Abstract
Aims
Adjustment of treatment based on remote monitoring of pulmonary artery (PA) pressure may reduce the risk of hospital admission for heart failure (HF). We have conducted a meta-analysis of large randomized trials investigating this question.
Methods and results
A systematic literature search was performed for randomized clinical trials with PA pressure monitoring devices in patients with HF. The primary outcome of interest was the total number of HF hospitalizations. Other outcomes assessed were urgent visits leading to treatment with intravenous diuretics, all-cause mortality, and composites. Treatment effects are expressed as hazard ratios, and pooled effect estimates were obtained applying random effects meta-analyses. Three eligible randomized clinical trials were identified that included 1898 outpatients in New York Heart Association functional classes II–IV, either hospitalized for HF in the prior 12 months or with elevated plasma NT-proBNP concentrations. The mean follow-up was 14.7 months, 67.8% of the patients were men, and 65.8% had an ejection fraction ≤40%. Compared to patients in the control group, the hazard ratio (95% confidence interval) for total HF hospitalizations in those randomized to PA pressure monitoring was 0.70 (0.58–0.86) (P = .0005). The corresponding hazard ratio for the composite of total HF hospitalizations, urgent visits and all-cause mortality was 0.75 (0.61–0.91; P = .0037) and for all-cause mortality 0.92 (0.73–1.16). Subgroup analyses, including ejection fraction phenotype, revealed no evidence of heterogeneity in the treatment effect.
Conclusion
The use of remote PA pressure monitoring to guide treatment of patients with HF reduces episodes of worsening HF and subsequent hospitalizations.
Keywords: Heart failure, Pulmonary artery pressure, Sensor, Monitoring, Trial
Structured Graphical Abstract
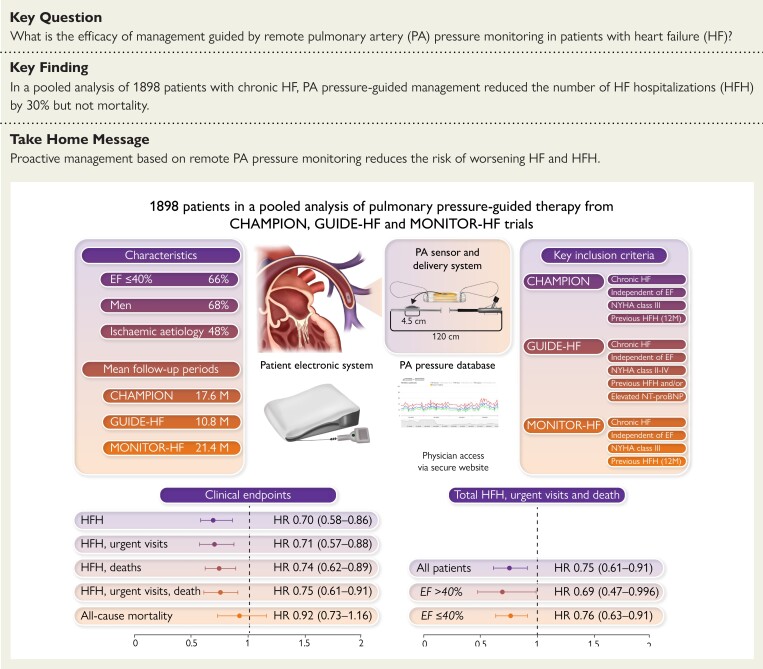
Structured Graphical Abstract.
The upper part of the figure shows the characteristics of the included RCTs at the sides, and the pulmonary artery sensor, patient electronics system, and the pulmonary artery pressure database in the middle. In the lower part of the figure, the x axis presents the risk ratio, the y axis presents the data points of clinical endpoints as addressed, the dot is the point estimate of the hazard ratio pooled estimate, and the bars correspond to the 95% confidence interval. CI, confidence interval; EF, ejection fraction; HR, hazard ratio; HF, heart failure; HFH, heart failure hospitalization; M, months; NT-proBNP, N-terminal pro-B-type natriuretic peptide; NYHA, New York Heart Association; PA, pulmonary artery.
See the editorial comment for this article ‘Remote heart failure management guided by pulmonary artery pressure home monitoring: rewriting the future?’, by C.E. Angermann and G. Ertl, https://doi.org/10.1093/eurheartj/ehad525.
Introduction
Hospital admission rates for heart failure (HF) are high and are mainly driven by congestion.1–3 Haemodynamic congestion, characterized by increasing pulmonary artery (PA) pressure, often precedes signs and symptoms of clinical congestion by several weeks, which may allow early detection and treatment to prevent hospitalization.4 Two devices that measure PA pressure are available but only one, the CardioMEMS HF System (Abbott, IL, USA), has efficacy data from randomized clinical trials.5–9 The first reported trial with this device, CHAMPION [CardioMEMS Heart Sensor Allows Monitoring of Pressure to Improve Outcomes in New York Heart Association (NYHA) Class III Heart Failure Patients], was conducted exclusively in USA and demonstrated a significant benefit of PA pressure-guided management in preventing HF hospitalization.6 The second trial, GUIDE-HF (haemodynamic-GUIDEd management of Heart Failure), carried out in USA and Canada, was neutral.7 The 2021 European Society of Cardiology (ESC) HF guideline, published before the results of GUIDE-HF were available, gave a Class II, Level B recommendation for PA pressure monitoring in patients with HF.1 Although the 2022 American Heart Association and American College of Cardiology guidelines made a similar recommendation after GUIDE-HF, it stated that the usefulness of this approach is uncertain and that further evidence was needed before it could be recommended for routine clinical care.10 A new and first European randomized controlled trial, MONITOR-HF, has just been published and showed that PA pressure-guided HF management resulted in a significant reduction of HF hospitalizations as compared to standard of care. A pooled analysis of these three trials is warranted and timely considering the uncertainty described above, in order to obtain more robust estimates of the effect of PA pressure-guided management on clinical endpoints with the larger number of patients and longer follow-up.
Methods
The reporting of this systematic review and meta-analysis adheres to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist and has been registered on PROSPERO with registration number CRD42023408739.11
This study was set up to estimate the effects of remote PA pressure monitoring on HF hospitalizations and mortality outcomes in a meta-analysis, by combining the results of the CHAMPION, GUIDE-HF, and MONITOR-HF.5–7,12 In contrast to earlier conducted meta-analyses assessing implantable haemodynamic telemonitoring devices,13,14 the focus of this meta-analysis was on the CardioMEMS HF System as at the moment of the PROSPERO registration, no efficacy data were available from other PA pressure devices based on randomized controlled trials. Nevertheless, we performed a systematic literature search to ensure no eligible studies were missed. Studies were eligible for inclusion if they had a randomized controlled trial design, prospective, compared the CardioMEMS HF System to a control group, included at least 100 patients, and reported on HF-related clinical endpoints. Medline, Web of Science, Embase, Cochrane, and Google Scholar were searched from inception until 28 February 2023. The systematic search was built and adapted for each database by an experienced information scientist (see Supplementary Material).15 No restrictions on language, study status, or time of publication were placed. Two independent teams of reviewers (P.C. and S.R.) screened the articles on eligibility in a title and abstract phase and a full-text phase.
Clinical endpoints of interest were HF hospitalizations, urgent visits with the need for intravenous diuretic therapy, all-cause mortality, and composites of these endpoints. For GUIDE-HF and MONITOR-HF, we accessed all follow-up data and for CHAMPION there were two reports, where we decided to use the extended follow-up analysis.6 The CHAMPION trial did not include urgent HF visits with the need for intravenous diuretics, which are presently considered as a comparable endpoint to HF hospitalizations. Urgent visits were included as endpoints in both the GUIDE-HF and MONITOR-HF trials. In the analysis of the composite endpoint consisting of total HF hospitalizations, urgent visits, and all-cause mortality, the CHAMPION data only included HF hospitalizations and all-cause mortality. Similarly, in the analysis of the composite endpoint of total HF hospitalizations and urgent visits, the CHAMPION data only included HF hospitalizations. This decision was made to ensure that data on these related endpoints were not missing, which was also the approach in an earlier meta-analysis on invasive haemodynamic monitoring.14 A summary of the population, intervantion, comparator, outcome, and study design (PICOTS) for this study is provided in Supplementary data online, Table S1.
Data extraction was performed by the same reviewers using a standardized data extraction sheet, which included study characteristics, baseline characteristics of the included patients for each treatment group, and clinical endpoints. Patient level data were available for MONITOR-HF. Hazard ratios (HRs) were the primary measure of effect, risk ratios and odds ratios were considered when HRs were not available. All effect sizes were extracted and reported as point estimates with 95% confidence intervals (CIs). Data were extracted from post hoc analyses, follow-up analyses, and Food and Drug Administration summary report when the included studies did not report on them.16–18 The numbers of patients in subgroups were calculated from available data where necessary. If the HR was not reported in the literature, the incidence rate ratio (IRR) was calculated using the number of events and study cohort time at risk. Study cohort time at risk was calculated by dividing the number of events by the event rate of the primary endpoint.
The risk of bias was assessed by the same independent reviewers; disagreements were resolved in a consensus meeting. To assess the risk of bias in the included studies, the Revised Cochrane Risk-of-Bias Tool for Randomized Trials (RoB 2 tool) was used.19
Meta-analyses were performed when outcomes were reported by at least two studies with similar effect measures (if only one trial reported on an outcome, we show the individual study data). For the meta-analyses, we used a random effects model with the DerSimonian and Laird estimator.20 Of note, the three trials analysed the total HF hospitalizations with the Andersen–Gill extension of the Cox model, which includes first and recurrent events. As a sensitivity analysis, we also included fixed effect models in Supplements. The presence of heterogeneity was quantified with I2 and P-values. The numbers of patients in subgroups were calculated from available data where necessary. The CHAMPION trial did not report on several subgroups included in this meta-analysis. If subgroups were reported, the investigators included HF hospitalizations only (deaths are not reported in subgroups). The GUIDE-HF reported many subgroups on the composite endpoint of HF hospitalizations, urgent visits, and mortality only. To follow this, we aligned with subgroups of GUIDE-HF (including endpoint) with the MONITOR-HF using individual patient level data. Subgroup analyses were performed for left ventricular ejection fraction (LVEF) (≤40% and >40%; <50% and ≥50%), NYHA class, sex, age, HF aetiology, and implantable cardioverter defibrillator (ICD) or cardiac resynchronization therapy (CRT) device implantation. Reported safety data on device- or system-related complications (DSRC) and sensor failures were presented and combined for total implant procedures in the trials. Complete data from all trials were used, also for the GUIDE-HF trial. Sensitivity analyses were performed using the data from the pre-specified COVID-19 analysis of GUIDE-HF.7 All calculations and analyses were performed with the Metafor package for R.21
Several outcomes were extracted and described in addition to the clinical endpoints described above. All trials described medication changes, changes in mean PA pressure and safety endpoints. The GUIDE-HF and MONITOR-HF also used the Kansas City Cardiomyopathy Questionnaire to described patient-reported outcomes after 12-month follow-up, which was not available in CHAMPION (which used the Minnesota Living with Heart Failure Questionnaire).
Results
Study and patient characteristics
The systematic search identified a total of 840 records of which the titles and abstracts were screened. Three studies met the eligibility criteria and were included in the meta-analysis: CHAMPION, GUIDE-HF, and MONITOR-HF (see Supplementary data online, Figure S1), of which only aggregated data were available for CHAMPION and GUIDE-HF. The trial design features and study characteristics are summarized in Table 1. In short, 67.8% of patients were men, and 15.6%, 81.6%, and 2.8% of patients were in NYHA functional class II, III, or IV, respectively. In CHAMPION and GUIDE-HF, all patients underwent implantation of a wireless PA pressure sensor and were subsequently randomized to receive standard HF care only or to PA pressure-guided management. In both trials, patients were blinded to the allocated treatment group while investigators were not. In MONITOR-HF, all enrolled patients were randomly allocated to either PA pressure-guided management or standard HF care without the implant. Both patients and investigators were unblinded to the allocated treatment group. All trials had an independent, masked, clinical event committee for adjudication of clinical endpoints.
Table 1.
Characteristics of included trials and patients
| CHAMPION | GUIDE-HF | MONITOR-HF | |
|---|---|---|---|
| Enrolment period | 2007–2009 | 2018–2019 | 2019–2022 |
| Number of randomized patients | 550 | 1000 | 348 |
| Number of participating sites | 64 in 1 country (USA) | 140 in 2 countries (USA and Canada) | 25 in 1 country (the Netherlands) |
| Design | Single-blind randomized clinical trial, all patients received the device | Single-blind randomized clinical trial, all patients received the device | Open-label randomized clinical trial, allocation to CM or SC (no device) |
| Blinding | Patients only | Patients only | None |
| Key inclusion criteria | NYHA III | NYHA II–IV | NYHA III |
| HFH <12 months | HFH <12 months and/or elevated natriuretic peptides levels | HFH <12 months | |
| Treatment according to guidelines (GDMT and/or device) | Treatment according to guidelines (GDMT and/or device) | Treatment according to guidelines (GDMT and/or device) | |
| Key exclusion criteria | eGFR <25 | eGFR <25 | eGFR <25 |
| Recurrent PE/DVT | Intolerance to all neurohormonal antagonists | Recurrent PE/DVT | |
| CRT implantation <3 months | Current/recurrent PE/DVT | CRT implantation <3 months | |
| CRT <3 months | |||
| Mean follow-up time | 17.6 months | 10.8 months | 21.4 months |
| Follow-up period | Entire study (randomized access period) | Fixed 12-month time-point | Entire study |
| Primary clinical endpoint | Total HFH (first and recurrent events) | Composite of total HF events (first and recurrent, including urgent HF visits) and mortality at 12 months | Quality of life (KCCQ) |
| Secondary: total HFH (first and recurrent events), urgent visits, mortality | |||
| Adjudication of clinical endpoints | Independent and masked CEC | Independent and masked CEC | Independent and masked CEC |
| Reports on the following clinical endpoints | HFH | HFH | HFH |
| Death | Urgent visits with i.v. diuretics | Urgent visit with i.v. diuretics | |
| Death | Death | ||
| Subgroup data available on | Total HFH only | Composite of HFH, urgent HF visits, and death | Composite of HFH, urgent HF visits, and death |
| Control group | Sensor implant, but no monitoring | Sensor implant, but no monitoring | No sensor implanted |
| Baseline characteristics | Treatment (n = 270) | Control (n = 280) | Treatment (n = 497) | Control (n = 503) | Treatment (n = 176) | Control (n = 172) |
|---|---|---|---|---|---|---|
| Age, years (mean with SD, or median with IQR) | 61 (13) | 62 (13) | 71 (64–76) | 70 (64–77) | 69 (61–75) | 70 (61–75) |
| Male sex | 194 (72%) | 205 (73%) | 310 (62%) | 315 (63%) | 138 (78%) | 125 (73%) |
| NYHA functional class | ||||||
| II | 0 (0%) | 0 (0%) | 146 (29%) | 150 (30%) | 0 (0%) | 0 (0%) |
| III | 270 (100%) | 280 (100%) | 322 (65%) | 328 (65%) | 176 (100%) | 172 (100%) |
| IV | 0 (0%) | 0 (0%) | 29 (6%) | 25 (5%) | 0 (0%) | 0 (0%) |
| Median EF | N.A. | N.A. | 38% (25–55) | 40% (25–55) | 30% (23–40) | 30% (22–43) |
| LVEF | ||||||
| ≤40% | 222 (82%) | 234 (84%) | 273 (55%) | 258 (51%) | 134 (76%) | 127 (74%) |
| >40% | 48 (18%) | 46 (16%) | 224 (45%) | 245 (49%) | 42 (24%) | 45 (26%) |
| NT-proBNP (pg/mL) | N.A. | N.A. | 1480 (686–2743) | 1274 (661–2318) | 2377 (837–5153) | 1905 (691–4444) |
| eGFR, mean (SD), or median (IQR) | 60 (23) | 62 (23) | 51 (39–65) | 49 (38–65) | 48 (35–60) | 48 (38–63) |
| Ischaemic aetiology | 158 (59%) | 174 (62%) | 207 (42%) | 190 (38%) | 93 (53%) | 81 (47%) |
| GDMT (all patients) | ||||||
| ACEi/ARB/ARNI | 205 (76%) | 222 (79%) | 319 (64%) | 320 (64%) | 144 (82%) | 139 (81%) |
| ARNI | N.A. | N.A. | 145 (29%) | 139 (28%) | 81 (46%) | 81 (47%) |
| Beta-blocker | 243 (90%) | 256 (91%) | 444 (89%) | 442 (88%) | 150 (85%) | 142 (83%) |
| MRA | 117 (43%) | 114 (41%) | 237 (48%) | 216 (43%) | 143 (81%) | 144 (84%) |
| Diureticsa | 248 (92%) | 258 (92%) | 474 (95%) | 478 (95%) | 168 (96%) | 167 (97%) |
| SGLT2 inhibitor | N.A. | N.A. | 2 (<1%) | 2 (<1%) | 12 (7%) | 21 (12%) |
| Device therapy | ||||||
| ICD | 88 (33%) | 98 (35%) | 213 (43%) | 205 (41%) | 94 (53%) | 102 (59%) |
| CRT | 91 (34%) | 99 (35%) | 142 (29%) | 163 (32%) | 46 (26%) | 46 (27%) |
CEC, clinical event committee; ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin-receptor blocker; ARNI, angiotensin-receptor-neprilysin inhibitor; MRA, mineralocorticoid receptor antagonist; ICD, implantable cardioverter defibrillator; CRT, cardiac resynchronisation therapy; eGFR, estimated glomerular filtration rate; NYHA, New York Heart Association; EF, ejection fraction; LVEF, left ventricular ejection fraction; PE, pulmonary embolism; DVT, deep venous thrombosis; NT-proBNP, N-terminal pro-B-type natriuretic peptide; HF, heart failure; HFH, heart failure hospitalization; SGLT2, sodium-glucose cotransporter 2; SC, standard care; GDMT, guideline-directed medical therapy; i.v., intravenous; N.A., not available; SD, standard deviation; IQR, interquartile range; KCCQ, Kansas City Cardiomyopathy Questionnaire.
Loop diuretics for CHAMPION and MONITOR-HF, unknown for GUIDE-HF.
Clinical efficacy of remote pulmonary artery pressure-guided treatment
The studies included a total of 1898 patients, and the mean follow-up was 14.7 months (which ranged from 10.8 months, 17.6 months, and 21.4 months across the trials, respectively). Only in the GUIDE-HF trial, the follow-up period was fixed at 12 months. The meta-analyses of all clinical endpoints are summarized in Figure 1. For the CHAMPION trial, no data were available on urgent visits.
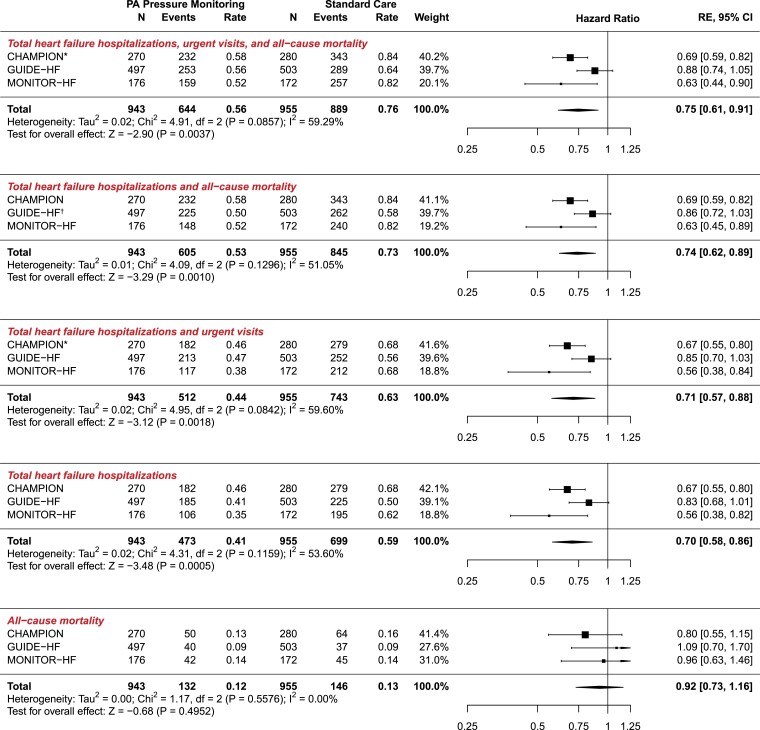
Figure 1.
Meta-analyses of clinical endpoints. All rates are reported as events per patient-year. PA, pulmonary artery; RE, random effects; CI, confidence interval. *CHAMPION did not report data on urgent visits; †Calculated and included as incidence rate ratio (IRR)
Composite of total heart failure hospitalizations, urgent heart failure visits, and all-cause mortality
The composite endpoint of total HF hospitalization, urgent visits, and all-cause mortality occurred 644 times among 943 patients in the PA pressure monitoring group (0.56 events per patient-year) and 889 times among 955 control group patients (0.76 events per patient-year), resulting in an HR of 0.75 and 95% CI 0.61–0.91; P = .0037 (moderate heterogeneity, I2 = 59.29%).
Composite of total heart failure hospitalizations and all-cause mortality
The composite endpoint of total HF hospitalizations and mortality occurred 605 times among 943 patients in the PA pressure monitoring group (0.53 events per patient-year) and occurred 845 times among 955 patients in the control group (0.73 events per patient-year), yielding an HR of 0.74 and 95% CI 0.62–0.89; P = .0010 (I2 = 51.05%).
Total heart failure hospitalizations and urgent heart failure visits
The composite endpoint HF hospitalizations and urgent HF visits occurred 512 times among 943 patients in the PA pressure monitoring group patients (0.44 events per patient-year) and 743 times among 955 control patients (0.63 events per patient-year), yielding an HR of 0.71 and 95% CI 0.57–0.88; P = .0018 (moderate heterogeneity, I2 = 59.60%).
Total heart failure hospitalizations
Heart failure hospitalizations occurred 473 times among 943 patients in the PA pressure monitoring group (0.41 events per patient-year) and 699 times among 955 control patients (0.59 events per patient-year), yielding an HR of 0.70 (95% CI 0.58–0.86; P = .0005) in favour of the PA pressure monitoring group (moderate heterogeneity, I2 = 53.60%).
All-cause mortality
Among 943 patients in the PA pressure monitoring group, 132 patients died (14.0%, 0.12 events per patient-year) and among 955 patients in the control group, 146 patients (15.3%, 0.13 events per patient-year) died, resulting in an HR of 0.92, 95% CI 0.73–1.16; P = .495 (I2 = 0%).
Subgroup analyses (heart failure hospitalizations, urgent visits, and all-cause mortality)
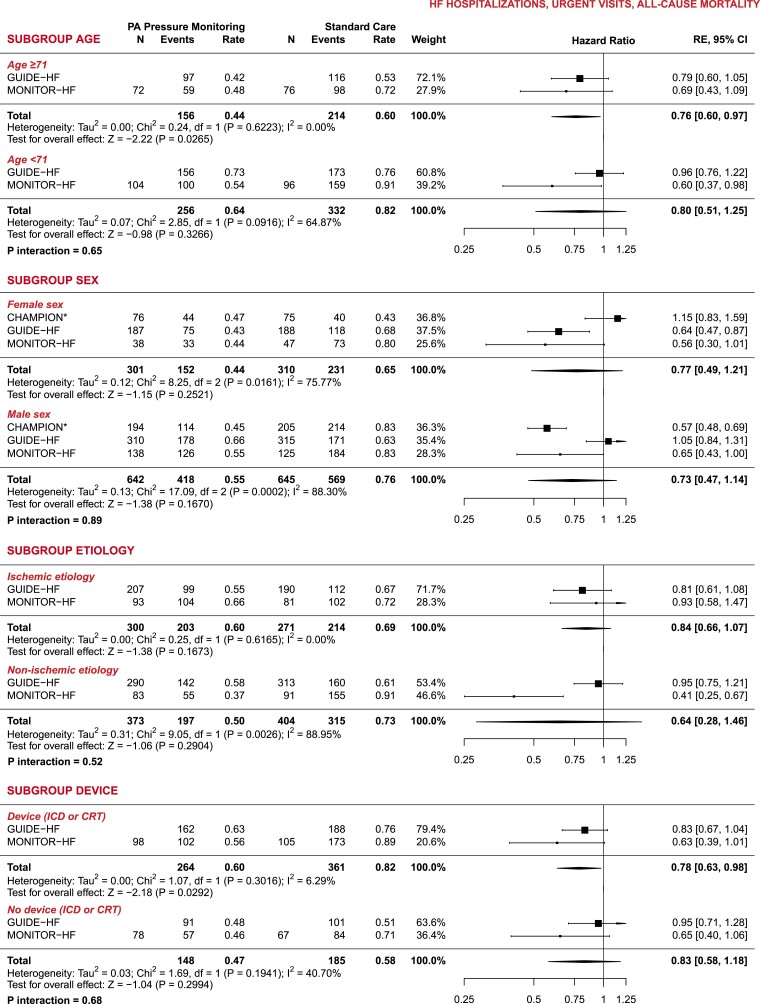
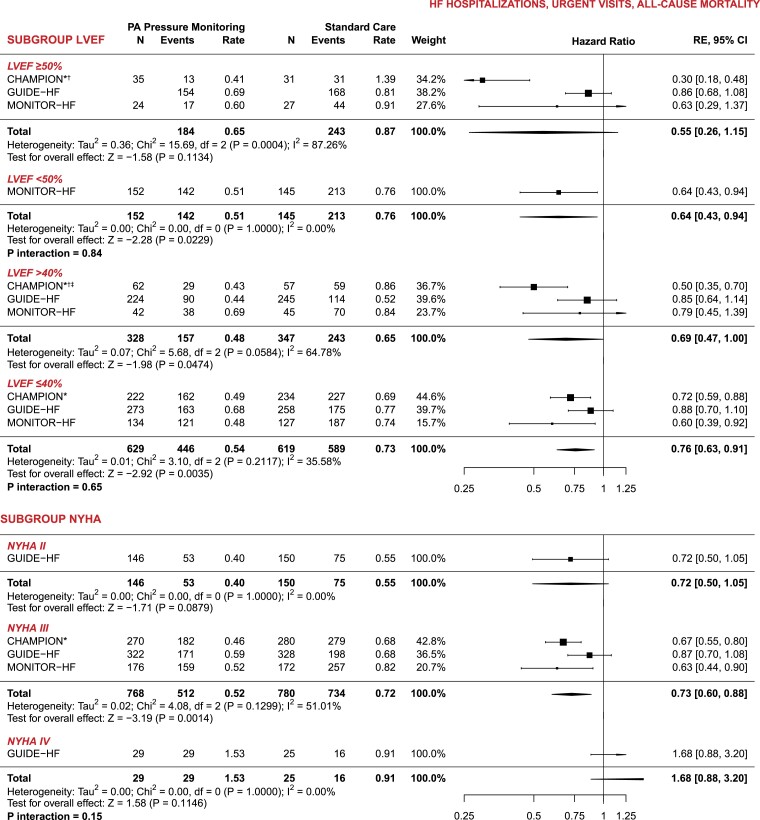
For the subgroup analyses, CHAMPION only included data on HF hospitalizations and reported on relatively few subgroups as compared to GUIDE-HF and MONITOR-HF. Pooled analyses of all three trials showed a consistent treatment benefit of remote PA pressure monitoring across the full spectrum of LVEF: among patients with LVEF ≤40% (n = 1248, 65.8%), we calculated an HR of 0.76 (95% CI 0.63–0.91), and an HR of 0.69 (95% CI 0.47–0.996) among patients with LVEF >40% (n = 675, 34.2%) (Figure 2) (P-value for interaction .65). Despite the presence of moderate heterogeneity, the effects of remote PA pressure monitoring were found to be largely consistent across clinically relevant subgroups (Figure 2 and Supplementary data online, Table S3).
Figure 2.
Subgroup analysis—meta-analyses of clinical endpoints (heart failure hospitalizations, urgent visits, and all-cause mortality). All rates are reported as events per patient-year. PA, pulmonary artery; RE, random effects; CI, confidence interval. *CHAMPION did not report data on urgent visits; †Calculated and included as incidence rate ratio (IRR); ‡CHAMPION only reported data for LVEF ≥40%
Exploratory endpoints
The results for these endpoints are summarized in Table 2. The freedom from DSRC was 98.9% and the freedom from sensor failure was 99.7% in the pooled analysis.
Table 2.
Overview of exploratory endpoints
| CHAMPION | GUIDE-HF | MONITOR-HF | ||||
|---|---|---|---|---|---|---|
| Treatment | Control | Treatment | Control | Treatment | Control | |
| Endpoint | ||||||
| Change in mean PAP (AUC) | −156 mmHg·days (6 months) | 33 mmHg·days (6 months) | −792.7 mmHg·days (12 months) | −582.9 mmHg·days (12 months) | −1623.8 mmHg·days (12 months) | N.A. |
| Change in average daily mean PAP | −0.6 mmHg | 0.1 mmHg | −2.4 mmHg | −1.8 mmHg | −4.7 mmHg | N.A. |
| Average mean PAP at 12 months | N.A. | N.A. | N.A. | N.A. | 24.9 mmHg | N.A. |
| Mean change in KCCQ at 12 months (SD) | N.A. | N.A. | 5 (21) | 4 (23) | 7 (25) | 0 (23) |
| Mean change in MLHFQ at 6 months (SD)a | −11 (25) | −7 (25) | N.A. | N.A. | N.A. | N.A. |
| Freedom from device or system related complications (%) | 98.6% | 99% | 97.7% | |||
| Freedom from sensor failure (%) | 100% | N.A. | 98.8% | |||
| Medication changes rate/month | 1.52 | 0.63 | 1.03 | 0.61 | 0.93b | 0.55b |
In combined analysis of the three trials, the freedom from device or system related complications was 98.9% and the freedom from sensor failure was 99.7% in implanted patients.
KCCQ, Kansas City Cardiomyopathy Questionnaire; MLHFQ, Minnesota Living with Heart Failure Questionnaire; PAP, pulmonary artery pressure; AUC, area under the curve; SD, standard deviation; N.A., not available.
Retrieved from the Food and Drug Administration Executive Summary (change not reported in main article).
Changes in guideline-recommended medical therapy and diuretics only (until 12 months of follow-up).
The full risk of bias assessment is included in Supplementary data online, Figure S2. Sensitivity analyses incorporating only the data from the pre-COVID-19 period of the GUIDE-HF trial (instead of all data in the main analysis) were performed. These analyses did not alter our overall findings (see Supplementary data online, Figs S3 and S4). Sensitivity analyses were performed with fixed effect models for the main analyses, and are presented in Supplementary data online, Table S2.
Discussion
This meta-analysis of three large randomized clinical trials including 1898 patients showed that adjusting treatment based on remote monitoring of PA pressures led to a 30% reduction in total HF hospitalizations. This beneficial effect of PA pressure-guided treatment was apparent in patients with LVEF ≤40% and >40%. However, PA pressure-guided treatment did not lead to a reduction in overall mortality. Importantly, the implantation of a PA sensor was safe and durable with a low number of device-related complications and sensor failures (Structured Graphical Abstract).
Although the CHAMPION trial suggested that PA pressure-guided management could substantially reduce rates of HF hospitalizations, that trial included a selected high-risk cohort enrolled exclusively in USA. Moreover, CHAMPION was conducted between 2007 and 2011 when guideline-recommended therapy was different than today.22 The GUIDE-HF, conducted between 2018 and 2021, extended the eligibility to patients in NYHA functional class II and patients with elevated NT-proBNP concentrations in case there was no HF hospitalization in the previous 12 months.7 However, the use of the same PA pressure-monitoring system to guide treatment did not lead to a significant reduction in the primary outcome or HF hospitalizations in GUIDE-HF compared to CHAMPION. While this may have been due to the impact of the COVID-19 pandemic on the conduct of GUIDE-HF, as suggested by the pre-specified COVID-19 sensitivity analysis of the trial that confirmed a significant treatment benefit, there were also concerns that this management approach might not work in a broader and lower-risk HF population. One of the potential reasons for the smaller difference between the treatment and control groups in GUIDE-HF as compared to CHAMPION is that the control group in GUIDE-HF had two weekly calls with their healthcare provider, which may not properly reflect the usual care HF patients receive.
The MONITOR-HF is the first European trial using the same implantable PA pressure monitor, and its results were largely consistent with CHAMPION and the pre-COVID-19 data from GUIDE-HF. The MONITOR-HF differed in that the control group did not have an implanted sensor that was not monitored (as in both prior trials) and did not receive two weekly calls (as in GUIDE-HF). Background pharmacological and device therapy in MONITOR-HF was excellent compared to both prior trials with the high use of renin-angiotensin system blockers (81% vs. 64% in GUIDE-HF), mineralocorticoid receptor antagonists (82% vs. 45% in GUIDE-HF), and ICD (56% vs. 42% in GUIDE-HF). Also, the uptake of angiotensin receptor–neprilysin inhibitor (ARNI) (47% vs. 28% in GUIDE-HF) and sodium-glucose cotransporter 2 inhibitors (12% vs. <1% in GUIDE-HF) was high and increased substantially to 60% and 30%, respectively, at 12 months in MONITOR-HF (which enrolled longer after the guideline updates). Interestingly, MONITOR-HF also showed the greatest effect of treatment on PA pressure. In GUIDE-HF, the impact on PA pressure was smaller, especially during the COVID-19 pandemic.23,24 In all three trials, there was a substantially higher number of cumulative drug changes during follow-up in the PA pressure monitoring arm, especially in diuretics, which likely explains the effect on PA pressure and congestion to avoid HF hospitalizations.
The combined evidence from the three trials indicates a significant and consistently positive outcome of PA pressure-guided treatment in reducing HF hospitalizations. The effects of PA pressure-guided therapy, observed across the three trials conducted in different periods with evolving background guideline-recommended medical therapy (and during the pandemic), demonstrate strong agreement in outcomes. These findings provide substantial support for PA pressure-guided HF management. Furthermore, this benefit remained consistent among patients with HF with reduced ejection fraction and those with an LVEF >40%. The aggregated data revealed a notable treatment effect in patients classified as NYHA class III, who are known to have high rates of HF hospitalizations. Based on the GUIDE-HF data, neither the NYHA class II nor IV patient groups exhibited a significant treatment effect on the primary outcome (HF hospitalization, urgent visits, and mortality), nor did NYHA class show a significant interaction of treatment effect. However, in GUIDE-HF, a significant reduction in the primary outcome was observed when combining patients in NYHA classes II and III. The accuracy of assigning NYHA class has its limitations, which should be kept in mind while interpreting these results. Although no reduction in mortality was observed, it is important to note that the overall number of deaths was relatively small, and even this meta-analysis had limited statistical power to detect an effect on mortality. We acknowledge that none of the trials were specifically designed or powered to assess mortality as a singular endpoint, and the follow-up time was limited.
Remote monitoring triggers an interaction between patient and healthcare provider to proactively optimize diuretic therapy based upon invasive markers of volume status. The potential benefit of this technique lies in optimizing and tailoring background therapy in patients, which is reflected by the higher rates of medication changes in the PA pressure-guided group. Although an important clinical question is in which patients PA pressure monitoring should be considered, the present meta-analysis shows consistent findings across subgroups tested including ejection fraction. While this reflects relative risk reductions related to PA pressure-guided treatment, higher risk groups such as NYHA class III patients and patients with recent HF hospitalization will most likely receive the larger absolute risk reductions. Despite the observed consistency in treatment effect, we underline that the procedure investigated is not without risk, although the complication rate was very low. The few complications were all easily manageable, and sensor failures were few, with a high reliability of the technology over several years.5–7 Similar rates of system-related adverse events were reported based upon post-marketing surveillance data in USA.25
The current meta-analysis has several limitations. First, individual data were only available from MONITOR-HF, and aggregate published data from CHAMPION and GUIDE-HF were used. Second, the overall neutral results from the full data of the GUIDE-HF trial were used in this meta-analysis and not the COVID-19 sensitivity analysis. Third, the trials included were performed in Northern America (predominantly USA and four sites in Canada) and in the Netherlands, and the technology and associated management may not be generalizable to all countries. Still, the additive effect on top of high levels of guideline-recommended medical therapy is reassuring for generalizability of these findings. Fourth, the three trials were underpowered to assess mortality, even combined in this meta-analysis. Fifth, moderate heterogeneity was present within the main and subgroup analyses. Nevertheless, the benefit of PA pressure monitoring remained consistent across most subgroups. Sixth, the lack of blinding in the three trials could have impacted the results through performance bias. Finally, the successful use of the technology depends on two factors as follows: (i) an adherent patient performing measurements at least several times a week, and (ii) an involved physician or healthcare provider responding to these pressure measurements.
In conclusion, the current meta-analysis of three randomized clinical trials demonstrated a substantial benefit of remote monitoring of PA pressures in patients with chronic HF. Total HF hospitalizations were reduced by 30%. This benefit was consistent among subgroups and independent of ejection fraction.
Supplementary data
Supplementary data is available at European Heart Journal online.
Pre-registered clinical trial number
CRD42023408739 (PROSPERO).
Ethical approval
Ethical Approval was not required.
Supplementary Material
Contributor Information
Pascal R D Clephas, Department of Cardiology, Erasmus MC University Medical Centre, Dr. Molewaterplein 40, 3015 GD Rotterdam, the Netherlands.
Sumant P Radhoe, Department of Cardiology, Erasmus MC University Medical Centre, Dr. Molewaterplein 40, 3015 GD Rotterdam, the Netherlands.
Eric Boersma, Department of Cardiology, Erasmus MC University Medical Centre, Dr. Molewaterplein 40, 3015 GD Rotterdam, the Netherlands.
John Gregson, Department of Medical Statistics, London School of Hygiene & Tropical Medicine, Keppel Street, London WC1E 7HT, UK.
Pardeep S Jhund, British Heart Foundation Cardiovascular Research Centre, School of Cardiovascular and Metabolic Health, University of Glasgow, 126 University Place, Glasgow G12 8TA, UK.
William T Abraham, Division of Cardiovascular Medicine, The Ohio State University, 410 W 10th Ave, Columbus, OH 43210, USA.
John J V McMurray, British Heart Foundation Cardiovascular Research Centre, School of Cardiovascular and Metabolic Health, University of Glasgow, 126 University Place, Glasgow G12 8TA, UK.
Rudolf A de Boer, Department of Cardiology, Erasmus MC University Medical Centre, Dr. Molewaterplein 40, 3015 GD Rotterdam, the Netherlands.
Jasper J Brugts, Department of Cardiology, Erasmus MC University Medical Centre, Dr. Molewaterplein 40, 3015 GD Rotterdam, the Netherlands.
Data availability
No aggregate or patient level collected in this study can be made available externally owing to internal regulations, patient consent, and data-regulations for outside Erasmus Medical Center. Yet, researchers interested in collaboration should contact the corresponding author.
Funding
None to report for this meta-analysis. For the individual trials, the CHAMPION and GUIDE HF trials were sponsored by Abbott Laboratories (Illinois, USA). The MONITOR-HF trial was an investigator-initiated study sponsored by the Dutch ministry of health with the innovation grant 2018 by the Health Care Institute for conditional reimbursement. Abbott Laboratories (Illinois, USA) was obligated to extend the grant by covering the clinical study costs with no part in the design, or conduct of the study or any of its components, analyses and/or writing.
References
- 1. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. . 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021;42:3599–3726. 10.1093/eurheartj/ehab368 [DOI] [PubMed] [Google Scholar]
- 2. Chioncel O, Lainscak M, Seferovic PM, Anker SD, Crespo-Leiro MG, Harjola V-P, et al. . Epidemiology and one-year outcomes in patients with chronic heart failure and preserved, mid-range and reduced ejection fraction: an analysis of the ESC heart failure long-term registry. Eur J Heart Fail 2017;19:1574–1585. 10.1002/ejhf.813 [DOI] [PubMed] [Google Scholar]
- 3. Ambrosy AP, Fonarow GC, Butler J, Chioncel O, Greene SJ, Vaduganathan M, et al. . The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. J Am Coll Cardiol 2014;63:1123–1133. 10.1016/j.jacc.2013.11.053 [DOI] [PubMed] [Google Scholar]
- 4. Adamson PB. Pathophysiology of the transition from chronic compensated and acute decompensated heart failure: new insights from continuous monitoring devices. Curr Heart Fail Rep 2009;6:287–292. 10.1007/s11897-009-0039-z [DOI] [PubMed] [Google Scholar]
- 5. Abraham WT, Adamson PB, Bourge RC, Aaron MF, Costanzo MR, Stevenson LW, et al. . Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet 2011;377:658–666. 10.1016/S0140-6736(11)60101-3 [DOI] [PubMed] [Google Scholar]
- 6. Abraham WT, Stevenson LW, Bourge RC, Lindenfeld JA, Bauman JG, Adamson PB. Sustained efficacy of pulmonary artery pressure to guide adjustment of chronic heart failure therapy: complete follow-up results from the CHAMPION randomised trial. Lancet 2016;387:453–461. 10.1016/S0140-6736(15)00723-0 [DOI] [PubMed] [Google Scholar]
- 7. Lindenfeld J, Zile MR, Desai AS, Bhatt K, Ducharme A, Horstmanshof D, et al. . Haemodynamic-guided management of heart failure (GUIDE-HF): a randomised controlled trial. Lancet 2021;398:991–1001. 10.1016/S0140-6736(21)01754-2 [DOI] [PubMed] [Google Scholar]
- 8. Mullens W, Sharif F, Dupont M, Rothman AMK, Wijns W. Digital health care solution for proactive heart failure management with the Cordella heart failure system: results of the SIRONA first-in-human study. Eur J Heart Fail 2020;22:1912–1919. 10.1002/ejhf.1870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sharif F, Rosenkranz S, Bartunek J, Kempf T, Assmus B, Mahon NG, et al. . Safety and efficacy of a wireless pulmonary artery pressure sensor: primary endpoint results of the SIRONA 2 clinical trial. ESC Heart Fail 2022;9:2862–2872. 10.1002/ehf2.14006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, et al. . 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. Circulation 2022;145:e895–e1032. 10.1161/CIR.0000000000001063 [DOI] [PubMed] [Google Scholar]
- 11. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. . The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brugts JJ, Radhoe SP, Clephas PRD, Aydin D, van Gent MWF, Szymanski MK, et al. . Remote haemodynamic monitoring of pulmonary artery pressures in patients with chronic heart failure (MONITOR-HF): a randomised clinical trial. Lancet 2023. 10.1016/S0140-6736(23)00923-6 [DOI] [PubMed] [Google Scholar]
- 13. Iaconelli A, Pellicori P, Caiazzo E, Rezig AO, Bruzzese D, Maffia P, et al. . Implanted haemodynamic telemonitoring devices to guide management of heart failure: a review and meta-analysis of randomised trials. Clin Res Cardiol 2022:1–13. 10.1007/s00392-022-02104-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Curtain JP, Lee MMY, McMurray JJ, Gardner RS, Petrie MC, Jhund PS, et al. . Efficacy of implantable haemodynamic monitoring in heart failure across ranges of ejection fraction: a systematic review and meta-analysis. Heart 2023;109:823–831. 10.1136/heartjnl-2022-321885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bramer WM, de Jonge GB, Rethlefsen ML, Mast F, Kleijnen J. A systematic approach to searching: an efficient and complete method to develop literature searches. J Med Libr Assoc 2018;106:531–541. 10.5195/jmla.2018.283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Adamson PB, Abraham WT, Bourge RC, Costanzo MR, Hasan A, Yadav C, et al. . Wireless pulmonary artery pressure monitoring guides management to reduce decompensation in heart failure with preserved ejection fraction. Circ Heart Fail 2014;7:935–944. 10.1161/CIRCHEARTFAILURE.113.001229 [DOI] [PubMed] [Google Scholar]
- 17. Givertz MM, Stevenson LW, Costanzo MR, Bourge RC, Bauman JG, Ginn G, et al. . Pulmonary artery pressure-guided management of patients with heart failure and reduced ejection fraction. J Am Coll Cardiol 2017;70:1875–1886. 10.1016/j.jacc.2017.08.010 [DOI] [PubMed] [Google Scholar]
- 18. Loh JP, Barbash IM, Waksman R. Overview of the 2011 Food and Drug Administration circulatory system devices panel of the medical devices advisory committee meeting on the CardioMEMS champion heart failure monitoring system. J Am Coll Cardiol 2013;61:1571–1576. 10.1016/j.jacc.2012.08.1035 [DOI] [PubMed] [Google Scholar]
- 19. Sterne JAC, Savovic J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. . Rob 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:l4898. 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 20. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–188. 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 21. Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw 2010;36:1–48. 10.18637/jss.v036.i03 [DOI] [Google Scholar]
- 22. Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, et al. . 2009 focused update incorporated into the ACC/AHA 2005 guidelines for the diagnosis and management of heart failure in adults: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines developed in collaboration with the International Society for Heart and Lung Transplantation. J Am Coll Cardiol 2009;53:e1–e90. . [DOI] [PubMed] [Google Scholar]
- 23. Zile MR, Desai AS, Costanzo MR, Ducharme A, Maisel A, Mehra MR, et al. . The GUIDE-HF trial of pulmonary artery pressure monitoring in heart failure: impact of the COVID-19 pandemic. Eur Heart J 2022;43:2603–2618. 10.1093/eurheartj/ehac114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cowie MR, Cleland JGF. The COVID-19 pandemic and heart failure: lessons from GUIDE-HF. Eur Heart J 2022;43:2619–2621. 10.1093/eurheartj/ehac226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vaduganathan M, DeFilippis EM, Fonarow GC, Butler J, Mehra MR. Postmarketing adverse events related to the CardioMEMS HF system. JAMA Cardiol 2017;2:1277–1279. 10.1001/jamacardio.2017.3791 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No aggregate or patient level collected in this study can be made available externally owing to internal regulations, patient consent, and data-regulations for outside Erasmus Medical Center. Yet, researchers interested in collaboration should contact the corresponding author.