Abstract
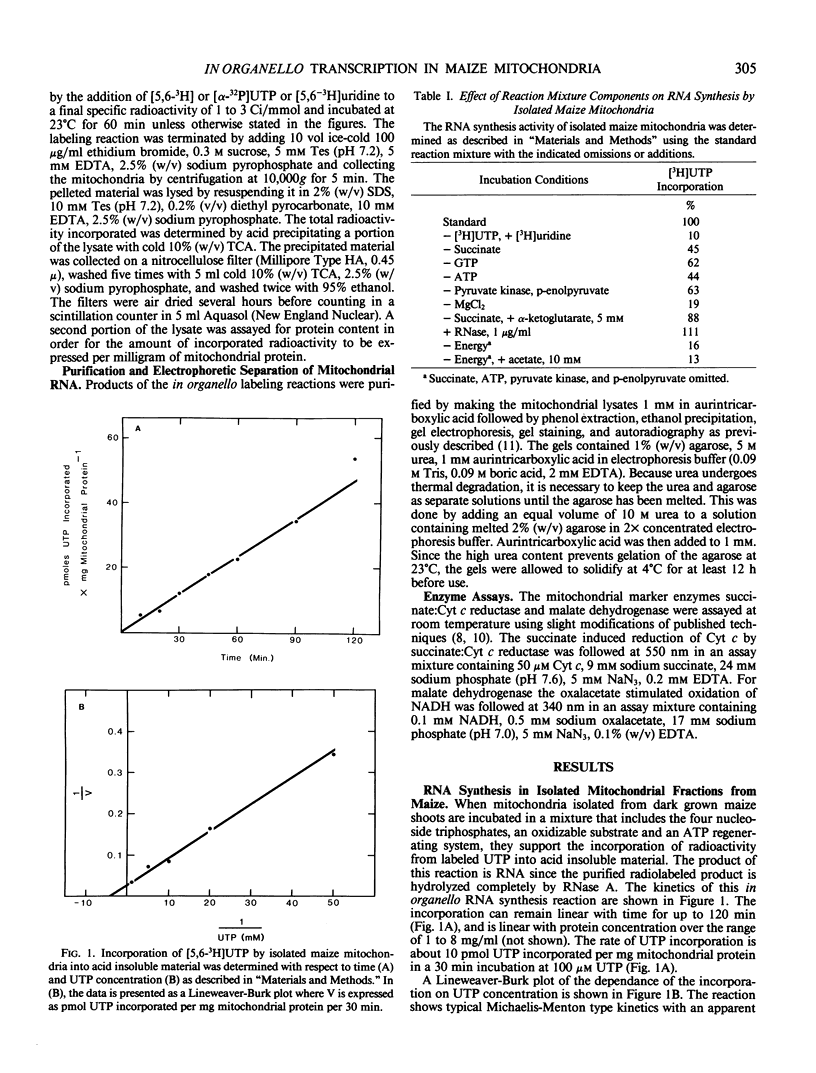
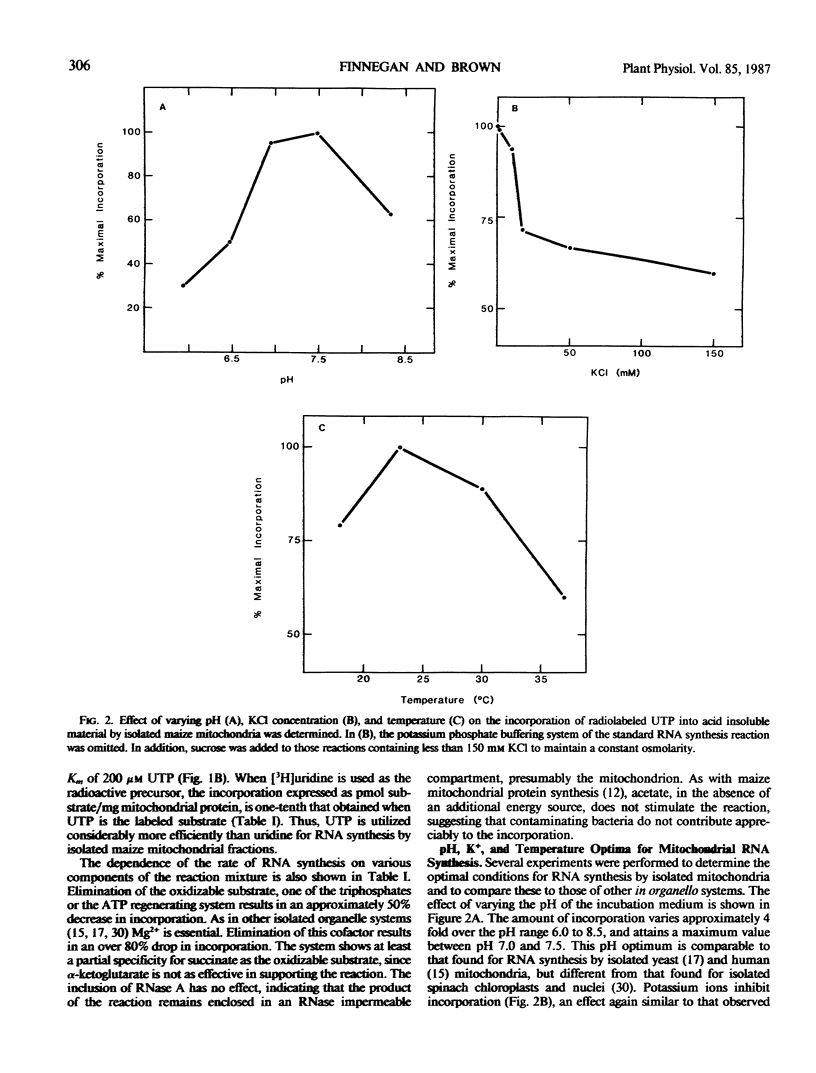
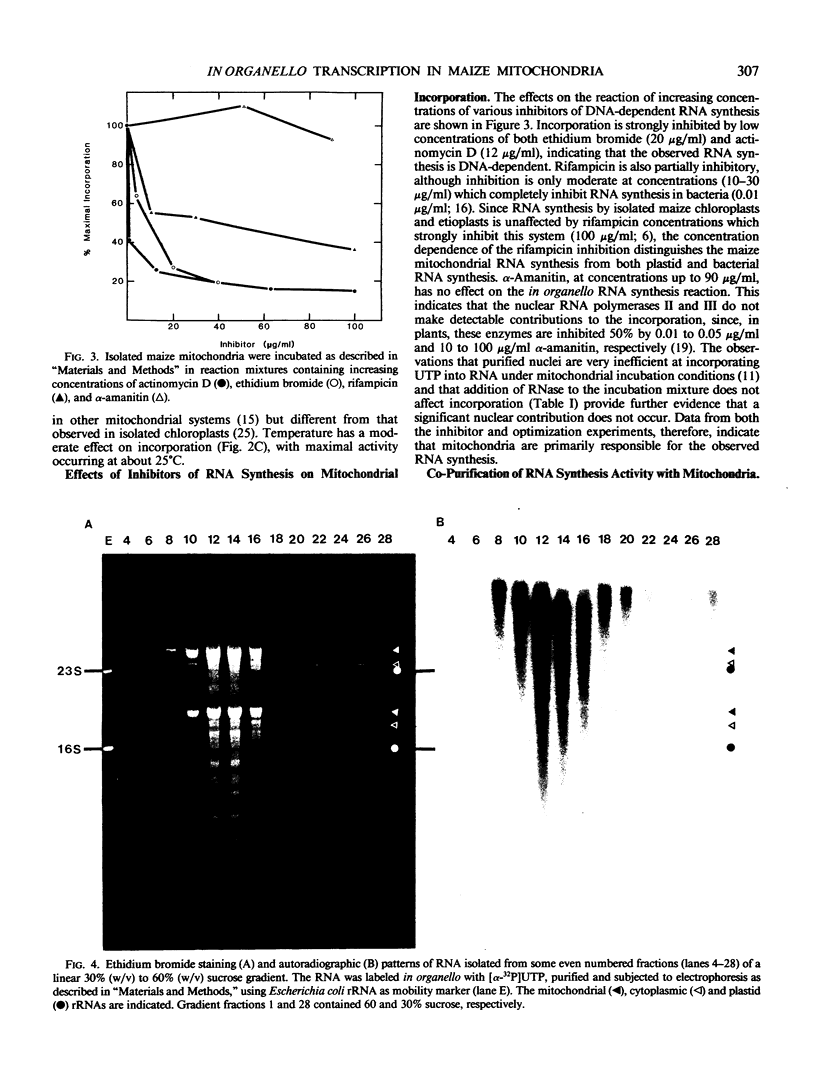
Purified mitochondrial preparations from etiolated maize shoots support the incorporation of radioactivity from labeled UTP into RNA. The incorporation is linear with time for up to 2 hours, shows Michaelis-Menton kinetics with respect to the concentration of the labeled substrate, UTP, and has salt and pH optima which are different than those previously reported for RNA synthesis by isolated chloroplasts. When a crude mitochondrial preparation is subjected to isopycnic sucrose gradient centrifugation, the bulk of the RNA synthetic activity co-sediments with mitochondrial marker enzymes and with the mitochondrial 26S and 18S rRNAs. Maize mitochondrial RNA synthesis is prevented by actinomycin D and ethidium bromide but unaffected by α-amanitin. It is strongly inhibited by rifampicin at concentrations which have no effect on nuclear and chloroplast RNA synthesis, but only moderately inhibited by rifampicin at concentrations which completely inhibit bacterial RNA synthesis. The optimization, cell fractionation, and inhibitor data all suggest that contaminating organelles and bacteria do not contribute appreciably to the RNA synthesis in purified mitochondrial preparations.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman A., Cohen B. N., Weissbach H., Brot N. Transcriptional activity of isolated maize chloroplasts. Arch Biochem Biophys. 1984 Nov 15;235(1):26–33. doi: 10.1016/0003-9861(84)90251-0. [DOI] [PubMed] [Google Scholar]
- Bhat K. S., Kantharaj G. R., Avadhani N. G. Nature of steady-state and newly synthesized mitochondrial messenger ribonucleic acids in mouse liver and Ehrlich ascites tumor cells. Biochemistry. 1984 Apr 10;23(8):1695–1701. doi: 10.1021/bi00303a018. [DOI] [PubMed] [Google Scholar]
- Boerner P., Mason T. L., Fox T. D. Synthesis and processing of ribosomal RNA in isolated yeast mitochondria. Nucleic Acids Res. 1981 Dec 11;9(23):6379–6390. doi: 10.1093/nar/9.23.6379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottomley W., Smith H. J., Bogorad L. RNA polymerases of maize: partial purification and properties of the chloroplast enzyme. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2412–2416. doi: 10.1073/pnas.68.10.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottomley W., Spencer D., Wheeler A. M., Whitfeld P. R. The effect of a range of RNA polymerase inhibitors on RNA synthesis in higher plant chloroplasts and nuclei. Arch Biochem Biophys. 1971 Mar;143(1):269–275. doi: 10.1016/0003-9861(71)90209-8. [DOI] [PubMed] [Google Scholar]
- Boutry M., Briquet M., Goffeau A. The alpha subunit of a plant mitochondrial F1-ATPase is translated in mitochondria. J Biol Chem. 1983 Jul 25;258(14):8524–8526. [PubMed] [Google Scholar]
- Brown G. G., Beattie D. S. Formation of the yeast mitochondrial membrane. V Differences in the assembly process of cytochrome oxidase and coenzyme QH2: cytochrome c reductase during respiratory adaptation. Biochim Biophys Acta. 1978 Jan 18;538(2):173–187. doi: 10.1016/0304-4165(78)90344-6. [DOI] [PubMed] [Google Scholar]
- Douce R., Mannella C. A., Bonner W. D., Jr The external NADH dehydrogenases of intact plant mitochondria. Biochim Biophys Acta. 1973 Jan 18;292(1):105–116. doi: 10.1016/0005-2728(73)90255-7. [DOI] [PubMed] [Google Scholar]
- Finnegan P. M., Brown G. G. Autonomously replicating RNA in mitochondria of maize plants with S-type cytoplasm. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5175–5179. doi: 10.1073/pnas.83.14.5175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forde B. G., Oliver R. J., Leaver C. J., Gunn R. E., Kemble R. J. Classification of normal and male-sterile cytoplasms in maize. I. Electrophoretic analysis of variation in mitochondrially synthesized proteins. Genetics. 1980 Jun;95(2):443–450. doi: 10.1093/genetics/95.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox T. D., Leaver C. J. The Zea mays mitochondrial gene coding cytochrome oxidase subunit II has an intervening sequence and does not contain TGA codons. Cell. 1981 Nov;26(3 Pt 1):315–323. doi: 10.1016/0092-8674(81)90200-2. [DOI] [PubMed] [Google Scholar]
- Gaines G., Attardi G. Highly efficient RNA-synthesizing system that uses isolated human mitochondria: new initiation events and in vivo-like processing patterns. Mol Cell Biol. 1984 Aug;4(8):1605–1617. doi: 10.1128/mcb.4.8.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaines G., Attardi G. Intercalating drugs and low temperatures inhibit synthesis and processing of ribosomal RNA in isolated human mitochondria. J Mol Biol. 1984 Feb 5;172(4):451–466. doi: 10.1016/s0022-2836(84)80017-0. [DOI] [PubMed] [Google Scholar]
- Groot G. S., van Harten-Loosbroek N., van Ommen G. J., Pijst H. L. RNA synthesis in isolated yeast mitochondria. Nucleic Acids Res. 1981 Dec 11;9(23):6369–6377. doi: 10.1093/nar/9.23.6369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruissem W., Greenberg B. M., Zurawski G., Prescott D. M., Hallick R. B. Biosynthesis of chloroplast transfer RNA in a spinach chloroplast transcription system. Cell. 1983 Dec;35(3 Pt 2):815–828. doi: 10.1016/0092-8674(83)90114-9. [DOI] [PubMed] [Google Scholar]
- Hack E., Leaver C. J. The alpha-subunit of the maize F(1)-ATPase is synthesised in the mitochondrion. EMBO J. 1983;2(10):1783–1789. doi: 10.1002/j.1460-2075.1983.tb01658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley M. R., Ellis R. J. Ribonucleic acid synthesis in chloroplasts. Biochem J. 1973 May;134(1):249–262. doi: 10.1042/bj1340249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantharaj G. R., Bhat K. S., Avadhani N. G. Mode of transcription and maturation of ribosomal ribonucleic acid in vitro in mitochondria from Ehrlich ascites cells. Biochemistry. 1983 Jun 21;22(13):3151–3156. doi: 10.1021/bi00282a018. [DOI] [PubMed] [Google Scholar]
- Kemble R. J., Gunn R. E., Flavell R. B. Classification of Normal and Male-Sterile Cytoplasms in Maize. II. Electrophoretic Analysis of DNA Species in Mitochondria. Genetics. 1980 Jun;95(2):451–458. doi: 10.1093/genetics/95.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leaver C. J., Harmey M. A. Higher-plant mitochondrial ribosomes contain a 5S ribosomal ribonucleic acid component. Biochem J. 1976 Jul 1;157(1):275–277. doi: 10.1042/bj1570275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita J. O., Rushlow K. E., Hallick R. B. Characterization of a Euglena gracilis chloroplast RNA polymerase specific for ribosomal RNA genes. J Biol Chem. 1985 Sep 15;260(20):11194–11199. [PubMed] [Google Scholar]
- Newman D., Martin N. Synthesis of RNA in isolated mitochondria from Saccharomyces cerevisiae. Plasmid. 1982 Jan;7(1):66–76. doi: 10.1016/0147-619x(82)90028-2. [DOI] [PubMed] [Google Scholar]
- Polya G. M., Jagendorf A. T. Wheat leaf RNA polymerases. I. Partial purification and characterization of nuclear, chloroplast and soluble DNA-dependent enzymes. Arch Biochem Biophys. 1971 Oct;146(2):635–648. doi: 10.1016/0003-9861(71)90172-x. [DOI] [PubMed] [Google Scholar]
- Spencer D., Whitfeld P. R. Ribonucleic acid synthesizing activity of spinach chloroplasts and nuclei. Arch Biochem Biophys. 1967 Aug;121(2):336–345. doi: 10.1016/0003-9861(67)90085-9. [DOI] [PubMed] [Google Scholar]