Summary
The epidemiology, natural history, and therapeutic responses of chronic liver diseases and liver lesions often vary by sex. In this review, we summarize available clinical and translational data on these aspects of the most common liver conditions encountered in clinical practice, including the potential contributions of sex hormones to the underlying pathophysiology of observed differences. We also highlight areas of notable knowledge gaps and discuss sex disparities in access to liver transplant and potential strategies to address these barriers. Given established sex differences in immune response, drug metabolism, and response to liver-related therapies, emerging clinical trials and epidemiological studies should prioritize dedicated analyses by sex to inform sex-specific approaches to liver-related care.
Keywords: Gender, Liver, Epidemiology, Sex disparities
Key points.
-
•
The epidemiology and natural history of many chronic liver diseases differs by sex.
-
•
Differences in endogenous sex hormones appear to contribute to less hepatic fibrosis and hepatocellular carcinoma in women, though hormonal contributions across all liver diseases are not consistent.
-
•
Given established sex differences in immune response, drug metabolism, and response to some liver-related therapies, emerging clinical trials must prioritize dedicated analyses by sex.
Introduction
The epidemiology, natural history, and therapeutic responses of chronic liver diseases and liver lesions often vary by sex. In this review, we summarize available clinical and translational data on these aspects of the most common liver conditions encountered in clinical practice, including the potential contributions of sex hormones to the underlying pathophysiology of observed differences. We also discuss sex disparities in access to liver transplant and potential strategies to address these barriers. Regarding terminology, the term “women” and “men” in this review will refer to individuals assigned female or male sex at birth, respectively, though dedicated studies within transgender populations are greatly needed, including on the influence of gender-affirming exogenous hormone use on the natural history of chronic liver disease.
Fibrotic diseases
The human liver expresses cellular receptors for several sex hormones, including androgens, oestrogens, and progesterone, with oestrogen being the most widely studied sex hormone with respect to hepatic fibrosis.1 Oestrogens have a direct inhibitory effect on hepatic stellate cells, which are activated in response to liver injury and subsequently produce collagen.2,3 Studies in preclinical and animal models have demonstrated the antifibrotic effects of oestrogens through various pathways involving, but not limited to, interleukin (IL)-6 activity, signal transducer and activator of transcription 3 (STAT-3) phosphorylation, transforming growth factor-β, and extracellular signal-regulated kinase.1 For example, in vitro studies using human hepatic stellate cells have demonstrated that increased oestrogen receptor expression reduces transforming growth factor-β production by inhibiting STAT-3 phosphorylation.4 Increased levels of phosphorylated STAT-3 in hepatocytes and hepatic stellate cells have been associated with increased inflammation, ballooning, and fibrosis.5
Interestingly, oestrogen has been shown to play a role in improving liver injury once present by restoring microRNA pathways that regulate hepatic stellate cells.6 Beyond mediating inflammatory markers and hepatic fibrosis, oestrogen also plays a key role in regulating lipid and glucose homeostasis as further discussed in the non-alcoholic fatty liver disease (NAFLD) section.
Oestrogen’s protective effects against liver injury and fibrosis likely account for the higher prevalence of advanced fibrosis in men compared to women for many chronic liver diseases. Likewise, the risk of fibrosis increases following the menopausal transition, with data from women co-infected with HIV/HCV showing an increased risk of fibrosis with reproductive aging, independent of chronologic aging.7 In the following sections we will review sex differences in the natural history of chronic liver diseases and liver lesions, including disease-specific data on the role of sex hormones in these processes.
NAFLD
NAFLD affects approximately one-third to one-quarter of individuals globally, though prevalence estimates differ by sex and reproductive status8,9 (Fig. 1). NAFLD prevalence is generally thought to be higher in men, although this finding dissipates when comparing estimates in men and post-menopausal women.10,11 The risk of advanced fibrosis related to non-alcoholic steatohepatitis (NASH) does appear to be greater in women aged over 50 than in men.9,12,13 Indeed, NASH is now the leading indication for liver transplant in women, and the second most common indication for transplant in men.14 As noted above, these epidemiologic trends likely relate in large part to the protective effects of oestrogens against hepatic stellate cell activity and subsequent fibrosis.15 Premature menopause and longer durations of oestrogen deficiency have been shown to increase the risk of advanced NASH fibrosis.16 In a large case-control study from Europe, oophorectomy in women aged under 50 was associated with a 50% increased risk of NAFLD.17 Data from cell culture and animal models have established the mechanistic roles of hepatic oestrogen receptors in regulating glucose and lipid homeostasis, as well as hepatic insulin sensitivity in both men and women,18 which likely contributes to age-related increases in NAFLD prevalence and fibrosis severity across sex.18 These basic and translational data are also supported by clinical findings of lower NAFLD prevalence among post-menopausal women receiving menopausal hormone therapy (MHT).19
Fig. 1.
Sex differences in NAFLD.
Left: Characteristics in women. Right: Characteristics in men. HCC, hepatocellular carcinoma; NAFLD, non-alcoholic fatty liver disease; PCOS, polycystic ovarian syndrome.
Interestingly, while oestrogens have been shown to have a protective effect on liver disease in both male and female models of liver disease, androgens have clear sexually dimorphic effects on the presence and severity of NAFLD, including on histologically confirmed NASH.[20], [21], [22], [23] Testosterone is traditionally considered a “male” sex hormone but has well-established sexually dimorphic effects on metabolic health, with higher levels promoting hepatic steatosis, as well as diabetes and dyslipidaemia in women, while opposite effects are apparent in men.21,[23], [24], [25] Data supporting these findings in women initially derived from patients with the common endocrinopathy polycystic ovary syndrome (PCOS), a typically hyperandrogenic condition in which over 50% of patients have imaging-confirmed NAFLD, as well as being at higher risk of NASH and advanced NASH fibrosis.[26], [27], [28], [29] Though 10% of women with PCOS have androgens in the normal range, the hyperandrogenic PCOS phenotype is associated with prevalent NAFLD.30,31 Beyond PCOS, higher testosterone levels within the normal range are also associated with increased risk of prevalent NAFLD in women, independent of comprehensive metabolic risk factors, while higher testosterone levels in younger patients without PCOS have also been shown to be independently associated with NASH severity.21,22 Such findings contrast with the observed increased risk of prevalent NAFLD, as well as increased histologic severity of NASH, associated with lower testosterone levels in men.20,23 Indeed, testosterone replacement in men improves insulin resistance, lipid profiles, and visceral adiposity, supporting a more direct role of testosterone on metabolic risk factors for NAFLD/NASH.32,33 Data from sex-specific animal models align with these clinical observations, including sexually dimorphic effects at the level of the liver in response to androgen receptor activity. This includes androgen receptor-mediated increases in hepatic gluconeogenesis, de novo lipogenesis, and impaired fatty acid oxidation in men/male animal models with low testosterone or impaired androgen receptor activity and in response to high testosterone in women/female animal models.18
Taken together, there is a clear need to recognize the relevance of sex- and gender-specific influences on the natural history of NAFLD, and tailor treatment studies accordingly. To date, few trials have evaluated sex-specific responses to treatment interventions, though differences in lifestyle exposures and lifestyle changes appear to differentially affect men and women. For example, the mainstay of NAFLD management is weight loss, with a goal of 7-10% total body weight loss advised to improve NAFLD histology, though women need at least 10% to achieve similar effects.34 Body composition also differs by sex with women having more subcutaneous fat, less visceral adiposity, and consequently higher insulin sensitivity, with less adipose tissue lipolysis and free fatty acid delivery to the liver. In response to excess dietary sugars and fat, women also tend to have greater insulin sensitivity, less hepatic lipid uptake, and more lipid export out of the liver than men, suggesting a more favourable adaptive response to these dietary insults.18,35
Alcohol-associated liver disease
Approximately 26 million people have cirrhosis related to alcohol-associated liver disease (ALD) globally, and the prevalence of those with decompensated liver disease is increasing.36 Hospital admissions and the burden of ALD have increased over time and to a greater extent in women over the last two decades.37 In general, women are likely more susceptible to the hepatotoxic effects of alcohol, but the prevalence of ALD is higher in men due to increased rates of consumption (Fig. 2).[38], [39], [40] Women do have more accelerated disease progression with ongoing alcohol use than men. For example, there is evidence that it takes 20 years on average for cirrhosis to develop from ALD in women compared to 35 years in men.41 Further, female sex is an independent risk factor for alcohol-associated hepatitis, progression to cirrhosis, liver-related death, and overall mortality in the setting of ALD.42,43 Women also die at earlier ages with a higher risk of mortality than men.41 Women with ALD are also more likely to be de-listed from the liver transplant waitlist than men and less likely to receive a transplant. Importantly, women with ALD are less likely to receive treatment for alcohol use disorder by way of face-to-face visits and medications for relapse prevention.44
Fig. 2.
Sex differences in ALD.
Left: Characteristics in women. Right: Characteristics in men. ADH, alcohol dehydrogenase; ALD, alcohol-associated liver disease; AUD, alcohol use disorder.
The reasons for the increased susceptibility to ALD in women are multifactorial. Women have smaller body mass than men which results in less alcohol dilution. Women also have decreased gastric alcohol dehydrogenase (ADH) activity, which is involved in first-pass alcohol metabolism, resulting in higher blood alcohol concentrations per unit alcohol consumed, independent of body mass.45 Furthermore, chronic alcohol use decreases gastric ADH activity to a greater extent in women than in men, resulting in higher levels of alcohol reaching the liver. Data on hepatic ADH levels are sparse, though one study demonstrated that women also have decreased hepatic ADH activity compared to men.46 While these data explain increased levels of blood alcohol in women compared to men, it is unclear if this affects hepatic fibrosis.
Oestrogen has been shown to increase the sensitivity of Kupffer cells to lipopolysaccharides, which results in inflammation and toxic byproduct production in preclinical models of ALD.47,48 The influence of alcohol on sex hormone expression may also play a role. Data from mouse models have shown increased alcohol-induced expression of oestrogen receptors in hepatocytes of male mice.49 Increased circulating oestrogen levels and hepatic oestrogen receptor expression were associated with improved hepatocyte proliferation in male mice. Alcohol exposure did not significantly alter oestrogen receptor expression in hepatocytes from female mice and hepatocyte apoptosis was more prominent in female mice.49 Recent data using mouse models showed that histone 3 lysine K4-specific demethylase enzymes known as KDM5B and KDM5C were impacted by alcohol exposure in a sex-dependent manner. These demethylases were shown to downregulate aryl hydrocarbon receptor signalling in hepatic stellate cells in female mice, which promoted stellate cell activation and fibrosis, a phenomenon that was not observed in male mice.50 These differences may relate to the role of oestrogen in regulating aryl hydrocarbon receptor-dependent gene expression.51 Data in human tissue are needed to understand whether findings from animal models may contribute to the sex disparities in clinical outcomes observed in ALD.
HBV
The global prevalence of HBV is between 2-7% (Fig. 3). Men are less likely to spontaneously clear HBV infection and are thus more likely to become chronically infected.52 Male sex is also an independent risk factor for HBV-associated complications including being associated with a 2.5x higher risk of cirrhosis, 3-6x higher risk of hepatocellular carcinoma (HCC), and 1.8x higher risk of death.[53], [54], [55] Sex differences in HBV outcomes may relate to interactions of the virus with sex hormones. Androgen activity is increased in the setting of HBV, which stimulates viral transcription, creating a positive feedback loop that results in higher viral replication and oncogenic potential.56 Higher testosterone levels have been associated with an increased HCC risk in patients infected with HBV.57 On the contrary, oestrogen is felt to play a synergistic role in the immune response to HBV, leading to improved viral clearance. CD107a expression by natural killer (NK) cells is considered a marker for degranulation, the process by which cytotoxic granules are released to kill a targeted cell. Degranulation of NK cells is crucial in controlling HBV infection. CD107+ intrahepatic NK cells are more prevalent in women than men, to an extent that correlates directly with oestradiol levels.58 Additionally, oestrogen may hinder HBV transcription by upregulating oestrogen receptors, which interfere with binding of the HBV enhancer.56 The protective effect of oestrogen in HBV is supported by data demonstrating lower risk of HBV-related complications, and HCC in particular, among post-menopausal women on MHT.57 Given sex differences in HCC risk, the American Association for the Study of Liver Diseases supports earlier initiation of HCC screening in Asian men with HBV at age 40 years, vs. age 50 years for Asian women with vertically acquired HBV.59 The European Association for the Study of the Liver and the Asian-Pacific Association for the Study of the Liver society guidelines do not provide HCC screening recommendations by sex, but do advise the use of risk calculators, such as the REACH-B or PAGE-B, that include sex as a variable.60,61
Fig. 3.
Sex differences in chronic viral hepatitis B and C.
Left: Characteristics in women. Right: Characteristics in men. SVR, sustained virologic response; Tx, treatment.
HCV
The global prevalence of chronic HCV is estimated to be 0.7%, with men affected twice as often as women (Fig. 3).62 Similar to HBV, women are more likely to spontaneously clear HCV infection. In addition, women with HCV are more likely to achieve sustained virologic responses with antiviral therapy.63,64 Oestrogen is felt to promote the immune response to HCV. For example, 17 beta-oestradiol has been shown to interfere with the life cycle of HCV through intracellular receptor signalling, inhibiting viral production.65 Further, male sex is an independent risk factor for fibrosis progression in HCV and men develop cirrhosis 10 years earlier than women on average.66,67 Even after direct-acting antiviral treatment with sustained virologic response, men have higher rates of liver-related mortality and need for transplantation than women.68 Similar to other aetiologies, oestrogen is known to have a protective effect on HCV fibrosis. Pre-menopausal women with HCV have decreased fibrosis progression compared to men, a difference that is not observed after menopause.67,69 Decreased fibrosis progression has been demonstrated in post-menopausal women receiving MHT.70 While oestrogens clearly have a protective effect against hepatic fibrosis, once cirrhosis is present, oestrogen levels have been shown to be higher in both men and women, which likely relates to increased peripheral conversion of androgens to oestrogens in the setting of advanced liver disease.[71], [72], [73]
Autoimmune liver diseases
Autoimmune hepatitis
Autoimmune hepatitis (AIH) affects <0.01% of the population, with a female to male ratio of 4 to 1 (Fig. 4).74 Overall fibrosis progression and risk of cirrhosis are similar by sex.75,76 However, men typically present at a younger age and may be more likely to have cirrhosis at the time of diagnosis.75,77 Treatment response and relapse rates are similar.76,78 However the largest study to date (N = 1,318) identified male sex as an independent predictor of increased mortality or need for liver transplant.79 Conversely, a study of 238 patients with biopsy-proven AIH found that women had lower overall survival over a 30-40-year follow-up (p = 0.02), but found no differences in the proportion with liver-related death or need for liver transplant.75 Interestingly, transplant need for AIH has been shown to be predicted by underlying genetic polymorphisms (e.g., human leukocyte antigen [HLA]-DR3, HLA-DR4) rather than sex itself.78 Women are more likely to have HLA-DR4, while men are more likely to have HLA-DR3. HLA-DR3 is associated with higher rates of disease progression and treatment failure compared to HLA-DR4, however, this is independent of sex.76,80 After liver transplant, AIH recurs in 25% of transplant recipients with no differences by sex.81
Fig. 4.
Sex differences in autoimmune and cholestatic liver diseases.
Left: Characteristics in women. Right: Characteristics in men. AILD, autoimmune liver diseases; LT, liver transplant; PBC, primary biliary cholangitis; PSC, primary sclerosing cholangitis; Tx, treatment.
The biology underlying the female predominance of AIH is complex, involving epigenetics, sex-chromosome factors, and hormonal influences on innate immunity.82,83 For example, oestrogen and androgen receptors are expressed on B lymphocytes, whereas CD8+ T lymphocytes, monocytes, neutrophils, and NK cells express oestrogen but not androgen receptors.84 Interestingly, disease quiescence during pregnancy and post-partum flares have been shown to relate to oestrogen-associated shifts in immunity.85 Earlier gestational age reflects a more immune suppressive state that enables the body to “tolerate” the growing fetus. Increased circulating oestrogen contributes to a change in T cell profiles from a predominance of type 1 to type 2 T helper cells, which lessens disease activity. Rising oestrogen levels and associated shifts toward immune activity, particularly in the third trimester and post-partum period, result in more predominant type 1 T helper cell activity and increased risk of AIH flares.85 Testosterone levels are less well studied in AIH, though testosterone reduces type 1 T helper cell activity and may contribute to lower AIH risk in men.86
Primary biliary cholangitis
PBC affects 20-40 per 100,000 adults, with a strong female predominance. Though the number of men diagnosed with PBC is increasing, 90% of cases are observed in women87 (Fig. 4). Of the autoimmune liver diseases, the most robust data on sex differences in presentation and outcomes are available for PBC. In terms of diagnostics, anti-mitochondrial antibody is absent in 15% of women with PBC while anti-mitochondrial antibody negativity typically excludes a diagnosis of PBC in men.88 Women are typically younger and more symptomatic than men at diagnosis. Women report more fatigue and pruritis, which may relate to hormonal differences.89 Pruritis increases with combined hormonal contraceptive pills and during pregnancy,89 which likely relates to oestrogenic effects on bile acid secretion. Women with PBC are also more likely to have other concomitant autoimmune diseases.90
At presentation, men often have higher bilirubin levels and more clinically significant liver disease, including cirrhosis, portal hypertension, and decompensation.91,92 In terms of treatment, men are less likely to respond to first-line treatment with ursodeoxycholic acid.93,94 To date, there are no available data regarding sex differences in response to obeticholic acid, a second-line agent. Men with PBC have increased pre-transplant mortality and are at a 3-fold greater risk of HCC.94 It is recommended to screen for HCC every 6 months regardless of fibrosis stage, while HCC screening is only indicated in women with advanced fibrosis.87
Liver transplant for PBC is now primarily reserved for those presenting with decompensated cirrhosis, HCC, and treatment non-responders with progression to advanced disease.93,95 Male sex is an independent risk factor for liver transplant, likely due to decreased responses to ursodiol and the increased risk of HCC.94 After liver transplant, Recurrent PBC occurs in 20-30% of patients within the first 10 years of transplant,87 with no sex differences in recurrence rate.96,97
The origins of sex differences in PBC are also multifactorial and include genetic, epigenetic, hormonal, and immune differences. An increased rate of X monosomy has been observed in female patients with PBC.82 Increased X monosomy in PBC and other autoimmune diseases suggests that haploinsufficiency of genes located on the X chromosome are related to disease pathogenesis. This has been supported by data in males showing that PBC is associated with loss of the Y chromosome in peripheral immune cells.82
Further, a preclinical model of PBC has demonstrated different levels of interferon type I and II activity in the livers of female compared to male mice. Interferons are proposed to increase the inflammatory response in the liver and occur more often in female mice due to increased interferon activity.98 The same research group completed a follow-up experiment where they knocked out the gene responsible for interferon-gamma and found that liver pathology was reduced and sex differences dissipated in the absence of type 1 interferon signalling.99
Oestrogen-related signalling of cytokines may promote activity and inflammation.99 Oestrogen receptors within the biliary epithelium of patients with PBC have been linked to increased inflammation through IL-6-mediated mechanisms that contribute to the progression of autoimmune liver diseases, specifically PBC and AIH.100 Conversely, testosterone has been shown to reduce cholangiocyte inflammation.101 Testosterone treatment has been shown to suppress liver inflammation in female mice while testosterone deprivation increased inflammation in male nice via IL-17-mediated pathways.101 At present, the causal role of either oestrogens or androgens on PBC-specific disease progression in men and women is not clear.
Primary sclerosing cholangitis
The prevalence of primary sclerosing cholangitis (PSC) varies worldwide and ranges from 6-16 per 100,000 adults102 (Fig. 4). PSC is unique amongst the autoimmune liver diseases in having a male predominance, with 60-70% of cases diagnosed in men.103,104 Historically the age of onset was considered to be similar in men and women,96 however more recent data suggest that women tend to be older at diagnosis, and more likely to have late-onset disease.105,106 Phenotypically, women are more likely to have small duct PSC, which is protective against cholangiocarcinoma risk, while men are more likely to have large duct PSC.105 Up to 80% of patients with PSC have concurrent inflammatory bowel disease, typically ulcerative colitis, which is associated with large duct PSC and male sex.107,108 Though smaller studies report similar survival between men and women,103,109 the largest study to date (N = 7,121) evaluated PSC progression over 30 years of follow-up and found female sex to be protective against liver fibrosis, with women having greater transplant-free survival.105 Severe complications of PSC, including cholangiocarcinoma and colorectal cancer, are less common in women and likely contribute to sex differences in survival.105,110 In terms of liver transplant for PSC, 70% of waitlist candidates are men.111 After transplantation, recurrent PSC occurs in 15-20% of recipients, with recent data suggesting that men are at greater risk of graft rejection and recurrent disease.111,112
Hereditary haemochromatosis
Hereditary haemochromatosis (HH) is a disorder of iron homeostasis that predominantly affects Caucasian individuals or those of Northern European descent, with a prevalence of about 1 in 200-300.113,114 The clinical manifestations of HH vary widely by the number (homozygous or heterozygous) and type of mutant alleles. The two most common pathogenic mutations are C282Y and H63D, though other mutations have less well-defined clinical significance.113,115 In terms of genotype, HH affects men and women equally. However, mouse models have shown that some HH phenotypes have reduced penetrance in females.116 As such, men with HH have higher serum ferritin and experience clinically significant iron overload more often than women.113,117 These differences are attributed to the protective effects of menses in reproductive age women, with regular blood loss via menses reducing risk of iron overload.114 In accordance with this, men with HH have higher rates of liver injury, fibrosis, cirrhosis, and HCC.115,118,119 In a large study comparing pathogenic to non-pathogenic HFE alleles, pathogenic alleles were associated with increased all-cause mortality in men but not women.119 This study also found that men with C282Y heterozygosity were at higher risk of liver injury due to alcohol than women. Finally, men with HH are transplanted more often than women.120 To our knowledge, there are no studies evaluating sex differences in post-transplant outcomes for HH.
Benign liver lesions
Haemangiomas and focal nodular hyperplasia
Liver haemangiomas and focal nodular hyperplasia (FNH) are the first and second most common benign liver lesions, respectively. Both have a strong female predominance with women accounting for 75-90% of cases.121,122 Limited reports have commented on the increased size of these lesions with oestrogen exposure.123,124 One study on haemangioma demonstrated increasing size in pre-menopausal women and men, but a decrease in size in post-menopausal women.125 However, in general, these lesions are not considered to be oestrogen respo-nsive and combined hormonal contraception is considered safe in this population.123 For FNH, there are no data to suggest sex-specific differences in FNH progression or outcomes,122 with surgical intervention reserved for the rare cases of symptomatic abdominal pain. For haemangioma management, liver transplant may be necessary for large and symptomatic lesions that are not amenable to hepatic resection, which is more common in women, reflecting the higher overall prevalence of the disease in women.126,127
Hepatocellular adenomas
Hepatocellular adenomas (HCAs) are 10-fold more common in women than men.123 Development and growth is promoted by oestrogen, as demonstrated by their association with combined hormonal contraceptive use and growth during pregnancy.128 Initial management, regardless of size, is cessation of exogenous oestrogens. Although HCAs express progesterone receptors, progestin-only contraception does not appear to promote HCA growth and can be used as an alternative to oestrogen-containing contraception in women with HCAs.123,129 Given the marked increase in circulating oestrogen levels during pregnancy, HCA growth is common. For lesions of ≥5 cm, intervention by way of embolization or resection can be considered, and monitoring of HCA in pregnancy should include a liver ultrasound per trimester and in the initial post-partum period.123
While HCAs are more common in women, the incidence is rising in both men and women. These epidemiologic trends mirror the increasing rates of obesity and metabolic syndrome, with adipose tissue representing a key site of oestrogen production.130 Weight loss is now a key approach to HCAs, with a recent retrospective study showing that at least 5% weight loss was associated with regression of HCA in female patients with recently discontinued combine hormonal contraception.130 A small case series also demonstrated HCA regression following bariatric surgery.131 Ultimately, weight loss should be prioritized in both men and women with HCAs.
In contrast to women, men have a 10-fold higher risk of malignant transformation,132 which is in part due to their higher risk of being affected by the β-catenin-activated subtype. A careful history should be taken in men for distinct hormonal risk factors that promote HCA development and growth, namely anabolic steroid use.133,134 Management in men, regardless of β-catenin positivity or size, includes resection or embolization due to their high risk of developing HCC.135,136 Liver transplant is reserved for men and women whose disease cannot be managed by embolization or resection. Consistent with the higher HCA prevalence in women, nearly 80% of waitlisted or transplanted patients with HCAs are female, although male sex is associated with worse post-transplant outcomes.137
Polycystic liver disease
Polycystic liver disease (PCLD) is a genetically inherited liver disease that is characterized by progressive cyst development and growth, affecting about one in one million adults.138 The autosomal dominant inheritance pattern of PCLD should confer similar risk to men and women,139 though data from clinical studies are conflicting. One study of 134 patients found similar incidence in men and women,140 while other data suggest a female predominance with a female to male ratio of 6 to 1 139. This discrepancy may relate to more sporadic lesions and clinically apparent disease in women. Female patients do have more symptomatic disease, likely due to the effects of oestrogen on cyst growth.141,142 The effects of oestrogen on PCLD contrast with its general lack of influence on the growth of simple hepatic cysts outside the context of PCLD. Thus, exogenous oestrogen use should be avoided in women with PCLD. Women with PCLD are also transplanted more often and have better post-transplant outcomes than men. In a recent study of adults transplanted for PCLD over a 20-year period, post-transplant mortality was 46% lower in women than men.143
Liver transplantation
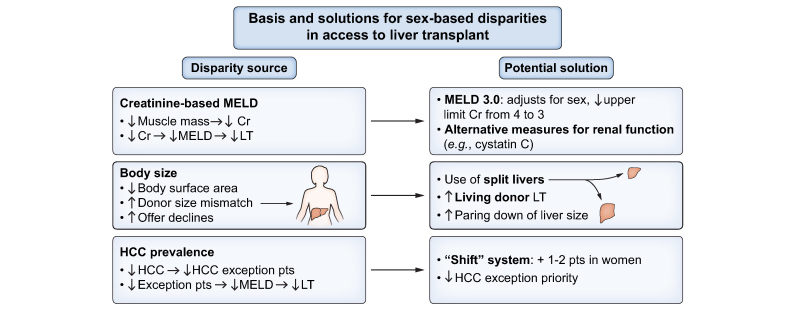
Sex disparities in access and outcomes in liver transplantation are well established (Fig. 5). Men account for 60% of liver transplant waitlist registries and almost two-thirds of liver transplants.144 Women experience more waitlist mortality, and are 20% less likely to undergo liver transplant.144 This disparity is multifactorial. First, the model for end-stage liver disease (MELD)-based allocation system does not accurately capture degree of renal dysfunction in women, as this system utilizes creatinine levels which are naturally lower in women due to lower muscle mass than men.145 Smaller height/body size in women also results in increased organ declines due to size mismatch, as deceased donors are more commonly men, with inherently larger organ size.144,146 Differential priority for HCC MELD exception points is also relevant as the prevalence of HCC is higher in men, who are thus more likely to access organs through this priority pathway.146,147
Fig. 5.
Basis and solutions for sex-based disparities in access to liver transplantation.
Cr, creatinine; HCC, hepatocellular carcinoma; LT, liver transplant(ation); MELD, model for end-stage liver disease; pts, points.
There are several proposed solutions to improving sex disparities in liver transplant access and waitlist mortality. Regarding body mass, options include increased use of split livers and paediatric livers (the latter after being declined by paediatric patients), and surgical paring down of larger livers to accommodate smaller recipients rather than declining these organs and waiting for smaller, size-appropriate organs to become available.145 Early discussion of living donor transplant is also critical to broadening pathways to transplant, including referral of patients with potential donors to centres that perform these surgeries if living donor transplantation is not available at the patient’s existing transplant centre. To address sex disparities in creatinine levels, use of glomerular filtration rate has been shown to help improve waitlist mortality while gender neutral biomarkers such as cystatin C may better capture true renal function in both men and women.145,148 Further, a variety of measures to improve the allocation system have been studied including using a shift system where women have 1-2 points added to their MELD score.149 Most notably, MELD 3.0 is an updated version of the current MELD system that has been proposed to address sex-based disparities in organ allocation by including female sex as a component, while capping serum creatinine at 3.0 mg/dl. Studies evaluating MELD 3.0 have predicted it would address much of the sex disparity in liver transplant access and reduce disparities in waitlist mortality for women.150
Conclusion
In summary, the epidemiology of many chronic liver diseases differs in men and women, which reflects sex-based differences in innate immunity, metabolism, and endogenous hormones, as well as epigenetic factors including exogenous hormone exposures. While most clinical trial data report composite outcomes in men and women, there is a need for sex-based stratification of treatment response and side effects to help tailor our therapeutic approach in men and women. A growing and robust basic science literature has shed light on potential mechanistic pathways underling sex disparities in liver disease. Evolving translational data using sex-stratified human tissue and clinical models are needed to understand whether such data can inform treatment approaches in humans with liver disease. Finally, there remains a gap in liver-related research among transgender populations, in whom the influence of gender-affirming hormone therapies on chronic liver disease needs to be determined, with the need to consider dedicated representation of this population in emerging clinical trials and epidemiologic studies.
Financial support
MS receives grant support from the NIDDK (DK111944/DK131238). DD receives grant support from the NIAAA (AA017986).
Authors’ contributions
KC: Drafting of manuscript, initial figure design and editing. MD: Drafting of manuscript. DD: Drafting of manuscript. MS: Drafting of manuscript, final manuscript review, editing and approval.
Conflict of interest
MS receives grant support from Zydus pharmaceuticals and GSK.
Please refer to the accompanying ICMJE disclosure forms for further details.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2023.100870.
Supplementary data
The following are the supplementary data to this article.
References
- 1.Xu L., Yuan Y., Che Z., Tan X., Wu B., Wang C., et al. The hepatoprotective and hepatotoxic roles of sex and sex-related hormones. Front Immunol. 2022:3521. doi: 10.3389/fimmu.2022.939631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ozdemir B.H., Ozgun G., Akdur A., Moray G., Haberal M. Hepatic estrogen receptor expression prevents liver fibrosis through decreasing the risk of early activation of hepatic stellate cells. Transplantation. 2018;102:S385. [Google Scholar]
- 3.Yasuda M., Shimizu I., Shiba M., Ito S. Suppressive effects of estradiol on dimethylnitrosamine-induced fibrosis of the liver in rats. Hepatology. 1999;29:719–727. doi: 10.1002/hep.510290307. [DOI] [PubMed] [Google Scholar]
- 4.Wang Y., Wu C., Zhou J., Fang H., Wang J. Overexpression of estrogen receptor β inhibits cellular functions of human hepatic stellate cells and promotes the anti-fibrosis effect of calycosin via inhibiting STAT3 phosphorylation. BMC Pharmacol Toxicol. 2022;23:77. doi: 10.1186/s40360-022-00617-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi E., Kim W., Joo S.K., Park S., Park J.H., Kang Y.K., et al. Expression patterns of STAT3, ERK and estrogen-receptor α are associated with development and histologic severity of hepatic steatosis: a retrospective study. Diagn Pathol. 2018;13:23. doi: 10.1186/s13000-018-0698-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang Y., Wu L., Wang Y., Zhang M., Li L., Zhu D., et al. Protective role of estrogen-induced miRNA-29 expression in carbon tetrachloride-induced mouse liver injury. J Biol Chem. 2012;287:14851–14862. doi: 10.1074/jbc.M111.314922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sarkar M., Dodge J.L., Greenblatt R.M., Kuniholm M.H., DeHovitz J., Plankey M., et al. Reproductive aging and hepatic fibrosis progression in human immunodeficiency virus/hepatitis C virus–coinfected women. Clin Infect Dis. 2017;65:1695–1702. doi: 10.1093/cid/cix643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paik J.M., Kabbara K., Eberly K.E., Younossi Y., Henry L., Younossi Z.M. Global burden of NAFLD and chronic liver disease among adolescents and young adults. Hepatology. 2022;75:1204–1217. doi: 10.1002/hep.32228. [DOI] [PubMed] [Google Scholar]
- 9.Lonardo A., Nascimbeni F., Ballestri S., Fairweather D., Win S., Than T.A., et al. Sex differences in nonalcoholic fatty liver disease: state of the art and identification of research gaps. Hepatology. 2019;70:1457–1469. doi: 10.1002/hep.30626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou Y.J., Li Y.Y., Nie Y.Q., Ma J.X., Lu L.G., Shi S.L., et al. Prevalence of fatty liver disease and its risk factors in the population of South China. World J Gastroenterol. 2007;13:6419–6424. doi: 10.3748/wjg.v13.i47.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Z., Xu M., Hu Z., Hultström M., Lai E. Sex-specific prevalence of fatty liver disease and associated metabolic factors in Wuhan, south central China. Eur J Gastroenterol Hepatol. 2014;26:1015–1021. doi: 10.1097/MEG.0000000000000151. [DOI] [PubMed] [Google Scholar]
- 12.Balakrishnan M., Patel P., Dunn-Valadez S., Dao C., Khan V., Ali H., et al. Women have a lower risk of nonalcoholic fatty liver disease but a higher risk of progression vs men: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2021;19:61–71.e15. doi: 10.1016/j.cgh.2020.04.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yuan L., Kardashian A., Sarkar M. NAFLD in women: unique pathways, biomarkers and therapeutic opportunities. Curr Hepatol Rep. 2019;18:425–432. doi: 10.1007/s11901-019-00495-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Noureddin M., Vipani A., Bresee C., Todo T., Kim I.K., Alkhouri N., et al. NASH leading cause of liver transplant in women: updated analysis of indications for liver transplant and ethnic and gender variances. Am J Gastroenterol. 2018;113:1649. doi: 10.1038/s41395-018-0088-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang B., Zhang C.G., Ji L.H., Zhao G., Wu Z.Y. Estrogen receptor beta selective agonist ameliorates liver cirrhosis in rats by inhibiting the activation and proliferation of hepatic stellate cells. J Gastroenterol Hepatol. 2018;33:747–755. doi: 10.1111/jgh.13976. [DOI] [PubMed] [Google Scholar]
- 16.Klair J.S., Yang J.D., Abdelmalek M.F., Guy C.D., Gill R.M., Yates K., et al. A longer duration of estrogen deficiency increases fibrosis risk among postmenopausal women with nonalcoholic fatty liver disease. Hepatology. 2016;64:85–91. doi: 10.1002/hep.28514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Florio A.A., Graubard B.I., Yang B., Thistle J.E., Bradley M.C., McGlynn K., et al. Oophorectomy and risk of non-alcoholic fatty liver disease and primary liver cancer in the Clinical Practice Research Datalink. Eur J Epidemiol. 2019;34(9):871–878. doi: 10.1007/s10654-019-00526-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Della Torre S. Beyond the X Factor: relevance of sex hormones in NAFLD pathophysiology. Cells. 2021:10. doi: 10.3390/cells10092502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clark J.M., Brancati F.L., Diehl A.M. Nonalcoholic fatty liver disease. Gastroenterology. 2002;122:1649–1657. doi: 10.1053/gast.2002.33573. [DOI] [PubMed] [Google Scholar]
- 20.Sarkar M., Yates K., Suzuki A., Lavine J., Gill R., Ziegler T., et al. Low testosterone is associated with nonalcoholic steatohepatitis (NASH) and severity of NASH fibrosis in men with NAFLD. Clin Gastroenterol Hepatol. 2021;19(2):400–402.e2. doi: 10.1016/j.cgh.2019.11.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sarkar M., Wellons M., Cedars M.I., VanWagnder L., Gunderson E.P., Ajmera V., et al. Testosterone levels in pre-menopausal women are associated with nonalcoholic fatty liver disease in midlife. Am J Gastroenterol. 2017;112:755–762. doi: 10.1038/ajg.2017.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sarkar M.A., Suzuki A., Abdelmalek M.F., Yates K.P., Wilson L.A., Bass N.M., et al. Testosterone is associated with nonalcoholic steatohepatitis and fibrosis in premenopausal women with NAFLD. Clin Gastroenterol Hepatol. 2021;19:1267–1274.e1. doi: 10.1016/j.cgh.2020.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jaruvongvanich V., Sanguankeo A., Riangwiwat T., Upala S. Testosterone, sex hormone-binding globulin and nonalcoholic fatty liver disease: a systematic review and meta-analysis. Ann Hepatol. 2017;16:382–394. doi: 10.5604/16652681.1235481. [DOI] [PubMed] [Google Scholar]
- 24.Schiffer L., Kempegowda P., Arlt W., O’Reilly M.W. Mechanisms in endocrinology: the sexually dimorphic role of androgens in human metabolic disease. Eur J Endocrinol. 2017;177:R125–R143. doi: 10.1530/EJE-17-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Navarro G., Allard C., Xu W., Mauvais-Jarvis F. The role of androgens in metabolism, obesity, and diabetes in males and females. Obesity (Silver Spring) 2015;23:713–719. doi: 10.1002/oby.21033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanchez-Garrido M.A., Tena-Sempere M. Metabolic dysfunction in polycystic ovary syndrome: pathogenic role of androgen excess and potential therapeutic strategies. Mol Metab. 2020;35 doi: 10.1016/j.molmet.2020.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salva-Pastor N., Lopez-Sanchez G.N., Chavez-Tapia N.C., Audifred-Salomón J.R., Niebla-Cárdenas D., Topete-Estrada R., et al. Polycystic ovary syndrome with feasible equivalence to overweight as a risk factor for non-alcoholic fatty liver disease development and severity in Mexican population. Ann Hepatol. 2020;19:251–257. doi: 10.1016/j.aohep.2020.01.004. [DOI] [PubMed] [Google Scholar]
- 28.Macut D., Tziomalos K., Bozic-Antic I., Bjekić-Macut J., Katsikis I., Papadakis E., et al. Non-alcoholic fatty liver disease is associated with insulin resistance and lipid accumulation product in women with polycystic ovary syndrome. Hum Reprod. 2016;31:1347–1353. doi: 10.1093/humrep/dew076. [DOI] [PubMed] [Google Scholar]
- 29.Maldonado S.S., Grab J., Wang C.W., Huddleston H., Cedars M., Sarkar M. Polycystic ovary syndrome is associated with nonalcoholic steatohepatitis in women of reproductive age. Hepatol Commun. 2022;6:2634–2639. doi: 10.1002/hep4.2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumarendran B., O’Reilly M.W., Manolopoulos K.N., Toulis K.A., Gokhale K.M., Sitch A.J., et al. Polycystic ovary syndrome, androgen excess, and the risk of nonalcoholic fatty liver disease in women: a longitudinal study based on a United Kingdom primary care database. Plos Med. 2018;15 doi: 10.1371/journal.pmed.1002542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sarkar M., Terrault N., Chan W., Cedars M.I., Huddleston H.G., Duwaerts C.C., et al. Polycystic ovary syndrome (PCOS) is associated with NASH severity and advanced fibrosis. Liver Int. 2020;40:355–359. doi: 10.1111/liv.14279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dhindsa S., Ghanim H., Batra M., Kuhadiya N.D., Abuaysheh S., Sandhu S., et al. Insulin resistance and inflammation in hypogonadotropic hypogonadism and their reduction after testosterone replacement in men with type 2 diabetes. Diabetes Care. 2016;39:82–91. doi: 10.2337/dc15-1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kapoor D., Goodwin E., Channer K.S., Jones T.H. Testosterone replacement therapy improves insulin resistance, glycaemic control, visceral adiposity and hypercholesterolaemia in hypogonadal men with type 2 diabetes. Eur J Endocrinol. 2006;154:899–906. doi: 10.1530/eje.1.02166. [DOI] [PubMed] [Google Scholar]
- 34.Vilar-Gomez E., Martinez-Perez Y., Calzadilla-Bertot L., Torres-Gonzalez A., Gra-Oramas B., Gonzalez-Fabian L., et al. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology. 2015;149:367–378. doi: 10.1053/j.gastro.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 35.Tramunt B., Smati S., Grandgeorge N., Lenfant F., Arnal J.F., Montagner A., et al. Sex differences in metabolic regulation and diabetes susceptibility. Diabetologia. 2020;63:453–461. doi: 10.1007/s00125-019-05040-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2020;5:245–266. doi: 10.1016/S2468-1253(19)30349-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bertha M., Shedden K., Mellinger J. Trends in the inpatient burden of alcohol-related liver disease among women hospitalized in the United States. Liver Int. 2022 doi: 10.1111/liv.15277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilsnack R.W., Wilsnack S.C., Kristjanson A.F., Vogeltanz-Holm N.D., Gmel G. Gender and alcohol consumption: patterns from the multinational GENACIS project. Addiction. 2009;104(9):1487–1500. doi: 10.1111/j.1360-0443.2009.02696.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rehm J., Samokhvalov A.V., Shield K.D. Global burden of alcoholic liver diseases. J Hepatol. 2013;59:160–168. doi: 10.1016/j.jhep.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 40.Becker U., Deis A., Sørensen T.I., Gronbaek M., Borch-Johnsen K., Muller C.F., et al. Prediction of risk of liver disease by alcohol intake, sex, and age: a prospective population study. Hepatology. 1996;23:1025–1029. doi: 10.1002/hep.510230513. [DOI] [PubMed] [Google Scholar]
- 41.Poynard T., Mathurin P., Lai C.L., Guyader D., Poupon R., Tainturier M.H., et al. A comparison of fibrosis progression in chronic liver diseases. J Hepatol. 2003;38:257–265. doi: 10.1016/s0168-8278(02)00413-0. [DOI] [PubMed] [Google Scholar]
- 42.Lackner C., Spindelboeck W., Haybaeck J., Douschan P., Rainer F., Terracciano L., et al. Histological parameters and alcohol abstinence determine long-term prognosis in patients with alcoholic liver disease. J Hepatol. 2017;66:610–618. doi: 10.1016/j.jhep.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 43.Naveau S., Giraud V., Borotto E., Aubert A., Capron F., Chaput J. Excess weight risk factor for alcoholic liver disease. Hepatology. 1997;25:108–111. doi: 10.1002/hep.510250120. [DOI] [PubMed] [Google Scholar]
- 44.Mellinger J.L., Fernandez A., Shedden K., Winder G.S., Fontana R.J., Volk M.L., et al. Gender disparities in alcohol use disorder treatment among privately insured patients with alcohol-associated cirrhosis. Alcohol Clin Exp Res. 2019;43:334–341. doi: 10.1111/acer.13944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Frezza M., di Padova C., Pozzato G., Terpin M., Baraona E., Lieber C.S. High blood alcohol levels in women. The role of decreased gastric alcohol dehydrogenase activity and first-pass metabolism. N Engl J Med. 1990;322:95–99. doi: 10.1056/NEJM199001113220205. [DOI] [PubMed] [Google Scholar]
- 46.Chrostek L., Jelski W., Szmitkowski M., Puchalski Z. Gender-related differences in hepatic activity of alcohol dehydrogenase isoenzymes and aldehyde dehydrogenase in humans. J Clin Lab Anal. 2003;17:93–96. doi: 10.1002/jcla.10076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Järveläinen H., Lukkari T., Heinaro S., Sippel H., Lindros K.O. The antiestrogen toremifene protects against alcoholic liver injury in female rats. J Hepatol. 2001;35:46–52. doi: 10.1016/s0168-8278(01)00050-2. [DOI] [PubMed] [Google Scholar]
- 48.Ikejima K., Enomoto N., Iimuro Y., Ikejima A., Fang D., Xu J., et al. Estrogen increases sensitivity of hepatic Kupffer cells to endotoxin. Am J Physiol. 1998;274:G669–G676. doi: 10.1152/ajpgi.1998.274.4.G669. [DOI] [PubMed] [Google Scholar]
- 49.Colantoni A., Emanuele M.A., Kovacs E.J., Villa E., Van Thiel D.H. Hepatic estrogen receptors and alcohol intake. Mol Cell Endocrinol. 2002;193:101–104. doi: 10.1016/s0303-7207(02)00102-8. [DOI] [PubMed] [Google Scholar]
- 50.Schonfeld M., Averilla J., Gunewardena S., Weinman S.A., Tikhanovich I. Alcohol-associated fibrosis in females is mediated by female-specific activation of lysine demethylases KDM5B and KDM5C. Hepatol Commun. 2022;6:2042–2057. doi: 10.1002/hep4.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ohtake F., Takeyama K.I., Matsumoto T., Kitagawa H., Yamamoto Y., Nohara K., et al. Modulation of oestrogen receptor signalling by association with the activated dioxin receptor. Nature. 2003;423:545–550. doi: 10.1038/nature01606. [DOI] [PubMed] [Google Scholar]
- 52.Terrault N.A., Wahed A.S., Feld J.J., Cooper S.L., Ghany M.G., Lisker-Melman M., et al. Incidence and prediction of HBsAg seroclearance in a prospective multi-ethnic HBeAg-negative chronic hepatitis B cohort. Hepatology. 2022;75:709–723. doi: 10.1002/hep.32231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Iloeje U.H., Yang H.I., Su J., Jen C.L., You S.L., Chen C.J. Predicting cirrhosis risk based on the level of circulating hepatitis B viral load. Gastroenterology. 2006;130:678–686. doi: 10.1053/j.gastro.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 54.Park B.K., Park Y.N., Ahn S.H., Lee K.S., Chon C.Y., Moon Y.M., et al. Long-term outcome of chronic hepatitis B based on histological grade and stage. J Gastroenterol Hepatol. 2007;22:383–388. doi: 10.1111/j.1440-1746.2007.04857.x. [DOI] [PubMed] [Google Scholar]
- 55.Iloeje U.H., Yang H.I., Jen C.L., Su J., Wang L.Y., You S.L., et al. Risk and predictors of mortality associated with chronic hepatitis B infection. Clin Gastroenterol Hepatol. 2007;5:921–931. doi: 10.1016/j.cgh.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 56.Wang S.H., Chen P.J., Yeh S.H. Gender disparity in chronic hepatitis B: mechanisms of sex hormones. J Gastroenterol Hepatol. 2015;30:1237–1245. doi: 10.1111/jgh.12934. [DOI] [PubMed] [Google Scholar]
- 57.Yu M.W., Yang Y.C., Yang S.Y., Cheng S.W., Liaw Y.F., Lin S.M., et al. Hormonal markers and hepatitis B virus-related hepatocellular carcinoma risk: a nested case-control study among men. J Natl Cancer Inst. 2001;93:1644–1651. doi: 10.1093/jnci/93.21.1644. [DOI] [PubMed] [Google Scholar]
- 58.Macek Jilkova Z., Decaens T., Marlu A., Marche H., Jouvin-Marche E., Marche P.N. Sex differences in spontaneous degranulation activity of intrahepatic natural killer cells during chronic hepatitis B: association with estradiol levels. Mediators Inflamm. 2017;2017 doi: 10.1155/2017/3214917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Terrault N.A., Lok A.S.F., McMahon B.J., Chang K.M., Hwang J.P., Jonas M.M., et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67:1560–1599. doi: 10.1002/hep.29800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sarin S.K., Kumar M., Lau G.K., Abbas Z., Chan H.L.Y., Chen C.J., et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. 2016;10:1–98. doi: 10.1007/s12072-015-9675-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370–398. doi: 10.1016/j.jhep.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 62.Global change in hepatitis C virus prevalence and cascade of care between 2015 and 2020: a modelling study. Lancet Gastroenterol Hepatol. 2022;7:396–415. doi: 10.1016/S2468-1253(21)00472-6. [DOI] [PubMed] [Google Scholar]
- 63.Page K., Hahn J.A., Evans J., Shiboski S., Lum P., Delwart E., et al. Acute hepatitis C virus infection in young adult injection drug users: a prospective study of incident infection, resolution, and reinfection. J Infect Dis. 2009;200:1216–1226. doi: 10.1086/605947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Holzmann I., Tovo C.V., Minmé R., Leal M.P., Kliemann M.P., Ubirajara C., et al. Effectiveness of chronic hepatitis C treatment with direct-acting antivirals in the Public Health System in Brazil. Braz J Infect Dis. 2018;22:317–322. doi: 10.1016/j.bjid.2018.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Magri A., Barbaglia M.N., Foglia C.Z., Boccato E., Burlone M.E., Cole S., et al. 17, β-estradiol inhibits hepatitis C virus mainly by interference with the release phase of its life cycle. Liver Int. 2017;37:669–677. doi: 10.1111/liv.13303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Thein H.H., Yi Q., Dore G.J., Krahn M.D. Estimation of stage-specific fibrosis progression rates in chronic hepatitis C virus infection: a meta-analysis and meta-regression. Hepatology. 2008;48:418–431. doi: 10.1002/hep.22375. [DOI] [PubMed] [Google Scholar]
- 67.Poynard T., Bedossa P., Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. The OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups. Lancet. 1997;349:825–832. doi: 10.1016/s0140-6736(96)07642-8. [DOI] [PubMed] [Google Scholar]
- 68.D’Ambrosio R., Degasperi E., Anolli M.P., Fanetti I., Borghi M., Soffredini R., et al. Incidence of liver- and non-liver-related outcomes in patients with HCV-cirrhosis after SVR. J Hepatol. 2022;76:302–310. doi: 10.1016/j.jhep.2021.09.013. [DOI] [PubMed] [Google Scholar]
- 69.Villa E., Vukotic R., Cammà C., Petta S., Di Leo A., Gitto S., et al. Reproductive status is associated with the severity of fibrosis in women with hepatitis C. PLoS One. 2012;7 doi: 10.1371/journal.pone.0044624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Di Martino V., Lebray P., Myers R.P., Paradis V., Charlotte F., et al. Progression of liver fibrosis in women infected with hepatitis C: long-term benefit of estrogen exposure. Hepatology. 2004;40:1426–1433. doi: 10.1002/hep.20463. [DOI] [PubMed] [Google Scholar]
- 71.Sarkar M., Lai J.C., Sawinski D., Zeigler T.E., Cedars M., Forde K.A. Sex hormone levels by presence and severity of cirrhosis in women with chronic hepatitis C virus infection. J Viral Hepat. 2019;26:258–262. doi: 10.1111/jvh.13027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.White D.L., Tavakoli-Tabasi S., Kuzniarek J., Pascua R., Ramsey D.J., El-Serag H.B. Higher serum testosterone is associated with increased risk of advanced hepatitis C-related liver disease in males. Hepatology. 2012;55:759–768. doi: 10.1002/hep.24618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tanaka K., Sakai H., Hashizume M., Hirohata T. Serum testosterone: estradiol ratio and the development of hepatocellular carcinoma among male cirrhotic patients. Cancer Res. 2000;60:5106–5110. [PubMed] [Google Scholar]
- 74.Mack C.L., Adams D., Assis D.N., Kerkar N., Manns M.P., Mayo M.J., et al. Diagnosis and management of autoimmune hepatitis in adults and children: 2019 practice guidance and guidelines from the American Association for the Study of Liver Diseases. Hepatology. 2020;72:671–722. doi: 10.1002/hep.31065. [DOI] [PubMed] [Google Scholar]
- 75.Al-Chalabi T., Underhill J.A., Portmann B.C., McFarlane I.G., Heneghan M.A. Impact of gender on the long-term outcome and survival of patients with autoimmune hepatitis. J Hepatol. 2008;48:140–147. doi: 10.1016/j.jhep.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 76.Kirstein M.M., Metzler F., Geiger E., Heinrich E., Hallensleben M., Manns M.P., et al. Prediction of short- and long-term outcome in patients with autoimmune hepatitis. Hepatology. 2015;62:1524–1535. doi: 10.1002/hep.27983. [DOI] [PubMed] [Google Scholar]
- 77.Ngu J.H., Gearry R.B., Frampton C.M., Stedman C.A. Predictors of poor outcome in patients w ith autoimmune hepatitis: a population-based study. Hepatology. 2013;57:2399–2406. doi: 10.1002/hep.26290. [DOI] [PubMed] [Google Scholar]
- 78.Czaja A.J., Donaldson P.T. Gender effects and synergisms with histocompatibility leukocyte antigens in type 1 autoimmune hepatitis. Am J Gastroenterol. 2002;97:2051–2057. doi: 10.1111/j.1572-0241.2002.05921.x. [DOI] [PubMed] [Google Scholar]
- 79.Grønbæk L., Vilstrup H., Jepsen P. Autoimmune hepatitis in Denmark: incidence, prevalence, prognosis, and causes of death. A nationwide registry-based cohort study. J Hepatol. 2014;60:612–617. doi: 10.1016/j.jhep.2013.10.020. [DOI] [PubMed] [Google Scholar]
- 80.Buzzetti E., Parikh P.M., Gerussi A., Tsochatzis E. Gender differences in liver disease and the drug-dose gender gap. Pharmacol Res. 2017;120:97–108. doi: 10.1016/j.phrs.2017.03.014. [DOI] [PubMed] [Google Scholar]
- 81.Montano-Loza A.J., Mason A.L., Ma M., Bastiampillai R.J., Bain V.G., Tandon P. Risk factors for recurrence of autoimmune hepatitis after liver transplantation. Liver Transplant. 2009;15:1254–1261. doi: 10.1002/lt.21796. [DOI] [PubMed] [Google Scholar]
- 82.Schwinge D., Schramm C. Seminars in immunopathology. Springer; 2019. Sex-related factors in autoimmune liver diseases. [DOI] [PubMed] [Google Scholar]
- 83.Invernizzi F., Cilla M., Trapani S., Guarino M., Cossiga V., Gambato M., et al. Gender and autoimmune liver diseases: relevant aspects in clinical practice. J Personalized Med. 2022;12:925. doi: 10.3390/jpm12060925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rubtsov A.V., Rubtsova K., Kappler J.W., Marrack P. Genetic and hormonal factors in female-biased autoimmunity. Autoimmun Rev. 2010;9:494–498. doi: 10.1016/j.autrev.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bozward A.G., Wootton G.E., Podstawka O., Oo Y.H. Autoimmune hepatitis: tolerogenic immunological state during pregnancy and immune escape in post-partum. Front Immunol. 2020;11:591380. doi: 10.3389/fimmu.2020.591380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Henze L., Schwinge D., Schramm C. The effects of androgens on T cells: clues to female predominance in autoimmune liver diseases? Front Immunol. 2020;11:1567. doi: 10.3389/fimmu.2020.01567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lindor K.D., Bowlus C.L., Boyer J., Levy C., Mayo M. Primary biliary cholangitis: 2018 practice guidance from the American association for the study of liver diseases. Hepatology. 2019;69:394–419. doi: 10.1002/hep.30145. [DOI] [PubMed] [Google Scholar]
- 88.Zakharia K., Robles J., Rasor M., He X., Altamimi B.A., Murali A., et al. 2793 primary biliary cholangitis (PBC): any differences between males and females? Official J Am Coll Gastroenterol ACG. 2019;114:S1543. [Google Scholar]
- 89.Smyk D.S., Rigopoulou E.I., Pares A., Billinis C., Burroughs A.K., Muratori L., et al. Sex differences associated with primary biliary cirrhosis. Clin Dev Immunol. 2012;2012:610504. doi: 10.1155/2012/610504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Marzioni M., Bassanelli C., Ripellino C., Urbinati D., Alvaro D. Epidemiology of primary biliary cholangitis in Italy: evidence from a real-world database. Dig Liver Dis. 2019;51:724–729. doi: 10.1016/j.dld.2018.11.008. [DOI] [PubMed] [Google Scholar]
- 91.Cheung A.C., Lammers W.J., Perez C.F.M., van Buuren H.R., Gulamhusein A., Trivedi P.J., et al. Effects of age and sex of response to ursodeoxycholic acid and transplant-free survival in patients with primary biliary cholangitis. Clin Gastroenterol Hepatol. 2019;17:2076–2084.e2. doi: 10.1016/j.cgh.2018.12.028. [DOI] [PubMed] [Google Scholar]
- 92.Adejumo A.C., Akhtar D.H., Dennis B.B., Cholankeril G., Alayo Q., Ogundipe O.A., et al. Gender and racial differences in hospitalizations for primary biliary cholangitis in the USA. Dig Dis Sci. 2021;66:1461–1476. doi: 10.1007/s10620-020-06402-3. [DOI] [PubMed] [Google Scholar]
- 93.Carbone M., Mells G.F., Pells G., Dawwas M.F., Newton J.L., Heneghan M.A., et al. Sex and age are determinants of the clinical phenotype of primary biliary cirrhosis and response to ursodeoxycholic acid. Gastroenterology. 2013;144:560–569.e7. doi: 10.1053/j.gastro.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 94.John B.V., Aitcheson G., Schwartz K.B., Khakoo N.S., Dahman B., Deng Y., et al. Male sex is associated with higher rates of liver-related mortality in primary biliary cholangitis and cirrhosis. Hepatology. 2021;74:879–891. doi: 10.1002/hep.31776. [DOI] [PubMed] [Google Scholar]
- 95.Aguilar M.T., Carey E.J. Current status of liver transplantation for primary biliary cholangitis. Clin Liver Dis. 2018;22:613–624. doi: 10.1016/j.cld.2018.03.011. [DOI] [PubMed] [Google Scholar]
- 96.Sarkar M., Watt K.D., Terrault N., Berenguer M. Outcomes in liver transplantation: does sex matter? J Hepatol. 2015;62:946–955. doi: 10.1016/j.jhep.2014.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Montano-Loza A.J., Hansen B.E., Corpechot C., Roccarina D., Thorburn D., Trivedi P., et al. Factors associated with recurrence of primary biliary cholangitis after liver transplantation and effects on graft and patient survival. Gastroenterology. 2019;156:96–107.e1. doi: 10.1053/j.gastro.2018.10.001. [DOI] [PubMed] [Google Scholar]
- 98.Bae H.R., Leung P.S., Tsuneyama K., Valencia J.C., Hodge D.L., Kim S., et al. Chronic expression of interferon-gamma leads to murine autoimmune cholangitis with a female predominance. Hepatology. 2016;64:1189–1201. doi: 10.1002/hep.28641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bae H.R., Hodge D.L., Yang G.X., Leung P.S., Chodisetti S.B., Valencia J.C., et al. The interplay of type I and type II interferons in murine autoimmune cholangitis as a basis for sex-biased autoimmunity. Hepatology. 2018;67:1408–1419. doi: 10.1002/hep.29524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Isse K., Specht S.M., Lunz J.G., III, Kang L.I., Mizuguchi Y., Demetris A.J. Estrogen stimulates female biliary epithelial cell interleukin-6 expression in mice and humans. Hepatology. 2010;51:869–880. doi: 10.1002/hep.23386. [DOI] [PubMed] [Google Scholar]
- 101.Schwinge D., Carambia A., Quaas A., Krech T., Wegscheid C., Tiegs G., et al. Testosterone suppresses hepatic inflammation by the downregulation of IL-17, CXCL-9, and CXCL-10 in a mouse model of experimental acute cholangitis. J Immunol. 2015;194:2522–2530. doi: 10.4049/jimmunol.1400076. [DOI] [PubMed] [Google Scholar]
- 102.Bowlus C.L., Arrivé L., Bergquist A., Deneau M., Forman L., Ilyas S., et al. AASLD practice guidance on primary sclerosing cholangitis and cholangiocarcinoma. Hepatology; Baltimore, Md: 2022. [DOI] [PubMed] [Google Scholar]
- 103.Carbone M., Kodra Y., Rocchetti A., Manno V., Minelli G., Gerussi A., et al. Primary sclerosing cholangitis: burden of disease and mortality using data from the national rare diseases registry in Italy. Int J Environ Res Public Health. 2020;17:3095. doi: 10.3390/ijerph17093095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lindkvist B., Benito de Valle M., Gullberg B., Björnsson E. Incidence and prevalence of primary sclerosing cholangitis in a defined adult population in Sweden. Hepatology. 2010;52:571–577. doi: 10.1002/hep.23678. [DOI] [PubMed] [Google Scholar]
- 105.Weismüller T.J., Trivedi P.J., Bergquist A., Imam M., Lenzen H., Ponsioen C.Y., et al. Patient age, sex, and inflammatory bowel disease phenotype associate with course of primary sclerosing cholangitis. Gastroenterology. 2017;152:1975–1984.e8. doi: 10.1053/j.gastro.2017.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Trivedi P.J., Crothers H., Mytton J., Bosch S., Iqbal T., Ferguson J., et al. Effects of primary sclerosing cholangitis on risks of cancer and death in people with inflammatory bowel disease, based on sex, race, and age. Gastroenterology. 2020;159:915–928. doi: 10.1053/j.gastro.2020.05.049. [DOI] [PubMed] [Google Scholar]
- 107.Zheng H.-H., Jiang X.-L. Increased risk of colorectal neoplasia in patients with primary sclerosing cholangitis and inflammatory bowel disease: a meta-analysis of 16 observational studies. Eur J Gastroenterol Hepatol. 2016;28:383–390. doi: 10.1097/MEG.0000000000000576. [DOI] [PubMed] [Google Scholar]
- 108.Núñez P., Quera R., Gomollón F. Primary sclerosing cholangitis and inflammatory bowel disease: intestine–liver interrelation. Gastroenterología y Hepatología (English Edition) 2019;42:316–325. doi: 10.1016/j.gastrohep.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 109.Boonstra K., Weersma R.K., van Erpecum K.J., Rauws E.A., Spanier B.M., Poen A.C., et al. Population-based epidemiology, malignancy risk, and outcome of primary sclerosing cholangitis. Hepatology. 2013;58:2045–2055. doi: 10.1002/hep.26565. [DOI] [PubMed] [Google Scholar]
- 110.Rupp C., Rössler A., Zhou T., Rauber C., Friedrich K., Wannhoff A., et al. Impact of age at diagnosis on disease progression in patients with primary sclerosing cholangitis. United Eur Gastroenterol J. 2018;6:255–262. doi: 10.1177/2050640617717156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Henson J.B., Patel Y.A., Wilder J.M., Zheng J., Chow S.C., King L.Y., et al. Differences in phenotypes and liver transplantation outcomes by age group in patients with primary sclerosing cholangitis. Dig Dis Sci. 2017;62:3200–3209. doi: 10.1007/s10620-017-4559-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Steenstraten I.C., Sebib Korkmaz K., Trivedi P.J., Inderson A., van Hoek B., Rodriguez Girondo M.D., et al. Systematic review with meta-analysis: risk factors for recurrent primary sclerosing cholangitis after liver transplantation. Aliment Pharmacol Ther. 2019;49:636–643. doi: 10.1111/apt.15148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Allen K.J., Gurrin L.C., Constantine C.C., Osborne N.J., Delatycki M.B., Nicoll A.J., et al. Iron-overload-related disease in HFE hereditary hemochromatosis. N Engl J Med. 2008;358:221–230. doi: 10.1056/NEJMoa073286. [DOI] [PubMed] [Google Scholar]
- 114.Bacon B.R., Adams P.C., Kowdley K.V., Powell L.W., Tavill A.S. Diagnosis and management of hemochromatosis: 2011 practice guideline by the American association for the study of liver diseases. Hepatol (Baltimore, Md. 2011;54:328. doi: 10.1002/hep.24330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Fleming R.E., Britton R.S., Waheed A., Sly W.S., Bacon B.R. Pathophysiology of hereditary hemochromatosis. Semin Liver Dis. 2005;25:411–419. doi: 10.1055/s-2005-923313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mura C., Le Gac G., Scotet V., Raguenes O., Mercier A.Y., Férec C. Variation of iron loading expression in C282Y homozygous haemochromatosis probands and sib pairs. J Med Genet. 2001;38:632–636. doi: 10.1136/jmg.38.9.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Adams P.C., Reboussin D.M., Barton J.C., McLaren C.E., Eckfeldt J.H., McLaren G.D., et al. Hemochromatosis and iron-overload screening in a racially diverse population. New Engl J Med. 2005;352:1769–1778. doi: 10.1056/NEJMoa041534. [DOI] [PubMed] [Google Scholar]
- 118.Harrison-Findik D.D. Gender-related variations in iron metabolism and liver diseases. World J Hepatol. 2010;2:302. doi: 10.4254/wjh.v2.i8.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Atkins J.L., Pilling L.C., Masoli J.A., Kuo C.L., Shearman J.D., Adams P.C., et al. Association of hemochromatosis HFE p. C282Y homozygosity with hepatic malignancy. JAMA. 2020;324:2048–2057. doi: 10.1001/jama.2020.21566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Dobrindt E.M., Keshi E., Neulichedl J., Schöning W., Öllinger R., Pratschke J., et al. Long-term outcome of orthotopic liver transplantation in patients with hemochromatosis: a summary of a 30-year transplant program. Transplant Direct. 2020;6:e560. doi: 10.1097/TXD.0000000000001007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Giannitrapani L., Soresi M., La Spada E., Cervello M., D’alessandro N., Montalto G. Sex hormones and risk of liver tumor. Ann N Y Acad Sci. 2006;1089:228–236. doi: 10.1196/annals.1386.044. [DOI] [PubMed] [Google Scholar]
- 122.Luciani A., Kobeiter H., Maison P., Cherqui D., Zafrani E.S., Dhumeaux D., et al. Focal nodular hyperplasia of the liver in men: is presentation the same in men and women? Gut. 2002;50:877–880. doi: 10.1136/gut.50.6.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sarkar M., Brady C.W., Fleckenstein J., Forde K.A., Khungar V., Molleston J.P., et al. Reproductive health and liver disease: practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2021;73:318–365. doi: 10.1002/hep.31559. [DOI] [PubMed] [Google Scholar]
- 124.Mathieu D., Kobeiter H., Maison P., Rahmouni A., Cherqui D., Zafrani E.S., et al. Oral contraceptive use and focal nodular hyperplasia of the liver. Gastroenterology. 2000;118:560–564. doi: 10.1016/s0016-5085(00)70262-9. [DOI] [PubMed] [Google Scholar]
- 125.Wang A., Deng J., Qian B., Chen H., Li M., Yang D., et al. Natural history of hepatic hemangioma: a follow-up analysis of 534 patients. Front Life Sci. 2019;12:27–32. [Google Scholar]
- 126.Zhao Y., Legan C.E. Liver transplantation for giant hemangioma complicated by kasabach-merritt syndrome: a case report and literature review. Am J Case Rep. 2022;23 doi: 10.12659/AJCR.936042. e936042-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Eghlimi H., Arasteh P., Azade N. Orthotopic liver transplantation for Management of a Giant Liver Hemangioma: a case report and review of literature. BMC Surg. 2020;20:1–6. doi: 10.1186/s12893-020-00801-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rooks J.B., Ory H.W., Ishak K.G., Strauss L.T., Greenspan J.R., Hill A.P., et al. Epidemiology of hepatocellular adenoma: the role of oral contraceptive use. Jama. 1979;242:644–648. [PubMed] [Google Scholar]
- 129.Qureshy Z., Lokken R.P., Kakar S., Grab J., Mehta N., Sarkar M. Influence of progestin-only hormonal use on hepatocellular adenomas: a retrospective cohort study. Contraception. 2022 doi: 10.1016/j.contraception.2022.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Demory A., Péron J.M., Calderaro J., Selves J., Mokrane F.Z., Amaddeo G., et al. Body weight changes and duration of estrogen exposure modulate the evolution of hepatocellular adenomas after contraception discontinuation. Hepatology. 2022:1–13. doi: 10.1002/hep.32734. [DOI] [PubMed] [Google Scholar]
- 131.Gevers T.J.G., Marcel Spanier B.W., Veendrick P.B., Veendrick P.B., Vrolijk J.M. Regression of hepatocellular adenoma after bariatric surgery in severe obese patients. Liver Int. 2018;38(12):2134–2136. doi: 10.1111/liv.13934. [DOI] [PubMed] [Google Scholar]
- 132.Farges O., Ferreira N., Dokmak S., Belghiti J., Bedossa P., Paradis V. Changing trends in malignant transformation of hepatocellular adenoma. Gut. 2011;60:85–89. doi: 10.1136/gut.2010.222109. [DOI] [PubMed] [Google Scholar]
- 133.Mounajjed T. Hepatocellular adenoma and focal nodular hyperplasia. Clin Liver Dis. 2021;17:244. doi: 10.1002/cld.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kato K., Abe H., Hanawa N., Fukuzawa J., Matsuo R., Yonezawa T., et al. Hepatocellular adenoma in a woman who was undergoing testosterone treatment for gender identity disorder. Clin J Gastroenterol. 2018;11:401–410. doi: 10.1007/s12328-018-0854-4. [DOI] [PubMed] [Google Scholar]
- 135.Dokmak S., Paradis V., Vilgrain V., Sauvanet A., Farges O., Valla D., et al. A single-center surgical experience of 122 patients with single and multiple hepatocellular adenomas. Gastroenterology. 2009;137:1698–1705. doi: 10.1053/j.gastro.2009.07.061. [DOI] [PubMed] [Google Scholar]
- 136.van Rosmalen B.V., Furumaya A., Klompenhouwer A.J., Tushuizen M.E., Braat A.E., Reinten R.J., et al. Hepatocellular adenoma in men: a nationwide assessment of pathology and correlation with clinical course. Liver Int. 2021;41:2474–2484. doi: 10.1111/liv.14989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ziogas I.A., Tasoudis P.T., Serifis N., Alexopoulos S.P., Montenovo M.I., Shingina A. Liver transplantation for hepatic adenoma: a UNOS database analysis and systematic review of the literature. Transpl Direct. 2022;8 doi: 10.1097/TXD.0000000000001264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kothadia J.P., Kreitman K., Shah J.M. StatPearls Publishing; Treasure Island (FL): 2022. Polycystic liver disease. StatPearls. Copyright © 2022, StatPearls Publishing LLC. [PubMed] [Google Scholar]
- 139.Gevers T.J., Drenth J.P. Diagnosis and management of polycystic liver disease. Nat Rev Gastroenterol Hepatol. 2013;10:101–108. doi: 10.1038/nrgastro.2012.254. [DOI] [PubMed] [Google Scholar]
- 140.Suwabe T., Chamberlain A.M., Killian J.M., King B.F., Gregory A.V., Madsen C.D., et al. Epidemiology of autosomal-dominant polycystic liver disease in Olmsted county. JHEP Rep. 2020;2:100166. doi: 10.1016/j.jhepr.2020.100166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.van Aerts R.M.M., Kievit W., de Jong M.E., Ahn C., Bañales J.M., Reiterová J., et al. Severity in polycystic liver disease is associated with aetiology and female gender: results of the International PLD Registry. Liver Int. 2019;39:575–582. doi: 10.1111/liv.13965. [DOI] [PubMed] [Google Scholar]
- 142.Aapkes S.E., Bernts L.H.P., Barten T.R.M., van den Berg M., Gansevoort R.T., Drenth J.P. Estrogens in polycystic liver disease: a target for future therapies? Liver Int. 2021;41:2009–2019. doi: 10.1111/liv.14986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chauhan M., Zhang T., Thuluvath P.J. Gender differences in liver transplantation outcomes in polycystic liver disease. Dig Dis Sci. 2022;67:3445–3454. doi: 10.1007/s10620-021-07125-9. [DOI] [PubMed] [Google Scholar]
- 144.Sawinski D., Lai J.C., Pinney S., Gray A.L., Jackson A.M., Stewart D., et al. Addressing sex-based disparities in solid organ transplantation in the United States–a conference report. Am J Transplant. 2023 doi: 10.1016/j.ajt.2022.11.008. [DOI] [PubMed] [Google Scholar]
- 145.Mindikoglu A.L., Regev A., Seliger S.L., Magder L.S. Gender disparity in liver transplant waiting-list mortality: the importance of kidney function. Liver Transplant. 2010;16(10):1147–1157. doi: 10.1002/lt.22121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Allen A.M., Heimbach J.K., Larson J.J., Mara K.C., Kim W.R., Kamath P.S. Reduced access to liver transplantation in women: role of height, MELD exception scores, and renal function underestimation. Transplantation. 2018;102:1710–1716. doi: 10.1097/TP.0000000000002196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Yeh S.H., Chen P.J. Gender disparity of hepatocellular carcinoma: the roles of sex hormones. Oncology. 2010;78(Suppl 1):172–179. doi: 10.1159/000315247. [DOI] [PubMed] [Google Scholar]
- 148.Mindikoglu A.L., Opekun A.R., Mitch W.E., Magder L.S., Christenson R.H., Dowling T.C., et al. Cystatin C is a gender-neutral glomerular filtration rate biomarker in patients with cirrhosis. Dig Dis Sci. 2018;63:665–675. doi: 10.1007/s10620-017-4897-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wood N.L., VanDerwerken D., Segev D.L., Gentry S.E. Correcting the sex disparity in MELD-Na. Am J Transplant. 2021;21:3296–3304. doi: 10.1111/ajt.16731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kim W.R., Mannalithara A., Heimbach J.K., Kamath P.S., Asrani S.K., Biggins S.W., et al. MELD 3.0: the model for end-stage liver disease updated for the modern era. Gastroenterology. 2021;161:1887–1895.e4. doi: 10.1053/j.gastro.2021.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.