Summary
Homoallenic alcohols are versatile building blocks to access complex molecules in synthetic chemistry. We present a protocol for accessing homoallenic alcohols by an allenyl radical-involved radical-polar crossover process. We describe steps for preparing solvents and the photoreactor and synthesizing 5-methyl-1-phenyl-3-tosylhexa-3,4-dien-1-ol 3 in 85% yield. In addition, 5-methyl-1-phenyl-3-tosylhexa-3,4-dien-1-ol 3 has been successfully synthesized in 2-mmol scale. This protocol is also applicable to the synthesis of homoallenic amides.
For complete details on the use and execution of this protocol, please refer to Wei et al. (2023).1
Subject areas: chemistry, energy, material sciences
Graphical abstract
Highlights
-
•
Allenyl radical-mediated difunctionalization of alkenes to generate homoallenic alcohols
-
•
Detailed steps to synthesize homoallenic alcohols
-
•
Detailed procedure for setting up a simplified photoreactor
Publisher’s note: Undertaking any experimental protocol requires adherence to local institutional guidelines for laboratory safety and ethics.
Homoallenic alcohols are versatile building blocks to access complex molecules in synthetic chemistry. We present a protocol for accessing homoallenic alcohols by an allenyl radical-involved radical-polar crossover process. We describe steps for preparing solvents and the photoreactor and synthesizing 5-methyl-1-phenyl-3-tosylhexa-3,4-dien-1-ol 3 in 85% yield. In addition, 5-methyl-1-phenyl-3-tosylhexa-3,4-dien-1-ol 3 has been successfully synthesized in 2-mmol scale. This protocol is also applicable to the synthesis of homoallenic amides.
Before you begin
Homoallenic alcohols and amides are versatile intermediates to construct diversely complex molecules by virtue of their consecutive C=C double bonds and a nucleophilic O- or N- center.2,3,4 Their preparations highly depend on structurally specific alkynyl or enynyl-containing precursors.5,6,7,8 These methods usually suffer from the need of specific precursors or reagents which are tedious to synthesize or with bad functional groups tolerance. It is of great interest to develop complementary routes for their preparations.
Up to now, radical-mediated alkene difunctionalization has gained significant progress and provides a robust toolbox to construct numerous high value-added products.9,10,11,12,13,14 In this protocol, we conceived that allenic radical-mediated difunctionalization of alkene should provide a direct solution to construct homoallenic alcohols and amides. However, the lack of efficient method to access allenic radicals impedes their utilization in synthetic chemistry.15 Moreover, allenic radical is a highly reactive species and is considered as a resonance hybrid with propargyl radical, which also restricts their utilization in intermolecular difunctionalization of alkenes.16,17 To solve the above challenge, we designed and synthesized allenic radical precursor 1 with a strong electron-withdrawing sulfonyl moiety, which was purposed to increase the electrophilicity of allenic radical and enabled the challenging intermolecular addition of allenyl radical to alkenes, affording diverse homoallenic alcohols and amides. Given that the procedures to synthesize homoallenic alcohols and amides are similar, we select homoallenic alcohols as an example to describe this protocol; for more details please refer to Wei et al. (2023).1
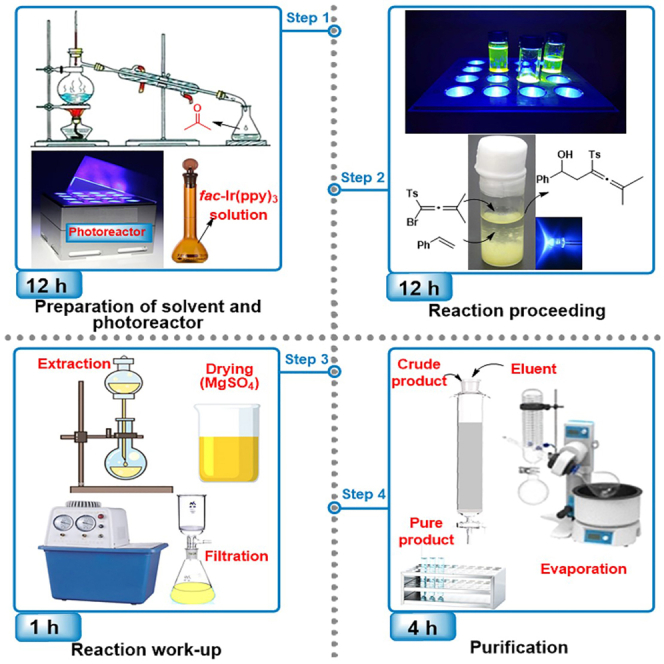
Preparation of reagent—fac-Ir(ppy)3 solution in 1,2-dichloroethane
Timing: 2 h
In this part, the preparation of 0.004 M fac-Ir(ppy)3 solution in 1,2-dichloroethane is described. Given to the low catalyst loading (0.2 mol%), it is difficult to weigh fac-Ir(ppy)3 for small scale reaction. The solubility, boiling point, and volatility of candidate solvents were taken into consideration. We evaluated various common solvents, including acetonitrile, ethyl acetate (EtOAc), toluene, dichloromethane (DCM), chloroform, finally 1,2-dichloroethane was selected as suitable solvent.
- 1.
-
2.
Put it into an ultrasound equipment and keep it at room temperature (22°C) for 1 h. (Figure 1C)
-
3.
Charge the flask with nitrogen gas or argon gas and store it in the dark at room temperature (22°C). (Figure 1D)
Note: The rate of gas flow should be carefully controlled. In our case, the flow rate is 0.3 L/min and maintains 30 s. For better dissolution, we prefer to keep it overnight before using.
Figure 1.
Overview of the preparation of photocatalyst solution
(A) Weigh fac-Ir(ppy)3.
(B) Add 1,2-dichloroethane.
(C) Dissolve under ultrasonic condition.
(D) Charge nitrogen gas or argon gas.
Purification of reagent—Acetone
Timing: 8 h
In this part, the purification of acetone is described. Commercially available acetone generally contains some nucleophilic alcohols such as methanol and isopropanol, which will slightly erosion the outcomes (1%–2%) of this protocol. KMnO4 is used as oxidant to remove the nucleophilic alcohols. This step can be skipped if one uses high quality acetone, such as chromatographic grade.
-
4.Preprocessing.
-
a.Add 60 mL of acetone into a 100 mL single-neck round bottom flask. Troubleshooting 1.
-
b.Add a stirring bar and 2 g potassium permanganate (KMnO4).
-
c.Prepare reflux device.
-
i.Add a spherical condenser on the flask.
-
ii.Lock the joint with a clamp and close the system with a rubber septum.
-
iii.Insert a balloon with an inlet needle (Figure 2B).
-
iv.Equip a pump for condensate water returning.
-
i.
-
d.Heat the mixture to reflux at 60°C for 4 h (Figure 2B).
-
a.
-
5.Distillation.
-
a.Cold down the system and remove the spherical condenser.
-
b.Equip distilling apparatus.
-
i.Equip a distilling head, a straight condenser, a tail nozzle and a 100 mL round bottom flask in sequence. Troubleshooting 2.Note: all of these glasses should be oven-dried.
-
ii.Then a balloon with a length of rubber pipe is used to close the system (Figure 2C).
-
iii.Connect the condenser with running water.
-
i.
-
c.Heat the system again to 60°C.
-
d.Cover the distilling head with cotton to maintain the temperature (Figure 2C).
-
e.Slowly heat to 65°C and collect the acetone.Note: To avoid any danger rising from KMnO4, it is necessary to retain about 10–15 mL acetone. The residual KMnO4 should be quenched with cold water and dealt with saturate sodium thiosulfate solution.
-
a.
-
6.Nitrogen-blowing process (Figure 2D).
-
a.Set up bubbling device.
-
i.Add a stirring bar once the collection is completed.
-
ii.Seal the bottle with a rubber stopper.
-
iii.Insert a nitrogen gas balloon with a long needle is into the bottle Seal the bottle (the needle tip is below the surface of acetone).
-
iv.Insert another short needle into the rubber stopper.
-
i.
-
b.Start the stirrer and keep 10 min.
-
c.Move the needles and seal the bottle with parafilm.
-
a.
Figure 2.
Overview of the purification system
(A) Required accessories.
(B) Set-up of refluxing.
(C) Set-up of distillation.
(D) Nitrogen-blowing process.
Preparation of photoreactor
Timing: 2 h
In this step, the construction of a simplified photoreactor is described. Generally, commercially available photoreactors are expensive and in some cases, there is no need to buy a photoreactor for seldom use. It is worth noting that this simplified photoreactor is only suitable for common conditions, such as room temperature, ordinary pressure, and small-scale reaction (2 mmol scale reaction has been demonstrated to be feasible). If you need a higher quality photoreactor or already have one, please skip this step.
-
7.Prepare a suitable cardboard box.
-
a.Cut two holes on the cardboard box, one for LED lamp and the other for blower (Figure 3B).Note: After long time irradiation, the temperature of inside cardboard box will increase. In order to maintain room temperature (22°C), a blower is necessary to cold down the system.
-
b.Attach tin foil paper on the inside of the cardboard box (Figure 3C).
-
a.
-
8.
Put a magnetic stirrer into the cardboard box.
-
9.
Install the blower (Figure 3B).
-
10.
Install the blue LED lamp (Liang Pai Lighting, 5 W) (Figure 3D).
Note: We have examined that in the absence of blower, after 11 hours’ irradiation, the internal temperature of the self-made photoreactor is 11° higher than the room temperature (22°C).
Figure 3.
Overview of the construction of photoreactor
(A) Required accessories.
(B) Install blower.
(C) Attach tin foil paper.
(D) Insert LED lamp.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals, peptides, and recombinant proteins | ||
| Acetone | Damao | CAS: 67-64-1 |
| 1,2-Dichloroethane | Kermel | CAS: 107-06-2 |
| Potassium permanganate (KMnO4) | Beijing Shiji | CAS: 7722-64-7 |
| AR ethyl acetate (EtOAc) | Macron | CAS: 141-78-6 |
| AR petroleum ether | Energy Chemical | CAS: 8032-32-4 |
| Styrene | Alfa Aesar | CAS: 100-42-5 |
| 1-((1-Bromo-3-methylbuta-1,2-dien-1-yl)sulfonyl)-4-methylbenzene | Synthesized by our laboratory | Wei et al.1 |
| Potassium dihydrogen phosphate (KH2PO4) | 3A | CAS: 7778-77-0 |
| Tris(2-phenylpyridine)iridium) [fac-Ir(ppy)3] | Energy Chemical | CAS: 94928-86-6 |
| Silica gel for chromatography | Haiyang | CAS: 112926-00-8 |
| Thin-layer chromatography plate (TLC plate) | Haiyang | |
| Other | ||
| Electronic balance | Ohaus | AR224CN |
| Magnetic stirrer | Guohua | 78 |
| Pump of rotary evaporator | Buchi | V-300 |
| Refrigerated circulator bath | Yuhua | CCA-20 |
| Rotary evaporator | Buchi | R-3 |
| Deionized water equipment | Proseers | Classic EU15 |
| Ultrasonic cleaner | Shumei | KQ-700DE |
| Lamp holder | Mingyuexing | QP-8889 |
| Blue LED lamp | Liang Pai Lighting | Dengbei Shedeng (5 W) |
| Heating system | Yuhua | DF-101D |
| Blower | Ve Mai | KS-2102 |
| Reaction vial | Agilent Technologies | 5183-4311 |
Step-by-step method details
Synthesize homoallenic alcohol
Timing: 12 h
In this part, the synthetic procedure of 5-methyl-1-phenyl-3-tosylhexa-3,4-dien-1-ol 3 has been carefully described (Scheme 1).
-
1.Setting up reaction.
-
a.Add catalyst
-
i.Charge 100 μL of the prepared fac-Ir(ppy)3 (0.0004 mmol, 0.2 mol%) solution into a 4 mL vial using micro-syringe.
-
ii.Seal the vial with a rubber stopper and insert a syringe needle.
-
iii.Evaporate the 1,2-dichloroethane under reduced pressure (Figures 4A and 4B).
-
i.
-
b.Add 60 mg starting material 1(0.2 mmol, 1 equiv.), 27.2 mg KH2PO4 (0.4 mmol, 2 equiv.), and a stirring bar respectively into the vial.
-
c.Seal the vial with an open type screw cover.
-
d.Subject it to evacuation/ flushing with nitrogen for three times (Figure 4C).
-
e.Add 1 mL distillated acetone, 1 mL deionized water and 36 μL styrene (0.3 mmol, 1.5 equiv.) respectively.Note: the acetone and deionized water are degassed via a nitrogen-blowing process in advance as shown in Before you begin 6.
-
f.Seal the vial with parafilm (Figure 4D).
-
g.Put the vial into the photoreactor (the length between the lamp and the vial is about 10 cm),
-
h.Then the reaction is irradiated and stirred (600 rpm) for 11 h (Figure 4E). Troubleshooting 3.
-
a.
Scheme 1.
General scheme of the reaction
Figure 4.
Overview of reaction set-up
(A–E) (A) Evaporate the 1,2-dichloroethane, (B) After evaporation, (C) Degas process, (D) Block the vial with parafilm, (E) Irradiate the reaction.
Tracking reactions and purification
Timing: 6 h
This part describes how to monitor the reaction, and how to obtain the desired homo-allenic alcohol 3 by purification.
-
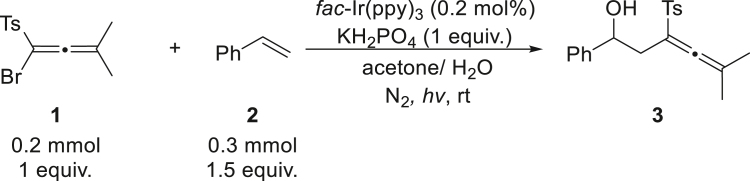
2.Tracking reaction.Note: Initially, the reaction mixture is heterogeneous (solid and liquid). With the reaction proceeding, the solid starting material 1 decreases slowly, meanwhile, the desired product (whose physical state is oil) appears and the reaction mixture changes to three phases (liquid, solid and oil).
-
a.Once the solid starting material 1 disappears, use a capillary to dip the reaction mixture for TLC analysis (Figure 5A).
-
b.The starting material 1 and reaction mixture are spotted on TLC plate. A mixture the starting material 1 and reaction mixture are also spotted on TLC plate.
-
i.Use a mixture of petroleum ether and EtOAc with 5 to 2 ratio as the developing solvents]
-
ii.Take the TLC plate out when the solvent run over about 80% of the TLC plate (Figure 5B)Note: There is no need to extraction. Given to the low solubility of the desired product 3, the true reaction result is better than the TLC presentation.
-
i.
-
c.The TLC plate is detected with 254 nm UV-light (Figure 5C). The reaction is terminated when the starting material 1 is well-consumed.Note: it should be very fast to dip the reaction mixture to prevent air enter into the vial. Generally, through observation, once the reaction mixture turns into two phases (liquid and oil), the substrate 1 is almost consumed, it is not necessary to recharge nitrogen again. In addition, if the reaction does not complete, the vial is sealed with parafilm again and put it back to the photoreactor.
-
a.
-
3.Extraction.
-
a.Transfer the reaction mixture to a separatory funnel.
-
i.Once the reaction completes, the reaction mixture is transferred into a 60 mL separatory funnel using a glue head dropper.
-
ii.Wash the vial with 2 mL EtOAc for three times, and the solvent is combined.
-
i.
-
b.Extract with EtOAc.
-
i.Add 5 mL water into the separatory funnel and seal it with a stopper.
-
ii.Shake the funnel vigorously, and then keep it quiescence until the organic phase separates from the aqueous one (Figure 6A).
-
iii.Pour the organic phase into a 50 mL beaker.
-
iv.Transfer the aqueous phase back into the separatory funnel.
-
v.Repeat the extraction with 3 mL EtOAc for three times.
-
vi.Transfer the combined organic phase back into the separatory funnel, and wash with 5 mL saturated brine.
-
vii.Pour the organic phase into a 50 mL beaker.
-
i.
-
c.Dry above resulting mixture.
-
i.Add 2 g of anhydrous magnesium sulfate (MgSO4) into the beaker.
-
ii.Stir 30 s with a glass rod and keep quiescence for 10 min (Figure 6B).
-
i.
- d.
-
a.
-
4.Purification.
-
a.Prepare sample mixed with silica gel.
-
i.Dissolve the residue with 10 mL DCM.
-
ii.Add 0.5 g of silica gel (300–400 mesh).
-
iii.Remove the solvent under rotary evaporation (200 mbar and 35°C) until the silica gel turn to white powder and attached on the wall of the bottle (Figure 7B).Note: Because the mixture is easy to erupt during rotary evaporation, it is better to stuff cotton into the beginning of anti-splash adapter to prevent sample damage (Figure 7A)
-
i.
-
b.Prepare a silica gel column.
-
i.Weigh 20 g of silica gel and pure into a 250 mL glass beaker with 50 mL petroleum ether and 5 mL EtOAc.
-
ii.Stir the mixture vigorously with a glass rod and transfer into the column.
-
iii.Compact the silica gel under pressure.
-
iv.Transfer the silica gel of above step 4a into the column with a glass funnel (Figure 7C).Note: In order to avoid the surface damage, about 2 cm of solvent should be retained when pouring the silica gel.
-
v.Compact the silica gel again, and add a layer of sea sand on the surface.
-
i.
-
c.Run the column chromatography
-
i.Run the column chromatography with petroleum ether and EtOAc (ratio 10:1) as eluent. Troubleshooting 4.
-
ii.Collect the sample with 20 mL test tube. Confirm the presence of 5-methyl-1-phenyl-3-tosylhexa-3,4-dien-1-ol 3, as shown in steps 2b and 2c (step-by-step method details) (Figure 7C).
-
i.
-
d.Remove the solvent
-
i.Collect the fractions containing the 5-methyl-1-phenyl-3-tosylhexa-3,4-dien-1-ol 3 into a 250 mL round bottom flask.
-
ii.Remove the solvent through rotary evaporation under 200 mbar and 40°C for 30 min.
-
iii.Finally remove the remaining solvent under high vacuum for 2 h to obtain the desired product 3 as white solid (58.1 mg, 85%). Troubleshooting 5.
-
i.
-
a.
Figure 5.
Overview of reaction tracing
(A–C) (A) Insert a needle on the cap, (B) Locate TLC plate in the camber, (C) Fluorescent TLC plate under UV-light (254 nm). And the desired product 3 is as shown.
Figure 6.
Overview of extraction
(A) Separation.
(B) Dehydration.
(C) Filtration.
(D) Evaporation.
Figure 7.
Overview of the purification
(A) Stuff carton.
(B) Evaporation.
(C) Purification.
Expected outcomes
5-methyl-1-phenyl-3-tosylhexa-3,4-dien-1-ol 3 obtained as a white solid in 85% yield.
Quantification and statistical analysis
1H NMR (400 MHz, CDCl3) δ 7.73-7.70 (m, 2H), 7.33-7.26 (m, 6H), 7.25-7.20 (m, 1H), 4.88 (dd, J = 6.8, 6.8 Hz, 1H), 2.78-2.63 (m, 3H), 2.43 (s, 3H), 1.53 (s, 3H), 1.53 (s, 3H).
13C NMR (100 MHz, CDCl3) δ 203.2, 144.3, 143.0, 137.2, 129.7, 128.5, 128.1, 127.7, 126.2, 107.6, 107.0, 72.7, 36.9, 21.7, 19.6, 19.5.
FT-IR: ν (cm-1) 3533, 2985, 2917, 2850, 2361, 1962, 1594, 1437, 1383, 1298, 1289, 1137, 1085, 1040, 1026.
HRMS [ESI] (m/z): [M+Na]+ calcd for C20H22O3SNa 365.1182, found 365.1180.
M.p.: 74.5°C–75.6°C.
TLC: Rf = 0.46 (petroleum ether/ethyl acetate = 5:2).
Limitations
This protocol is less efficient to aliphatic alkenes. For example, when methylenecyclohexane was used as starting material, it only afforded the desired product with 32% yield (more detail, please see Wei et al., 2023).1
Troubleshooting
Problem 1
Step 4a (before you begin): acetone is hazardous because of its low boiling point and might cause in some health discomforts.
Potential solution
The boiling point of acetone is only 56.5°C. Given to its volatile and inflammable property, it is better to perform the purification in a well-ventilated fume hood.
Problem 2
Step 5b (before you begin): airtightness is crucial for distillation.
Potential solution
Acetone is volatile, and the pressure of the distillation system is a little higher than ordinary pressure, therefore, all joints should be smeared with Vaseline and locked by clamp. To be noted, Vaseline cannot be used too much to avoid the acetone polluted by Vaseline.
Problem 3
Step 1h (Step-by-step method details): long time of blue LED light exposure is harmful to the eyes.
Potential solution
The photoreactor should be placed in a confine space to prevent light leak. Before any operations during the reaction running, the operator should turn off the photoreactor or wear sunglasses.
Problem 4
Step 4c (Step-by-step method details): impurity.
Potential solution
The reason to select petroleum ether and ethyl acetate (10:1) as eluent is that from the TLC analysis there is some impurity with quite similar Rf with the 5-methyl-1-phenyl-3-tosylhexa-3,4-dien-1-ol 3, so it is better to keep the polarity of eluent until the presence of 5-methyl-1-phenyl-3-tosylhexa-3,4-dien-1-ol 3.
Problem 5
Step 4d (Step-by-step method details): Initially, the physical state of 5-methyl-1-phenyl-3-tosylhexa-3,4-dien-1-ol 3 is light yellow oil.
Potential solution
The melting point of 5-methyl-1-phenyl-3-tosylhexa-3,4-dien-1-ol 3 is only 74.5°C. It is difficult to solidify at room temperature (22°C). To obtain solid state of product 3, one should store it in fridge for a long time (generally overnight).
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Chen Zhu (chzhu@sjtu.edu.cn).
Materials availability
This study did not generate new unique reagents.
Data and code availability
-
•
Original data for substrate scope and the detail please refer to our previous article (Wei et al., 2023).1
-
•
This study did not generate code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
Acknowledgments
The authors are grateful for the financial support from the High-level Talent Research Funding Project of Hebei University (050001/521100222058), the National Natural Science Foundation of China (21971173 and 22171201), the Fundamental Research Funds for the Central Universities (22X010201631), and the Program of Shanghai Academic/Technology Research Leader (23XD1421900).
Author contributions
Y.W. and C.Z. conceived and designed this study and wrote the protocol. Y.W. performed the experiment. C.Z. directed the project.
Declaration of interests
The authors declare no competing interests.
Contributor Information
Yunlong Wei, Email: ylwei@hbu.edu.cn.
Chen Zhu, Email: chzhu@sjtu.edu.cn.
References
- 1.Wei Y., Wu X., Chen Y., Zhu C. Radical-Polar Crossover Allenylation of Alkenes: Divergent Synthesis of Homoallenic Alcohols and Amides. Chem Catal. 2023;3 doi: 10.1016/j.checat.2023.100551. [DOI] [Google Scholar]
- 2.Krause N., Hashmi A.S.K. Wiley-VCH; 2004. Modern Allene Chemistry”. [DOI] [Google Scholar]
- 3.Krause N., Winter C. Gold-catalyzed nucleophilic cyclization of functionalized allenes: A powerful access to carbo- and heterocycles. Chem. Rev. 2011;111:1994–2009. doi: 10.1021/cr1004088. [DOI] [PubMed] [Google Scholar]
- 4.Alonso J.M., Almendros P. Deciphering the chameleonic chemistry of allenols: Breaking the taboo of a onetime esoteric functionality. Chem. Rev. 2021;121:4193–4252. doi: 10.1021/acs.chemrev.0c00986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Posevins D., Bermejo-López A., Bäckvall J.E. Iron-catalyzed crosscoupling of propargyl ethers with grignard reagents for the synthesis of functionalized allenes and allenols. Angew. Chem. Int. Ed. 2021;60:22178–22183. doi: 10.1002/anie.202106742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poh J.-S., Tran D.N., Battilocchio C., Hawkins J.M., Ley S.V. A versatile room-temperature route to di- and trisubstituted allenes using flow generated diazo compounds. Angew. Chem. Int. Ed. 2015;54:7920–7923. doi: 10.1002/anie.201501538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bayeh-Romero L., Buchwald S.L. Copper hydride catalyzed enantioselective synthesis of axially chiral 1,3-disubstituted allenes. J. Am. Chem. Soc. 2019;141:13788–13794. doi: 10.1021/jacs.9b07582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang F., Wang D., Zhou Y., Liang L., Lu R., Chen P., Lin Z., Liu G. Divergent synthesis of CF3-substituted allenyl nitriles by ligand-controlled radical 1,2- and 1,4-addition to 1,3-enynes. Angew. Chem. Int. Ed. 2018;57:7140–7145. doi: 10.1002/anie.201803668. [DOI] [PubMed] [Google Scholar]
- 9.Koike T., Akita M. New Horizons of Photocatalytic Fluoromethylative Difunctionalization of Alkenes. Chem. 2018;4:409–437. doi: 10.1016/j.chempr.2017.11.004. [DOI] [Google Scholar]
- 10.Badir S.O., Molander G.A. Developments in Photoredox/Nickel Dual-Catalyzed 1,2-Difunctionalizations. Chem. 2020;6:1327–1339. doi: 10.1016/j.chempr.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nandi S., Das P., Das S., Mondal S., Jana R. Visible-light-mediated β-acylative divergent alkene difunctionalization with Katritzky salt/CO2. Green Chem. 2023;25:3633–3643. doi: 10.1039/D3GC00143A. [DOI] [Google Scholar]
- 12.Wu X., Zhu C. Radical-mediated remote functional group migration. Acc. Chem. Res. 2020;53:1620–1636. doi: 10.1021/acs.accounts.0c00306. [DOI] [PubMed] [Google Scholar]
- 13.Wei Y., Zhang H., Wu X., Zhu C. Alkene difunctionalization triggered by a stabilized allenyl radical: concomitant installation of two unsaturated C−C bonds. Angew. Chem. Int. Ed. 2021;60:20215–20219. doi: 10.1002/anie.202106145. [DOI] [PubMed] [Google Scholar]
- 14.Li Z.L., Fang G.C., Gu Q.S., Liu X.-Y. Recent advances in coppercatalysed radical-involved asymmetric 1,2-difunctionalization of alkenes. Chem. Soc. Rev. 2020;49:32–48. doi: 10.1039/C9CS00681H. [DOI] [PubMed] [Google Scholar]
- 15.Alameda-Angulo C., Quiclet-Sire B., Zard S.Z. An expedient approach to allenes and polycyclic structures using propargyl radicals. Tetrahedron Lett. 2006;47:913–916. doi: 10.1016/j.tetlet.2005.11.154. [DOI] [Google Scholar]
- 16.Fantazier R.M., Poutsma M.L. Reduction of propargylic chlorides with tri-n-butyltin hydride. The ambident behavior of propargylic radicals. J. Am. Chem. Soc. 1968;90:5490–5498. doi: 10.1021/ja01022a029. [DOI] [Google Scholar]
- 17.Walling C., Thaler W. Positive halogen compounds. III. Allylic chlorination with t-butyl hypochlorite the stereochemistry of allylic radicals. J. Am. Chem. Soc. 1961;83:3877–3884. doi: 10.1021/ja01479a033. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
-
•
Original data for substrate scope and the detail please refer to our previous article (Wei et al., 2023).1
-
•
This study did not generate code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.