Abstract
Human mercury (Hg) exposure is mostly caused by eating fish. However, there are major differences between the measured and predicted mercury concentration on Hg bioavailability. This study investigated the effects of cooking (steaming, baking, frying, marinating, and smoking) and selected components' co-ingestion on Hg bioaccessibility. Baking and frying reduced Hg bioaccessibility compared to the raw sample. The bioaccessible Hg fraction in fish was assessed through in vitro digestion method. Hg bioaccessibility varied from 4.31 to nearly 24.95% and the Hg recovery rate varied from 63.44 to 78.74%. Co-ingested garlic and broccoli with pontic shad had a positive effect on decreasing fish Hg bioaccessibility. The antioxidant activity of co-ingested food items was also calculated and correlated with mercury bioaccessibility. These results highlighted a possible positive role of plant-based foods and other food processing techniques in the bioaccessibility reduction of other chemical contaminants found in food sources.
Keywords: Mercury, Bioaccessibility, Cooking methods, Shad, Co-ingestion, Antioxidant activity
Graphical abstract
Highlights
-
•
Bioaccessibility of mercury from pontic shad was performed using in vitro digestion.
-
•
Cooking methods and selected components' co-ingestion decrease Hg bioaccessibility.
-
•
The effect on mercury reduction increased with the severity of the heat treatment.
-
•
Garlic has ameliorating potential against heavy metal toxicity.
-
•
Risk assessments should consider nutritional habits and mercury bioavailability.
1. Introduction
According to earlier studies, the direct discharge of urban waste, untreated industrial effluents, and agricultural chemicals into rivers and tributaries has a major negative impact on aquatic ecosystems. Worldwide, both natural water sources and aquaculture systems are being severely contaminated by heavy metals due to increasing anthropogenic activities (Islam et al., 2018). The presence of chemical contaminants in the environment and feed in aquaculture areas may cause significant metal accumulation in aquaculture food products (Tahity et al., 2022). Heavy metals are considered to be the most severe source of pollution in the aquatic environment due to their toxicity and absorption (Senthamils et al., 2015). Heavy metal toxicity is determined by a number of parameters, including the dose, route of exposure, as well as age, gender, genetics, and nutritional state of those who have been exposed. Arsenic, cadmium, chromium, lead, and mercury comprise the most significant metals of public health concern due to their high toxicity. Since toxicity and heaviness are considered to be connected to one another, heavy metals can cause toxicity even at minimal exposure levels (Tchounwou et al., 2012). The Commission Regulation (EC) No 1881/2006 2006 sets maximum levels for metals in fish muscle meat, which present the highest potential for toxicity to human consumers. Therefore, aiming to protect the health of citizens, the European Commission has established toxicologically acceptable levels in fish muscle meat for lead (Pb) at 0.30 mg/kg, for cadmium (Cd) at 0.05 mg/kg and for mercury (Hg) at 0.50 mg/kg. However, there are some exceptions in terms of fish species. For instance, species such as Engraulis and Trachurus have a maximum level for Cd set at 0.10 mg/kg, while species such as Anguilla, Mullus and Acipenser have a maximum level for Hg set at 1 mg/kg. Moreover, the maximum levels for metals in the fish meat is based on the provisional tolerable weekly intake (PTWI) endorsed by the European Food Safety Authority (EFSA). For example, the PTWI for Hg is established at 1.6 μg/kg body weight. Consequently, aquatic living organisms are adversely affected by heavy metals. Due to their non-biodegradability, they remain for a very long period once discharged into aquatic environments. Metals are spread throughout the water column, deposited in sediments, and consumed by biota in the aquatic environment (Sneddon et al., 2017).
Fishes are a significant component of the human diet because they have many health benefits. As a result, much research on the contamination of diverse fish species by heavy metals has been conducted intensively. Due to fish's higher trophic status, or tertiary level in the food chain, humans are significantly dependent on fish for their nutritional needs. It is a rich source of protein and abundant in essential minerals, vitamins, and unsaturated fatty acids (Tahity et al., 2022). Fish oil contains vitamins A, D, E, and K, which have been shown to be effective in the treatment of heart disease, arthritis, asthma, atherosclerosis, cancer, and autoimmune deficiency illnesses (Nădejde et al., 2019). Nevertheless, consuming fish may expose people to heavy metals (Merola et al., 2021; Shahjahan et al., 2022; Shiry et al., 2021). Fish have the potential to accumulate heavy metals in their tissues at increased doses than the environment due to absorption along the gill surface, kidney, liver, and gastrointestinal tract wall (Cahova et al., 2023; Filice et al., 2023; Impellitteri et al., 2023; Pradhoshini et al., 2023; Saha et al., 2023). Some dangerous heavy metals are not metabolized by the fish body and build up in the soft tissues, where they are amplified by the biomagnification process (Tahity et al., 2022). Fish consumption can provide a health risk to humans if the concentration of metals in fish tissues exceeds the maximum permitted levels because particular metals, including Hg, chromium (Cr), cadmium (Cd), and arsenic (As), can harm the kidneys, liver, and neurological system. Therefore, warnings against pollutants could be in opposition to dietary suggestions. The human body is significantly affected by the increasing content of heavy metals in fish (Ali and Khan, 2018). Mercury is one of the rarest elements on Earth, placing 74th on a list of 90 elements, and is categorized as a rare heavy metal due to its density of higher than 5 g/cm3 (Łuczyńska et al., 2022).
At increasing concentrations, heavy metals and metalloids are significant pollutants for all forms of life, including humans (Jebara et al., 2021). Long-term inhalation of excessive amounts of Hg, for instance, is dangerous to living cells and can result in disease or death. The Joint FAO/WHO Expert Committee on Food Additives. Meeting. 73rd & Organization, 2011; WHO | JECFA evaluates and assesses the safety of contaminants in foods, by using tools such as PTWI to highlight toxicants that may accumulate in the body. Thus, JECFA established PTWI for several heavy metals, including for Hg at 4 μg/kg body weight, for Pb at 25 μg/kg body weight, for Cd at 6.25 μg/kg body weight, and for Al at 2 mg/kg body weight. Industrial emissions and/or concentrations in the lower links of the food chain must be regulated by defining acceptable limits in order to prevent the accumulation of hazardous metals in food designed for human consumption. This also needs to apply to fish, because eating them can be detrimental to human health (Łuczyńska et al., 2022).
The total concentration of pollutants in food is commonly utilized in human health risk assessment (Aliko et al., 2022; Azadikhah et al., 2023; Costa et al., 2022; Kannan et al., 2023; Ravi et al., 2023). Nonetheless, the overall concentration of pollutants may not always accurately reflect the amount of contaminants ingested with foods. The degree of harmful effects generated by contaminants is defined not by their overall concentration, but rather by metal forms that may efficiently interact with sites on biological ligands (Azenha and Vasconcelos, 2000). These forms may exist in food and are not transformed during digestion, but only in a limited way. In most cases, they are caused by interactions between elements and various functional groups of food in the gastrointestinal system. As a result, only a percentage of these dietary components are absorbed and used. Several hypotheses have been presented to explain the differences between measured and anticipated mercury concentrations. One of the concepts is that the oral bioavailability of mercury from fish may be diminished due to the complexation of mercury by chelating compounds such as fibers and phytates found in other co-ingested food items during digestion (Shim et al., 2009). Only a portion of the ingested food components may thus be accessible following oral exposure (Ouédraogo and Amyot, 2011). Thus, bioaccessibility is defined as the maximum concentration soluble in the simulated gastrointestinal environment that is available for further absorption processes into the intestinal mucosa (Costa et al., 2022). Bioavailability refers to the concentration of compounds that completely pass through the digestive system, are absorbed, and reach the target tissues in the intact or metabolized form to exert their function (Rodrigues et al., 2022). Bioavailability is commonly defined as the proportion of a substance's active form that reaches systemic circulation unchanged (Price and Patel, 2023). Thus, bioavailability includes the term bioaccessibility. Since bioaccessibility is a theoretical maximum potential bioavailability, it may be used as a conservative estimation for bioavailability, for instance (Bradley et al., 2017). According to certain research, some dietary components, such as fiber and phytochemicals, can have a comparable effect on the bioavailability of mercury (Shim et al., 2009). By way of example, in animal trials, co-consumption of wheat bran with methylmercury simultaneously enhanced fecal mercury excretion and lowered levels of methylmercury in the blood and brain (Rowland et al., 1986). Additionally, bioactive elements found in tea and coffee have been linked to a potential inhibition of the adsorption of heavy metals including cadmium and lead (Minamisawa et al., 2004). Phytochemical-rich foods may be equally effective in preventing chronic long-term mercury exposure in fish-eating communities as synthetic chelating treatments because they decrease mercury's bioavailability (Shim et al., 2009). Phytochemical compounds, the majority of which are polyphenols and flavonoids, can act as chelating and scavenging agents for redox-active metals (for example, the capacity to bind and precipitate specific molecules). They may be reducing Hg absorption in intestinal tracts, therefore, are responsible for variations in Hg and MeHg bioaccessibility from fish (Anacleto et al., 2020). Furthermore, food containing phytochemicals may contribute to the daily intake of important minerals such as Se, which can help to minimize MeHg bioaccessibility and interfere with Hg toxicity (Girard et al., 2018). For example, in vivo studies show that tea extracts may reduce oxidative stress caused by MeHg in rats and change its pharmacokinetics (Black et al., 2011). Seafood items may be consumed raw or cooked, depending on dietary habits, along with additives like vinegar and lemon, whose acidic nature might alter the bioaccessibility of components. Furthermore, cooking and co-ingestion of other foods can modify the bioaccessibility of mussels' metals, metalloids, and radionuclides.
The fish specie Alosa immaculata has a high economic value generated by abundant cacthes in the Danube River area. According to a previous study, Alosa immaculata registered the most abundant fish catches in the Lower Danube River area, in the year 2017 and 2019, with 263 and 348 tons respectively (Simionov et al., 2020). During the spring period, the pontic shad, an anadromous fish that lives in the Black Sea, migrates up the Danube to spawn, in the river's marshes or on the shore, where the water turbidity is lower.
The pontic shad is an important commercial species from the Clupeidae family exploited in the Black Sea and Danube River. The catches of this species has been reported at 263 tones in 2017, 348 tons in 2019, and 364 tones in 2021 (George et al., 2023; Simionov et al., 2020). Since this specie is characterised by a high lipid content (minimum 11%) and cannot be produced in aquaculture systems, the wild stocks are highly valuable. Moreover, the smoked pontic shad received protected geographical indication product (PGI) from the European Commission. This is the first study to approach the in vitro digestibility of the pontic shad both raw and heat-treated, with or without various co-ingested food products. As well, the research provides information in accordance to the European Union Regulation 2019/1020, which mandates that each member country provide a monitoring strategy for ensuring food products safety.
The study hereunder detailed uses an in vitro digestive model to evaluate the impact of cooking techniques and dietary factors on the bioaccessibility of Hg in shad species. The first experiment objective was to find out whether various cooking techniques affected mercury concentrations in fish tissues, before digestion. Heat-treated fish was chosen because there is no tradition for raw fish consumption in Europe. For instance, a study conducted by Golden et al. (2022) highlighted that the population in Portugal consumes raw fish which only accounts for 10% from the total fish consumed. The next purpose was to assess Hg bioavailability following simulated digestion of steamed, baked, fried, marinated, and smoked fish muscles. Forwards, the effect of co-ingested food items (garlic, broccoli, and lemon juice) on mercury concentration in heat-treated fish was assessed using an in vitro model of digestion. Some medicinal plants such as broccoli and garlic have been shown to reduce the harmful effects of heavy metals. They contain a variety of polyphenols, iron, selenium, and potassium and helps regulate the body's ionic balance (Raeeszadeh et al., 2022). A final step of this research was to study the antioxidant activity of some food components, as a potential sustainer for mercury bioaccessibility.
2. Materials and methods
2.1. Materials and reagents
Pontic shad (Alosa immaculata Bennet, 1835) was sacrificed before purchase. The fish, garlic, lemon, and broccoli were randomly achieved from a local market in Galati, Romania, in March 2023. Enzymes were achieved from Sigma-Aldrich and included salivary α-amylase, porcine pepsin, and porcine pancreatin. The following used reagents were of analytical or reagent grade: Trizma Base, hydrochloric acid, sodium carbonate, 6-Hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid (Trolox), 2, 2′-azino-bis (3-ethylbenzothiazoline-6-sulfonate. All reagents were purchased from SIGMA-ALDRICH, Co., 3050 Spruce Street Saint Louis.
2.2. Sample preparation
The fish (Alosa immaculata) was first eviscerated, beheaded, washed, and trimmed into four pieces. The portioned fish was subjected to different cooking methods, which were carried out in an electric pressure cooker Tefal CY505E30 One Pot. Except for the smoked shad (Sk), which was purchased already smoked, the other cooking methods were chosen in accordance with conventional home preparation: steaming (S), baking (B), frying (F), and vinegar boiling (M) (marinade). The steaming was performed using 200 mL of water, at 104 °C for 3 min. For the baking process, the fish was greased with sunflower oil and covered with parchment paper, after that was heat-treated at 150 °C for 10 min. The frying was carried out using 200 mL sunflower for each fish portion, at 160 °C temperature, for 6 min and for the marinade, the fish was boiled in vinegar for 30 min, at 90 °C. Fish pieces were weighed both before and after cooking, and the results were represented in wet weight. Moisture content was measured using a thermogravimetric method (using Binder Drying and heating chambers with forced convection Model FD 115) in which the samples were heated, and dried, and the weight loss caused by moisture evaporation was recorded. The length of cooked fish was 10 cm and the weight varied between 24 and 38 g. Broccoli was first portioned and boiled in salted water for 5 min. Garlic was crushed before use and lemon was squeezed to obtain fresh lemon ju0069ce.
2.3. In vitro digestion protocol
An improved in vitro digestion model (INFOGEST 2.0) was applied to this study according to (Brodkorb et al., 2019). The analysis was carried out in triplicate. Food is exposed to three sequential stages during the digestion process: oral, gastric, and intestinal. Briefly, the oral phase involved dilution and maintenance of the meal 1:1 (w/w) with simulated oral fluid (SOF) at pH = 7.0, containing salivary amylase. Immediately following that, the oral bolus was diluted 1:1 (v/v) with simulated gastric fluid (SGF) at pH 2.0 and stomach enzymes (pepsin from porcine gastric mucosa), and the solution was continuously stirred for 2 h. The gastric chyme was then again mixed (1:1 - v/v) and incubated at pH 7.0 for another 2 h with simulated intestinal fluid (SIF) containing pancreatin from the porcine pancreas. The selected diet components were added to cooked fish in order to study their effects after in vitro digestion. The portions of examined samples consisted each time of two components in a ratio of 1:1 cooked fish: co-ingested food (5 g of baked fish + 5 g of boiled broccoli, 5 g of baked fish + 5 g of garlic, and 5 g of baked fish + 5 g of lemon juice). At the end of the digestion simulation, the samples were placed on ice and then centrifuged at 4500 rpm, 15 min, at 4 °C using OHAUS FC5916R Laboratory Centrifuge to separate the supernatant from the pellets.
2.4. Total mercury (THg) determination
Total mercury (THg) in the fresh and cooked samples was determined from the muscle tissue in a direct mercury analyzer (DMA-80 EVO Milestone Inc.). The analysis involved burning samples at 750 °C and capturing mercury vapor on a gold foil for atomic absorption spectrometry measurement. Moreover, total mercury concentrations in cooked fish samples (S, C, F, M, Sk) were evaluated during each step of the digestive process in the aqueous phase, in the remaining pellet, and in the final mixture. Each sample used in the experiment had its mercury content measured in raw, boiled, and fried samples to ascertain the effects of the cooking method on fish mercury levels and their effect on mercury bioaccessibility. To determine the influence of other food components on mercury bioaccessibility during digestion, a mixed meal consisting of baked fish and other food items was subjected to the same protocol, and total mercury was assessed.
2.5. Total mercury bioaccessibility in digested cooked fish with/without other food items
After following the digestion process, the digested fish meals were centrifuged and the collected supernatant was separated from the pellet component. Each part was both, separately and together analyzed and the total mercury from the supernatant was defined as bioaccessibility (% BA), and the total mercury existing in the pellet after gastrointestinal digestion was relevant for the determination of the recovery rate (% RR). Bioaccessibility and recovery rate was calculated using the following equations:
| (1) |
where Caq is the total mercury concentration in the aqueous phase (ng/mL), V is the total volume of simulated gastrointestinal fluids (mL), Ci is the total mercury concentration in the test meals (ng/mL), and m is the total mass of the food digested through in vitro digestion method (g).
| (2) |
where Caq is the total mercury concentration in the aqueous phase (ng/g), Cp is the total mercury concentration in the pellet (ng/g), and Cpd is the total mercury concentration in the test food before the digestion process (ng/g).
2.6. Antioxidant activity of co-ingested food components
The extraction of antioxidant compounds of each food component was carried out by ultrasound-assisted method (Ultrasonic bath model Elmasonic S 60 (220–240 V)), using 99.98% methanol as solvent extraction. One gram of the previously prepared sample was mixed with 10 mL of methanol 99.98%, then subjected to ultrasound treatment for 30 min, at 30 °C and 37 kHz (150 W power). After the extraction procedure, the final mixtures were centrifuged for 14000 rpm, 15 min, at 4 °C (OHAUS FC5916R Laboratory Centrifuge). The supernatant was collected in order to determine the antioxidant activity of each extract. The 2,2′-azinobis-3-ethylbenzotiazoline-6-sulfonic acid (ABTS•+) protocol, with some modifications by Re et al. (1999), was used for assessing antioxidant activity. Potassium persulfate solution 140 mM and ABTS•+ solution 7 mM were mixed to obtain the ABTS•+ radical, which was then incubated at 25 °C in the darkness for 16 h. After that, the stock solution was diluted with ethanol to have an absorbance of 0.700 ± 0.020 at 734 nm. A volume of 0.02 mL of each food extract was transferred to test tubes with 1.98 mL of ABTS•+ radical in the dark. The absorbance was measured at 734 nm after 30 min of the reaction by using a Spectrophotometer UV–Vis–NIR, Model Cary 5000, Agilent. The results were represented as mM Trolox/mL sample.
2.7. Statistical analysis
Each experiment was performed in triplicate (three-point measurements of the same experiment). The results were expressed as means ± standard error means. After running the normality and homoscedasticity tests, the analysis of variance (ANOVA), was used for statistical analysis, followed by Tukey's posthoc test with a 95% confidence interval for each parameter examined. The statistical analysis was carried out using Minitab 18 software.
3. Results and discussion
3.1. Effect of cooking methods on total mercury concentrations
The concentration of total mercury in raw and various heat-treated fish samples is presented in Table 1. The effect on mercury reduction was seen to increase with the severity of the heat treatment. Compared with raw fish mercury concentration (214.15 ± 1.45 ng/g dry weight), it can be clearly observed that the baked and fried samples showed a good effect in terms of mercury content reduction across the four different types of heat treatment, respectively 192.62 ± 2.83 and 195.25 ± 7.47 ng/g dry weight. Moreover, the oil resulting from the fish frying was analyzed and presented a small concentration of 1.47 ± 0.07 ng mercury/g dry weight. It is known that mercury has a high affinity for proteins. Ouédraogo and Amyot (2011) stated that heat from different cooking techniques alter the mineral concentration and protein structure, changing Hg bioaccessibility. Afonso et al. (2015) suggested that the loss in bio-accessibility was due to the protein denaturation caused by cooking, which destroys the tertiary and quaternary structure. Liao et al. (2019) studied the discarded water after the cooking process and revealed that washing and soaking released only about 0.2% and 0.3% of the original THg. Schmidt et al. (2015) found that neither water nor oil had substantial THg concentrations either before or after cooking. According to some studies, evaporation and moisture loss caused the concentrations of Hg in fish muscle to reduce after cooking (Hajeb et al., 2014; Mieiro et al., 2016). As can be also seen in our present study, the baked and fried samples' moisture loss correlates with the decreasing mercury concentration. On the other hand, in the case of steamed and marinated fish samples, a higher concentration of mercury in the cooked sample is observed than in the non-thermally treated sample. Both values are very similar, recording an increase of almost 20% over the initial uncooked sample. Ouédraogo and Amyot (2011) declared that for all studied fish, THg concentrations (dry weight) in boiled fish were slightly but not significantly higher than those in fried or raw fish. Furthermore, in accordance with our study, they found water loss in the fried sample. The preparation of fish including heating and cooking can certainly have a major impact on fluctuations in Hg concentrations (Liao et al., 2019). There are numerous data in the literature regarding the high concentrations of heavy metals in raw fish. However, the majority of these investigations reported the levels of chemical contaminants on an uncooked basis, and information on how cooking methods may affect the quantities of metals in foods must be also studied (Perelló et al., 2008). Regarding metals, it has been observed that some foods can absorb metals if the cooking water or other present food items are contaminated, even if cooking can reduce their level (Morgan, 1999). As Bradley et al. (2017) stated in their review, seafood that has been cooked tends to have a higher wet-weight concentration of mercury than seafood that hasn't been cooked; however, this is probably definitely due to moisture loss during cooking rather than a change in the mercury content of the seafood. Unfortunately, most studies are still unclear regarding this topic because mercury concentration is calculated on a wet-weight basis. From the present study, it can be concluded that at least two (baking and frying) of the four applied cooking methods may certainly decrease the mercury concentration.
Table 1.
Effect of cooking methods on THg concentrations.
| Sample/Determination | THg concentration, ng/g dry weight | Moisture, % | Raw to Cooked weight conversion |
|---|---|---|---|
| Raw | 214.15 ± 1.45b | 61.95 ± 2.10a | – |
| Steamed | 258.07 ± 7.57a | 61.53 ± 1.98a | 1.18 |
| Baked | 192.62 ± 2.83c | 57.00 ± 2.01ab | 1.08 |
| Fried (Oil resulted from frying) | 195.25 ± 7.47c (1.47 ± 0.07) | 55.77 ± 1.87b | 1.17 |
| Marinated | 258.23 ± 9.89a | 58.06 ± 1.66ab | 1.34 |
The average values on the same row that do not share a letter (a,b,c) are significantly different, based on Tukey method and 95% confidence (p < 0.05).
Measuring raw food is more accurate and removes the possibility of fluctuation caused by the cooking process. However, occasionally weighing prepared food is more useful. Cooked fish tend to shrink in size and weight due to the evaporation of moisture from fish (Blikra et al., 2020). In the present study, the fish samples were weighed before and after cooking in order to calculate a raw-to-cooked weight conversion parameter. This conversion parameter provides the estimated raw weight of food based on the cooked weight. Raw vs. cooked food measurement errors can lead to under-reporting or over-reporting results Fig. 1.
Fig. 1.
Effect of cooking methods (steaming, baking, frying, and marinating) on THg concentration.
3.2. Total mercury bioaccessibility and recovery rate after in vitro digestion
The results presented in Table 2 reveal that the highest concentration of mercury in the liquid phase after centrifuging the digested sample shows up in the smoked sample (8.79 ± 1.14 ng/g sample) and the smallest concentration of 1.07 ± 0.16 ng/g appears in the liquid phase of the baked sample. Regarding the sediment resulting from the digested samples, significant differences were also observed for the smoked sample (80.86 ± 7.67 ng/g) when compared with other ones. These results can be correlated with the initial and final tested concentration and prove that after passing through in vitro digestion, significant concentrations of mercury could be found in both the liquid and sediment parts of the smoked sample. In baked, fried, and marinated samples, no significant differences were obtained between values in terms of liquid mercury concentration. The total mercury values from sediment analysis are much higher in all the cooked samples than the total mercury found in the aliquot phase in all samples suggesting that the highest concentration remains in the digesta. Fortunately, liquid identification of total mercury has the highest relevance with respect to bioaccessibility and absorption. It is assumed that the mercury released during digestion from the food matrix and transferred (solubilized) to the aqueous phase is available (bioaccessible) for future absorption by absorptive epithelial cells of the small intestine (Shim et al., 2009). This finding suggests that although mercury in the solid phase could be resolubilized in the lower intestine, it may not be bioaccessible. Ouédraogo and Amyot (2011) assessed the analysis of total mercury in both dissolved and particulate (pellet) phases after digestion and reported quantities up to 10 times higher in sediment than in the liquid samples.
Table 2.
Determination of mercury concentration in liquid and sediment.
| Sample | Steamed | Baked | Fried | Marinated | Smoked |
|---|---|---|---|---|---|
| Liquid, ng/g | 1.21 ± 0.26b | 1.07 ± 0.16b | 1.75 ± 0.42b | 2.45 ± 1.07b | 8.79 ± 1.14a |
| Sediment, ng/g | 62.55 ± 3.70b | 60.13 ± 4.03b | 58.14 ± 5.50b | 66.95 ± 2.18b | 80.86 ± 3.44a |
| Total, ng/g | 67.14 ± 7.45b | 58.53 ± 2.38b | 56.07 ± 2.99b | 95.15 ± 2.96a | 99.29 ± 1064a |
The average values on the same row that do not share a letter (a,b,c) are significantly different, based on Tukey method and 95% confidence (p < 0.05).
The main human exposure route for mercury (Hg) is considered to be through fish consumption, however, it can be difficult to determine how much Hg is actually bioavailable. Risk assessors frequently use 95%–100% bioavailability in their models when estimating human mercury exposure (Siedlikowski et al., 2016). Recent studies, however, indicate that it might be incorrect to assume that all or the majority of the mercury consumed is absorbed into the circulatory system. In this study, the Hg in vitro bioaccessibility from ingested fish was developed, using a gastrointestinal digestion procedure. Overall, Hg bioaccessibility ranged from 4.31 to nearly 24.95% and the Hg recovery rate varied from 63.44 to 78.74% (Fig. 2). Less bioaccessible Hg was found in baked samples, followed by steamed, fried, marinated, and smoked fish. Besides having greater protein contents, baked and fried fish samples may also have reduced THg bioaccessibility due to higher levels of fat content. Moreover, the study was conducted by analyzing the total mercury concentration at each step of gastrointestinal digestion in the digestive mixture. In all samples, an increase in mercury concentration was observed after passing through gastric conditions, of approximately 30% compared to the oral digestion phase. Contrariwise, the intestinal transition of the digestive mixture led to a decrease in mercury concentration of about 30%, excepting the marinated fish, where the mercury concentration remained constant in both, gastric and intestinal steps. The marinated fish involved the use of vinegar during the heat treatment, and therefore decreased the acidity during the experimental process, similar to gastric conditions, which appears to support mercury bioaccessibility, in this study. In the scientific literature, there is a wide range of values regarding mercury bioaccessibility and it is difficult to establish the cause of such a large variation. For example, Bradley et al. (2017) reviewed that in unspecified tuna species, Hg bioaccessibility ranged from 9% to 78% (raw), 6%–39% (grilled), and 48% (boiled), 18% (canned in olive oil), and 20% (canned in water). In agreement with this study are also Torres-Escribano et al. (2010) which indicate there is a wide range (9–85%) in the amount of Hg that is solubilized from seafood during gastrointestinal digestion and thereafter accessible for subsequent absorption. These varied results may be due to many factors such as methodological differences, differences in location, fish sources, fish species, or other ones. Beyond the aforementioned methodological considerations, there is emerging knowledge of other elements (such as diet, gut flora, and genetics) that might also affect the fluctuation of Hg bioavailability. In their study, Laird et al. (2009) hypothesized that HgT bioaccessibility is independent of mercury concentration food, indicating that the same percentage of dietary Hg is theoretically accessible for absorption regardless of the initial Hg content. In vitro Hg bioaccessibility is sometimes shown to be independent of total Hg concentration, and sometimes it is found to be negatively correlated with Hg concentration (He and Wang, 2011; Laird et al., 2009; Laird and Chan, 2013; Siedlikowski et al., 2016). It is probable that the heating that occurs during cooking techniques lowers the bioaccessibility of Hg from fish due to the significant affinity of mercury for proteins and the tendency of protein denaturation by heat to alter the reactivity of protein-bound mercury (Bradley et al., 2017). Fish mercury levels can be highly variable and determined by many different kinds of environmental parameters (Mills et al., 2018). Size, age, trophic position, physical and chemical environmental factors, and the place of capture have all been connected to inter- and intra-specific differences in mercury levels in fish (Choy et al., 2009). The mercury bioaccessibility in the gastrointestinal gut may be affected by cooking effects, the presence of the divalent metals, mainly selenium, protein and glutathione content, dietary fibers effect, gut microflora, and fat content (Abdullah, 2020). In the present study, an accelerated decrease in the bioaccessibility of thermally treated mercury can be observed, independent of the cooking method, because shad has a high fat concentration. On the other hand, the increased amounts of fat content in fried fish samples compared to raw or other cooked samples, according to Ou'edraogo & Amyot (2011), may also reduce Hg bioaccessibility. The investigations reveal that the baking process associated with the simulation of gastrointestinal parameters supports the idea that mercury concentration decreases when bioaccessibility and cooking methods reasons are applied. The results are supported by the total mercury concentration found in the liquid phase.
Fig. 2.
Total mercury bioaccessibility and recovery rate after in vitro digestion.
3.3. In vitro evaluation of other foods co-ingestion to reduce mercury bioaccessibility
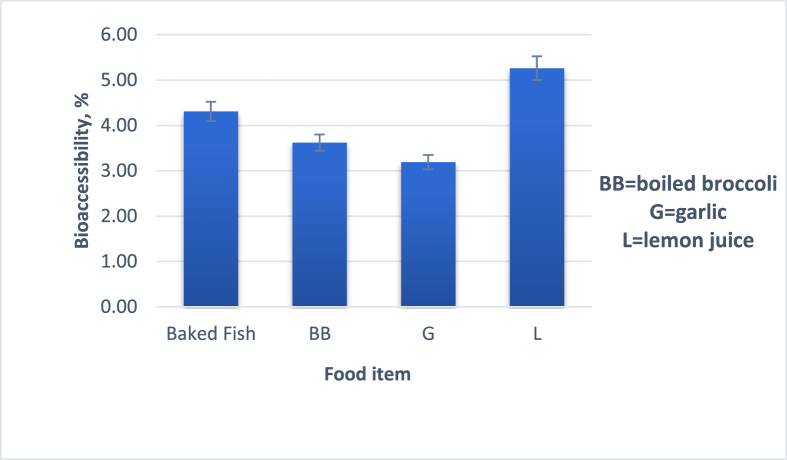
Current concern has led researchers to investigate alternatives to limit Hg dietary exposure (Jadán-Piedra et al., 2018). It is suggested that consuming food rich in natural antioxidants simultaneously with mercury-containing fish may potentially minimize mercury absorption in comparison to eating fish alone (Shim et al., 2009). Therefore, there are dietary elements that regulate the amount of Hg that passes through the systemic circulation and accumulates in certain organs (Jadán Piedra et al., 2016). Tea, coffee, as well as several types of fiber (wheat bran, Oat bran, and psyllium) have all been correlated to decreased Hg bioaccessibility in vitro (Ouédraogo and Amyot, 2011; Shim et al., 2009). According to some studies, the addition of dietary fiber and polyphenols in sort beverages, which may function as metal chelators, may be the cause of this decrease. Ouédraogo and Amyot (2011) showed that the bioaccessibility of Hg in raw fish was reduced by 50–60% in the presence of black coffee and green and black tea. It is suggested that some dietary elements may operate during the digestive process to interact with mercury to generate less soluble complexes, which would diminish the metal's absorption and decrease its pathway into the systemic circulation (Jadán Piedra et al., 2016). This study used a three-stage in vitro digestion approach to assess how foods rich in bioactives affected mercury bioaccessibility from fish tissue. Baked fish and additional dietary components (1:1) such as broccoli (BB), garlic (G), and lemon juice (L) were co-ingested in the small intestine and the stomach, respectively. According to Fig. 3, the total concentration of mercury in the aqueous phase following simulated in vitro digestion of baked fish with added food items was used to measure mercury bioaccessibility. Boiled broccoli and fresh garlic decreased the mercury bioaccessibility from 4.31 ± 0.75% to 3.62 ± 0.02% and 3.19 ± 0.29, respectively, meanwhile, lemon juice slightly increased mercury concentration to 5.26 ± 0.10%. Firstly, plant-based foods might bind to mercury and produce insoluble complexes that decrease bioaccessibility (Jadán Piedra et al., 2016; Shim et al., 2009). Secondly, it is already known that the presence of minerals such as zinc and selenium helps in detoxifying metals which also is a reason why garlic for mercury detox is a potent cure. Garlic, almost in fresh state has antioxidant properties. Due to its presence of compounds containing organo-sulfur groups, volatile oils, enzymes, carbohydrates, and amino acids, it has remarkable ameliorating potential against heavy metal toxicity (Nwokocha et al., 2012). The highest mercury binding activity was observed in juices extracted from broccoli and garlic in the study of Jovel et al. (2018). Similar to our results, Shim et al. (2009) stated that grapefruit juice did not reduce mercury in the aqueous phase. It was unexpected to notice that grapefruit juice (Shim et al., 2009) and lemon juice (from the present study) had no positive impact on mercury reduction. This may be due to other components of grapefruit and lemon juice, such as sugar, vitamin C, and citric acid, which may interfere with interactions between mercury and other phytochemicals. The co-ingestion of green tea extract, black tea extract, and soy protein significantly reduced mercury bioaccessibility by 82–92%, 88–91%, and 44–87%, respectively. In the same research, they observed that wheat bran, oat bran, and psyllium reduced bioaccessibility. Ouédraogo and Amyot (2011) did not observe any positive effect on mercury bioaccessibility in mackerel and shark when added corn starch. According to (Girard et al., 2018) the most important natural antioxidant in green tea, catechin has stronger chelating capabilities and may form insoluble complexes with MeHg, reducing its bioaccessibility. In another study, Janle et al. (2015) stated that the Hg in swordfish is absorbed more efficiently in rats when it is co-ingested with green tea extract.
Fig. 3.
Hg bioaccessibility after co-ingestion with different food items.
The total mercury concentration in the aqueous phase and in the solid phase following simulated in vitro digestion of baked fish with added food items was used to determine the mercury recovery rate. As Fig. 4 shows, the Hg recovery rate for co-ingested foods and shad ranged from 85.13 ± 8.48% in the sample with garlic co-ingestion to 136.59 ± 13.72 and 137.35 ± 1.38% in the samples with broccoli and lemon juice co-ingested sample, respectively. The digested baked shad recorded the lowest recovery rate of 78.78 ± 5.03%. These results were similar to Ouédraogo and Amyot (2011) which calculated the recovery rate of THg and MeHg after digestion and obtained 93%–144% and from 95% to 111%, respectively. Moreover, the same authors highlight that MeHg concentrations in pellets resulting from digested boiled and fried tuna were 2 and 12 times greater than in the liquid phase, respectively.
Fig. 4.
Hg recovery rate after co-ingestion with different food items.
3.4. Antioxidant activity of co-ingested food items
Several substances, including selenium, tannic acid, cellulose, lignin, pectin, and microorganisms such as lactic acid bacteria and yeast, may lower the toxicity of mercury (Jadán-Piedra et al., 2016). Additionally, meals high in polyphenols, such as coffee and tea, might affect the bioaccessibility of Hg and MeHg (Anacleto et al., 2020). Plant-derived foods include important large amounts of natural antioxidants, such as phytochemical substances that have positive health effects (Girard et al., 2018). Such food items may help people consume the recommended daily amounts of important minerals like selenium, which can help decrease MeHg bioaccessibility (Anacleto et al., 2020). The current experiment examined the effect of broccoli, garlic, and lemon juice on the mercury bioaccessibility from baked shad. Additionally, the antioxidant activity of these food items was calculated in order to correlate with mercury bioaccessibility. Table 3(Supplementary material) contains the values for antioxidant activity obtained in boiled broccoli, garlic, and lemon juice. Regarding the solid samples (boiled broccoli and garlic), it can be noted that the highest antioxidant activity for garlic (10.03 ± 0.14 mM Trolox/g sample) is correlated with the clearest decrease in mercury bioaccessibility. Unfortunately, the addition of lemon juice with significant antioxidant activity of 15.31 ± 0.10 mM Trolox/mL did not influence positive mercury bioaccessibility at the selected ratio. Some studies explain that, in most cases, an increase in the component's concentration results in a reduction of mercury's bioaccessibility (Jadán Piedra et al., 2016). Considering broccoli, assuming that it was previously heat treated, the antioxidant activity values presented a 4.54 ± 0.10 mM Trolox/g value and exhibited a slight decrease in mercury bioaccessibility.
4. Conclusions and perspectives
It is proposed that consuming meals rich in phytochemicals concurrently with mercury-containing fish may potentially minimize mercury absorption in comparison to eating fish individually. The aforementioned results show that heat treatment and food co-ingestion have a cumulative influence on fish Hg bioaccessibility. In this study, frying and baking resulted in the loss of the highest concentration of mercury in shad. Regarding mercury bioaccessibility, it could be observed that the option selected for co-ingestion, the baked fish, presented the lowest value. Likewise, the association of baked fish with fresh garlic revealed the best results. The interaction noticed during digestion may be influenced by other substances present in the lumen, particularly by the activity of intestinal bacteria, therefore it is important to evaluate if this impact found in vitro will be confirmed in vivo. Notwithstanding, investigating in vivo bioaccessibility involves direct access to gastrointestinal fluids, which asks for invasive and unethical treatments. On the other hand, the advantages of the outlined methods in this work relate to a system that may provide an alternative for both determining mercury bioaccessibility and screening food component impacts on mercury bioaccessibility. The in vitro digestive models offer a quick, easy, and cost-effective way to estimate the real maximum internal exposure without using animals for risk assessment. The risk assessor's exposure scenarios, which are based on real-world situations, may be totally adapted to the experimental design. Apart from the methodological considerations, there is emerging knowledge of variables (such as diet, gut flora, and genetics) that may also affect the variability of Hg bioavailability. In conclusion, it can be stated that a variety of factors, including the source of mercury, cooking techniques, and nutrients, can impact the bioavailability of mercury. Furthermore, it is also suggested that risk assessments for each population should consider nutritional habits and mercury bioavailability.
Funding
This research was funded by Fondo Proserpina S.R.L., grant number 52062022, “The impact of heavy metals and microplastics from aquatic organisms on human health".
Data statement
Not applicable.
CRediT authorship contribution statement
Ștefania-Adelina Milea: Conceptualization, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. Nina-Nicoleta Lazăr: Conceptualization, Formal analysis, Investigation, Writing – original draft. Ira-Adeline Simionov: Software, Validation. Ștefan-Mihai Petrea: Methodology, Validation. Mădălina Călmuc: Data curation, Supervision. Valentina Călmuc: Data curation, Software. Puiu-Lucian Georgescu: Funding acquisition, Project administration. Cătălina Iticescu: Funding acquisition, Resources, Supervision.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared inappropriately influence the work reported in this paper.
Acknowledgments
The technical support was provided by the Rexdan Research Infrastructure, the infrastructure created through the project An Integrated System for the Complex Environmental Research and Monitoring in the Danube River Area, REXDAN, SMIS code 127065, project co-financed by the European Regional Development Fund through the Competitiveness Operational Programme 2014–2020, contract no. 309/10.07.2020.
Handling Editor: Dr. Quancai Sun
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.crfs.2023.100599.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- Abdullah N. Mercury in the diet, absorption and bioaccessibility. Open Access Libr. J. 2020;7(10) doi: 10.4236/oalib.1106666. Article 10. [DOI] [Google Scholar]
- Afonso C., Costa S., Cardoso C., Oliveira R., Lourenço H.M., Viula A., Batista I., Coelho I., Nunes M.L. Benefits and risks associated with consumption of raw, cooked, and canned tuna (Thunnus spp.) based on the bioaccessibility of selenium and methylmercury. Environ. Res. 2015;143:130–137. doi: 10.1016/j.envres.2015.04.019. [DOI] [PubMed] [Google Scholar]
- Ali H., Khan E. Bioaccumulation of non-essential hazardous heavy metals and metalloids in freshwater fish. Risk to human health. Environ. Chem. Lett. 2018;16(3):903–917. doi: 10.1007/s10311-018-0734-7. [DOI] [Google Scholar]
- Aliko V., Multisanti C.R., Turani B., Faggio C. Get rid of marine pollution: bioremediation an innovative, attractive, and successful cleaning strategy. Sustainability. 2022;14(18) doi: 10.3390/su141811784. Article 18. [DOI] [Google Scholar]
- Anacleto P., Barbosa V., Alves R.N., Maulvault A.L., Bronze M.R., Marques A. Green tea infusion reduces mercury bioaccessibility and dietary exposure from raw and cooked fish. Food Chem. Toxicol.: Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2020;145 doi: 10.1016/j.fct.2020.111717. [DOI] [PubMed] [Google Scholar]
- Azadikhah D., Yalsuyi A.M., Saha S., Saha N.C., Faggio C. Biochemical and pathophysiological responses in capoeta capoeta under lethal and sub-lethal exposures of silver nanoparticles. MDPI AG. 2023 https://doaj.org/article/aadbb0f369b14bab96ddfee05688dc9e [Google Scholar]
- Azenha M.A., Vasconcelos M.T. Assessment of the Pb and Cu in vitro availability in wines by means of speciation procedures. Food Chem. Toxicol.: Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2000;38(10):899–912. doi: 10.1016/s0278-6915(00)00082-x. [DOI] [PubMed] [Google Scholar]
- Black P., Niu L., Sachdeva M., Lean D., Poon R., Bowers W.J., Chan H.M., Arnason J.T., Pelletier G. Modulation of the effects of methylmercury on rat neurodevelopment by co-exposure with Labrador Tea (Rhododendron tomentosum ssp. Subarcticum) Food Chem. Toxicol.: Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2011;49(9):2336–2342. doi: 10.1016/j.fct.2011.06.035. [DOI] [PubMed] [Google Scholar]
- Blikra M.J., Hodnefjell Å.V., Feyissa A.H., Skipnes D. Dimensional change and cook loss during heating of fish: problem formulation and semi-empirical modeling approach. J. Food Eng. 2020;281 doi: 10.1016/j.jfoodeng.2020.110004. [DOI] [Google Scholar]
- Bradley M., Barst B., Basu N. A review of mercury bioavailability in humans and fish. Int. J. Environ. Res. Publ. Health. 2017;14(2):169. doi: 10.3390/ijerph14020169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodkorb A., Egger L., Alminger M., Alvito P., Assunção R., Ballance S., Bohn T., Bourlieu-Lacanal C., Boutrou R., Carrière F., Clemente A., Corredig M., Dupont D., Dufour C., Edwards C., Golding M., Karakaya S., Kirkhus B., Le Feunteun S., et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019;14(4) doi: 10.1038/s41596-018-0119-1. Article 4. [DOI] [PubMed] [Google Scholar]
- Cahova J., Blahova J., Plhalova L., Marsalek P., Doubkova V., Hostovsky M., Divisova L., Mares J., Faggio C., Svobodova Z. Long-term exposure to polycyclic musk tonalide—a potential threat to juvenile zebrafish (Danio rerio)? Vet. Med. 2023;68(5):218–224. doi: 10.17221/40/2023-VETMED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choy C.A., Popp B.N., Kaneko J.J., Drazen J.C. The influence of depth on mercury levels in pelagic fishes and their prey. Proc. Natl. Acad. Sci. USA. 2009;106(33):13865–13869. doi: 10.1073/pnas.0900711106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commission Regulation (EC) No 1881/2006 of 19 December 2006 Setting Maximum Levels for Certain Contaminants in Foodstuffs (Text with EEA Relevance), vol. 364. OJ L, 2006. http://data.europa.eu/eli/reg/2006/1881/oj/eng.
- Costa F., Mieiro C.L., Pereira M.E., Coelho J.P. Mercury bioaccessibility in fish and seafood: effect of method, cooking and trophic level on consumption risk assessment. Mar. Pollut. Bull. 2022;179 doi: 10.1016/j.marpolbul.2022.113736. [DOI] [PubMed] [Google Scholar]
- Filice M., Reinero F.R., Cerra M.C., Faggio C., Leonetti F.L., Micarelli P., Giglio G., Sperone E., Barca D., Imbrogno S. Contamination by trace elements and oxidative stress in the skeletal muscle of Scyliorhinus canicula from the central tyrrhenian Sea. Antioxidants. 2023;12(2) doi: 10.3390/antiox12020524. Article 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George T., Daniel G., Aurel N., Catalin P., Madalina G. Assessing of pontic shad (Alosa immaculata, Bennett 1835) stock status from Romanian Black Sea coast. Turk. J. Fish. Aquat. Sci. 2023;23(3) doi: 10.4194/TRJFAS23217. [DOI] [Google Scholar]
- Girard C., Charette T., Leclerc M., Shapiro B.J., Amyot M. Cooking and co-ingested polyphenols reduce in vitro methylmercury bioaccessibility from fish and may alter exposure in humans. Sci. Total Environ. 2018;616–617:863–874. doi: 10.1016/j.scitotenv.2017.10.236. [DOI] [PubMed] [Google Scholar]
- Golden O., Caldeira A.J.R., Santos M.J. Raw fish consumption in Portugal: a survey on trends in consumption and consumer characteristics. Food Control. 2022;135 doi: 10.1016/j.foodcont.2022.108810. [DOI] [Google Scholar]
- Hajeb P., Sloth J.J., Shakibazadeh S., Mahyudin N.A., Afsah-Hejri L. Toxic elements in food: occurrence, binding, and reduction approaches. Compr. Rev. Food Sci. Food Saf. 2014;13(4):457–472. doi: 10.1111/1541-4337.12068. [DOI] [PubMed] [Google Scholar]
- He M., Wang W.-X. Factors affecting the bioaccessibility of methylmercury in several marine fish species. J. Agric. Food Chem. 2011;59(13):7155–7162. doi: 10.1021/jf201424g. [DOI] [PubMed] [Google Scholar]
- Impellitteri F., Multisanti C.R., Rusanova P., Piccione G., Falco F., Faggio C. Exploring the impact of contaminants of emerging concern on fish and invertebrates physiology in the mediterranean Sea. Biology. 2023;12(6) doi: 10.3390/biology12060767. Article 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam M.S., Hossain M.B., Matin A., Islam Sarker M.S. Assessment of heavy metal pollution, distribution and source apportionment in the sediment from Feni River estuary, Bangladesh. Chemosphere. 2018;202:25–32. doi: 10.1016/j.chemosphere.2018.03.077. [DOI] [PubMed] [Google Scholar]
- Jadán Piedra C., Sánchez V., Vélez D., Devesa V. Reduction of mercury bioaccessibility using dietary strategies. LWT - Food Sci. Technol. (Lebensmittel-Wissenschaft -Technol.) 2016;71:10–16. doi: 10.1016/j.lwt.2016.03.015. [DOI] [Google Scholar]
- Jadán-Piedra C., Vélez D., Devesa V. In vitro evaluation of dietary compounds to reduce mercury bioavailability. Food Chem. 2018;248:353–359. doi: 10.1016/j.foodchem.2017.12.012. [DOI] [PubMed] [Google Scholar]
- Janle E.M., Freiser H., Manganais C., Chen T.-Y., Craig B.A., Santerre C.R. Green tea increases the concentration of total mercury in the blood of rats following an oral fish tissue bolus. BioMed Res. Int. 2015;2015 doi: 10.1155/2015/320936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jebara A., Lo Turco V., Faggio C., Licata P., Nava V., Potortì A.G., Crupi R., Mansour H.B., Di Bella G. Monitoring of environmental Hg occurrence in Tunisian coastal areas. Int. J. Environ. Res. Publ. Health. 2021;18(10) doi: 10.3390/ijerph18105202. Article 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joint FAO/WHO Expert Committee on Food Additives. Meeting. 73th, & Organization, W. H. World Health Organization; 2011. Safety Evaluation of Certain Food Additives and Contaminants: Prepared by the Seventy-Third Meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA)https://apps.who.int/iris/handle/10665/44521 [Google Scholar]
- Jovel E., Abramowski Z., Pakalnis E., Marshall B., Veiga M. Mercury (II) binding activity of vegetable and fruit juices: identifying potential detoxifying juices for the citizens of portovelo-zaruma, Ecuador. Aspect Min. Miner. Sci. 2018;2(1):1–15. [Google Scholar]
- Kannan M., Bojan N., Swaminathan J., Zicarelli G., Hemalatha D., Zhang Y., Ramesh M., Faggio C. Nanopesticides in agricultural pest management and their environmental risks: a review. Int. J. Environ. Sci. Technol. 2023 doi: 10.1007/s13762-023-04795-y. [DOI] [Google Scholar]
- Laird B.D., Chan H.M. Bioaccessibility of metals in fish, shellfish, wild game, and seaweed harvested in British Columbia, Canada. Food Chem. Toxicol.: Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2013;58:381–387. doi: 10.1016/j.fct.2013.04.033. [DOI] [PubMed] [Google Scholar]
- Laird B.D., Shade C., Gantner N., Chan H.M., Siciliano S.D. Bioaccessibility of mercury from traditional northern country foods measured using an in vitro gastrointestinal model is independent of mercury concentration. Sci. Total Environ. 2009;407(23):6003–6008. doi: 10.1016/j.scitotenv.2009.08.014. [DOI] [PubMed] [Google Scholar]
- Liao W., Wang G., Zhao W., Zhang M., Wu Y., Liu X., Li K. Change in mercury speciation in seafood after cooking and gastrointestinal digestion. J. Hazard Mater. 2019;375:130–137. doi: 10.1016/j.jhazmat.2019.03.093. [DOI] [PubMed] [Google Scholar]
- Łuczyńska J., Łuczyński M.J., Nowosad J., Kowalska-Góralska M., Senze M. Total mercury and fatty acids in selected fish species on the polish market: a risk to human health. Int. J. Environ. Res. Publ. Health. 2022;19(16) doi: 10.3390/ijerph191610092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merola C., Bisegna A., Angelozzi G., Conte A., Abete M.C., Stella C., Pederiva S., Faggio C., Riganelli N., Perugini M. Study of heavy metals pollution and vitellogenin levels in Brown trout (Salmo trutta trutta) wild fish populations. Appl. Sci. 2021;11(11) doi: 10.3390/app11114965. Article 11. [DOI] [Google Scholar]
- Mieiro C.L., Coelho J.P., Dolbeth M., Pacheco M., Duarte A.C., Pardal M.A., Pereira M.E. Fish and mercury: influence of fish fillet culinary practices on human risk. Food Control. 2016;60:575–581. doi: 10.1016/j.foodcont.2015.09.006. [DOI] [Google Scholar]
- Mills N., Cashatt D., Weber M.J., Pierce C.L. A case study and a meta-analysis of seasonal variation in fish mercury concentrations. Ecotoxicology. 2018;27(6):641–649. doi: 10.1007/s10646-018-1942-4. [DOI] [PubMed] [Google Scholar]
- Minamisawa M., Minamisawa H., Yoshida S., Takai N. Adsorption behavior of heavy metals on biomaterials. J. Agric. Food Chem. 2004;52(18):5606–5611. doi: 10.1021/jf0496402. [DOI] [PubMed] [Google Scholar]
- Morgan J.N. In: Impact of Processing on Food Safety. Jackson L.S., Knize M.G., Morgan J.N., editors. Springer US; 1999. Effects of processing on heavy metal content of foods; pp. 195–211. [DOI] [Google Scholar]
- Nădejde M.I., Bran E.-P., Ureche D., Alexa I.-C., Lazăr G., Lazăr I.M. 2019. CORRELATIONS BETWEEN MORPHOLOGICAL CHARACTERISTICS AND HEAVY METALS CONCENTRATION IN THREE SPECIES OF FRESHWATER FISH. [Google Scholar]
- Nwokocha C.R., Owu D.U., Nwokocha M.I., Ufearo C.S., Iwuala M.O.E. Comparative study on the efficacy of Allium sativum (garlic) in reducing some heavy metal accumulation in liver of wistar rats. Food Chem. Toxicol. 2012;50(2):222–226. doi: 10.1016/j.fct.2011.11.003. [DOI] [PubMed] [Google Scholar]
- Ouédraogo O., Amyot M. Effects of various cooking methods and food components on bioaccessibility of mercury from fish. Environ. Res. 2011;111(8):1064–1069. doi: 10.1016/j.envres.2011.09.018. [DOI] [PubMed] [Google Scholar]
- Perelló G., Martí-Cid R., Llobet J.M., Domingo J.L. Effects of various cooking processes on the concentrations of arsenic, cadmium, mercury, and lead in foods. J. Agric. Food Chem. 2008;56(23):11262–11269. doi: 10.1021/jf802411q. [DOI] [PubMed] [Google Scholar]
- Pradhoshini K.P., Priyadharshini M., Santhanabharathi B., Ahmed M.S., Parveen M.H.S., War M.U.D., Musthafa M.S., Alam L., Falco F., Faggio C. Biological effects of ionizing radiation on aquatic biota—a critical review. Environ. Toxicol. Pharmacol. 2023;99 doi: 10.1016/j.etap.2023.104091. [DOI] [PubMed] [Google Scholar]
- Price G., Patel D.A. StatPearls. StatPearls Publishing; 2023. Drug bioavailability.http://www.ncbi.nlm.nih.gov/books/NBK557852/ [Google Scholar]
- Raeeszadeh M., Karimi P., Khademi N., Mortazavi P. The effect of broccoli extract in arsenic-induced experimental poisoning on the hematological, biochemical, and electrophoretic parameters of the liver and kidney of rats. Evid. base Compl. Alternative Med. 2022;2022 doi: 10.1155/2022/3509706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravi R., Athisuyambulingam M., Kanagaraj S., Tresnakova N., Impellitteri F., Viswambaran G., Faggio C. Impact of chlorpyrifos on cytopathological indices in mangrove crab, Episesarma tetragonum (Fabricius) Vet. Sci. 2023;10(1) doi: 10.3390/vetsci10010053. Article 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Re R., Pellegrini N., Proteggente A., Pannala A., Yang M., Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biol. Med. 1999;26(9–10):1231–1237. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Rodrigues D.B., Marques M.C., Hacke A., Loubet Filho P.S., Cazarin C.B.B., Mariutti L.R.B. Trust your gut: bioavailability and bioaccessibility of dietary compounds. Curr. Res. Food Sci. 2022;5:228–233. doi: 10.1016/j.crfs.2022.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland I.R., Mallett A.K., Flynn J., Hargreaves R.J. The effect of various dietary fibres on tissue concentration and chemical form of mercury after methylmercury exposure in mice. Arch. Toxicol. 1986;59(2):94–98. doi: 10.1007/BF00286730. [DOI] [PubMed] [Google Scholar]
- Saha S., Chandra Saha N., Chatterjee A., Banerjee P., Garai P., Sharma P., Patnaik L., Nayak S., Dhara K., Chukwuka A.V., Faggio C. Integrated multi-biomarker responses in Mozambique tilapia, Oreochromis mossambicus under acute and chronic Diazinon® exposures. Chem. Ecol. 2023;39(3):235–255. doi: 10.1080/02757540.2023.2178649. [DOI] [Google Scholar]
- Schmidt L., Bizzi C.A., Duarte F.A., Muller E.I., Krupp E., Feldmann J., Flores E.M.M. Evaluation of Hg species after culinary treatments of fish. Food Control. 2015;47:413–419. doi: 10.1016/j.foodcont.2014.07.040. [DOI] [Google Scholar]
- Senthamils D., Chezhian A., Suresh E. Synergistic effect of nickel and mercury on fatty acid composition in the muscle of fish Lates calcarifer. J. Fish. Aquat. Sci. 2015;11(1):77–84. doi: 10.3923/jfas.2016.77.84. [DOI] [Google Scholar]
- Shahjahan M., Taslima K., Rahman M.S., Al-Emran M., Alam S.I., Faggio C. Effects of heavy metals on fish physiology – a review. Chemosphere. 2022;300 doi: 10.1016/j.chemosphere.2022.134519. [DOI] [PubMed] [Google Scholar]
- Shim S.-M., Ferruzzi M.G., Kim Y.-C., Janle E.M., Santerre C.R. Impact of phytochemical-rich foods on bioaccessibility of mercury from fish. Food Chem. 2009;112(1):46–50. doi: 10.1016/j.foodchem.2008.05.030. [DOI] [Google Scholar]
- Shiry N., Derakhshesh N., Gholamhosseini A., Pouladi M., Faggio C. Heavy metal concentrations in Cynoglossus arel (Bloch & Schneider, 1801) and sediment in the Chabahar Bay, Iran. Int. J. Environ. Res. 2021;15 doi: 10.1007/s41742-021-00352-y. [DOI] [Google Scholar]
- Siedlikowski M., Bradley M., Kubow S., Goodrich J.M., Franzblau A., Basu N. Bioaccessibility and bioavailability of methylmercury from seafood commonly consumed in North America: In vitro and epidemiological studies. Environ. Res. 2016;149:266–273. doi: 10.1016/j.envres.2016.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simionov I.-A., Petrea S.-M., Mogodan A., Nica A., Cristea D., Neculita M. Effect of changes in the Romanian lower sector Danube River hydrological and hydrothermal regime ON FISH diversity. Earth Obs. 2020 [Google Scholar]
- Sneddon E.J., Hardaway C.J., Sneddon J., Boggavarapu K., Tate A.S., Tidwell S.L., Gary D.P., Douvris C. Determination of selected metals in rice and cereal by inductively coupled plasma-optical emission spectrometry (ICP-OES) Microchem. J. 2017;134:9–12. doi: 10.1016/j.microc.2017.04.009. [DOI] [Google Scholar]
- Tahity T., Islam Md.R.U., Bhuiyan N.Z., Choudhury T.R., Yu J., Noman Md.A., Hosen M.M., Quraishi S.B., Paray B.A., Arai T., Hossain M.B. Heavy metals accumulation in tissues of wild and farmed Barramundi from the northern Bay of Bengal coast, and its estimated human health risks. Toxics. 2022;10(8):410. doi: 10.3390/toxics10080410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchounwou P.B., Yedjou C.G., Patlolla A.K., Sutton D.J. Heavy metals toxicity and the environment. EXS. 2012;101:133–164. doi: 10.1007/978-3-7643-8340-4_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Escribano S., Vélez D., Montoro R. Mercury and methylmercury bioaccessibility in swordfish. Food Addit. Contam. 2010;27(3):327–337. doi: 10.1080/19440040903365272. [DOI] [PubMed] [Google Scholar]
- WHO | JECFA. (n.d.). Retrieved August 28, 2023, from https://apps.who.int/food-additives-contaminants-jecfa-database/.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.