Abstract
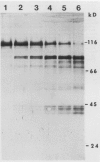
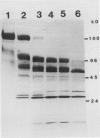
The sensitivity of the two forms of nitrate reductase, NRI and NRII, obtained from the primary leaf of corn, to a limited action corn root proteinase has been examined. The corn inactivating protein (CIP) inhibited the overall reaction (NADH-NR) and the two partial reactions, cytochrome c reductase and reduced methyl viologen NR (MV-NR) of both forms of NR. NADH-cytochrome c reductase was more sensitive to the protease than MV-NR. NRII was less sensitive to inactivation than NRI. When NRI and NRII were inactivated and then subjected to native gel electrophoresis the protein bands associated with MV-NR activity shifted from an Rm value of 0.32 to 0.61 for NRI and from an Rm of 0.28 to 0.60 for NRII. For Chlorella NR these values are 0.32 and 0.70. The initial cleavage of the 116 kilodalton subunit of NRI yielded fragments of 84 and 80 kilodaltons after a 5 minute incubation with CIP. With longer incubation times smaller fragments were also identified. For the Chlorella NR the initial cleavage products are approximately 68 and 25 kilodaltons. Longer incubation times also led to smaller fragments. The products of hydrolysis by this limited action protease are quite different for the corn and Chlorella NRs.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aslam M., Oaks A. Comparative studies on the induction and inactivation of nitrate reductase in corn roots and leaves. Plant Physiol. 1976 Apr;57(4):572–576. doi: 10.1104/pp.57.4.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolly S. O., Tolbert N. E. NADH-Nitrate Reductase Inhibitor from Soybean Leaves. Plant Physiol. 1978 Aug;62(2):197–203. doi: 10.1104/pp.62.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Nakagawa H., Poulle M., Oaks A. Characterization of Nitrate Reductase from Corn Leaves (Zea mays cv W64A x W182E) : Two Molecular Forms of the Enzyme. Plant Physiol. 1984 Jun;75(2):285–289. doi: 10.1104/pp.75.2.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon J. D., Wallace W. Isolation and characterisation of peptide hydrolases from the maize root. Eur J Biochem. 1979 Dec 17;102(2):399–408. doi: 10.1111/j.1432-1033.1979.tb04255.x. [DOI] [PubMed] [Google Scholar]
- Solomonson L. P., Barber M. J., Robbins A. P., Oaks A. Functional domains of assimilatory NADH:nitrate reductase from Chlorella. J Biol Chem. 1986 Aug 25;261(24):11290–11294. [PubMed] [Google Scholar]
- Solomonson L. P., Howard W. D., Yamaya T., Oaks A. Mode of action of natural inactivator proteins from corn and rice on a purified assimilatory nitrate reductase. Arch Biochem Biophys. 1984 Sep;233(2):469–474. doi: 10.1016/0003-9861(84)90469-7. [DOI] [PubMed] [Google Scholar]
- Solomonson L. P., Lorimer G. H., Hall R. L., Borchers R., Bailey J. L. Reduced nicotinamide adenine dinucleotide-nitrate reductase of Chlorella vulgaris. Purification, prosthetic groups, and molecular properties. J Biol Chem. 1975 Jun 10;250(11):4120–4127. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace W. Comparison of a nitrate reductase-inactivating enzyme from the maize root with a protease form yeast which inactivates tryptophan synthase. Biochim Biophys Acta. 1978 Jun 9;524(2):418–427. doi: 10.1016/0005-2744(78)90179-1. [DOI] [PubMed] [Google Scholar]
- Wallace W. Effects of a nitrate reductase inactivating enzyme and NAD(P)H on the nitrate reductase from higher plants and Neurospora. Biochim Biophys Acta. 1975 Feb 19;377(2):239–250. doi: 10.1016/0005-2744(75)90306-x. [DOI] [PubMed] [Google Scholar]
- Wallace W. Purification and properties of a nitrate reductase-inactivating enzyme. Biochim Biophys Acta. 1974 Mar 21;341(1):265–276. doi: 10.1016/0005-2744(74)90087-4. [DOI] [PubMed] [Google Scholar]
- Yamaya T., Oaks A., Boesel I. L. Characteristics of Nitrate Reductase-inactivating Proteins Obtained from Corn Roots and Rice Cell Cultures. Plant Physiol. 1980 Jan;65(1):141–145. doi: 10.1104/pp.65.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaya T., Solomonson L. P., Oaks A. Action of Corn and Rice-inactivating Proteins on a Purified Nitrate Reductase from Chlorella vulgaris. Plant Physiol. 1980 Jan;65(1):146–150. doi: 10.1104/pp.65.1.146. [DOI] [PMC free article] [PubMed] [Google Scholar]