroscopy. Postharvest Biol Technol. 2022;192:112029. doi: 10.1016/j.postharvbio.2022.112029. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data supporting the findings of the study are included in the article and can be submitted to researchers who request it.
Not applicable.