Abstract
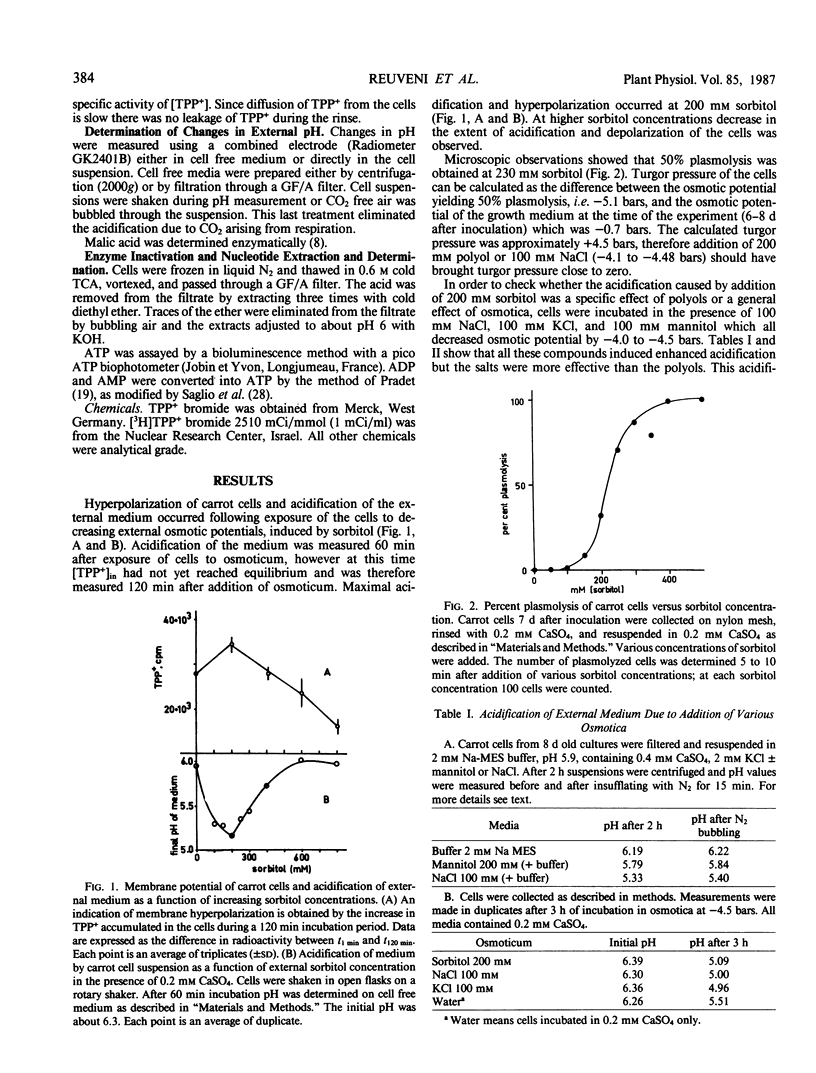
Addition of 200 mm of a polyol to anthocyanin containing carrot (Daucus carota L.) cells in suspension culture decreased turgor pressure to zero and induced hyperpolarization of the membrane potential and acidification of the medium due to H+ extrusion. These changes were shown to be slightly affected by vanadate. In parallel, a decrease in intracellular ATP and total adenylate concentrations were observed. However, when the osmoticum was NaCl acidification of the medium occurred in the absence of considerable changes in intracellular ATP concentration. These results are interpreted as indicating that a drop of turgor, by addition of a polyol, triggers a proton extrusion activity which is only slightly inhibited by vanadate but apparently ATP utilizing. The observed decrease in ATP level occurs without a change in respiration rate and is accompanied by a drop in total adenylate pool. However when NaCl is the osmoticum it is assumed that ΔμH+ is enhanced through a Na+/H+ antiporter. The difference between the two types of osmotica as related to their ability to penetrate through the cellular membrane is discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Gamborg O. L., Miller R. A., Ojima K. Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res. 1968 Apr;50(1):151–158. doi: 10.1016/0014-4827(68)90403-5. [DOI] [PubMed] [Google Scholar]
- Kinraide T. B., Wyse R. E. Electrical evidence for turgor inhibition of proton extrusion in sugar beet taproot. Plant Physiol. 1986 Dec;82(4):1148–1150. doi: 10.1104/pp.82.4.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch J., Cramer G. R., Läuchli A. Salinity reduces membrane-associated calcium in corn root protoplasts. Plant Physiol. 1987 Feb;83(2):390–394. doi: 10.1104/pp.83.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocquot B., Prat C., Mouches C., Pradet A. Effect of anoxia on energy charge and protein synthesis in rice embryo. Plant Physiol. 1981 Sep;68(3):636–640. doi: 10.1104/pp.68.3.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhold L., Seiden A., Volokita M. Is modulation of the rate of proton pumping a key event in osmoregulation? Plant Physiol. 1984 Jul;75(3):846–849. doi: 10.1104/pp.75.3.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuveni M., Lerner H. R., Poljakoff-Mayber A. Changes in Membrane Potential as a Demonstration of Selective Pore Formation in the Plasmalemma by Poly-l-Lysine Treatment. Plant Physiol. 1985 Oct;79(2):406–410. doi: 10.1104/pp.79.2.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein B. Use of lipophilic cations to measure the membrane potential of oat leaf protoplasts. Plant Physiol. 1978 Dec;62(6):927–929. doi: 10.1104/pp.62.6.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willsky G. R., White D. A., McCabe B. C. Metabolism of added orthovanadate to vanadyl and high-molecular-weight vanadates by Saccharomyces cerevisiae. J Biol Chem. 1984 Nov 10;259(21):13273–13281. [PubMed] [Google Scholar]


