Abstract
We characterized previously identified RNA viruses (L-A, L-BC, 20S, and 23S), L-A–dependent M satellites (M1, M2, M28, and Mlus), and M satellite–dependent killer phenotypes in the Saccharomyces cerevisiae 100-genomes genetic resource population. L-BC was present in all strains, albeit in 2 distinct levels, L-BChi and L-BClo; the L-BC level is associated with the L-BC genotype. L-BChi, L-A, 20S, 23S, M1, M2, and Mlus (M28 was absent) were in fewer strains than the similarly inherited 2µ plasmid. Novel L-A–dependent phenotypes were identified. Ten M+ strains exhibited M satellite–dependent killing (K+) of at least 1 of the naturally M0 and cured M0 derivatives of the 100-genomes strains; in these M0 strains, sensitivities to K1+, K2+, and K28+ strains varied. Finally, to complement our M satellite–encoded killer toxin analysis, we assembled the chromosomal KHS1 and KHR1 killer genes and used naturally M0 and cured M0 derivatives of the 100-genomes strains to assess and characterize the chromosomal killer phenotypes.
Keywords: RNA virus, Saccharomyces cerevisiae, killer, phenotypes, associations, 100-genomes strains
Introduction
Like many other species of yeasts and fungi (McCabe et al. 1999; Nuss 2005; Ghabrial and Suzuki 2009; Pearson et al. 2009; Roossinck 2011; Xie and Jiang 2014; Ghabrial et al. 2015), the yeast Saccharomyces cerevisiae can host symbiotic cytoplasmically transmitted RNA viruses, which include 2 narnaviruses, 20S (2,514 bases) and 23S (2,891 bases), and 2 totiviruses, L-A (4,579 bp) and L-BC (4,615 bp), reviewed in (Wickner 1992, 1996a, 1996b; Wickner et al. 2013; Rowley 2017). In addition, L-A–containing S. cerevisiae strains also may host any 1 of 4 satellite (that is, requiring L-A for replication) RNAs—M1 (1.6 kbp), M2 (1.5 kbp), M28 (1.75 kbp), and Mlus (2.1–2.3 kbp). Fully functional M1, M2, M28, and Mlus satellites encode secreted K1, K2, K28, and Klus killer toxins (K+), respectively, and corresponding killer toxin–specific resistance (R+) functions, resulting in S. cerevisiae strains with 4 distinct M satellite–dependent killer phenotypes (Magliani et al. 1997; Schmitt and Breinig 2002, 2006; Rodriguez-Cousiño et al. 2011).
Although the RNA viruses, M satellites, and M satellite–dependent killer phenotypes of S. cerevisiae have been extensively characterized (Wickner 1992, 1996a, 1996b; Magliani et al. 1997; Schmitt and Breinig 2002, 2006; Rodriguez-Cousiño et al. 2011; Wickner et al. 2013; Rodriguez-Cousino and Esteban 2017; Rowley 2017), most of this characterization appears to have been done in laboratory strains rather than population studies. Of the previous population studies, most focused on assessing the frequencies in S. cerevisiae strains of killer phenotypes (Philliskirk and Young 1975; Rosini 1983; Nakayashiki et al. 2005; Baeza et al. 2008; Rodriguez-Cousiño et al. 2011; Pieczynska et al. 2013; Chang et al. 2015) but did so using different techniques and to different extents. With respect to killer phenotypes, some of these previous population studies identified killer S. cerevisiae strains using only 1 killer toxin–sensitive strain, some identified the killer type(s), some determined the M satellite dependence of killer phenotype(s), and some assayed M satellite–independent resistance to known killer strain(s). With respect to the RNA viruses, previous population studies used northern analysis to assess the frequencies of 20S and 23S (Lopez et al. 2002) and PCR with 1 primer pair each to the L-A, L-BC, 20S, and 23S viruses to assess their frequencies (Nakayashiki et al. 2005). Nevertheless, no single study has used multiple, consistent techniques to explore RNA viruses, M satellites, and killer phenotypes across a broad set of genetically diverse, well-characterized strains.
Our S. cerevisiae 100-genomes population studies described their nuclear genome sequences, population structure, phenotypes, and genotype associations (Strope et al. 2015b), as well as their 2µ plasmid (Strope et al. 2015a) and mitochondrial genomes (Vijayraghavan et al. 2019). Unlike these DNA genome components, however, RNA viruses and M satellites are not assessed by genome sequencing. Thus, in this study, we assessed the presence in the 100-genomes strains of the abovementioned RNA viruses and M satellites, as well as their M satellite–dependent killer phenotypes. We cured L-A from most L-A+ strains and determined the phenotypes of isogenic L-A+ vs L-A0 strains, finding novel L-A–dependent phenotypes. In naturally M satellite–free and M satellite–cured 100-genomes strains, we determined M satellite–independent K1, K2, K28, and Klus killer toxin resistance phenotypes.
Finally, in contrast to the extensive work in S. cerevisiae on M satellites and their encoded killer toxins–antitoxins (Magliani et al. 1997; Schmitt and Breinig 2002, 2006; Rodriguez-Cousiño et al. 2011), there has been much less work on chromosomally encoded killer genes and their killer toxins, KHR1 (killer toxin, heat resistant) (Goto et al. 1990b) and KHS1 (killer toxin, heat sensitive) (Goto et al. 1991). Thus, we assembled and analyzed the KHR1 and KHS1 genes in the 100-genomes strains. In naturally M satellite–free and M satellite–cured 100-genomes strains, we determined chromosomal killer phenotypes and performed a detailed analysis of KHS1. We discuss the implications of our results on RNA virus, M satellite, and chromosomal killer effects on fitness, balancing selection on resistance to killer toxins, the analysis of host virus–satellite interactions, and the analysis of host phenotypes.
Methods
Strains
The S. cerevisiae strains listed in Supplementary Table 1 have been deposited in and should be requested from the Fungal Genetics Stock Center (McCluskey et al. 2010): http://www.fgsc.net. Unless explicitly stated otherwise, all strains were MATa/MATα diploids. For additional descriptions of the sequenced 100-genomes S. cerevisiae strains, or genetic backgrounds, listed in Supplementary Table 1, see (Strope et al. 2015a, 2015b; Vijayraghavan et al. 2019). The reference M28-containing K28 killer strain MS300c (Schmitt and Tipper 1990) and the reference Mlus-containing Klus killer strain EX229 (Rodriguez-Cousiño et al. 2011) were obtained and should be requested from Manfred Schmitt and Manuel Ramirez, respectively. The type strain of Candida glabrata (ATCC 2001 = IFO 0622 = CBS 138 = NRRL Y-65), which previously was used to test for and characterize KHS1-dependent (Goto et al. 1991) and KHR1-dependent (Goto et al. 1990a, 1990b) killing and to isolate the corresponding genes, should be requested from the ATCC, NBRC, CBS-KNAW, or NRRL culture collections. Isogenic derivatives of YJM189 (HO; self-diploidized) were constructed by deleting 1 copy of a gene (i.e. KHS1, KHR1, KEX1, and KEX2), sporulating the resulting +/Δ diploids and dissecting tetrads. From each of the +/Δ diploids, the segregants from 1 tetrad were phenotypically tested for Khr1- and/or Khs1-mediated killing.
KHS1 and KHR1 assemblies
The chromosomal killer genes KHS1 (Goto et al. 1991), as corrected by (Frank and Wolfe 2009), and KHR1 (Goto et al. 1990a, 1990b) were assembled and annotated using 100-genomes strains sequence data (Strope et al. 2015b).
Media and phenotypic analysis
YPD and YPE (1% yeast extract, 2% bacto peptone) contained 2% dextrose and 2% ethanol (added after autoclaving), respectively; plates contained 2% agar. To perform high-throughput nonkiller phenotypic analysis of S. cerevisiae strains, we used an S&P Robotics BM5-BC colony handling robot with imaging scanner/software for quantitative colony diameter phenotypes, as described previously (Strope et al. 2015b). We performed low-throughput nonkiller phenotypic analysis on subsets of strains by spot dilutions onto 100-mm diameter plates. That is, starting with cell suspensions of each tested strain (108 cells/ml), we serially diluted 1:10 in sterile deionized H2O and spotted 5 µl from each dilution onto control and experimental media to assess phenotypes.
Killer media and killer phenotypic analysis
We used MB medium [YPD: 1% yeast extract, 2% bacto peptone, 2% dextrose, and 2% agar, plus 0.003% methylene blue (methylene blue stains dead yeast cells; see citation in (Fink and Styles 1972))] (Fink and Styles 1972; Schmitt and Tipper 1990; Rodriguez-Cousiño et al. 2011) to determine killer phenotypes. When testing for K1, K2, Klus, and Khs1 killer phenotypes at pH 4.7 (final pH), both methylene blue (3 ml of 1% filter sterile solution) and 100 ml of 1 M citrate (sterile, adjusted to pH 4.5 with K3PO4) were added to 900 ml of medium after autoclaving. When testing for K28 and Khr1 killer phenotypes at pH6 (final pH), both methylene blue (3 ml of 1% filter sterile solution) and 100 ml of 1 M citrate (adjusted to pH6 with K3PO4; sterile) were added to 900 ml of medium after autoclaving. Like Goto et al. (Goto et al. 1990a, 1990b), we also used a synthetic defined medium (SD + MB) (final concentrations: 0.67% yeast nitrogen base, 2% dextrose, 2% agar, and 0.003% methylene blue) to test chromosomal KHR1 killer phenotypes.
In addition to 1 reference L-A+ M28+ K28+ strain (MS300c (Schmitt and Tipper 1990)) and 1 reference L-A+ Mlus+ Klus+ strain (EX229 (Rodriguez-Cousiño et al. 2011)), we determined the killer phenotypes of L-A+ M+ 100-genomes strains (M1+: n = 5; M2+: n = 3; Mlus+: n = 7; unknown M+: n = 2), as well as their isogenic L-A0 M0 derivatives. Briefly, strains being tested for killer activity were patched onto MB plates that had been separately seeded with approximately 105 cells/plate of each of the 100-genomes strains that were naturally M0, as well as isogenic L-A0 (M0) derivatives of L-A+ 100-genomes strains, or with C. glabrata (ATCC 2001), to create lawns. These plates were incubated in the dark at 20°C (K1, K2, Khs1, and Khr1) and 30°C (K28, Klus) for 1 to 3 days after which killing was scored by killing zone sizes (qualitative killing zone bin sizes in decreasing order of killing: 2, 1, 0.5, and 0) and killing staining [qualitative cell killing staining bins: 2 (clear killing zone), 1 (turbid killing zone), 0.5 (dark blue ring), and 0 (no staining)], around the patched strains on the lawns.
Virus and M satellite presence/absence determination by PCR and gel electrophoresis
Total nucleic acids were extracted using a slight modification of the phenol-mediated method described by (Maqueda et al. 2010), as follows. Cells of 5-ml overnight YPD cultures were collected by centrifugation and washed once with 50 mM Na2EDTA (pH 7.5). Cell pellets were then resuspended in 1 ml of 50 mM Tris-H2SO4 (pH 9.3)/1% 2-mercaptoethanol solution and incubated for 15 minutes at room temperature. Cells were subsequently collected by centrifugation and resuspended in 1 ml of 0.1 M NaCl/10 mM Tris-HCl (pH 7.5)/10 mM Na2EDTA/0.2% sodium dodecyl sulfate solution. 0.7 ml of phenol (pH 8.0) was added, and samples were incubated on a shaking platform for 1 h at room temperature, after which 0.7 ml of the aqueous phase was recovered by 5-minute centrifugation. Nucleic acids were precipitated by the addition of 70 µl of 3 M potassium acetate and 0.7 ml of isopropanol, followed by incubation for 5 minutes at room temperature and centrifugation at 14,000 rpm for 10 minutes. The precipitated nucleic acids were washed with 70% ethanol, dried using a Speedvac (Eppendorf), and dissolved in 100 µl of water. These samples were stored at −80°C.
PCR analysis of previously described RNA viruses and M satellites was performed as follows. 15-µl aliquots of total nucleic acid samples were incubated for 2 minutes at 98°C and then placed on ice. 5-µl aliquots of these samples were used as a template for cDNA synthesis performed using the Maxima First-Strand cDNA Synthesis Kit (Thermo Fisher Scientific) in accordance with the manufacturer's protocol. One-tenth of the cDNA reaction volume was used in virus-diagnostic PCR performed with OneTaq DNA Polymerase (New England Biolabs) in accordance with the manufacturer's protocol. The primers used to detect the presence of previously described RNA viruses and M satellites are listed in Supplementary Table 2.
PCRs of each of the known RNA viruses and M satellites were performed with multiple primer pairs to determine the presence/absence (Supplementary Table 3 and Supplementary Fig. 1). In addition, for a given primer pair, strain-to-strain variation in PCR product formation (+ vs −), which may be influenced by the template level as well as template sequence variation, was observed, which is referred to as a genotype or PCR genotype (Supplementary Table 3). For all RNA viruses and M satellites (high abundance: L-A; low abundance: M satellites, 20S, 23S; high and low abundance: L-BC), PCR genotypes were reproducible.
Relative quantification of L-BC levels in YJM1463 and YJM1529 (L-A+ strains that could not be cured of L-A by any technique, see below) was determined using the Luna Universal One-Step RT-qPCR Kit (New England Biolabs) using SYBR chemistry according to the manufacturer's recommendations. Real-time PCR was performed using QuantStudio 6 (Applied Biosystems). L-BC quantification was performed relative to UBC6 using primers described in Supplementary Table 2, with relative expression calculated as 2−ΔΔEqCq using the QuantStudio Design and Analysis software (Applied Biosystems).
For gel electrophoresis, 5-μl aliquots of total nucleic acid samples (to detect abundant RNA viruses and satellites) or qPCR-derived nucleic acid samples (to detect PCR products of sequenced RNA viruses and M satellites) were mixed with 1× gel-loading buffer and loaded on 1.3% agarose gels prestained with Green Gene dye (Southern Biolabs) (and/or ethidium bromide; BioRad) according to the manufacturer's instructions. Electrophoresis was carried out in 1× TAE buffer at room temperature at a constant voltage of 6 V/cm for 45–60 minutes. Gels were subsequently imaged under UV light using the Alpha Innotech Red gel documentation system.
Nuclease treatments
Nucleic acid samples were treated with DNaseI (New England Biolabs) at 37°C, and samples were analyzed on 1.2% TAE agarose gel stained with ethidium bromide. For subsequent analyses, treated samples were column purified using standard PCR purification kits (Qiagen), reverse transcribed with Maxima First-Strand cDNA Synthesis Kit (Thermo Fisher Scientific), and subjected to PCR to test for the presence/absence of various genomic and viral elements, as described above. PCR samples were analyzed on 2% TBE (Tris-borate EDTA) gels at 4–6 V/cm for 75–90 minutes in prechilled 0.5× TBE buffer.
Curing L-A
We used previously described techniques (Valle and Wickner 1993; Dinman et al. 1997; Esteban et al. 2008; Drinnenberg et al. 2011) and a novel technique described below, to cure 28 of 30 L-A+ strains of L-A; the remaining 2 L-A+ strains, YJM1463 and YJM1529, were refractory to curing by all methods. In the case of L-A curing with plasmid-borne L-A cDNA, we made and used a nourseothricin resistance (Nat) containing derivative of pI2L2 (Wickner et al. 1991; Valle and Wickner 1993) (obtained from J. Dinman as pJD1223), pJD1223-NAT (Supplementary Table 1, Technique I). Because these previously described techniques failed to cure L-A from some strains, we also used the requirement of Mak3 and Mak10 (Ball et al. 1984), components of the NatC N-terminal acetyltransferase, for L-A propagation to cure L-A. We crossed isogenic haploid mak3Δ and mak10Δ derivatives and, after sporulation and tetrad dissection, isolated Mak+ segregants that were then diploidized using the HO-containing plasmid pHS3 (Supplementary Table 2) (Supplementary Table 1, Technique II). We also developed a novel technique to cure L-A that combines the requirement of Mak10p for L-A propagation (Ball et al. 1984) with the positive/negative selection amdS cassette (Solis-Escalante et al. 2013), which we obtained from EUROSCARF. Specifically, we cured L-A by introducing a recombinationally reversible mak10 disruption mutation. That is, we integrated a (amdS + internal fragment of mak10 ORF)–containing plasmid (pUG-amdSYM-mak10TR) into haploid MAK10 strains, selecting for acetamide utilization and subsequently for recombinational plasmid pop outs, selecting for fluoroacetamide resistance; that is, MAK10 L-A+ → mak10::amdS L-A0 → MAK10 L-A0; these Mak+ derivatives were then diploidized as described above. pUG-amdSYM-mak10TR is a pUG-amdSYM (Solis-Escalante et al. 2013) derivative. An NdeI-cloned 734-bp internal fragment of the S288C-derived MAK10 open reading frame was amplified using primers mak10TR-Nde-F and mak10TR-Nde-R (Supplementary Table 2) (Supplementary Table 1, Technique III) and cloned into an NdeI site of pUG-amdSYM. In all cases, curing of L-A (and cocuring of M, if present) was determined by gel electrophoresis (in L-BClo strains) and/or by PCR. Plasmids described above (with the exception of pJD1223-NAT, the loss of which was not realized until after the shutdown of the McCusker laboratory) have been deposited with, and should be requested from, Addgene http://www.addgene.org/John_McCusker/.
Curing M satellites
M satellites from L-A+ M+ strains were cured essentially as described (Fink and Styles 1972; Carroll and Wickner 1995; Rodriguez-Cousiño et al. 2011). Briefly, overnight log-phase cultures of L-A+ M+ strains were obtained by growth in liquid YPD at 30°C. Cultures were subsequently serially passaged twice for 12 hours each in fresh YPD containing 0.05 mg/L cycloheximide at 30°C. Cells from cycloheximide-treated cultures were streaked on YPD plates without cycloheximide to isolate single colonies, which were tested by PCR for retention of L-A and for curing of M.
Testing correlations between RNA viruses, M satellites, and narnaviruses
Fisher's exact test was used to test for correlation between the L-A, L-BC, 20S, and 23S viruses, as well as the M1 (n = 5) and Mlus (n = 7) satellites (Supplementary Table 4), with previously determined (Strope et al. 2015b) population ancestry and clinical/nonclinical origin using Prism (GraphPad).
M satellite–independent K1, K2, K28, and Klus toxin sensitivity/resistance phenotypes
To avoid the confounding effects of M-dependent killer toxin resistance, we determined the killer toxin sensitivity/resistance phenotypes of 100-genomes strains that were naturally M0 and of L-A0 M0 derivatives of the 100-genomes strains. We tested for M satellite–independent killer toxin sensitivity/resistance phenotypes with M1+ (K1+: YJM1077, YJM1290, and YJM1307; K1±: YJM1387; and K−: YJM1419), M2+ (K2+: YJM1341, YJM453, and YJM1574 that have different levels of K2 activity), M28+ (the reference K28+ strain, MS300c), and Mlus+ Klus+ (the reference Klus+ EX229 strain) strains (Supplementary Table 5).
Genotype associations
Based on the genome sequences of the 100 yeast genome strains (Strope et al. 2015b), we identified 142,313 variable sites with MAF ≥ 0.05. The “–indep” option of PLINK (1.90b6.26) (Chang et al. 2015) was used to prune sites in linkage disequilibrium (R2 > 0.5), resulting in a final set of 19,061 biallelic sites. These pruned sites were used to estimate a genetic relationship matrix using the GTCA software package (1.94.1) (Yang et al. 2011). Association analysis was carried out using the mixed linear model GWAS command in GCTA (fastGWA-MLM) (Jiang et al. 2019), with account for population structure using a sparse genetic relationship matrix derived from the full relationship matrix using a threshold of 0.05. The ordinal scores for each phenotype of interest (Supplementary Tables 5 and 6) were normalized to a standard scale (0, 1, 2, and 3) before carrying out GWAS. Thresholds for significance were established by generating 1,000 permutations of the original phenotypes and refitting the fastGWA model to each permutation. The 0.05 quantile of the distribution of minimum P-values from each permutation was used to estimate P-value thresholds of 2.96 × 10−6 and 1.44 × 10−9 for C. glabrata killing and killing by YJM189, respectively. To account for the possibility that small differences in the rank ordering of phenotypes might lead to false positive associations, we fit the GWAS model to “jittered” replicates of each phenotypic data set in which the phenotypes were adjusted by adding normally distributed random values (mean = 0, SD = 0.25) to each phenotypic score. This has the effect of randomizing rank-order within each phenotypic class, while maintaining rank-order between classes. We generated 100 such jittered data sets and here report only those peaks that exceed the significance threshold in at least 70% of the jittered replicates.
Analyses of statistical significance were performed using chi square analysis or Fisher's exact test, where applicable.
Results and discussion
L-A, L-BC, 20S, and 23S RNA viruses in the S. cerevisiae 100-genomes strains
Integrated RNA virus and satellite cDNAs, for which there are precedents in yeast nuclear genomes (Frank and Wolfe 2009), might confound PCR detection of RNA viruses and M satellites. We found no sequences with high homology to L-A, L-BC, 20S, 23S, M1, M2, or Mlus in our previous analysis of the nuclear genomes of the 100-genomes strains (Strope et al. 2015b). However, we identified a 713-bp sequence with 82% similarity to the S. cerevisiae M28 1.75-kbp dsRNA (Schmitt and Tipper 1995) in the nuclear genome of 1 strain, YJM195 (Strope et al. 2015b). Therefore, except for the partial M28 cDNA in the nuclear genome of YJM195, RNA virus and M satellite cDNAs did not confound PCR detection of RNA viruses or M satellites in the 100-genomes strains.
We used PCR to test the 100-genomes strain population (Strope et al. 2015b) for the presence/absence and, where present, the PCR genotypes of the L-A and L-BC totiviruses (Table 1 and Supplementary Tables 2 and 3). Because PCR would not detect novel totiviruses and might not detect sequence variants of the L-A and L-BC totiviruses, we also used gel electrophoresis to test for the presence/absence of bands corresponding in size to the abundant L-A and L-BC totiviruses both before and after curing L-A.
Table 1.
Summary of PCR analysis of the 100-genomes strains for RNA viruses and M satellites.
| Virus/M | Strains | Primer pairs | PCR genotypes |
|---|---|---|---|
| L-A | 30 | 7 | 6 |
| L-BC | 100 | 3 | 6 |
| 20S | 26 | 2 | 3 |
| 23S | 14 | 2 | 3 |
| M1 | 5 | 2 | 1 |
| M2 | 3 | 2 | 1 |
| M28 | 0 | 2 | NA |
| Mlus | 7 | 5 | 1 |
Virus/M: previously described RNA viruses (L-A, L-BC, 20S, and 23S) and L-A–dependent M satellites (M1, M2, M28, and Mlus); strains: number of 100-genomes strains containing a specific RNA virus/M satellite as determined by PCR product(s); primer pairs: number of virus- or M satellite–specific primer pair(s) (Supplementary Table 2); PCR genotypes: for ≥2 primer pairs, virus genotype–specific combinations of PCR products. One strain, YJM195, which contains a partial M28 cDNA (Strope et al. 2015b), yielded a PCR product with 1 of 2 M28-specific primer pairs. NA, not applicable. Individual strain data (Supplementary Table 3).
By PCR with multiple primer pairs, 30 of the 100-genomes strains were L-A+ and all were L-BC+ (Table 1). However, despite all the strains being L-BC+, gel electrophoresis of total RNA revealed a 4.6-kb band in only 47 strains. (There were no gel bands greater than 4.6 kb, the approximate size of L-A and L-BC, in any of the 100-genomes strains.) L-BC abundance in the naturally L-A0 strains (n = 70) and L-A–cured (L-A+ → L-A0; n = 28) strains fell into 2 categories—L-BChi (readily discernable 4.6-kb gel band: n = 31) and L-BClo (no discernable 4.6-kb gel band: n = 67) (Supplementary Table 3). For the 2 L-A+ L-BC+ strains where L-A could not be cured, real-time PCR was performed, from which it was determined that YJM1529 was L-BChi and YJM1463 was L-BClo (Supplementary Table 3 and Supplementary Fig. 5). We found that the L-BC PCR genotype and L-BC level are associated (Table 2; Supplementary Table 4), consistent with the L-BC genotype having a major effect on the L-BC level. The combined results of gel electrophoresis and PCR with multiple primer pairs suggest that we identified all L-A and L-BC totiviruses and that there were no novel, abundant totiviruses in the 100-genomes strains.
Table 2.
Summary of L-BC PCR genotypes and L-BC levels.
| L-BC PCR genotype |
L-BChi | L-BClo |
|---|---|---|
| 0,0,1 | 1 | 2 |
| 0,1,0 | 0 | 49 |
| 0,1,1 | 13 | 3 |
| 1,0,0 | 0 | 0 |
| 1,0,1 | 5 | 2 |
| 1,1,0 | 0 | 1 |
| 1,1,1 | 14 | 10 |
| Total: | 33 | 67 |
L-BC PCR genotype: for each of the 3 L-BC primer pairs (Supplementary Tables 2 and 3)—1, PCR product; 0, no PCR product; 6 of the 7 possible L-BC PCR genotypes were observed. L-BChi and L-BClo: gel analysis of naturally L-A0 (n = 70) and L-A+ → L-A0 (cured: n = 28) strains assessed the presence (L-BChi: n = 32) vs absence (L-BClo: n = 66) of an abundant 4.6-kb gel band. L-BC levels in the 2 remaining L-A+ L-BC+ strains were assessed by real-time PCR. L-BC level associated with the PCR genotype (Supplementary Table 4). Individual strain data (Supplementary Table 3).
L-BC, like L-A and its variants (Sommer and Wickner 1982; El-Sherbeini et al. 1984; Rodriguez-Cousino et al. 2013; Rodriguez-Cousino and Esteban 2017), appears to have been presumed to exist only at high copy numbers sufficient to be readily observable on gels. However, a previous study described low-level L-BC in some strains and hypothesized this as being due to a complex chromosomal defect [chromosomal L-0 (Clo−)] (Wesolowski and Wickner 1984). Consistent with such complexity, we suggest that both the L-BC genotype and the host genotype contribute to the L-BC level.
In a previous work, we identified multiple examples of introgression from other Saccharomyces species in the S. cerevisiae 100-genomes strains (Strope et al. 2015b). Because species-specific XRN1-L-A interactions have been identified (Rowley et al. 2016), we considered the hypothesis that XRN1 introgression, or loss of function polymorphisms, might contribute to L-A presence/absence or, possibly, L-BC levels in the 100-genomes strains. However, we did not identify any cases of introgression, LTR insertions, or ORF length polymorphisms (indels/frameshifts, premature stop codons) in the XRN1 genes, nor in any of the SKI genes, in the 100-genomes strains (Strope et al. 2015b).
Strains were grown under nonstress conditions (YPD at 30°C) where 20S and 23S were expected to be present in low abundance and hence not detectable by gel. Thus, as above, PCR on total nucleic acids isolated from the 100-genomes strains was used to assess the presence of the 20S and 23S narnaviruses. Twenty-six strains were 20S+ and 14 were 23S+, with both narnaviruses having multiple PCR genotypes (Table 1; Supplementary Table 3).
In S. cerevisiae, RNA viruses and 2µ plasmid are inherited in a similar fashion. Specifically, after the mating of haploid “+” and “−” strains followed by meiosis, there is 4+:0− segregation. The 2µ plasmid has low loss rates and deleterious effects on fitness, either alone or in combination with specific nuclear genome mutations (Holm 1982a, 1982b; Futcher and Cox 1983; Mead et al. 1986; Dobson et al. 2005; Harrison et al. 2012). However, we found 2µ plasmid in more of the 100-genomes strains (n = 72) (Strope et al. 2015a) than the L-A (n = 30), L-BChi (n = 33), 20S (n = 26), and 23S (n = 14) RNA viruses. The higher frequency of 2µ plasmid relative to the RNA viruses may be influenced by population structure and/or by time(s) of entry into the S. cerevisiae population. Alternatively, the higher frequency of 2µ plasmid may be due to these S. cerevisiae RNA viruses having higher loss rates, possibly due to the high mutation frequency characteristic of RNA viruses (Sanjuan et al. 2010), and/or higher fitness costs.
L-A–dependent M satellites, M satellite–dependent killer phenotypes, and M satellite–independent killer resistance phenotypes in the S. cerevisiae 100-genomes strains
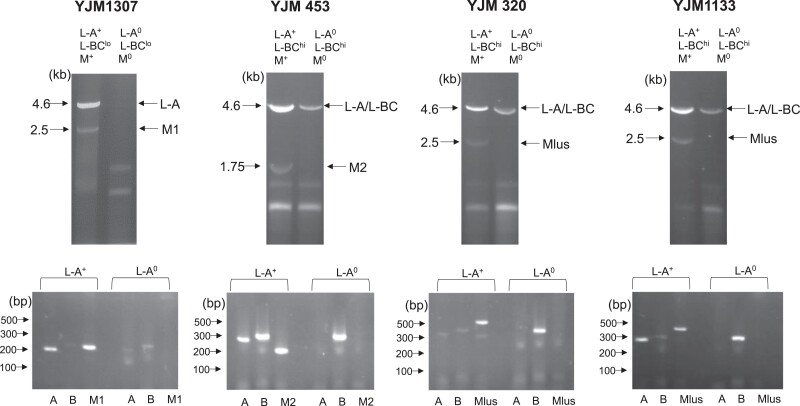
Using multiple primer pairs, we used PCR to test the 100-genomes strains population for the presence/absence of the L-A–dependent satellites M1, M2, M28, and Mlus. Sixteen strains, all of which were L-A+, were M+ by PCR; 5 strains were M1+, 3 were M2+, 7 were Mlus+, and 1 was M28+ (Table 3). The 1 L-A+ M28+ strain, YJM195, contained a previously identified partial M28 cDNA in its nuclear genome (Strope et al. 2015b). In YJM195, curing L-A cocured an unknown L-A–dependent M satellite (see below) but not the chromosomally encoded M28 cDNA-dependent PCR product (Supplementary Fig. 2). In contrast, in the remaining 15 L-A+ M+ strains, curing L-A cocured the known species of M, as determined by PCR; that is, L-A+ M+ → L-A0 M0 (Table 1; Supplementary Table 3; Fig. 1. Each of these 15 M+ strains contained a single species of M satellite, consistent with M exclusion (Schmitt and Tipper 1992). In contrast to L-A and L-BC, no PCR genotype variation was observed for these M satellites.
Table 3.
Summary of L-A–dependent M satellite–containing 100-genomes strains and M satellite–dependent killer phenotypes.
| Strain | M species | Gel band (<4.6 kb) |
Killer |
|---|---|---|---|
| YJM1077 | M1+ | — | K1+ |
| YJM1290 | M1+ | — | K1+ |
| YJM1307 | M1+ | 2.5 kb | K1+ |
| YJM1387 | M1+ | — | K1+/− |
| YJM1419 | M1+ | — | K1− |
| YJM453 | M2+ | 1.7 kb | K2+ |
| YJM1341 | M2+ | — | K2+ |
| YJM1574 | M2+ | — | K2+ |
| YJM145 | Mlus+ | 2.5 kb | Klus− |
| YJM320 | Mlus+ | 2.5 kb | Klus+ |
| YJM975 | Mlus+ | 2.5 kb | Klus− |
| YJM978 | Mlus+ | 2.5 kb | Klus− |
| YJM993 | Mlus+ | 2.5 kb | Klus− |
| YJM1133 | Mlus+ | 2.5 kb | Klus+ |
| YJM1526 | Mlus+ | 2 kb | Klus+ |
| YJM195 | 0 | 3 kb | K− |
| YJM681 | 0 | 2.5 kb | K− |
All listed strains were L-A+. M species (+): the presence of a previously described species of M as determined by PCR; by PCR, each M+ strain contained 1 M species. 0, no PCR products with any M primers (Supplementary Table 2). Gel band: the presence of a <4.6-kb dsRNA gel band. Upon curing of L-A, all listed L-A+ M+ strains were L-A0 M0 by gel and/or PCR and all K+ strains were K−, consistent with an L-A–dependent M satellite. YJM195, which contains a partial M28 cDNA (Strope et al. 2015b), yielded a PCR product with 1 of 2 M28-specific primer pairs. In an isogenic L-A0 derivative, this M28-specific PCR product was produced while the 3.0-kb gel band was absent. Killer: killer phenotype of a strain. Individual strain data (Supplementary Tables 3 and 5).
Fig. 1.
Representative gels (top) of strains carrying different species of the dsRNA totiviruses (L-A and L-BC) and satellites (M1, M2, and Mlus). Shown are isogenic strain pairs of native and L-A–cured strains. (Bottom) Corresponding PCRs from cDNAs of RNA derived from the indicated strains, probing for presence/absence of L-A (A), L-BC (B), or M (M1, M2, and Mlus). All samples were treated with DNase prior to gel and PCR analysis. Primers for PCR analysis LA-F4 + LA-R5 (L-A), LBC-F2 + LBC-R2 (L-BC), M1-F + M1-R (M1), M2-F + M2-R (M2), and Mlus-F2 + Mlus-R2 (Mlus).
Gel electrophoresis also was used to determine the presence, if at sufficiently high levels, of smaller gel bands, such as M satellites. Eleven L-A+ strains had 1 small (1.75–3 kb) gel band (Table 3; Supplementary Table 3; Fig. 1). Seven of these 11 L-A+ strains, all of which were Mlus+ by PCR, had gel band sizes (2–2.5 kbp) approximately consistent with the previously reported 2.1–2.3 kbp size range of Mlus (Rodriguez-Cousiño et al. 2011). One of these 11 L-A+ strains (YJM453; PCR: M2+) had a small gel band size of 1.75 kbp approximately consistent with M2 (1.5 kbp). The 3 remaining S. cerevisiae L-A+ strains with a small gel band were YJM1307 (PCR: M1+), YJM681 (PCR: M0), and YJM195 (PCR: M0). The 2.5-kbp gel band in YJM1307 was larger than the 1.6 kb of the canonical M1. The sizes of the 2.5-kbp gel band in YJM681 and the 3.0-kbp gel band in YJM195 were inconsistent with M1, M2, and M28. For these 15 L-A+ M+ strains, curing L-A cocured M, consistent with their being L-A–dependent M satellites (Table 3; Supplementary Table 3; Fig. 1).
The reference M28-containing K28 killer (MS300c) (Schmitt and Tipper 1990) and the reference Mlus-containing Klus killer (EX229) (Rodriguez-Cousino et al. 2011) strains as well as the L-A+ M+ 100-genomes strains [M1+ (n = 5); M2+ (n = 3); Mlus+ (n = 7); unknown M+ (n = 2)]; also, for L-A+ M1+ and L-A+ M2+, their isogenic L-A0 M0 derivatives were tested for their abilities to kill 100-genomes strains that were naturally M0 (n = 83) and isogenic L-A0 (M0) derivatives (n = 28). None of the naturally M0 and isogenic L-A0 (M0) derivatives of the 100-genomes strains were killed by the partial M28 genomic cDNA-containing strain YJM195 (Strope et al. 2015b) or by YJM681, the 2 L-A+ strains with L-A–dependent, unknown M satellites, or by their isogenic L-A0 M0 derivatives (Supplementary Table 5 and Supplementary Fig. 3).
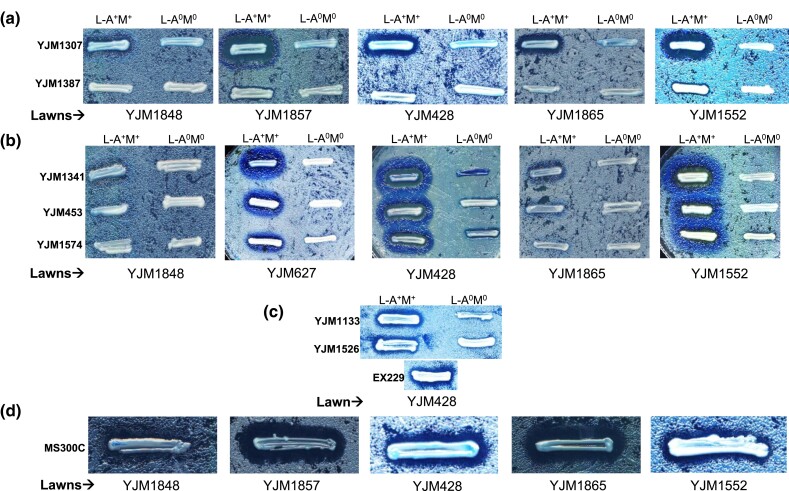
Of the 100-genomes strains that were naturally M0 and isogenic L-A0 (M0) derivatives, only 1 strain, YJM428 (L-BChi 20S+), was killed by EX229, the reference L-A+ Mlus+ Klus+ strain (Rodriguez-Cousiño et al. 2011) (Supplementary Table 5). Of the 100-genomes strains that were L-A+ Mlus+ (n = 7), 4 strains (YJM145, YJM975, YJM978, and YJM993) did not kill YJM428; that is, they were Klus−. The 3 remaining L-A+ Mlus+ strains (YJM320, YJM1133, and YJM1526) killed YJM428; that is, they were Klus+ since isogenic L-A0 M0 derivatives of these 3 strains did not kill YJM428 (Supplementary Table 5; Fig. 2).
Fig. 2.
Killer activity in M-containing strains. Isogenic strain pairs of native and L-A–cured strains were tested on lawns of M0 strains. Representative strains displaying a) K1, b) K2, c) Klus, and d) K28 killer activity are shown. For Klus, only 1 strain background (YJM428) showed sensitivity to the Mlus toxin. For K28, a corresponding M0 strain was not tested.
In contrast to Klus, many of the 100-genomes strains that were naturally M0 and isogenic L-A0 (M0) derivatives were killed by at least 1 of the 3 L-A+ M2+ strains (YJM1341, YJM453, and YJM1574); cocuring L-A and M2 eliminated killing. The 3 L-A+ M2+ K2+ strains differed in their K2 killing activity: YJM1341 >YJM453 > YJM1574 (Supplementary Table 5). Like K2, many of the 100-genomes strains that were naturally M0 and isogenic L-A0 (M0) derivatives were killed by at least 3 of the 5 L-A+ M1+ strains. The 5 L-A+ M1+ strains differed in their K1 killing activity [YJM1077 = YJM1290 = YJM1307 > YJM1387 (K1±) > YJM1419 (K1−)]; for the 4 L-A+ M1+ K1+ (or K1±) strains, cocuring L-A and M1 eliminated killing (Supplementary Table 5). Finally, many of the 100-genomes strains that were naturally M0 and isogenic L-A0 (M0) derivatives were killed by the reference L-A+ M28+ K28+ strain MS300c (Schmitt and Tipper 1990), including the partial M28 cDNA-containing strain YJM195 (Supplementary Table 5).
The rarity of M satellite–dependent killer strains in the 100-genomes S. cerevisiae strains, which is consistent with previous studies (Nakayashiki et al. 2005; Pieczynska et al. 2013; Chang et al. 2015), suggests that some and perhaps all the M satellites have significant fitness costs. Indeed, M1 has a fitness cost (reduced growth rate) in wild-type strains that is alleviated by [Kil-d]-mediated mutation of M1 from K1+ to K1− (Suzuki et al. 2015). In addition, when present in combination with specific nuclear genome mutations or polymorphisms, M1 and M2 have significant fitness costs (Ball et al. 1984; Ridley et al. 1984; Edwards et al. 2014).
Previous studies have identified strains with M satellite–independent resistance to some killer toxins (Pieczynska et al. 2013; Chang et al. 2015). Our results suggest that there may be selection for M satellite–independent resistance to killer toxins, with near-complete fixation for Mlus-independent Klus resistance. However, the lack of fixation for M satellite–independent resistance to K1, K2, and K28 toxins suggests that there also may be balancing selection; that is, M satellite–independent resistance to K1, K2, and/or K28 toxins may have fitness costs. M satellite–independent resistance to killer toxins would reduce selection for maintenance of killer toxin-/killer toxin resistance–encoding M satellites that, together with the M-dependent deleterious effects on host fitness and phenotypes referenced above, may contribute to the low frequencies of M satellites in S. cerevisiae.
Unlike fully functional K+ R+ M satellites, mutant M satellites may encode partially functional or nonfunctional killer toxin (K± or K−, respectively) and functional killer toxin–specific resistance (R+) functions (Talloczy et al. 1998, 2000; Suzuki et al. 2015). Thus, for YJM1387 (L-A+ M1+ K1±) and YJM1419 (L-A+ M1+ K1−), we compared isogenic L-A+ M+ vs L-A0 M0 derivatives from both backgrounds to determine whether these M1 confer resistance to K1 toxin. While parental YJM1387 M1+ K1± and YJM1419 M1+ K1− strains were resistant to K1 toxin (R1+), isogenic L-A0 M0 strains were sensitive to K1 toxin (R1−), consistent with the R1+ phenotypes of both L-A+ M1+ parent strains being M1 dependent.
Like the L-A+ M1+ K1− R1+ strain YJM1419, neither YJM195 nor YJM681, L-A+ strains with L-A–dependent, unknown M satellites, killed any of the naturally M0 and isogenic L-A0 (M0) derivatives of the 100-genomes strains (Supplementary Table 5). To test the hypothesis that 1 or both M satellites might be K− R+ sequence variants (that is, not detectable by PCR with multiple primer pairs) of known M satellites, we compared the K1, K2, and K28 toxin resistance phenotypes of isogenic L-A+ M+ vs L-A0 M0 derivatives of the YJM195 and YJM681 backgrounds. (Neither L-A+ M+ nor L-A0 M0 derivatives of the YJM195 and YJM681 backgrounds were killed by Klus+ strains; thus, the corresponding Klus experiment was not possible.) In the YJM195 background, the unknown M satellite and/or L-A may confer slight resistance to K1 and K2 killer toxins; however, neither the unknown M satellite nor L-A affected resistance to K28 killer toxin (Supplementary Fig. 2). In the YJM681 background, neither the unknown M satellite nor L-A affected resistance to K1, K2, or K28 killer toxins (Supplementary Fig. 2).
If these L-A–dependent unknown M satellites, like all known M satellites, encode a killer toxin–antitoxin, there are 3 hypotheses for the K− phenotypes of the L-A+ M+ YJM195 and YJM681 backgrounds. First, the K− phenotypes of 1 or both backgrounds may be [Kil-d]/M satellite genotype (Talloczy et al. 1998,2000; Suzuki et al. 2015) or nuclear genotype dependent (e.g. kex1 and kex2 (Wickner and Leibowitz 1976)). Second, the K− phenotypes of 1 or both backgrounds may be due to the activity of their putative M satellite–encoded killer toxin(s) requiring environmental conditions, such as temperature and/or pH, different from the conditions used to test for the activities of K1, K2, K28, and Klus toxins. Finally, the K− phenotypes of 1 or both parental backgrounds may be due to all the 100-genomes strains being resistant to their putative M satellite–encoded killer toxin(s), like 99 of the 100-genomes strains having Mlus-independent resistance to Klus toxin. Because the ability to detect killer strains depends on both the testing environment (e.g. pH and temperature) and the genotypes of the strains being tested for killing, novel potentially toxin-encoding M satellites may exist in S. cerevisiae.
There were significant associations of totiviruses with 2µ plasmid (Strope et al. 2015a), as well as with 2 site-specific, mobile mitochondrial introns, SCE1 and COX1-intron 1 (Vijayraghavan et al. 2019) (Supplementary Table 4). There were also significant associations between the different viruses (Supplementary Table 4). While there were no significant L-A associations with either population or clinical/nonclinical origin, there was significant association of the L-BC level with population (the large wine/European population and mosaic group), as well as significant associations of the L-BC level, 20S and 23S with clinical/nonclinical origin (Supplementary Table 4).
As assayed in M satellite–free 100-genomes strains, there were no significant genotype associations with killer (K1, K2, and K28) toxin resistance phenotypes. Similarly, there were no significant 100-genomes strains genotype associations with the virus (L-A, 20S, and 23S) presence or with the L-BC level (data not shown), with the exceptions of the abovementioned SCE1 and COX1-intron 1. The lack of significant genotype associations may be due to small population size (n = 100), population structure, and/or complex genetic architecture. In addition, the lack of significant host genotype associations with the L-BC level also may be confounded by the significant L-BC genotype–L-BC level association (Table 2; Supplementary Table 3).
Phenotypic effects of L-A
We recently described a novel narnavirus, N1199, that, when present in a high copy number, has major effects on S. cerevisiae host phenotypes (Vijayraghavan et al. 2023). However, despite extensive characterization (e.g. (Wickner 1992,1996a, 1996b; Wickner et al. 2013)), to our knowledge, the L-BC, 20S, and 23S RNA viruses have no previously described effects on S. cerevisiae phenotypes and the L-A virus has few previously described effects on S. cerevisiae host phenotypes (Dihanich et al. 1987, 1989; Liu and Dieckmann 1989; Gao et al. 2019; Chau et al. 2023). The near complete absence of previously described RNA virus effects on S. cerevisiae host phenotypes stands in sharp contrast to the many effects of RNA viruses, including dsRNA totiviruses, on host phenotypes in other species of fungi (McCabe et al. 1999; Dawe and Nuss 2001; Nuss 2005; Ghabrial and Suzuki 2009; Bhatti et al. 2011; Xie and Jiang 2014; Ghabrial et al. 2015). However, in our analysis, there were no virus or M satellite associations with 100-genomes strain nonkiller phenotypes (listed in Supplementary Table 4); hypotheses for the lack of association include the population size, population structure, choice of tested phenotypes, host genotype–virus epistasis, and RNA virus genotype variation.
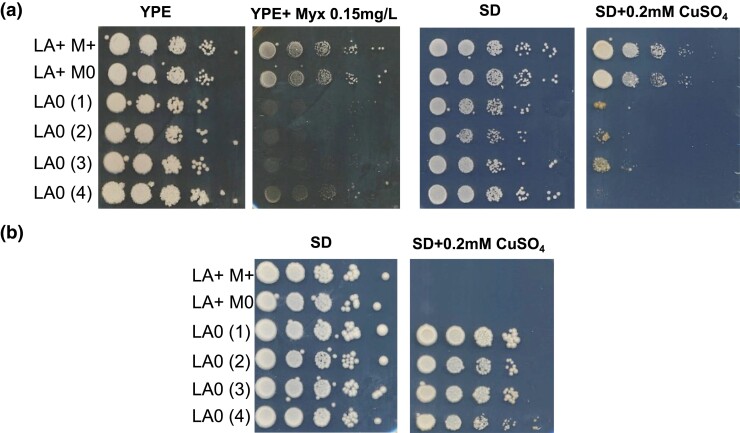
An independent method to identify virus-dependent phenotypes is to compare the phenotypes of isogenic virus-containing and virus-free strain sets, which we did for L-A. For the 28 L-A+ parental genetic backgrounds where L-A could be cured, we compared the phenotypes (Strope et al. 2015b; Vijayraghavan et al. 2019) of isogenic L-A+ and L-A0 strain sets. In 2 of these 28 genetic backgrounds [YJM1133 (parental): L-A+ Mlus+ Klus+; YJM1419 (parental): L-A+ M1+ K1−], there were differences in copper (YJM1133, YJM1419) and myxothiazol (a cytochrome b oxidase inhibitor; YJM1133) resistance phenotypes between the parental L-A+ M+ strains and multiple independently cured, isogenic L-A0 M0 derivatives (Fig. 3). Because these L-A+ M+ parent vs L-A0 M0 derivative comparisons could not distinguish between L-A– and M-dependent phenotypes, YJM1133 and YJM1419 background strains were cured of their M satellites to generate L-A+ M0 derivatives. (Gel and PCR analyses of parental and representative cured derivatives of both backgrounds are shown in Supplementary Fig. 4.) Upon testing, parental YJM1133 and YJM1419 background L-A+ M+ strains and isogenic L-A+ M0 strains had the same copper and myxothiazol resistance phenotypes, which differed from the phenotypes of isogenic L-A0 M0 strains (Fig. 3), consistent with these phenotypes being L-A dependent.
Fig. 3.
Phenotypic analysis of a) YJM1133 and b) YJM1419 L-A+ M+ parental as well as isogenic L-A+ M0 and L-A0 M0 derivatives. Ten-fold dilutions of isogenic strains differing only in the presence/absence of L-A and/or M were spotted on media with the indicated compounds and monitored for growth.
Our previous 100-genomes strains study identified the CUP1 locus, where the copy number ranges from 1 to 18 tandem copies, as the major effect contributor to copper resistance (Strope et al. 2015b). The YJM1419 (CUP1: n = 4) and YJM1133 (CUP1: n = 2) backgrounds are at the low end of the CUP1 copy number range, which may have facilitated the identification of L-A–dependent effects on copper resistance. Interestingly, while the copper resistance phenotypes in the YJM1133 and YJM1419 backgrounds were L-A dependent, the phenotypic effect of L-A was opposite in the 2 backgrounds. One hypothesis for the opposite effects of L-A on copper resistance in the YJM1419 and YJM1133 backgrounds is that they are L-A genotype dependent; however, while not definitive, the L-A in YJM1419 and YJM1133 have the same PCR genotypes (Supplementary Table 3). A second hypothesis for the opposite effects of L-A on copper resistance in the YJM1419 and YJM1133 backgrounds is that they are host genotype dependent. In either case, unless accounted for, L-A is a confounding factor for studies of copper resistance as well as, at a minimum, myxothiazol resistance. More generally, RNA viruses have the potential to be confounding factors for multiple host phenotypes.
Chromosomal killer genes and killer phenotypes
Some yeast killer toxin–encoding genes are not in viruses, or virus satellites, but are instead chromosomally encoded (Magliani et al. 1997; Liu et al. 2015). Thus, we complemented our analysis of M satellite–encoded killer toxins/antitoxins and M satellite–independent resistance to M satellite–encoded killer toxins by analyzing chromosomal killer genes and killer phenotypes in the 100-genomes strains. In S. cerevisiae, although less studied than M satellite–dependent killers, 2 chromosomally encoded killer genes have been described, KHR1 on chromosome 9 (Goto et al. 1990b) and KHS1 on chromosome 5 (Goto et al. 1991). We screened the 100-genomes sequences for KHR1 (Goto et al. 1990a, 1990b). The KHR1 ORF was not present in 31 strains, including the canonical S288c genome, and was present in 69 of the 100-genomes strains, 1 of which (YJM1326) had a premature stop codon polymorphism (Supplementary Table 6). Similarly, we screened the 100-genomes sequences for KHS1 (Goto et al. 1991), using the KHS1 ORF sequence from YJM789 (Wei et al. 2007) because, as noted by Frank and Wolfe (Frank and Wolfe 2009), the previously determined KHS1 sequence (Goto et al. 1991) was likely misassembled. While absent from 1 strain (YJM326), KHS1 was present in 99 of the 100-genomes strains. Of the 99 KHS1-containing strains, 46 had S. cerevisiae KHS1 and 53 had KHS1 introgressed from S. paradoxus. Of the 46 strains with S. cerevisiae KHS1, 9 had full-length ORFs and 35 strains, including the canonical S288c, had a premature stop codon polymorphism (W66 to opal) with a further 2 strains also having a second premature stop codon polymorphism (Y88 to ochre) (Supplementary Table 6). Our KHS1 premature stop codon polymorphism and introgression results are consistent with previous analysis of KHS1 (Frank and Wolfe 2009), and both are consistent with khs1 being present and misannotated in the canonical S288c genome.
As a first step in assessing chromosomal killers, we tested L-A+ M+ and isogenic L-A0 M0 derivatives of the 100-genomes strains for their abilities to kill C. glabrata ATCC 2001, the same strain used to isolate and characterize the S. cerevisiae chromosomal killer genes KHR1 (Goto et al. 1990a, 1990b) and KHS1 (Goto et al. 1991). In contrast to their loss of S. cerevisiae killing activity (Supplementary Table 5), many M0 derivatives retained at least some of their C. glabrata killing activity (Supplementary Table 6), consistent with the presence in many S. cerevisiae strains of functional chromosomal killer gene(s). Thus, to eliminate confounding by M satellite–dependent killing, we utilized M satellite–free 100-genomes S. cerevisiae strains to characterize chromosomal killer genes and phenotypes for all subsequent experiments.
None of the M satellite–free 100-genomes S. cerevisiae strains killed C. glabrata ATCC 2001 under conditions specific for Khr1-dependent killing (Goto et al. 1990a, 1990b) (Supplementary Table 6), an example of which is shown (Supplementary Fig. 6a). Thus, KHR1 is not further described or discussed. In contrast, 70 of the M satellite–free and L-A– and M satellite–cocured derivatives of the 100-genomes strains killed C. glabrata ATCC 2001 to varying degrees under conditions specific for Khs1-dependent killing (Goto et al. 1991) (Supplementary Table 6). Testing for associations between killing of C. glabrata and S. cerevisiae genotypes showed the sole significant association to be a peak on the right arm of chromosome 5, including YER187w (Supplementary Fig. 7 and Supplementary Table 7) that corresponds to the 3′ region of the KHS1 ORF, as identified by (Frank and Wolfe 2009).
Similarly, we used YJM189, which has the strongest Khs1C. glabrata killing phenotype (Supplementary Table 6), to test for killing of the M satellite–free 100-genomes strains and, subsequently, association with S. cerevisiae genotypes and again found the sole significant association corresponded to KHS1 (Supplementary Fig. 8 and Supplementary Table 8). However, relative to the association between M satellite–free killing of C. glabrata and S. cerevisiae genotypes, the YJM189 KHS1 killing of the M satellite–free 100-genomes strains association was not as clear-cut, for which we suggest a hypothesis. S. cerevisiae strains have an unavoidable ancestral history of exposure to S. cerevisiae killer toxins, with the resulting selective pressure likely affecting sensitivity to these toxins, possibly analogous to the M satellite–independent resistance to M satellite–encoded toxins described above. In contrast to S. cerevisiae, its close relative C. glabrata (Roetzer et al. 2011; Gabaldón et al. 2013) may be viewed as a relative blank slate with respect to ancestral history of exposure to S. cerevisiae killer toxins that may affect sensitivity to S. cerevisiae killer toxins; indeed, this may have been an unstated rationale for the previous use of C. glabrata ATCC 2001 to isolate and characterize the S. cerevisiae chromosomal killer genes KHR1 (Goto et al. 1990a, 1990b) and KHS1 (Goto et al. 1991). Thus, while unlikely to be the sole explanation, 1 hypothesis is that heterogeneity in the 100-genomes strains for Khs1 antitoxin–independent resistance to Khs1 toxin contributes to the less clear-cut KHS1 association. Evidence consistent with Khs1 antitoxin–independent resistance to Khs1 toxin is described below.
We tested the KHS1 genotype association by deleting KHS1 in YJM189 and found that the isogenic khs1Δ strain YJM1896 did not kill C. glabrata (Supplementary Fig. 6a) (Supplementary Table 6) or Khs1-sensitive S. cerevisiae strains (Supplementary Table 6). Thus, both the killing of C. glabrata and Khs1-sensitive S. cerevisiae strains was entirely KHS1 dependent, at least for the YJM189 KHS1 genetic background. Because M satellite–derived killer toxin(s) require processing by host-encoded killer expression protease(s) for their activity (Wickner and Leibowitz 1976; Wickner 1992; Wickner et al. 2013), we tested KEX1 and KEX2 for their roles in Khs1 killer activity. While kex1Δ had no effect, kex2Δ eliminated Khs1 killer activity in the KHS1 YJM189 background (Supplementary Fig. 6b). Because each of the M satellites encodes a toxin–antitoxin, we tested the sensitivity of the khs1Δ strain YJM1896 to killing by the isogenic KHS1 strain YJM189; the khs1Δ strain YJM1896 was sensitive to exogenous Khs1 toxin from the isogenic KHS1 parent strain (Supplementary Fig. 6c). That is, like the M satellite–encoded toxins–antitoxins, KHS1 encodes both Khs1 toxin and Khs1 antitoxin, with resistance to exogenous Khs1 toxin being dependent on endogenous Khs1 antitoxin, at least in the YJM189 genetic background.
While the killing of C. glabrata/resistance to killing by YJM189 KHS1 phenotypes of most of the 100-genomes strains were consistent with their KHS1 genotypes, the phenotypes of some strains were inconsistent, including 17 of the 34 strains having the khs1 single premature opal stop codon polymorphism. One hypothesis for the phenotypic inconsistencies of these 17 khs1 opal strains would be translational read-through that should result in the production of both Khs1 toxin and antitoxin. However, these 17 khs1 opal strains had phenotype(s) inconsistent with such translational read-through (see below).
The choice of YJM189 for phenotypic testing was serendipitous as this genetic background allowed both KHS1-dependent and KHS1-independent killer phenotypes, as well as KHS1-independent Khs1 toxin resistance phenotypes, to be identified, as described below. Based on KHS1 genotypes and killing of C. glabrata/resistance to killing by YJM189 KHS1 phenotypes, classes are described and hypotheses (other than opal translational read-through) for inconsistent phenotypes are discussed below.
Class 1 strains (n = 20), which did not kill C. glabrata and were killed by YJM189 KHS1, consisted of 17 strains with the khs1 opal polymorphism, 2 strains with 2 khs1 premature stop codons (66 opal, 88 ochre), and YJM326, the sole khs1 null (not present in genome) strain. The phenotypes of all class 1 strains were consistent with their presumed inability to produce Khs1 toxin and Khs1 antitoxin.
Class 2 strains (n = 10), which did not kill C. glabrata but were resistant to killing by YJM189 KHS1, consisted of 10 strains with the khs1 opal polymorphism. Like class 1 strains, the inability to kill the C. glabrata phenotype of class 2 strains was consistent with their presumed inability to produce Khs1 toxin. However, in contrast to class 1 strains, the resistance to killing by YJM189 KHS1 of class 2 strains was inconsistent with their presumed inability to produce Khs1 antitoxin. The resistance of the khs1 class 2 strains to killing by YJM189 KHS1 is hypothesized to be due to KHS1-independent resistance to exogenous Khs1 toxin, like the M satellite–independent resistance to each of the M satellite–encoded killer toxins described above.
Class 3 strains (n = 60), which killed C. glabrata and were resistant to killing by YJM189 KHS1, consisted of 58 strains with full-length KHS1 ORFs (introgressed S. paradoxus KHS1: n = 52; S. cerevisiae KHS1: n = 6) and 2 strains with the khs1 opal polymorphism. Based on KHS1 genotypes, class 3 strains were split into 2 subclasses. For class 3A strains with full-length KHS1 ORFs (n = 58), both the C. glabrata and YJM189 phenotypes were consistent with their presumed production of functional Khs1 toxin and Khs1 antitoxin. For class 3B strains with the khs1 opal polymorphism (n = 2), both the C. glabrata phenotypes and the YJM189 phenotypes were inconsistent with nonfunctional khs1 genotypes. The resistance to killing by the YJM189 KHS1 phenotype of the 2 khs1 class 3B strains is hypothesized to be due to KHS1-independent resistance to exogenous Khs1 toxin, as in class 2 strains. The C. glabrata killing phenotype of the 2 khs1 class 3B strains (YJM1383 and YJM1450) is hypothesized to be due to their production of a non-Khs1 toxin. This hypothesized non-Khs1 toxin is not Khr1 because, like all the 100-genomes strains, neither strain shows Khr1 killer activity; in addition, YJM1383 has no KHR1 in its genome (Supplementary Table 6). The hypothesized non-Khs1 toxin may be antimicrobial glyceraldehyde-3-phosphate dehydrogenase–derived peptides that have been shown to kill some bacteria and some non-Saccharomyces yeasts (Branco et al. 2014, 2017, 2018, 2019).
Finally, class 4 strains (n = 10), which killed C. glabrata and were killed by YJM189 KHS1, consisted of 2 strains with the introgressed S. paradoxus KHS1 ORF, 3 strains with the full-length S. cerevisiae KHS1 ORF, and 5 strains with the khs1 opal polymorphism. Based on KHS1 genotypes, the class 4 strains were split into 2 subclasses. For class 4A strains with the khs1 opal polymorphism (n = 5), while the C. glabrata phenotypes were inconsistent, the YJM189 phenotypes were consistent with nonfunctional khs1 genotypes. The C. glabrata killing phenotype of class 4A khs1 strains is hypothesized to be due to their production of a non-Khs1 toxin, as in the class 3B strains. For class 4B strains with full-length KHS1 ORFs (n = 5), while the C. glabrata killing phenotypes were consistent, those killed by YJM189 phenotypes were inconsistent with those by functional KHS1 genotypes. Those killed by the YJM189 phenotype of class 4B strains is hypothesized to be due to KHS1 being functionally null, possibly due to cis-acting KHS1 noncoding polymorphisms or trans-acting polymorphisms in transcription factor(s) and/or by silencing of the subtelomeric KHS1 gene. The killing of the C. glabrata phenotype of class 4B strains is hypothesized to be due to their production of a non-Khs1 toxin, as in class 3B and class 4A strains.
Thus, S. cerevisiae can produce multiple toxins and antitoxins, both L-A–dependent M satellite–encoded and chromosomally encoded, as well as chromosomally encoded, presumably non–antitoxin-mediated toxin resistance mechanisms, each of which exhibits variation in the 100-genomes strains. Like the variation in M satellite–independent resistance to K1, K2, and K28 toxins described above, variation in Khs1 production and for Khs1 antitoxin–independent resistance to exogenous Khs1 toxin, as well as for variation for production of non-Khs1 toxin(s), suggests balancing selection.
Conclusion
When present in an S. cerevisiae strain, a functional killer toxin–antitoxin–encoding gene, whether chromosomally or M satellite encoded, can be antimicrobial, killing strains that lack that gene, with the caveat that such killing is affected by genotype (i.e. antitoxin-independent effects on killer toxin resistance) and by the environment. Thus, secreted killer toxin–encoding genes may have fitness benefits via their antimicrobial activities in naturally occurring mixed genotype/heterogeneous cultures but may be confounding in mixed genotype/heterogeneous culture experiments. These same killer toxin–encoding genes also may have deleterious effects on fitness, as has been shown for the L-A–dependent M1 satellite that confers a severe growth defect or lethality when present in specific genetic backgrounds (Ridley et al. 1984; Sommer and Wickner 1987; Edwards et al. 2014). Indeed, even in backgrounds where it does not confer a severe growth defect or lethality, M1 confers a slight growth deficit (Suzuki et al. 2015). Notably, rather than mixed genotype/heterogeneous cultures, the cited cases are in clonal/uniform cultures, which may suggest an intracellular/cell autonomous phenotypic effect of M1/K1; other M satellites may have similarly deleterious effects on host fitness. Given the abundance of khs1 alleles, KHS1 also may have deleterious effects on fitness. We suggest that killer toxin–encoding M satellites and KHS1 may confound the analysis of host phenotypes, including quantitative traits.
As with genotype variation in nuclear genes that often affects organismal phenotypes, genotype variation in viruses can affect viral phenotypes. For example, although the mechanism(s) and phenotypically relevant sequence difference(s) are not known, genotypically distinct L-A viruses differ in their abilities to compete with each other, to resist curing, and to support different M satellites (Wickner 1980; Wickner and Toh-e 1982; Wickner 1983, 1992; Rodriguez-Cousino et al. 2013, 2017). RNA viral genotype–dependent effects on S. cerevisiae host phenotypes, if such exist, could contribute to the lack of virus presence/absence associations with host phenotypes. Similarly, high-frequency RNA viral mutation that improves viral adaptation to host genotypes could also contribute to the lack of virus presence/absence associations with host phenotypes, as further discussed below.
Like M-dependent effects on host phenotypes that are only seen in ski mutant strains with elevated levels of M (Ridley et al. 1984; Sommer and Wickner 1987), RNA virus–dependent effects on host phenotypes may also depend on RNA virus levels. RNA virus levels may be environment dependent, as has been reported for 20S and 23S (Garvik and Haber 1978; Wesolowski and Wickner 1984; Matsumoto et al. 1990) and L-A (Oliver et al. 1977). RNA virus levels also may be host genotype dependent, as has been reported for L-A in por1 (Dihanich et al. 1987; Dihanich et al. 1989), nuc1 (Liu and Dieckmann 1989), lcb2 (Garnepudi et al. 1997), and ski mutants (Wickner 1992, 1996a). Given the high mutation frequencies of RNA viruses in other species (Sanjuan et al. 2010), mutations in S. cerevisiae RNA viruses also may affect virus levels. Interestingly, the reported increased L-A level in por1 mutants was accompanied, after a lag, by L-A–dependent suppression of the por1 respiration defect (Dihanich et al. 1987, 1989). In principle, the increased L-A levels may be due to secondary host mutation(s) and/or to L-A mutation(s). We suggest that RNA virus levels may contribute to RNA virus–dependent effects on host phenotypes.
Of the 100-genomes strains, 67 were L-BClo and 70 were L-A0. Of the 30 L-A+ 100-genomes strains, while 17 were M+, only 10 were K+. In contrast, 5 S. cerevisiae genetic backgrounds commonly used in the laboratory are quite distinct: S288c (isogenic with YJM1552) is L-A+ L-BChi M0; RM11 (isogenic with YJM1293) is L-A0 L-BChi M0; Σ1278b (isogenic with YJM1290) is L-A+ L-BChi M1+ K1+; SK1 (isogenic with YJM1077) is L-A+ L-BChi M1+ K1+; and YJM789 (isogenic with YJM145) is L-A+ L-BChi Mlus+ Klus−. Clearly, these commonly used genetic backgrounds are not representative for L-BC level, L-A, L-A–dependent M satellites, or M satellite–dependent killer phenotypes in S. cerevisiae. To our knowledge, the only study that has examined interactions between the host and viral (or, in this case, M1 satellite) genomes found profound effects (Edwards et al. 2014). In addition to M satellites, we suggest that L-A and, possibly, L-BC may be confounding factors in the analysis of both qualitative and quantitative traits.
In numerous species, RNA viruses have very high mutation rates (Sanjuan et al. 2010). Thus, the identification of RNA virus and M satellite effects on S. cerevisiae host phenotypes may be confounded by high rates of RNA virus and M satellite mutation and loss that are likely to be far higher than S. cerevisiae host mutation. While the mutation rates for S. cerevisiae RNA viruses are not known, there is strong evidence that the mutation/loss rates for M satellites are high due, for example, to the prion-like [KIL-d] element (Talloczy et al. 1998, 2000; Suzuki et al. 2015). Mutation of RNA viruses and L-A–dependent M satellites may result in their loss, which would result in virus- and M satellite–dependent effects on host phenotypes also being lost. Alternatively, mutation of RNA viruses and L-A–dependent dsRNA M satellites may result in virus and M satellite adaptation to environment(s) and/or to S. cerevisiae host genotype(s). Such RNA viral and M satellite adaptation may result in the modification of viral- and M satellite–dependent effects on host phenotypes. In sum, the presence of RNA viruses and M satellites varied considerably across the 100-genomes population resource. Virus-dependent and M satellite–dependent phenotypes were characterized and extend our understanding of the phenotypic effects of these non–Mendelian-inherited factors. Given their effects on host phenotype and the potential for virus/satellite mutation and loss, the contributions of RNA viruses and L-A–dependent M satellites should be considered in S. cerevisiae studies.
Supplementary Material
Acknowledgments
The authors thank Joseph Heitman and the Department of Microbiology and Molecular Genetics for covering the publication costs. The authors thank J. Dinman for the L-A cDNA–containing plasmid pJD1223 (= pI2L2) (Valle and Wickner 1993), M. Schmitt for the M28+ K28+ reference strain MS300c (Schmitt and Tipper 1990), and M. Ramirez for the Mlus+ Klus+ reference strain EX229 (Rodriguez-Cousiño et al. 2011).
Contributor Information
Sriram Vijayraghavan, Department of Molecular Genetics and Microbiology, Duke University Medical Center, Durham, NC 27710, USA.
Stanislav G Kozmin, Department of Molecular Genetics and Microbiology, Duke University Medical Center, Durham, NC 27710, USA.
Pooja K Strope, Department of Molecular Genetics and Microbiology, Duke University Medical Center, Durham, NC 27710, USA.
Daniel A Skelly, Department of Biology, Duke University, Durham, NC 27708, USA.
Paul M Magwene, Department of Biology, Duke University, Durham, NC 27708, USA.
Fred S Dietrich, Department of Molecular Genetics and Microbiology, Duke University Medical Center, Durham, NC 27710, USA.
John H McCusker, Department of Molecular Genetics and Microbiology, Duke University Medical Center, Durham, NC 27710, USA.
Data availability
The 100-genomes strains (and derivatives) and plasmids described in this work have been deposited in Fungal Genetics Stock Center (http://www.fgsc.net) and Addgene (http://www.addgene.org/John_McCusker/), respectively.
Supplemental material available at G3 online.
Funding
National Institutes of Health grant R01 GM098287 supported this work. The National Institutes of Health grant F32 GM110997 supported DAS.
Literature cited
- Baeza ME, Sanhueza MA, Cifuentes VH. Occurrence of killer yeast strains in industrial and clinical yeast isolates. Biol Res. 2008;41(2):173–182. doi: 10.4067/S0716-97602008000200007. [DOI] [PubMed] [Google Scholar]
- Ball SG, Tirtiaux C, Wickner RB. Genetic control of L-A and L-(BC) dsRNA copy number in killer systems of Saccharomyces cerevisiae. Genetics. 1984;107(2):199–217. doi: 10.1093/genetics/107.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatti MF, Jamal A, Petrou MA, Cairns TC, Bignell EM, Coutts RH. The effects of dsRNA mycoviruses on growth and murine virulence of Aspergillus fumigatus. Fungal Genet Biol. 2011;48(11):1071–1075. doi: 10.1016/j.fgb.2011.07.008. [DOI] [PubMed] [Google Scholar]
- Branco P, Albergaria H, Arneborg N, Prista C. Effect of GAPDH-derived antimicrobial peptides on sensitive yeasts cells: membrane permeability, intracellular pH and H+-influx/-efflux rates. FEMS Yeast Res. 2018;18(3). doi: 10.1093/femsyr/foy030. [DOI] [PubMed] [Google Scholar]
- Branco P, Francisco D, Chambon C, Hébraud M, Arneborg N, Almeida MG, Caldeira J, Albergaria H. Identification of novel GAPDH-derived antimicrobial peptides secreted by Saccharomyces cerevisiae and involved in wine microbial interactions. Appl Microbiol Biotechnol. 2014;98(2):843–853. doi: 10.1007/s00253-013-5411-y. [DOI] [PubMed] [Google Scholar]
- Branco P, Kemsawasd V, Santos L, Diniz M, Caldeira J, Almeida MG, Arneborg N, Albergaria H. Saccharomyces cerevisiae accumulates GAPDH-derived peptides on its cell surface that induce death of non-Saccharomyces yeasts by cell-to-cell contact. FEMS Microbiol Ecol. 2017;93(5). doi: 10.1093/femsec/fix055. [DOI] [PubMed] [Google Scholar]
- Branco P, Sabir F, Diniz M, Carvalho L, Albergaria H, Prista C. Biocontrol of Brettanomyces/Dekkera bruxellensis in alcoholic fermentations using saccharomycin-overproducing Saccharomyces cerevisiae strains. Appl Microbiol Biotechnol. 2019;103(7):3073–3083. doi: 10.1007/s00253-019-09657-7. [DOI] [PubMed] [Google Scholar]
- Carroll K, Wickner RB. Translation and M1 double-stranded RNA propagation: MAK18 = RPL41B and cycloheximide curing. J Bacteriol. 1995;177(10):2887–2891. doi: 10.1128/jb.177.10.2887-2891.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SL, Leu JY, Chang TH. A population study of killer viruses reveals different evolutionary histories of two closely related Saccharomyces sensu stricto yeasts. Mol Ecol. 2015;24(16):4312–4322. doi: 10.1111/mec.13310. [DOI] [PubMed] [Google Scholar]
- Chau S, Gao J, Diao AJ, Cao SB, Azhieh A, Davidson AR, Meneghini MD. Diverse yeast antiviral systems prevent lethal pathogenesis caused by the L-A mycovirus. Proc Natl Acad Sci U S A. 2023;120(11):e2208695120. doi: 10.1073/pnas.2208695120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawe AL, Nuss DL. Hypoviruses and chestnut blight: exploiting viruses to understand and modulate fungal pathogenesis. Annu Rev Genet. 2001;35(1):1–29. doi: 10.1146/annurev.genet.35.102401.085929. [DOI] [PubMed] [Google Scholar]
- Dihanich M, Suda K, Schatz G. A yeast mutant lacking mitochondrial porin is respiratory-deficient, but can recover respiration with simultaneous accumulation of an 86-kd extramitochondrial protein. EMBO J. 1987;6(3):723–728. doi: 10.1002/j.1460-2075.1987.tb04813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dihanich M, van Tuinen E, Lambris JD, Marshallsay B. Accumulation of viruslike particles in a yeast mutant lacking a mitochondrial pore protein. Mol Cell Biol. 1989;9(3):1100–1108. doi: 10.1128/mcb.9.3.1100-1108.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinman JD, Ruiz-Echevarria MJ, Czaplinski K, Peltz SW. Peptidyl-transferase inhibitors have antiviral properties by altering programmed −1 ribosomal frameshifting efficiencies: development of model systems. Proc Natl Acad Sci U S A. 1997;94(13):6606–6611. doi: 10.1073/pnas.94.13.6606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson MJ, Pickett AJ, Velmurugan S, Pinder JB, Barrett LA, Jayaram M, Chew JS. The 2 micron plasmid causes cell death in Saccharomyces cerevisiae with a mutation in Ulp1 protease. Mol Cell Biol. 2005;25(10):4299–4310. doi: 10.1128/MCB.25.10.4299-4310.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drinnenberg IA, Fink GR, Bartel DP. Compatibility with killer explains the rise of RNAi-deficient fungi. Science. 2011;333(6049):1592. doi: 10.1126/science.1209575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards MD, Symbor-Nagrabska A, Dollard L, Gifford DK, Fink GR. Interactions between chromosomal and nonchromosomal elements reveal missing heritability. Proc Natl Acad Sci U S A. 2014;111(21):7719–7722. doi: 10.1073/pnas.1407126111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sherbeini M, Tipper DJ, Mitchell DJ, Bostian KA. Virus-like particle capsid proteins encoded by different L double-stranded RNAs of Saccharomyces cerevisiae: their roles in maintenance of M double-stranded killer plasmids. Mol Cell Biol. 1984;4(12):2818–2827. doi: 10.1128/mcb.4.12.2818-2827.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban R, Vega L, Fujimura T. 20S RNA narnavirus defies the antiviral activity of SKI1/XRN1 in Saccharomyces cerevisiae. J Biol Chem. 2008;283(38):25812–25820. doi: 10.1074/jbc.M804400200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink GR, Styles CA. Curing of a killer factor in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1972;69(10):2846–2849. doi: 10.1073/pnas.69.10.2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank AC, Wolfe KH. Evolutionary capture of viral and plasmid DNA by yeast nuclear chromosomes. Eukaryot Cell. 2009;8(10):1521–1531. doi: 10.1128/EC.00110-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futcher AB, Cox BS. Maintenance of the 2 microns circle plasmid in populations of Saccharomyces cerevisiae. J Bacteriol. 1983;154(2):612–622. doi: 10.1128/jb.154.2.612-622.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabaldón T, Martin T, Marcet-Houben M, Durrens P, Bolotin-Fukuhara M, Lespinet O, Arnaise S, Boisnard S, Aguileta G, Atanasova R, et al. Comparative genomics of emerging pathogens in the Candida glabrata clade. BMC Genomics. 2013;14(1):623. doi: 10.1186/1471-2164-14-623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Chau S, Chowdhury F, Zhou T, Hossain S, McQuibban GA, Meneghini MD. Meiotic viral attenuation through an ancestral apoptotic pathway. Proc Natl Acad Sci U S A. 2019;116(33):16454–16462. doi: 10.1073/pnas.1900751116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnepudi VR, Zhao C, Beeler T, Dunn T. Serine palmitoyltransferase (scs1/lcb2) mutants have elevated copy number of the L-A dsRNA virus. Yeast. 1997;13(4):299–304. doi:. [DOI] [PubMed] [Google Scholar]
- Garvik B, Haber JE. New cytoplasmic genetic element that controls 20S RNA synthesis during sporulation in yeast. J Bacteriol. 1978;134(1):261–269. doi: 10.1128/jb.134.1.261-269.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghabrial SA, Caston JR, Jiang D, Nibert ML, Suzuki N. 50-plus years of fungal viruses. Virology. 2015;479–480:356–368. doi: 10.1016/j.virol.2015.02.034. [DOI] [PubMed] [Google Scholar]
- Ghabrial SA, Suzuki N. Viruses of plant pathogenic fungi. Annu Rev Phytopathol. 2009;47(1):353–384. doi: 10.1146/annurev-phyto-080508-081932. [DOI] [PubMed] [Google Scholar]
- Goto K, Fukuda H, Kichise K, Kitano K, Hara S. Cloning and nucleotide sequence of the KHS killer gene of Saccharomyces cerevisiae. Agric Biol Chem. 1991;55(8):1953–1958. doi: 10.1271/bbb1961.55.1953. [DOI] [PubMed] [Google Scholar]
- Goto K, Iwase T, Kichise K, Kitano K, Totuka A, Obata T, Hara S. Isolation and properties of a chromosome-dependent KHR killer toxin in Saccharomyces cerevisiae. Agric Biol Chem. 1990a;54(2):505–509. doi: 10.1271/bbb1961.54.505. [DOI] [PubMed] [Google Scholar]
- Goto K, Iwatuki Y, Kitano K, Obata T, Hara S. Cloning and nucleotide sequence of the KHR killer gene of Saccharomyces cerevisiae. Agric Biol Chem. 1990b;54(4):979–984. doi: 10.1271/bbb1961.54.979. [DOI] [PubMed] [Google Scholar]
- Harrison E, Koufopanou V, Burt A, MacLean RC. The cost of copy number in a selfish genetic element: the 2-μm plasmid of Saccharomyces cerevisiae. J Evol Biol. 2012;25(11):2348–2356. doi: 10.1111/j.1420-9101.2012.02610.x. [DOI] [PubMed] [Google Scholar]
- Holm C. Clonal lethality caused by the yeast plasmid 2 mu DNA. Cell. 1982a;29(2):585–594. doi: 10.1016/0092-8674(82)90174-X. [DOI] [PubMed] [Google Scholar]
- Holm C. Sensitivity to the yeast plasmid 2mu DNA is conferred by the nuclear allele nibl. Mol Cell Biol. 1982b;2(8):985–992. doi: 10.1128/mcb.2.8.985-992.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Zheng Z, Qi T, Kemper KE, Wray NR, Visscher PM, Yang J. A resource-efficient tool for mixed model association analysis of large-scale data. Nat Genet. 2019;51:1749–1755. doi: 10.1038/s41588-019-0530-8. [DOI] [PubMed] [Google Scholar]
- Liu GL, Chi Z, Wang GY, Wang ZP, Li Y, Chi ZM. Yeast killer toxins, molecular mechanisms of their action and their applications. Crit Rev Biotechnol. 2015;35(2):222–234. doi: 10.3109/07388551.2013.833582. [DOI] [PubMed] [Google Scholar]
- Liu YX, Dieckmann CL. Overproduction of yeast viruslike particles by strains deficient in a mitochondrial nuclease. Mol Cell Biol. 1989;9(8):3323–3331. doi: 10.1128/mcb.9.8.3323-3331.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez V, Gil R, Vicente Carbonell J, Navarro A. Occurrence of 20S RNA and 23S RNA replicons in industrial yeast strains and their variation under nutritional stress conditions. Yeast. 2002;19(6):545–552. doi: 10.1002/yea.848. [DOI] [PubMed] [Google Scholar]
- Magliani W, Conti S, Gerloni M, Bertolotti D, Polonelli L. Yeast killer systems. Clin Microbiol Rev. 1997;10(3):369–400. doi: 10.1128/CMR.10.3.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maqueda M, Zamora E, Rodriguez-Cousino N, Ramirez M. Wine yeast molecular typing using a simplified method for simultaneously extracting mtDNA, nuclear DNA and virus dsRNA. Food Microbiol. 2010;27(2):205–209. doi: 10.1016/j.fm.2009.10.004. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y, Fishel R, Wickner RB. Circular single-stranded RNA replicon in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1990;87(19):7628–7632. doi: 10.1073/pnas.87.19.7628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe PM, Pfeiffer P, Van Alfen NK. The influence of dsRNA viruses on the biology of plant pathogenic fungi. Trends Microbiol. 1999;7(9):377–381. doi: 10.1016/S0966-842X(99)01568-1. [DOI] [PubMed] [Google Scholar]
- McCluskey K, Wiest A, Plamann M. The fungal genetics stock center: a repository for 50 years of fungal genetics research. J Biosci. 2010;35(1):119–126. doi: 10.1007/s12038-010-0014-6. [DOI] [PubMed] [Google Scholar]
- Mead DJ, Gardner DC, Oliver SG. The yeast 2 micron plasmid: strategies for the survival of a selfish DNA. Mol Gen Genet. 1986;205(3):417–421. doi: 10.1007/BF00338076. [DOI] [PubMed] [Google Scholar]
- Nakayashiki T, Kurtzman CP, Edskes HK, Wickner RB. Yeast prions [URE3] and [PSI+] are diseases. Proc Natl Acad Sci U S A. 2005;102(30):10575–10580. doi: 10.1073/pnas.0504882102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuss DL. Hypovirulence: mycoviruses at the fungal-plant interface. Nat Rev Microbiol. 2005;3(8):632–642. doi: 10.1038/nrmicro1206. [DOI] [PubMed] [Google Scholar]
- Oliver SG, McCREADY SJ, Holm C, Sutherland PA, McLaughlin CS, Cox BS. Biochemical and physiological studies of the yeast virus-like particle. J Bacteriol. 1977;130(3):1303–1309. doi: 10.1128/jb.130.3.1303-1309.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson MN, Beever RE, Boine B, Arthur K. Mycoviruses of filamentous fungi and their relevance to plant pathology. Mol Plant Pathol. 2009;10(1):115–128. doi: 10.1111/j.1364-3703.2008.00503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philliskirk G, Young TW. The occurrence of killer character in yeasts of various genera. Antonie Van Leeuwenhoek. 1975;41(1):147–151. doi: 10.1007/BF02565046. [DOI] [PubMed] [Google Scholar]
- Pieczynska MD, de Visser JA, Korona R. Incidence of symbiotic dsRNA ‘killer’ viruses in wild and domesticated yeast. FEMS Yeast Res. 2013;13(8):856–859. doi: 10.1111/1567-1364.12086. [DOI] [PubMed] [Google Scholar]
- Ridley SP, Sommer SS, Wickner RB. Superkiller mutations in Saccharomyces cerevisiae suppress exclusion of M2 double-stranded RNA by L-A-HN and confer cold sensitivity in the presence of M and L-A-HN. Mol Cell Biol. 1984;4(4):761–770. doi: 10.1128/mcb.4.4.761-770.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Cousino N, Esteban R. Relationships and evolution of double-stranded RNA totiviruses of yeasts inferred from analysis of L-A-2 and L-BC variants in wine yeast strain populations. Appl Environ Microbiol. 2017;83(4):e02991–e02916. doi: 10.1128/AEM.02991-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Cousino N, Gomez P, Esteban R. L-A-lus, a new variant of the L-A totivirus found in wine yeasts with Klus killer toxin-encoding Mlus double-stranded RNA: possible role of killer toxin-encoding satellite RNAs in the evolution of their helper viruses. Appl Environ Microbiol. 2013;79(15):4661–4674. doi: 10.1128/AEM.00500-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Cousino N, Gomez P, Esteban R. Variation and distribution of L-A helper totiviruses in Saccharomyces sensu stricto yeasts producing different killer toxins. Toxins (Basel). 2017;9(10):313. doi: 10.3390/toxins9100313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Cousiño N, Maqueda M, Ambrona J, Zamora E, Esteban R, Ramírez M. A new wine Saccharomyces cerevisiae killer toxin (Klus), encoded by a double-stranded RNA virus, with broad antifungal activity is evolutionarily related to a chromosomal host gene. Appl Environ Microbiol. 2011;77(5):1822–1832. doi: 10.1128/AEM.02501-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roetzer A, Gabaldon T, Schuller C. From Saccharomyces cerevisiae to Candida glabrata in a few easy steps: important adaptations for an opportunistic pathogen. FEMS Microbiol Lett. 2011;314(1):1–9. doi: 10.1111/j.1574-6968.2010.02102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roossinck MJ. The good viruses: viral mutualistic symbioses. Nature reviews. Microbiology. 2011;9(2):99–108. doi: 10.1038/nrmicro2491. [DOI] [PubMed] [Google Scholar]
- Rosini G. The occurrence of killer characters in yeasts. Can J Microbiol. 1983;29(10):1462–1464. doi: 10.1139/m83-224. [DOI] [PubMed] [Google Scholar]
- Rowley PA. The frenemies within: viruses, retrotransposons and plasmids that naturally infect Saccharomyces yeasts. Yeast. 2017;34(7):279–292. doi: 10.1002/yea.3234. [DOI] [PubMed] [Google Scholar]
- Rowley PA, Ho B, Bushong S, Johnson A, Sawyer SL. XRN1 is a species-specific virus restriction factor in yeasts. PLoS Pathog. 2016;12(10):e1005890. doi: 10.1371/journal.ppat.1005890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanjuan R, Nebot MR, Chirico N, Mansky LM, Belshaw R. Viral mutation rates. J Virol. 2010;84(19):9733–9748. doi: 10.1128/JVI.00694-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt MJ, Breinig F. The viral killer system in yeast: from molecular biology to application. FEMS Microbiol Rev. 2002;26(3):257–276. doi: 10.1111/j.1574-6976.2002.tb00614.x. [DOI] [PubMed] [Google Scholar]
- Schmitt MJ, Breinig F. Yeast viral killer toxins: lethality and self-protection. Nat Rev Microbiol. 2006;4(3):212–221. doi: 10.1038/nrmicro1347. [DOI] [PubMed] [Google Scholar]
- Schmitt MJ, Tipper DJ. K28, a unique double-stranded RNA killer virus of Saccharomyces cerevisiae. Mol Cell Biol. 1990;10(9):4807–4815. doi: 10.1128/mcb.10.9.4807-4815.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt MJ, Tipper DJ. Genetic analysis of maintenance and expression of L and M double-stranded RNAs from yeast killer virus K28. Yeast. 1992;8(5):373–384. doi: 10.1002/yea.320080505. [DOI] [PubMed] [Google Scholar]
- Schmitt MJ, Tipper DJ. Sequence of the M28 dsRNA: preprotoxin is processed to an alpha/beta heterodimeric protein toxin. Virology. 1995;213(2):341–351. doi: 10.1006/viro.1995.0007. [DOI] [PubMed] [Google Scholar]
- Solis-Escalante D, Kuijpers NG, Bongaerts N, Bolat I, Bosman L, Pronk JT, Daran JM, Daran-Lapujade P. amdSYM, a new dominant recyclable marker cassette for Saccharomyces cerevisiae. FEMS Yeast Res. 2013;13(1):126–139. doi: 10.1111/1567-1364.12024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer SS, Wickner RB. Yeast L dsRNA consists of at least three distinct RNAs; evidence that the non-Mendelian genes [HOK], [NEX] and [EXL] are on one of these dsRNAs. Cell. 1982;31(2):429–441. doi: 10.1016/0092-8674(82)90136-2. [DOI] [PubMed] [Google Scholar]
- Sommer SS, Wickner RB. Gene disruption indicates that the only essential function of the SKI8 chromosomal gene is to protect Saccharomyces cerevisiae from viral cytopathology. Virology. 1987;157(1):252–256. doi: 10.1016/0042-6822(87)90338-2. [DOI] [PubMed] [Google Scholar]
- Strope PK, Kozmin SG, Skelly DA, Magwene PM, Dietrich FS, McCusker JH. 2µ plasmid in Saccharomyces species and in Saccharomyces cerevisiae. FEMS Yeast Res. 2015a;15(8):fov090. doi: 10.1093/femsyr/fov090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strope PK, Skelly DA, Kozmin SG, Mahadevan G, Stone EA, Magwene PM, Dietrich FS, McCusker JH. The 100-genomes strains, an S. cerevisiae resource that illuminates its natural phenotypic and genotypic variation and emergence as an opportunistic pathogen. Genome Res. 2015b;25(5):762–774. doi: 10.1101/gr.185538.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki G, Weissman JS, Tanaka M. [KIL-d] protein element confers antiviral activity via catastrophic viral mutagenesis. Mol Cell. 2015;60(4):651–660. doi: 10.1016/j.molcel.2015.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talloczy Z, Mazar R, Georgopoulos DE, Ramos F, Leibowitz MJ. The [KIL-d] element specifically regulates viral gene expression in yeast. Genetics. 2000;155(2):601–609. doi: 10.1093/genetics/155.2.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talloczy Z, Menon S, Neigeborn L, Leibowitz MJ. The [KIL-d] cytoplasmic genetic element of yeast results in epigenetic regulation of viral M double-stranded RNA gene expression. Genetics. 1998;150(1):21–30. doi: 10.1093/genetics/150.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valle RP, Wickner RB. Elimination of L-A double-stranded RNA virus of Saccharomyces cerevisiae by expression of gag and gag-pol from an L-A cDNA clone. J Virol. 1993;67(5):2764–2771. doi: 10.1128/jvi.67.5.2764-2771.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayraghavan S, Kozmin SG, Strope PK, Skelly DA, Lin Z, Kennell J, Magwene PM, Dietrich FS, McCusker JH. Mitochondrial genome variation affects multiple respiration and non-respiration phenotypes in Saccharomyces cerevisiae. Genetics. 2019;211(2):773–786. doi: 10.1534/genetics.118.301546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayraghavan S, Kozmin SG, Xi W, McCusker JH. A novel narnavirus is widespread in Saccharomyces cerevisiae and impacts multiple host phenotypes. G3 (Bethesda). 2023;13(2):jkac337. doi: 10.1093/g3journal/jkac337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, McCusker JH, Hyman RW, Jones T, Ning Y, Cao Z, Gu Z, Bruno D, Miranda M, Nguyen M, et al. Genome sequencing and comparative analysis of Saccharomyces cerevisiae strain YJM789. Proc Natl Acad Sci U S A. 2007;104(31):12825–12830. doi: 10.1073/pnas.0701291104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesolowski M, Wickner RB. Two new double-stranded RNA molecules showing non-Mendelian inheritance and heat inducibility in Saccharomyces cerevisiae. Mol Cell Biol. 1984;4(1):181–187. doi: 10.1128/mcb.4.1.181-187.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner RB. Plasmids controlled exclusion of the K2 killer double-stranded RNA plasmid of yeast. Cell. 1980;21(1):217–226. doi: 10.1016/0092-8674(80)90129-4. [DOI] [PubMed] [Google Scholar]
- Wickner RB. Killer systems in Saccharomyces cerevisiae: three distinct modes of exclusion of M2 double-stranded RNA by three species of double-stranded RNA, M1, L-A-E, and L-A-HN. Mol Cell Biol. 1983;3(4):654–661. doi: 10.1128/mcb.3.4.654-661.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner RB. Double-stranded and single-stranded RNA viruses of Saccharomyces cerevisiae. Annu Rev Microbiol. 1992;46(1):347–375. doi: 10.1146/annurev.mi.46.100192.002023. [DOI] [PubMed] [Google Scholar]
- Wickner RB. Double-stranded RNA viruses of Saccharomyces cerevisiae. Microbiol Rev. 1996a;60(1):250–265. doi: 10.1128/mr.60.1.250-265.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner RB. Prions and RNA viruses of Saccharomyces cerevisiae. Annu Rev Genet. 1996b;30(1):109–139. doi: 10.1146/annurev.genet.30.1.109. [DOI] [PubMed] [Google Scholar]
- Wickner RB, Fujimura T, Esteban R. Viruses and prions of Saccharomyces cerevisiae. Adv Virus Res. 2013;86:1–36. doi: 10.1016/B978-0-12-394315-6.00001-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner RB, Icho T, Fujimura T, Widner WR. Expression of yeast L-A double-stranded RNA virus proteins produces derepressed replication: a ski- phenocopy. J Virol. 1991;65(1):155–161. doi: 10.1128/jvi.65.1.155-161.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner RB, Leibowitz MJ. Two chromosomal genes required for killing expression in killer strains of Saccharomyces cerevisiae. Genetics. 1976;82(3):429–442. doi: 10.1093/genetics/82.3.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner RB, Toh-e A. [HOK], a new yeast non-Mendelian trait, enables a replication-defective killer plasmid to be maintained. Genetics. 1982;100(2):159–174. doi: 10.1093/genetics/100.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J, Jiang D. New insights into mycoviruses and exploration for the biological control of crop fungal diseases. Annu Rev Phytopathol. 2014;52(1):45–68. doi: 10.1146/annurev-phyto-102313-050222. [DOI] [PubMed] [Google Scholar]
- Yang J, Lee SH, Goddard ME, Visscher PM. GCTA: a tool for genome-wide complex trait analysis. Am J Hum Genet. 2011;88(1):76–82. doi: 10.1016/j.ajhg.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The 100-genomes strains (and derivatives) and plasmids described in this work have been deposited in Fungal Genetics Stock Center (http://www.fgsc.net) and Addgene (http://www.addgene.org/John_McCusker/), respectively.
Supplemental material available at G3 online.