Abstract
One of the main strategies of neutrophils in responding to microbial infections is the formation of neutrophil extracellular traps (NETs). NETs are web-like structures of decondensed chromatin associated with antimicrobial proteins. Citrullination plays an important role during NET formation and a substantial fraction of NET-associated proteins appeared to be citrullinated. The release of citrullinated intracellular proteins from netting neutrophils led to the hypothesis that the production of anti-citrullinated protein autoantibodies by autoimmune patients, in particular patients with rheumatoid arthritis, might be initiated when citrullinated NET components are not properly cleared and are exposed to the immune system. Here, we discuss the processes that lead to NET formation, including the role of peptidylarginine deiminase activation and our current knowledge on citrullinated NET-associated proteins. Citrulline-dependent epitopes do not appear to play a major role in the recognition of NETs by autoantibodies from rheumatoid arthritis and systemic lupus erythematosus patients, even though anti-NET autoantibodies are frequently observed in sera from these patients. The neutrophil proteases associated with NETs have a major impact on the integrity of NET-associated proteins when NET formation is induced by activating isolated human neutrophils. Cleavage/degradation of these proteins also resulted in a strong reduction of the reactivity with autoantibodies.
This article is part of the Theo Murphy meeting issue ‘The virtues and vices of protein citrullination’.
Keywords: neutrophil extracellular traps, NETs, autoantibodies, rheumatoid arthritis
1. Introduction
(a) . Neutrophil extracellular traps
Neutrophil extracellular traps (NETs) are fibrous networks of chromatin and antibacterial proteins that are formed by neutrophils in order to capture pathogens and prevent them from spreading. Since the identification of NETs in 2004 [1], researchers have speculated that these structures might be the source of autoantigens capable of inducing autoimmune responses. NETs contain many intracellular autoantigens, such as DNA and histones, which become exposed when NETs are formed. In this process, termed NETosis, the neutrophil decondenses its chromatin, which leads to rupture of the nuclear envelope. At the same time, granular membranes also rupture, allowing their antimicrobial protein content to bind to the chromatin. When the mechanical forces exerted by the expanding chromatin become so high that it ruptures the cell membrane, the chromatin decorated with antimicrobial proteins is extruded [2]. Pathogens become entrapped in these NET structures and will be eliminated by the action of NET components and phagocytic cells.
Various stimuli have been found to induce NET formation. Roughly, these can be divided into chemical stimuli (for example phorbol 12-myristate 13-acetate (PMA) and the calcium ionophore A23187) [1,3,4] and physiological stimuli (for example bacteria, such as Staphylococcus aureus [5,6], yeast, such as Candida albicans [7], and cytokines, such as TNFα, IL-8 and IFNγ [8]. In general, PMA appeared to be a potent inducer of NET formation and for this reason PMA is often used as a positive control in NET-related experiments [9]. PMA mimicks NET-induction by bacteria and fungi, because it activates phosphokinase C and ERK signalling [10].
The antimicrobial proteins present on NETs are mostly positively charged, which aids their binding to the negatively charged DNA [11]. The most abundant NET proteins are histones and they have been implicated as antimicrobial proteins because of their highly basic properties [7,12]. Other proteins reported to be vital for the bacterial killing capacities of NETs are neutrophil elastase (NE), myeloperoxidase (MPO) and calprotectin [13,14].
Although almost two decades have passed since the first description of NETosis, the exact mechanism by which NETs are formed is still not completely understood. This might be partly due to the fact that there seem to be different mechanisms that lead to NET formation. The activation of a specific intracellular pathway depends on the stimulus that is used to form NETs [3]. PMA induces NET formation and death of the neutrophil in about three hours and the production of reactive oxygen species (ROS) was shown to be crucial. Studies with other stimuli showed NET formation within half an hour and this was not dependent on ROS production [15]. The two most well-characterized molecular mechanisms of NET formation, ROS-dependent NET formation (as stimulated by PMA) and ROS-independent NET formation (as stimulated by A23187 and most types of microbes) are described in more detail below.
(b) . ROS-dependent NET formation
PMA is a small molecule that is able to cross the cell membrane and to directly activate intracellular protein kinase C [10,11]. This initiates a reaction cascade that includes phosphorylation of RAF, MEK, Akt and ERK [16]. This signalling pathway leads to the activation of the NADPH oxidase complex, also called the NOX complex, which leads to the production of ROS. When ROS scavengers or NADPH oxidase inhibitors are present in the medium, neutrophils will not form NETs in response to PMA [11,17]. Vice versa, neutrophils will form NETs when exposed to ROS species such as superoxide and H2O2. ROS production activates MPO, which is present in the granules together with NE [18]. It is not completely clear how, but activation of MPO leads to release of NE from the granules. A possible mechanism involves gasdermin D, which creates pores in the granular membranes to free their contents [19]. Upon release, NE travels to the nucleus and on its way degrades the actin cytoskeleton of the cell [20]. The breakdown of the actin cytoskeleton is a pivotal process in the formation of NETs [21]. In the nucleus, NE degrades histones, which leads to decondensation of the DNA [22]. At this point, the cells have passed a ‘point of no return’: there is no way back and the NET will inevitably be formed [2]. First, the nuclear and granular membranes are breached by unknown mechanisms, although the involvement of pore formation by gasdermin D has been proposed [19]. When the membranes are compromised, granule and cytosolic proteins are able to bind the expanding chromatin. At a certain point the pressure of the decondensing chromatin becomes so high that the cell membrane bursts and the NET is casted.
(c) . ROS-independent NET formation
A23187 is a compound that is able to increase the intracellular calcium concentration in neutrophils via activation of the small conductance potassium channel member SK3 [4]. This mimics multiple bacteria that are able to induce NET formation by producing pore-forming toxins, such as pneumolysin of Streptococcus pneumoniae and autolysin and Protein A of S. aureus [15]. The resulting pores in the plasma membrane allow entry of extracellular calcium, leading to increased intracellular calcium concentrations [4,23]. Due to the rise in intracellular calcium PAD4 (peptidylarginine deiminase 4), an enzyme able to citrullinate proteins, becomes activated [24]. Citrullination is the process in which a positively charged arginine residue is deiminated, creating a neutral citrulline residue [25]. When activated, PAD4 migrates to the nucleus and citrullinates histones, which loosens the binding of the histones to the negatively charged DNA and in this way stimulates chromatin decondensation [26]. The decondensing chromatin will disrupt the nuclear membrane, allowing cytosolic and granular proteins to bind, before bursting through the plasma membrane to form NETs. By contrast to the mechanism described above, this process is not dependent on ROS production. It has been shown that activated PAD4 citrullinates subunits of the NOX complex, which interferes with the production of ROS [27]. There are fewer granule proteins associated with ROS-independent NETs compared to ROS-dependent NETs, and these granule proteins are also less active on the NETs [15,28].
In spite of this distinction between ROS-dependent and -independent NET formation, there is still some controversy among researchers on the processes involved in NET formation [9,23]. This may at least in part be due to the lack of standardized protocols, the diversity of stimuli, the presence or absence of serum and variation of incubation times [9,29,30]. Also the ways by which NET formation is visualized and/or quantified (i.e. immunofluorescence or detection of NET fragments with ELISA) in different laboratories are very diverse and may have caused additional discrepancies.
It is also important to note that conflicting results exist on the involvement of PAD4 and citrullination in the process of NET formation upon PMA stimulation. In an early study, Wang and co-workers found that PAD4 activity is indispensable for NET formation in mice [31]. In first instance, this finding was corroborated by many other research groups, but later it was reported that PAD4 activity is not required for PMA-induced NET formation [32,33]. A critical reappraisal of NET formation followed, hypothesizing that citrullination occurs as a side effect of PMA-induced NETosis, but that it is not a critical molecular feature of NET formation induced by PMA [23]. Another important aspect is that all studies using mouse models to study NET formation without exception show that PAD4 is essential for NET formation, whereas failures to demonstrate the dependence on PAD4 almost exclusively involved human material.
Some researchers have suggested a role for autophagy in the formation of NETs [34,35]. Autophagy is the cellular process in which the cell catabolizes its own organelles or proteins in order to generate energy, or to get rid of excess cellular content. After PMA stimulation, large vacuoles that resemble autophagosomes appear in the cytosol of neutrophils, and their formation is not dependent on ROS production [34]. Indeed, inhibition of autophagy led to a decrease in NET formation for different stimuli [36]. However, additional studies suggested that autophagy inhibitors might actually inhibit ROS production, indirectly inhibiting NET formation [37].
These are just a few of the controversies that exist about NET formation. A joint effort of several research groups resulted in a critical discussion of controversies that exist in this field and an attempt to reach more consensus [9]. Although this paper is a good start, there is still a long way to go before the mechanisms of NET formation will be completely understood and standardized methods for NET generation and quantification are established.
(d) . Autoimmunity
The release of intracellular material during NET formation triggered researchers to speculate about a role in breaking immunological tolerance to self-components. Although Brinkmann and colleagues suggested that the biological function of NETs is mainly antibacterial, soon it was recognized that NETs can also have detrimental effects on the host when not properly cleared by DNases and macrophages [38]. The NET-associated antibacterial proteins were shown to inflict collateral tissue damage and endothelial cells respond by producing inflammatory mediators [39]. Furthermore, NETs were shown to form obstructions in veins or ducts, leading to several pathogenic conditions such as infarcts and nephritis [40]. The association between NETs and autoimmune diseases is probably the best-documented downside of NETs [5]. In addition to the increased availability of intracellular molecules for presentation to the immune system, NETs are usually formed in a response against pathogens, which creates a co-stimulatory milieu in which microbial molecules can act as adjuvant-like danger signals in activation of the immune system [41]. When NETs are formed uncontrollably, or when they are not cleared properly, the immune system might recognize NETs as harmful and this might trigger the generation of an autoimmune response against NET components. Once an autoimmune reaction against NETs is initiated, they can contribute to an inflammatory feed-forward loop because the inflammatory milieu will prime neutrophils to form NETs, and autoantibodies against NETs might lead to increased inflammation. NETs have been implicated in several autoimmune diseases, including rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), systemic sclerosis (SSc) and anti-neutrophil cytoplasmic antibody-associated vasculitis (AAV) [5,42,43].
(e) . Autoimmunity to citrullinated proteins
RA patients frequently have anti-citrullinated protein antibodies (ACPA) in their sera. Based on the idea that PAD4 activation and citrullination are indispensable for NET formation, it was speculated that NETs may expose citrullinated epitopes to which ACPA can bind. Several research groups identified citrullinated peptides on NETs by proteomic analyses [29,44]. Vimentin, azurocidin, NE, myeloid cell nuclear differentiation antigen (MNDA), histidine decarboxylase, histone 1 and histone 4 were identified as NET proteins containing citrullinated epitopes [8,29]. Furthermore, citrullinated vimentin, histone 1, histone 3 and histone 4 were also detected on NETs via immunofluorescent techniques using citrullination site-specific antibodies [8,41,45]. It was shown that synovial fibroblasts can take up NETs in the inflamed synovium and present citrullinated peptides to T-cells and this may stimulate the generation of an ACPA response [46]. Therefore, it is possible that NETs play a role in autoimmunity against citrullinated proteins.
Neutrophils of RA patients were found to be more prone to form NETs spontaneously and respond more vigorously to NET-inducing stimuli than healthy control neutrophils [8,46,47]. Moreover, sera of RA patients were able to induce NET formation in neutrophils from healthy individuals, in contrast to sera of healthy individuals [8,47], and these effects appeared to be (ACPA) IgG-dependent. Also immune complexes formed in RA were able to stimulate NET production [48]. Besides stimulating neutrophils to form NETs, the (ACPA) antibodies in RA sera are also able to bind to NETs and thereby activate the immune system via the classical arm of the complement system [49]. These investigators also showed that RA NETs are a more potent inducer of macrophage activity than healthy control NETs. Furthermore, NETs in the synovium can be taken up by synovial fibroblasts and macrophages, which then become activated and start to produce inflammatory cytokines [8,46,49]. Therefore, NETs may maintain a vicious inflammatory cycle in the synovium of affected joints. Due to the inflammatory milieu in the joints and the presence of autoantibodies and cytokines, neutrophils may be more prone to form NETs, which will boost the formation of more antibodies against NETs, leading to the recruitment of additional immune cells to fuel the inflammation and, again, more NET formation.
(f) . Objective
Here, we address the hypothesis that citrullinated epitopes associated with NETs are among the major determinants of the B cell response in autoimmune diseases, in particular RA and SLE.
2. Heterogeneity of human NET formation
To study the reactivity of autoimmune patient sera with NETs, it is important to take the heterogeneity of NETs into account. Different stimuli and different pathways leading to NET formation may result in NETs with partially different compositions, both at the level of protein composition and with respect to post-translational modifications. To better understand the differences in the formation of NETs, neutrophils isolated from peripheral blood from healthy human donors were subjected to different NET-inducing stimuli, PMA, lipopolysaccharide (LPS) and the calcium ionophore A23187. The applied methods are described in the electronic supplementary material.
Disruption of the plasma membrane was visualized by the uptake of the plasma membrane impermeable dye NucGreen (SYTOX Green) that emits bright green fluorescence when bound to DNA [50]. While loss of plasma membrane integrity was already observed after 40 min of exposure to A23187, DNA staining in neutrophils treated with LPS was first observed after approximately 90 min and after about 100 min after adding PMA (figure 1). Decondensation of chromatin in the neutrophils was much more prominent upon treatment with PMA and LPS than upon exposure to A23187. As expected, A23187 resulted in a rapid rise in intracellular calcium concentrations, as detected by the Ca2+ sensor fura-2. PMA and LPS, on the other hand, induced early calcium oscillations that were followed by a gradual increase in calcium levels until the plasma membrane integrity was lost. ROS appeared to be produced early in neutrophils exposed to PMA, relatively late in cells exposed to LPS, and was not or hardly detectable in neutrophils treated with A23187. The release of two of the most abundant neutrophil proteins, MPO and NE, which are both also associated with NETs, was monitored by their enzymatic activities using colorimetric substrates. Both MPO and NE activity was readily detected in NET harvests, which were obtained by subjecting NETs to micrococcal nuclease treatment (see §3), irrespective of the inducer of NET formation. By contrast, in the culture supernatants MPO and NE activity was only detected upon treatment with A23187, and not or hardly upon exposure to PMA and LPS [50]. The results of these analyses are summarized in figure 1. These data show that there are important differences in the cellular and molecular features of NET formation induced by different signals.
Figure 1.
Inducer-dependent differences in the execution of NET formation. Schematic overview of morphological and molecular changes of isolated human neutrophils in vitro after the induction of NET formation by PMA, LPS and A23187. The same features for non-stimulated neutrophils were analysed in parallel. n.a.: not applicable.
3. Autoantibody reactivity to NET components in autoimmune patient sera
To study the reactivity of autoantibodies in patient sera with NET components, two assays were developed. For one of these assays NET formation was induced by exposing isolated human neutrophils to NET-inducing stimuli and harvesting NETs after three hours by treatment with micrococcal nuclease. The nuclease cleaves the DNA strands of NETs into small fragments, which are released from the neutrophils together with the associated proteins. The NET harvests were subsequently immobilized in the wells of microtiter plates and the reactivity of patient sera was assessed by an enzyme-linked immunosorbent assay. The applied methods are described in the electronic supplementary material. For the analysis of RA patient sera, NETs were induced by both PMA and A23187, using neutrophils from both healthy individuals and RA patients. No statistically significant differences were observed between the recognition of NETs from healthy or RA neutrophils by RA autoantibodies, nor between the recognition of NETs originating from PMA or A23187 induction (figure 2). This suggests that potential differences in the composition of NETs generated by different mechanisms do not have a major impact on the recognition by RA autoantibodies. In total, 51% of the RA sera showed reactivity with at least one of the NET preparations (figure 2) [51]. Highly reactive sera showed reactivity with all NET preparations; more weakly reactive sera recognized only one or a subset of these NET preparations.
Figure 2.
Reactivity of RA patient sera with PMA- and A23187-induced NETs. Isolated neutrophils from healthy individuals (HC) and RA patients were treated with PMA or A23187 and after 3 h incubation NETs were harvested. NET harvests were used to coat microtiter plates, which were used to analyse the reactivity with anti-NET antibodies (abs) positive RA sera by ELISA. (a) Box plot; (b) results for individual sera with reactivities higher than two times the cut-off value, showing the pairing of reactivities with NETs induced as indicated. arb. units, arbitrary units. Note that these data in part are reproduced from [49].
As an alternative assay, the reactivity of RA patient sera with NETs was assessed on glass slides by immunofluorescence. After NET formation the cells were fixed, incubated consecutively with patient sera and fluorophore-labelled secondary antibodies and visualized by fluorescence microscopy (figure 3). To quantify the reactivity of patient autoantibodies with NETs, the images were analysed by FIJI software, which allowed the subtraction of cell body signals from the overall signals, resulting in the selective quantification of NET signals [51]. In agreement with the ELISA data, again no significant difference was observed between the signals obtained with NETs originating from RA neutrophils and from healthy control neutrophils. In the immunofluorescence assay 48% of the RA sera showed reactivity with NETs and the immunofluorescence scores correlated reasonably well with the ELISA scores.
Figure 3.
Reactivity of RA patient sera with NETs analysed by immunofluorescence. Isolated neutrophils from healthy individuals were treated with PMA and after 3 h incubation cells were fixed and incubated with RA patient sera. Fluorescent secondary antibodies were used to visualize bound antibodies. DNA was stained with DAPI (a,d,g). The results for a strongly (b), moderately (e) and non-reactive (h) RA serum are shown. (c,f,i): merged images.
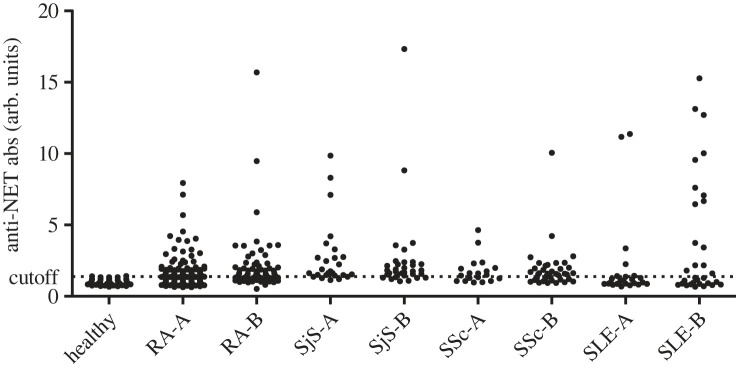
To further assess anti-NET autoantibody reactivity in autoimmune patients, sera from two additional cohorts of established RA patients, early RA patients, SLE, Sjögren's syndrome (SjS) and SSc patients were analysed in the NET-ELISA assay. Anti-NET reactivity was detected in 22%–76% of these cohorts (figure 4). No correlation between anti-NET reactivity and age, gender and disease activity parameters was observed [51]. These data show that anti-NET autoantibodies are frequently present in the sera of autoimmune patients and that their production does not appear to be disease-specific.
Figure 4.
Anti-NET antibodies in autoimmune patient sera. The reactivity of RA, SjS, SSc and SLE patient sera with NET components was analysed using the anti-NET ELISA (PMA; neutrophils from healthy subjects; see legend to figure 2). In parallel, sera from healthy individuals were analysed to define a cut-off value. Two cohorts (termed A and B; samples collected in different clinics and/or in different time periods) were analysed for all disease groups. arb. units: arbitrary units. Note that these data in part are reproduced from [49].
The main epitopes recognized by anti-NET autoantibodies remain to be identified. The frequent presence of such antibodies in SLE, SjS and SSc sera argues against a major role for citrullinated epitopes, in spite of the fact that it is known that NETs contain various citrullinated proteins (see §4) [8,29,45]. Although citrullination may be important for at least some of the epitopes recognized by RA patient sera, the lack of a correlation with ACPA indicates that other epitopes are likely to play a more prominent role.
4. Citrullinated proteins in NETs
Several proteomics studies have identified a number of proteins consistently associated with NETs. Petretto and co-workers analysed the protein composition of NETs produced by neutrophils from healthy donors upon exposure to PMA, LPS and A23187 [44]. In total 330 proteins were identified, 74 of which were detected in all NET preparations. Histones, proteins from neutrophil granules and cytoskeletal and cytoplasmic proteins appeared to be most abundant (table 1). These data are in agreement with the results from earlier proteomic analyses of NETs [13,52,53] and were further substantiated by the results of an extensive NET proteomics study performed by Chapman et al. [29]. The most commonly detected proteins associated with NETs, irrespective of the mode of NET induction and of the source of the neutrophils (from healthy or diseased donors), are listed in table 1. The proteins are categorized based upon the subcellular compartment from which they originate.
Table 1.
NET-associated proteins.
| subcellular localization | protein names | gene names | protein IDs | Urban et al. 2009 [13] | O'Donoghue et al. 2013 [52] | Lim et al. 2018 [53] | Petretto et al. 2019 [44] | Chapman et al. 2019 [29] |
|---|---|---|---|---|---|---|---|---|
| nucleus | histone H1 | H1 | P10412 | ✓ | ✓ | ✓ | Cit | |
| histone H2A | H2A | Q99878 | ✓ | ✓ | ✓ | ✓ | ✓ | |
| histone H2B | H2B | Q16778 | ✓ | ✓ | ✓ | ✓ | ✓ | |
| histone H3 | H3 | K7EK07 | ✓ | ✓ | ✓ | ✓ | Cit | |
| histone H4 | H4 | P62805 | ✓ | ✓ | ✓ | Cit | ✓ | |
| high mobility group protein B2 | HMGB2 | P26583 | ✓ | ✓ | Cit | |||
| myeloid cell nuclear differentiation antigen | MNDA | P41218 | ✓ | ✓ | ✓ | ✓ | Cit | |
| non-histone chromosomal protein HMG-17 | HMGN2 | P05204 | ✓ | ✓ | Cit | |||
| putative high mobility group protein B1-like 1 | HMGB1 | Q5T7C4 | ✓ | ✓ | ||||
| granules | azurocidin | AZU1 | P20160 | ✓ | ✓ | ✓ | ✓ | Cit |
| cathelicidin antimicrobial peptide | CAMP | J3KNB4 | ✓ | ✓ | ||||
| cathepsin G | CTSG | P08311 | ✓ | ✓ | ✓ | Cit | Cit | |
| eosinophil cationic protein | RNASE3 | P12724 | ✓ | ✓ | ✓ | ✓ | ||
| lactotransferrin | LTF | E7EQB2 | ✓ | ✓ | ✓ | Cit | Cit | |
| leucocyte proteinase 3 | PRTN3 | P24158 | ✓ | ✓ | ✓ | ✓ | ✓ | |
| lipocalin-2 | LCN2 | P80188 | ✓ | ✓ | ✓ | |||
| lysozyme C | LYZ | P61626 | ✓ | ✓ | ✓ | ✓ | ✓ | |
| myeloperoxidase | MPO | P05164-2 | ✓ | ✓ | ✓ | Cit | Cit | |
| neutrophil defensin | DEFA1 | P59665 | ✓ | ✓ | ✓ | ✓ | ✓ | |
| neutroplil elastase | ELANE | P08246 | ✓ | ✓ | ✓ | ✓ | Cit | |
| non-secretory ribonuclease | RNASE2 | P10153 | ✓ | ✓ | ||||
| serotransferrin | TF | P02787 | ✓ | ✓ | ✓ | |||
| cytoskeleton | actin | ACTB | P60709 | ✓ | ✓ | ✓ | ✓ | |
| actin-related protein 2 | ACTR2 | F5H6T1 | ✓ | ✓ | ||||
| α-actinin-1 | ACTN1 | P12814 | ✓ | ✓ | ✓ | ✓ | ||
| α-actinin-4 | ACTN4 | Q43707 | ✓ | ✓ | ✓ | ✓ | ||
| coactosin-like protein | COTL1 | H3BT58 | Cit | ✓ | ||||
| filamin-A | FLNA | Q60FE5 | ✓ | ✓ | ||||
| myosin-9 | MYH9 | P35579 | ✓ | ✓ | ✓ | ✓ | ✓ | |
| plastin-2 | LCP1 | P13796 | ✓ | ✓ | ✓ | ✓ | ✓ | |
| profilin-1 | PFN1 | P07737 | ✓ | ✓ | ✓ | |||
| vimentin | VIM | B0YJC4 | ✓ | ✓ | Cit | |||
| cytosol | α-enolase | ENO1 | P06733 | ✓ | ✓ | ✓ | ✓ | ✓ |
| calmodulin | CALM2 | P0DP25 | ✓ | ✓ | ||||
| fructose-bisphosphate aldolase | ALDOA | H3BQN4 | ✓ | ✓ | ||||
| glyceraldehyde-3-phosphate dehydrogenase | GAPDH | P04406-2 | ✓ | ✓ | ✓ | |||
| L-lactate dehydrogenase A chain | LDHA | P00338 | ✓ | ✓ | ||||
| 6-phosphogluconate dehydrogenase, decarboxylating | PGD | K7EMN2 | ✓ | ✓ | ||||
| S100-A8 | S100A8 | P05109 | ✓ | ✓ | ✓ | Cit | ✓ | |
| S100-A9 | S100A9 | P06702 | ✓ | ✓ | ✓ | ✓ | ✓ | |
| S100-A11 | S100A11 | P31949 | ✓ | ✓ | ||||
| S100-A12 | S100A12 | P80511 | ✓ | ✓ | ✓ | ✓ | ||
| rho GDP-dissociation inhibitor 2 | ARHGDIB | F5H2R5 | ✓ | ✓ | ||||
| serpin B1 | SERPINB1 | P30740 | ✓ | ✓ | ✓ | |||
| transketolase | TKT | P29401 | ✓ | ✓ | ✓ | ✓ | ✓ | |
| plasma membrane | carcinoembryonic antigen-related cell adhesion molecule 6 | CEACAM6 | P40199 | ✓ | ✓ | |||
| carcinoembryonic antigen-related cell adhesion molecule 8 | CEACAM8 | P31997 | ✓ | ✓ | ||||
| integrin α-M | ITGAM | P11215 | Cit | ✓ | ||||
| protein-tyrosine-phosphatase | PTPRC | A0A140TA77 | ✓ | ✓ | ||||
| membrane associated | annexin A1 | ANXA1 | P04083 | ✓ | ✓ | |||
| annexin A3 | ANXA3 | P12429 | ✓ | ✓ | ||||
| endoplasmic reticulum | calnexin | CANX | D6RGY2 | ✓ | ✓ | |||
| calreticulin | CALR | K7EJB9 | ✓ | ✓ | ||||
| peroxisome | catalase | CAT | P04040 | ✓ | ✓ | ✓ | ✓ |
✓ indicates that protein was detected in the proteomic dataset of the respective study; Cit indicates that the protein was detected and that evidence for its citrullination was obtained.
The quantitative proteomics approach applied by Chapman and co-workers [29] allowed a detailed comparison of the NET proteomes obtained in response to PMA and A23187 and this showed significant differences in NET proteins. This also includes quantitative differences between common NET proteins that are listed in table 1. These investigators also compared NETs from RA and SLE neutrophils and observed only minor differences.
The presence of post-translational modifications, a.o. citrullination, in the NET proteomes was addressed in the studies of and Chapman et al. and Petretto et al. [29,44]. Citrullinated residues were detected in 15 of the 54 common NET proteins (table 1). This indicates that citrullination is a common type of post-translational modification of NET proteins, since it also has to be taken into account that the sequence coverage in protein characterization by mass spectrometry in general is much less than 100%. In addition to the nuclear proteins like histones, neutrophil granule proteins seem to be especially frequently citrullinated. However, as already mentioned above, the citrullinated epitopes associated with NET proteins do not seem to play a prominent role in the recognition of NETs by RA and SLE autoantibodies.
5. Proteolytic degradation of NET components
During our studies we noticed that the addition of protease inhibitors often had strong effects on the efficiency with which a subset of NET-associated proteins was detected. We argued that this might be due to the activity of neutrophil proteases that are NET-associated, such as cathepsin G, leucocyte proteinase 3 and NE. All of these belong to the family of serine proteases and serine protease inhibitors like phenylmethylsulfonyl fluoride (PMSF) can therefore be used to interfere with the proteolytic activity of these proteases. It should, however, be noted that proper NET formation is also dependent on the activity of (some of) these proteases, which means that the inhibitor should not be added too early. In the case of PMA stimulation PMSF was added to the neutrophil cultures after 2 h, when NET formation was irreversible in most of the neutrophils. The applied methods are described in the electronic supplementary material.
Indeed, the presence of PMSF severely affected the integrity of NET-associated proteins [54]. When PMSF was added during the last stages of NET formation and during NET harvesting, many NET proteins appeared to be more abundant than in the absence of PMSF, as demonstrated by total protein staining of SDS-PAGE gels. The analysis of specific proteins by immunoblotting confirmed that many proteins could be efficiently detected unless NETs were prepared in the absence of PMSF, which resulted in a severe reduction in the signals or a complete disappearance (figure 5). Not all proteins appeared to be equally susceptible to degradation by neutrophil proteases. Full-length histones H2B, H3 and H4 are examples of proteins that were strongly affected. In addition to the analysis of histones by immunoblotting, also specifically reactive antibodies with citrullinated H3 were used. Citrullinated H3 is commonly used as a NET citrullination marker and was reduced to undetectable levels when the NETs were prepared in the absence of PMSF [54].
Figure 5.
Degradation of NET-associated proteins by neutrophil proteases. Isolated neutrophils from healthy individuals were treated with PMA and after 3 h incubation NETs were harvested and analysed by immunoblotting using antibodies to specific NET-associated proteins. During the third hour after the addition of PMA the serine protease inhibitor was added every 15 min to a parallel batch of neutrophils. Signal intensities of the indicated proteins on the blots were quantified and the fraction of these proteins that was detected in the absence of PMSF was determined. Note that these data in part are reproduced from [52].
Other proteins that appeared to be degraded by neutrophil proteases are the NET-associated proteins HMG-B2, MNDA, actin and myosin. Interestingly, neutrophil proteins that are directly involved in the antimicrobial activity of NETs, such as MPO and NE, were only marginally affected when PMSF was absent. Cytosolic proteins α-enolase and S100A9 were moderately affected by the neutrophil proteases. Similar results were obtained with alternative serine protease inhibitors (pefabloc; α-1-antitrypsin). As discussed above, patients with autoimmune diseases often have antibodies against NET components in their sera. When RA and SLE sera were used to analyse the association of autoantigenic proteins with NETs, many reactivities appeared to be dependent on the preparation of NETs in the presence of PMSF (figure 6). These data indicate that a substantial fraction of autoreactive epitopes associated with NETs are disrupted by the action of neutrophil proteases. Currently, it is not known whether autoepitope disruption occurs during the process of NET formation or after NET formation when these proteases, which are also NET-associated, are in close proximity to the other NET-associated proteins.
Figure 6.
Neutrophil proteases degrade autoepitopes recognized by RA and SLE sera. Reactivity of RA (1–7) and SLE (8–14) sera with NET-associated proteins produced without PMSF (a) or with PMSF (b). NET harvests from PMA-activated neutrophils were analysed by immunoblotting. The positions of molecular weight markers (× 1000) are indicated on the left. Note that these data in part are reproduced from [52].
6. Conclusion
Until recently citrullinated epitopes were considered to play a major role in the recognition of NETs by autoantibodies in the sera of autoimmune patients. Indeed, citrullination has been shown to be among the most prominent forms of post-translational modification of NET-associated proteins. Moreover, anti-citrullinated protein antibodies are frequently produced by RA patients and can also be detected in the sera of patients with related disorders.
Our results indicate that autoantibody reactivity to NET components is not restricted to RA, but is also frequently seen in other autoimmune diseases. This strongly suggests that the vast majority of anti-NET autoantibodies are not targeting citrullinated epitopes. A weakness of our study is that we did not substantiate this with competition experiments. It remains to be determined what are the most important NET-associated antigens recognized by autoantibodies. Most likely, these are common NET-associated proteins, which are shared by NETs induced with different stimuli or via distinct pathways.
Neutrophil serine proteases play an important role during NET formation and also become associated with NETs. When NETs are produced by isolated human neutrophils, these proteases appeared to cleave many NET proteins, which not only affected the total protein composition of NETs, but also disrupted many autoepitopes. It is unknown to what extent this phenomenon affects NET composition in vivo, where endogenous protease inhibitors may modulate these processes. In any case, the addition of serine protease inhibitors should be seriously considered when isolated neutrophils are used to study the structure and properties of NETs.
Acknowledgements
We are very grateful to all colleagues who voluntarily donated blood for neutrophil isolations.
Ethics
These studies were carried out in accordance with the recommendations of the National Research Ethics Service (NRES) Committee Northwest (Greater Manchester West), UK and were approved by the ‘Commissie Mensgebonden Onderzoek’ region Arnhem Nijmegen, with written informed consent from all subjects in accordance with the Declaration of Helsinki.
Data accessibility
The data that support the findings of this study are available from the corresponding author, G.J.M.P., upon reasonable request.
The applied methods are provided in electronic supplementary material [55].
Declaration of AI use
We have not used AI-assisted technologies in creating this article.
Authors' contributions
C.B.: conceptualization, data curation, formal analysis, investigation, methodology, validation, visualization, writing—review and editing; G.J.M.P.: conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, project administration, resources, supervision, validation, visualization, writing—original draft, writing—review and editing.
Both authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
We declare we have no competing interests.
Funding
This work was supported in part by the Dutch Technology Foundation STW (grant no. 05188).
References
- 1.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. 2004. Neutrophil extracellular traps kill bacteria. Science. 303, 1532-1535. ( 10.1126/science.1092385) [DOI] [PubMed] [Google Scholar]
- 2.Neubert E, et al. 2018. Chromatin swelling drives neutrophil extracellular trap release. Nat. Commun.. 9, 3767. ( 10.1038/s41467-018-06263-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kenny EF, Herzig A, Kruger R, Muth A, Mondal S, Thompson PR, Brinkmann V, Bernuth H, Zychlinsky A. 2017. Diverse stimuli engage different neutrophil extracellular trap pathways. Elife 6, e24437. ( 10.7554/eLife.24437) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Douda DN, Khan MA, Grasemann H, Palaniyar N. 2015. SK3 channel and mitochondrial ROS mediate NADPH oxidase-independent NETosis induced by calcium influx. Proc. Natl Acad. Sci. USA 112, 2817-2822. ( 10.1073/pnas.1414055112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaplan MJ, Radic M. 2012. Neutrophil extracellular traps: double-edged swords of innate immunity. J. Immunol. 189, 2689-2695. ( 10.4049/jimmunol.1201719) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoppenbrouwers T, et al. 2018. Staphylococcal protein A is a key factor in neutrophil extracellular traps formation. Front. Immunol. 9, 165. ( 10.3389/fimmu.2018.00165) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Urban CF, Reichard U, Brinkmann V, Zychlinsky A. 2006. Neutrophil extracellular traps capture and kill Candida albicans yeast and hyphal forms. Cell. Microbiol. 8, 668-676. ( 10.1111/j.1462-5822.2005.00659.x) [DOI] [PubMed] [Google Scholar]
- 8.Khandpur R, et al. 2013. NETs are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis. Sci. Transl. Med. 5, 178ra40. ( 10.1126/scitranslmed.3005580) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boeltz S, et al. 2019. To NET or not to NET: current opinions and state of the science regarding the formation of neutrophil extracellular traps. Cell Death Diff. 26, 395-408. ( 10.1038/s41418-018-0261-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Castagna M, Takai Y, Kaibuchi K, Sano K, Kikkawa U, Nishizuka Y. 1982. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J. Biol. Chem. 257, 7847-7851. ( 10.1016/S0021-9258(18)34459-4) [DOI] [PubMed] [Google Scholar]
- 11.Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V, Weinrauch Y, Brinkmann V, Zychlinsky A. 2007. Novel cell death program leads to neutrophil extracellular traps. J. Cell Biol. 176, 231-241. ( 10.1083/jcb.200606027) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brinkmann V, Zychlinsky A. 2012. Neutrophil extracellular traps: is immunity the second function of chromatin? J. Cell Biol. 198, 773-783. ( 10.1083/jcb.201203170) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Urban CF, Ermert D, Schmid M, Abu-Abed U, Goosmann C, Nacken W, Brinkmann V, Jungblut PR, Zychlinsky A. 2009. Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans. PLoS Pathog. 5, e1000639. ( 10.1371/journal.ppat.1000639) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parker H, Albrett AM, Kettle AJ, Winterbourn CC. 2012. Myeloperoxidase associated with neutrophil extracellular traps is active and mediates bacterial killing in the presence of hydrogen peroxide. J. Leukoc. Biol. 91, 369-376. ( 10.1189/jlb.0711387) [DOI] [PubMed] [Google Scholar]
- 15.Pilsczek FH, et al. 2010. A novel mechanism of rapid nuclear neutrophil extracellular trap formation in response to Staphylococcus aureus. J. Immunol. 185, 7413-7425. ( 10.4049/jimmunol.1000675) [DOI] [PubMed] [Google Scholar]
- 16.Hakkim A, Fuchs TA, Martinez NE, Hess S, Prinz H, Zychlinsky A, Waldmann H. 2011. Activation of the Raf-MEK-ERK pathway is required for neutrophil extracellular trap formation. Nat. Chem. Biol. 7, 75-77. ( 10.1038/nchembio.496) [DOI] [PubMed] [Google Scholar]
- 17.Al-Khafaji AB, Tohme S, Yazdani HO, Miller D, Huang H, Tsung A. 2016. Superoxide induces neutrophil extracellular trap formation in a TLR-4 and NOX-dependent mechanism. Mol. Med. 22, 621-631. ( 10.2119/molmed.2016.00054) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Metzler KD, Fuchs TA, Nauseef WM, Reumaux D, Roesler J, Schulze I, Wahn V, Papayannopoulos V, Zychlinsky A. 2011. Myeloperoxidase is required for neutrophil extracellular trap formation: implications for innate immunity. Blood 117, 953-959. ( 10.1182/blood-2010-06-290171) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sollberger G, et al. 2018. Gasdermin D plays a vital role in the generation of neutrophil extracellular traps. Sci. Immunol. 3, eaar6689. ( 10.1126/sciimmunol.aar6689) [DOI] [PubMed] [Google Scholar]
- 20.Metzler KD, Goosmann C, Lubojemska A, Zychlinsky A, Papayannopoulos V. 2014. A myeloperoxidase-containing complex regulates neutrophil elastase release and actin dynamics during NETosis. Cell Rep. 8, 883-896. ( 10.1016/j.celrep.2014.06.044) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neeli I, Dwivedi N, Khan S, Radic M. 2009. Regulation of extracellular chromatin release from neutrophils. J. Innate Immun. 1, 194-201. ( 10.1159/000206974) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Papayannopoulos V, Metzler KD, Hakkim A, Zychlinsky A. 2010. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J. Cell Biol. 191, 677-691. ( 10.1083/jcb.201006052) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Konig MF, Andrade F. 2016. A critical reappraisal of neutrophil extracellular traps and NETosis mimics based on differential requirements for protein citrullination. Front. Immunol. 7, 461. ( 10.3389/fimmu.2016.00461) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lewis HD, et al. 2015. Inhibition of PAD4 activity is sufficient to disrupt mouse and human NET formation. Nat. Chem. Biol. 11, 189-191. ( 10.1038/nchembio.1735) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vossenaar ER, Zendman AJ, Van Venrooij WJ, Pruijn GJ. 2003. PAD, a growing family of citrullinating enzymes: genes, features and involvement in disease. Bioessays. 25, 1106-1118. ( 10.1002/bies.10357) [DOI] [PubMed] [Google Scholar]
- 26.Leshner M, Wang S, Lewis C, Zheng H, Chen XA, Santy L, Wang Y. 2012. PAD4 mediated histone hypercitrullination induces heterochromatin decondensation and chromatin unfolding to form neutrophil extracellular trap-like structures. Front. Immunol. 3, 307. ( 10.3389/fimmu.2012.00307) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou Y, et al. 2018. Evidence for a direct link between PAD4-mediated citrullination and the oxidative burst in human neutrophils. Sci. Rep. 8, 15228. ( 10.1038/s41598-018-33385-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pieterse E, et al. 2018. Cleaved N-terminal histone tails distinguish between NADPH oxidase (NOX)-dependent and NOX-independent pathways of neutrophil extracellular trap formation. Ann. Rheum. Dis. 77, 1790-1798. ( 10.1136/annrheumdis-2018-213223) [DOI] [PubMed] [Google Scholar]
- 29.Chapman EA, Lyon M, Simpson D, Mason D, Beynon RJ, Moots RJ, Wright HL. 2019. Caught in a trap? Proteomic analysis of neutrophil extracellular traps in rheumatoid arthritis and systemic lupus erythematosus. Front. Immunol. 10, 423. ( 10.3389/fimmu.2019.00423) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neubert E, Senger-Sander SN, Manzke VS, Busse J, Polo E, Scheidmann SEF, Schön MP, Kruss S, Erpenbeck L. 2019. Serum and serum albumin inhibit in vitro formation of neutrophil extracellular traps (NETs). Front. Immunol. 10, 12. ( 10.3389/fimmu.2019.00012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Y, et al. 2009. Histone hypercitrullination mediates chromatin decondensation and neutrophil extracellular trap formation. J. Cell Biol. 184, 205-213. ( 10.1083/jcb.200806072) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Claushuis TAM, et al. 2018. Role of peptidylarginine deiminase 4 in neutrophil extracellular trap formation and host defense during Klebsiella pneumoniae-induced pneumonia-derived sepsis. J. Immunol. 201, 1241-1252. ( 10.4049/jimmunol.1800314) [DOI] [PubMed] [Google Scholar]
- 33.Guiducci E, Lemberg C, Kung N, Schraner E, Theocharides APA, Leibundgut-Landmann S. 2018. Candida albicans-induced NETosis is independent of peptidylarginine deiminase 4. Front. Immunol. 9, 1573. ( 10.3389/fimmu.2018.01573) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Remijsen Q, et al. 2011. Neutrophil extracellular trap cell death requires both autophagy and superoxide generation. Cell Res. 21, 290-304. ( 10.1038/cr.2010.150) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Skendros P, Mitroulis I, Ritis K. 2018. Autophagy in neutrophils: from granulopoiesis to neutrophil extracellular traps. Front. Cell Dev. Biol. 6, 109. ( 10.3389/fcell.2018.00109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kenno S, Perito S, Mosci P, Vecchiarelli A, Monari C. 2016. Autophagy and reactive oxygen species are involved in neutrophil extracellular traps release induced by C. albicans morphotypes. Front. Microbiol. 7, 879. ( 10.3389/fmicb.2016.00879) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Romao S, Puente ET, Nytko KJ, Siler U, Munz C, Reichenbach J. 2015. Defective nuclear entry of hydrolases prevents neutrophil extracellular trap formation in patients with chronic granulomatous disease. J. Allergy Clin. Immunol. 136, 1703-6 e1-5. ( 10.1016/j.jaci.2015.09.007) [DOI] [PubMed] [Google Scholar]
- 38.Hakkim A, Furnrohr BG, Amann K, Laube B, Abed UA, Brinkmann V, Herrmann M, Voll RE, Zychlinsky A. 2010. Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proc. Natl Acad. Sci. USA 107, 9813-9818. ( 10.1073/pnas.0909927107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gupta AK, Joshi MB, Philippova M, Erne P, Hasler P, Hahn S, Resink TJ. 2010. Activated endothelial cells induce neutrophil extracellular traps and are susceptible to NETosis-mediated cell death. FEBS Lett. 584, 3193-3197. ( 10.1016/j.febslet.2010.06.006) [DOI] [PubMed] [Google Scholar]
- 40.Jimenez-Alcazar M, et al. 2017. Host DNases prevent vascular occlusion by neutrophil extracellular traps. Science. 358, 1202-1206. ( 10.1126/science.aam8897) [DOI] [PubMed] [Google Scholar]
- 41.Dwivedi N, Radic M. 2014. Citrullination of autoantigens implicates NETosis in the induction of autoimmunity. Ann. Rheum. Dis. 73, 483-491. ( 10.1136/annrheumdis-2013-203844) [DOI] [PubMed] [Google Scholar]
- 42.Maugeri N, et al. 2018. Platelet microparticles sustain autophagy-associated activation of neutrophils in systemic sclerosis. Science Transl. Med. 10, eaao3089. ( 10.1126/scitranslmed.aao3089) [DOI] [PubMed] [Google Scholar]
- 43.Didier K, Giusti D, Le Jan S, Terryn C, Muller C, Pham BN, Le Naour R, Antonicelli FD, Servettaz A. 2020. Neutrophil extracellular traps generation relates with early stage and vascular complications in systemic sclerosis. J. Clin. Med. 9, 2136. ( 10.3390/jcm9072136) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Petretto A, Bruschi M, Pratesi F, Croia C, Candiano G, Ghiggeri G, Migliorini P. 2019. Neutrophil extracellular traps (NET) induced by different stimuli: a comparative proteomic analysis. PLoS ONE 14, e0218946. ( 10.1371/journal.pone.0218946) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pratesi F, et al. 2014. Antibodies from patients with rheumatoid arthritis target citrullinated histone 4 contained in neutrophils extracellular traps. Ann. Rheum. Dis. 73, 1414-1422. ( 10.1136/annrheumdis-2012-202765) [DOI] [PubMed] [Google Scholar]
- 46.Carmona-Rivera C, et al. 2017. Synovial fibroblast-neutrophil interactions promote pathogenic adaptive immunity in rheumatoid arthritis. Sci. Immunol. 2, eaag3358. ( 10.1126/sciimmunol.aag3358) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sur Chowdhury C, Giaglis S, Walker UA, Buser A, Hahn S, Hasler P. 2014. Enhanced neutrophil extracellular trap generation in rheumatoid arthritis: analysis of underlying signal transduction pathways and potential diagnostic utility. Arthritis Res. Ther. 16, R122. ( 10.1186/ar4579) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aleyd E, Al M, Tuk CW, Van Der Laken CJ, Van Egmond M. 2016. IgA complexes in plasma and synovial fluid of patients with rheumatoid arthritis induce neutrophil extracellular traps via FcαRI. J. Immunol. 197, 4552-4559. ( 10.4049/jimmunol.1502353) [DOI] [PubMed] [Google Scholar]
- 49.Ribon M, Seninet S, Mussard J, Sebbag M, Clavel C, Serre G, Boissier M-C, Semerano L, Decker P. 2019. Neutrophil extracellular traps exert both pro- and anti-inflammatory actions in rheumatoid arthritis that are modulated by C1q and LL-37. J. Autoimmun. 98, 122-131. ( 10.1016/j.jaut.2019.01.003) [DOI] [PubMed] [Google Scholar]
- 50.De Bont CM, Koopman WJH, Boelens WC, Pruijn GJM. 2018. Stimulus-dependent chromatin dynamics, citrullination, calcium signalling and ROS production during NET formation. Biochim. Biophys. Acta Mol. Cell. Res. 1865, 1621-1629. ( 10.1016/j.bbamcr.2018.08.014) [DOI] [PubMed] [Google Scholar]
- 51.De Bont CM, Stokman MEM, Faas P, Thurlings RM, Boelens WC, Wright HL, Pruijn GJM. 2020. Autoantibodies to neutrophil extracellular traps represent a potential serological biomarker in rheumatoid arthritis. J. Autoimmun. 113, 102484. ( 10.1016/j.jaut.2020.102484) [DOI] [PubMed] [Google Scholar]
- 52.O'Donoghue AJ, Jin Y, Knudsen GM, Perera NC, Jenne DE, Murphy JE, Craik CS, Hermiston TW. 2013. Global substrate profiling of proteases in human neutrophil extracellular traps reveals consensus motif predominantly contributed by elastase. PLOS ONE 8, e75141. ( 10.1371/journal.pone.0075141) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lim CH, Adav SS, Sze SK, Choong YK, Saravanan R, Schmidtchen A. 2018. Thrombin and plasmin alter the proteome of neutrophil extracellular traps. Front. Immunol. 9, 1554. ( 10.3389/fimmu.2018.01554) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.De Bont CM, Eerden N, Boelens WC, Pruijn GJM. 2020. Neutrophil proteases degrade autoepitopes of NET-associated proteins. Clin. Exp. Immunol. 199, 1-8. ( 10.1111/cei.13392) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.de Bont C, Pruijn GJM. 2023. Citrulline is not a major determinant of autoantibody reactivity to neutrophil extracellular traps. Figshare. ( 10.6084/m9.figshare.c.6806617) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- de Bont C, Pruijn GJM. 2023. Citrulline is not a major determinant of autoantibody reactivity to neutrophil extracellular traps. Figshare. ( 10.6084/m9.figshare.c.6806617) [DOI] [PMC free article] [PubMed]
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, G.J.M.P., upon reasonable request.
The applied methods are provided in electronic supplementary material [55].