Abstract
INTRODUCTION:
Chronic idiopathic constipation (CIC) is a common disorder associated with significant impairment in quality of life. This clinical practice guideline, jointly developed by the American Gastroenterological Association and the American College of Gastroenterology, aims to inform clinicians and patients by providing evidence-based practice recommendations for the pharmacological treatment of CIC in adults.
METHODS:
The American Gastroenterological Association and the American College of Gastroenterology formed a multidisciplinary guideline panel that conducted systematic reviews of the following agents: fiber, osmotic laxatives (polyethylene glycol, magnesium oxide, lactulose), stimulant laxatives (bisacodyl, sodium picosulfate, senna), secretagogues (lubiprostone, linaclotide, plecanatide), and serotonin type 4 agonist (prucalopride). The panel prioritized clinical questions and outcomes and used the Grading of Recommendations Assessment, Development, and Evaluation framework to assess the certainty of evidence for each intervention. The Evidence to Decision framework was used to develop clinical recommendations based on the balance between the desirable and undesirable effects, patient values, costs, and health equity considerations.
RESULTS:
The panel agreed on 10 recommendations for the pharmacological management of CIC in adults. Based on available evidence, the panel made strong recommendations for the use of polyethylene glycol, sodium picosulfate, linaclotide, plecanatide, and prucalopride for CIC in adults. Conditional recommendations were made for the use of fiber, lactulose, senna, magnesium oxide, and lubiprostone.
DISCUSSION:
This document provides a comprehensive outline of the various over-the-counter and prescription pharmacological agents available for the treatment of CIC. The guidelines are meant to provide a framework for approaching the management of CIC; clinical providers should engage in shared decision making based on patient preferences as well as medication cost and availability. Limitations and gaps in the evidence are highlighted to help guide future research opportunities and enhance the care of patients with chronic constipation.
Keywords: Fiber, Polyethylene Glycol, Magnesium Oxide, Lactulose, Docusate, Bisacodyl, Senna, Sodium Picosulfate, Lubiprostone, Linaclotide, Plecanatide, Prucalopride
Executive Summary
Chronic idiopathic constipation (CIC) is a common clinical diagnosis that affects approximately 8%–12% of the US population.1 Nonpharmacological therapies often represent the initial steps in management and may include dietary recommendations (such as increased fluid intake and increased dietary fiber) and behavioral changes (such as exercise). Pharmacological treatment may include the use of over-the-counter (OTC) or prescription medications, such as polyethylene glycol (PEG), secretagogues, or prokinetic agents.2 This joint evidence-based guideline from the American Gastroenterological Association (AGA) and the American College of Gastroenterology (ACG) aims to provide recommendations for the pharmacological management of CIC in adults.
How to Read These Guidelines
Table 1 provides an overview of each guideline recommendation along with the associated certainty of evidence and the strength of recommendation. Additional information about the background, methods, evidence reviews, and detailed justifications for each recommendation is provided after Table 1 for readers wishing to read the full guideline. Corresponding forest plots for each intervention and evidence profiles which provide a synthesis of the evidence as well as Evidence to Decision (EtD) framework tables that summarize the panel’s detailed judgments supporting each recommendation are provided in the Supplementary Material. Each recommendation is accompanied by clinical practice considerations (based on the collective experience of the panel members) that are meant to help guideline users implement the recommendations. The term “recommend” was used to indicate strong recommendations, and the term “suggest” was used to indicate conditional recommendations. The interpretation of certainty of evidence and implications of strong and conditional recommendations for healthcare providers, patients, and policymakers are presented in Tables 2 and 3, respectively. For all the recommendations, the alternative approach was management of CIC without the intervention.
Table 1.
Summary of Recommendations and Implementation Considerations
| Recommendations | Strength of recommendation | Certainty of evidence |
|---|---|---|
| Fiber | ||
| Recommendation 1: In adults with CIC, the panel suggests the use of fiber supplementation over management without fiber supplements Implementation considerations
|
Conditional | Low |
| Osmotic laxatives | ||
| Recommendation 2: In adults with CIC, the panel recommends the use of PEG compared with management without PEG Implementation considerations
|
Strong | Moderate |
| Recommendation 3: In adults with CIC, the panel suggests the use of magnesium oxide over management without magnesium oxide Implementation considerations
|
Conditional | Very low |
| Recommendation 4: In adults with CIC who fail or are intolerant to OTC therapies, the panel suggests the use of lactulose over management without lactulose Implementation considerations
|
Conditional | Very low |
| Stimulant laxatives | ||
| Recommendation 5: In adults with CIC, the panel recommends the use of bisacodyl or sodium picosulphate short term or as rescue therapy over management without bisacodyl or sodium picosulphate Implementation considerations
|
Strong | Moderate |
| Recommendation 6: In adults with CIC, the panel suggests the use of senna over management without senna Implementation considerations
|
Conditional | Low |
| Secretagogues | ||
| Recommendation 7: In adults with CIC who do not respond to OTC agents, the panel suggests the use of lubiprostone over management without lubiprostone Implementation considerations
|
Conditional | Low |
| Recommendation 8: In adults with CIC who do not respond to OTC agents, the panel recommends the use of linaclotide over management without linaclotide Implementation considerations
|
Strong | Moderate |
| Recommendation 9: In adults with CIC who do not respond to OTC agents, the panel recommends the use of plecanatide over management without plecanatide Implementation considerations
|
Strong | Moderate |
| 5-HT4 agonist | ||
| Recommendation 10: In adults with CIC who do not respond to OTC agents, the panel recommends the use of prucalopride over management without prucalopride Implementation considerations
|
Strong | Moderate |
NOTE. The implementation considerations are based on the collective experience of the panel members, and evidence may not be available for each of the implementation considerations.
5-HT4, serotonin type 4; CIC, chronic idiopathic constipation; OTC, over-the-counter; PEG, polyethylene glycol.
Table 2.
Interpretation of the Certainty of Effects Using the GRADE Framework
| Certainty of evidence | Definition |
|---|---|
| High | We are very confident that the true effect lies close to that of the estimate of the effect. |
| Moderate | We are moderately confident that the true effect lies close to that of the estimate of the effect. There is a possibility that it is substantially different. |
| Low | Our confidence that the true effect lies close to that of the estimate of the effect is low. The true effect may be substantially different from the estimate of the effect. |
| Very low | Our confidence that the true effect lies close to that of the estimate of the effect is very low. The true effect is likely substantially different from the estimate of the effect. |
GRADE, Grading of Recommendations Assessment, Development, and Evaluation.
Table 3.
Interpretation of a Strong and Conditional Recommendation
| Implications | Strong recommendation | Conditional recommendation |
|---|---|---|
| For patients | Most individuals in this situation would want the recommended course of action and only a small proportion would not. | The majority of individuals in this situation would want the suggested course of action, but many would not. |
| For clinicians | Most individuals should receive the intervention. Formal decision aids are not likely to be needed to help individuals make decisions consistent with their values and preferences. | Different choices will be appropriate for individual patients consistent with his or her values and preferences. Use shared decision making. Decision aids may be useful in helping patients make decisions consistent with their individual risks, values, and preferences. |
| For policy makers | The recommendation can be adapted as policy or performance measure in most situations. | Policy making will require substantial debate and involvement of various stakeholders. Performance measures should assess whether decision making is appropriate. |
Introduction
Description of the Health Problem
CIC is a common clinical diagnosis that affects approximately 8%–12% of the US population.1 CIC is a lower gastrointestinal (GI) tract disorder of gut-brain interaction and can be associated with symptoms such as infrequent and incomplete defecation in the absence of mucosal or structural abnormalities.2,3 The medical costs related to the management of constipation are estimated to be between approximately $2,000 and $7,500 US dollars per patient per year, and the effects on quality of life can be similar to those associated with conditions such as chronic obstructive pulmonary disease, diabetes, and depression.4,5 Nonpharmacological interventions often represent the initial step in management and may include dietary (such as increased fluid intake and increased dietary fiber) and behavioral changes (such as exercise). Pharmacological treatment may include the use of PEG, secretagogues, and prokinetic agents.2,6,7 Overall, a significant proportion of patients with CIC are not satisfied with their treatment and may use multiple OTC medications, followed by prescription medications before they have improvement in their symptoms.8-10
Objective of the Review and Guideline
The AGA and ACG jointly developed this systematic review and clinical guideline to provide evidence-based recommendations for the pharmacological management of CIC in adults.
Target Audience
The target audience for these guidelines includes primary care, internal medicine, family medicine, and gastroenterology healthcare providers; patients; and policymakers. The recommendations in this document are not intended to be used as the standard of care. Instead, they can be used to guide the management of adult patients with CIC. Although no single recommendation can encompass every individual circumstance and context, it can be used to address the benefits and harms of treatments and support the processes of shared decision making so that patients are treated based on their values and preferences.
Methods
Overview
This document represents the official recommendations of the AGA and ACG. These recommendations were developed using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach.
Organization and Panel Composition
The guideline panel members from the AGA and ACG were selected based on their clinical and methodological expertise. Each member underwent a vetting process that required disclosing all conflicts of interest. The panel included 3 guideline committee members specializing in general gastroenterology, motility, and primary care. Panel members comprising the evidence review team (divided into 3 subcommittees) included gastroenterologists with expertise in CIC, 1 senior methodologist, and 3 junior methodologists. All included interventions were divided among the 3 subcommittees (see Supplementary Table 1). The senior methodologist supervised the evidence synthesis for all the interventions across the 3 subcommittees. Members of the guideline committee helped review all the synthesized evidence, contributed to discussion, and helped develop the clinical decision support tool. A librarian assisted with designing and executing the relevant literature searches. An executive committee of members of the AGA and ACG were responsible for oversight of this collaborative guideline (S.S., W.D.C., L.C., A.L.).
Management of Conflict of Interest and Guideline Funding
Panel members disclosed all potential conflicts of interest. Conflicts were managed according to AGA and ACG policies, the National Academy of Medicine, and Guidelines International Network standards.11-13 Panel members determined to have a potential conflict of interest with a specific intervention or agent were allocated to a subcommittee that did not include the specific intervention(s). Development of this guideline was wholly funded by the AGA and ACG with no support from the industry.
Scope
The guideline panel and evidence review team formulated clinically relevant questions on the pharmacological therapies for CIC in adults. The last position paper by the AGA on CIC included guidance on clinical evaluation, diagnostics tests, and medical and surgical management.6 This document does not specifically address considerations related to special populations such as those with malignancy, pregnancy, or opioid-induced constipation.
Formulation of Clinical Questions and Determining Outcomes of Interest
PICO format
For each guideline question, the evidence review team conducted a systematic review. The systematic review was based on specific Population, Intervention, Comparison, Outcome (PICO) questions developed by the guidelines committee and approved by the Boards of both organizations. A protocol guided the systematic review process and is registered at the international Prospective Register of Systematic Reviews website (CRD42021254673). In summary, we included individual randomized controlled trials (RCTs). We also considered the trials with multiple arms and included comparisons where the only difference between the 2 groups was the intervention of interest. If a study included multiple treatment arms but only 1 comparison group, we combined the treatment arms when appropriate so that the comparison group was not counted twice in the meta-analysis.
Population
The population of interest was adults (18 years or older) diagnosed with CIC. We excluded studies where individuals were diagnosed with other similar conditions such as opioid-induced constipation or constipation due to other medical conditions such as hypothyroidism and celiac disease. We also excluded studies in patients with irritable bowel syndrome with constipation (IBS-C) because the pharmacological management of IBS-C was covered in another recent AGA14 and ACG guideline.15
Intervention
We included the following interventions: fiber: psyllium, bran, methylcellulose, and inulin; osmotic or surfactant laxatives: PEG, magnesium oxide (MgO), lactulose, and docusate; stimulant laxatives: bisacodyl, senna, and sodium picosulfate; secretagogues: lubiprostone, linaclotide, and plecanatide; and serotonin type 4 (5-HT4) agonist: prucalopride. We considered studies that included the above interventions, regardless of the dose or route of administration. We included studies in which the intervention duration was at least 4 weeks. We analyzed all the interventions separately.
Comparison
The comparison group included placebo, no intervention, or standard of care. We excluded studies that compared different doses or frequencies of the same drug and did not include a comparison group that did not receive the drug. We also excluded studies that compared different pharmacological agents for CIC, and there was no placebo group.
Outcomes
We considered the following outcomes: complete spontaneous bowel moments (CSBMs) per week; spontaneous bowel movements (SBMs) per week; responder rate, defined as CSBM per week of equal or greater than 3 and increased by at least 1 from baseline; diarrhea (adverse event) leading to discontinuation of treatment; serious adverse events; global relief outcome; quality-of-life scores (using the Patient Assessment of Constipation-Quality of Life, or PAC-QOL); and stool form. We considered the outcomes of CSBMs per week, SBMs per week, and adverse events leading to discontinuation of medication as the critical outcomes.
Search Strategy
A comprehensive search was conducted on the following databases: EMBASE, MEDLINE, Cochrane, Scopus, Web of Science, ClinicalTrials.gov, Centre for Reviews and Dissemination, and PubMed. We also reviewed the reference sections of available systematic reviews7,8,16,17 and updated the searches if a recent systematic review was available. The literature was first searched on May 15, 2021, and the search was updated on November 5, 2022. The search terms used can be found in Appendix 1 (see Supplementary Material).
Study Selection, Data Collection, and Analysis
Searches from all the databases were combined in bibliographic software (EndNote),18 and duplicates were removed. Two reviewers screened the titles and conducted a full-text review of the eligible studies (using a reference software Covidence), and a consensus was reached on inclusion.19 Any conflicts were resolved with the help of a senior member of the team. Data were extracted from each study, including study characteristics, such as year of publication, study site, study population, dose and frequency of intervention, comparison group, outcomes and methods for risk-of-bias assessment. Meta-analysis was conducted when more than 1 study contributed data for the same intervention and outcome. We combined the dichotomous outcomes to obtain a relative risk (RR) and 95% confidence interval (CI). We used the mean difference (MD) to pool the continuous outcomes.20 For the meta-analysis, we used the generic inverse variance method of weighting and applied the random-effects model. We assessed the statistical heterogeneity by using the I2 index and χ2 statistic. We used funnel plots to assess the small study effect and publication bias when at least 10 studies were available in a pooled analysis. We used RevMan21 software for all the statistical analyses. We used the Cochrane Risk of Bias tool to assess the risk of bias in the included studies.22 This tool assesses the risk of bias in the following domains: sequence generation, allocation concealment, blinding, incomplete outcome data, selective reporting, and other biases.22
Certainty of the Evidence
We used the GRADE approach to assess the certainty of evidence for the effect of the intervention on each outcome using the software GradePro.23 The GRADE approach considers factors such as study design, risk of bias, inconsistency, indirectness, imprecision, and risk of publication bias to rate the certainty of evidence as high, moderate, low, or very low (Table 2).24 The results of certainty assessment are reported in evidence profiles available in the Supplementary Material for all the interventions included in this review.
Development of Recommendations
The process of translation of evidence into guideline recommendations followed the GRADE EtD framework25 and was achieved by discussion during virtual meetings of the guideline committee. The EtD framework considers the certainty of evidence, balance of benefits and harm, patient values and preferences, feasibility, acceptability, equity, and resource use.25 All 10 EtD tables are presented in the Supplementary Material. Consensus was reached for all the recommendations among the group. The interpretation of strength of recommendations is summarized in Table 3.
Document Review
The guideline underwent expedited internal and external peer review. The guideline document was revised to address pertinent comments, but no changes were made to the direction or strength of recommendations.
Recommendations
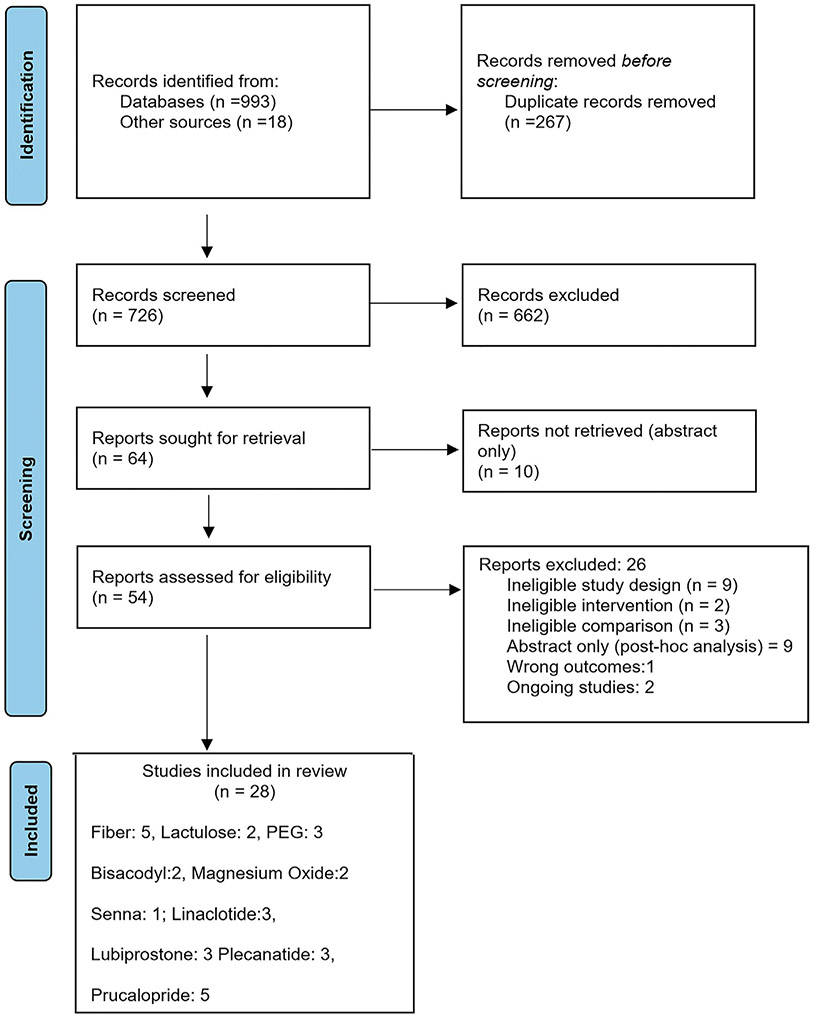
The literature search yielded 993 titles, and a total of 726 titles and abstracts were screened after the duplicates were removed. Of 54 studies reviewed with full text, 28 studies were included in evidence synthesis and 14 studies were excluded (Figure 1). Supplementary Table 2 gives the reason for exclusion of studies. The summary estimates for effect of a specified intervention on each of the prespecified outcome are included as forest plots and evidence profiles for each PICO question and are available in the Supplementary Material.
Figure 1.
PRISMA flow diagram. Six studies on fiber supplements included studies on the use of insulin (1 study), bran (1 study), and psyllium (3 studies). One study addressed magnesium oxide and senna in the same trial, so the total number of included studies is 28 and not 29. PEG, polyethylene glycol.
Table 1 provides a summary of the recommendations presented in this guideline. More detailed information regarding the medication indication, dosing, availability, and mechanism of action is summarized in Table 4.
Table 4.
Overview of Interventions for the Pharmacological Management of Chronic Idiopathic Constipation
| What medications can be used to treat chronic idiopathic constipation? |
Mechanism of action | Recommended initial dose |
Guidance for dose titration | Maximum dose | Estimated monthly cost, USDa |
Additional comments |
|---|---|---|---|---|---|---|
| Fiber | Soluble fiber traps water in the intestine and softens stool Insoluble fiber increases stool bulk |
The Academy of Nutrition and Dieteticsa recommends 14 g/1,000 kcal intake per day Total daily fiber intake (dietary + supplement) 20–30 g/d |
Per response to symptoms and side effects Common side effects include bloating and abdominal discomfort |
Usually no benefit to increasing total fiber intake over 25–30 g | <$50 | Ensure adequate hydration as fiber intake increases No clear evidence that soluble or insoluble fiber is more effective Soluble fiber includes psyllium, inulin, oats, fruit, barley, and legumes Insoluble fiber includes wheat bran, methylcellulose, wheat, rye, and other grains |
| Polyethylene glycol | Osmotic laxative | 17 g daily | Per symptom response and side effects Common side effects include bloating, abdominal discomfort, and cramping |
No clear maximum dose | $10-$45 | Response to PEG has been shown to be durable over 6 mo |
| Magnesium oxide | Osmotic laxative | 400–500 mg daily | Per symptom and response and side effects | No clear maximum dose. Prior studies used 1,000–1,500 mg daily | <$50 | Use with caution in patients with renal insufficiency and in pregnancy |
| Lactulose | Osmotic laxative | 15 g daily | Per symptom response and side effects Bloating and flatulence may be limiting if preexisting symptoms or at higher doses |
No clear maximum dose. May cause hypernatremia and hypokalemia if patients experience significant diarrhea | <$50 | Only osmotic agent studied in pregnancy |
| Bisadocyl and picosulfate | Stimulant laxative | Bisacodyl 5 mg daily | Per symptom response and side effects Side effects limited by cramping and abdominal discomfort |
10 mg orally daily | <$50 | Recommended for short-term use or rescue therapy Prolonged or excessive use can cause diarrhea and electrolyte imbalance Long-term safety and efficacy unknown |
| Senna | Stimulant laxative | 8.6–17.2 mg daily | Per symptom response and side effects Side effects most commonly cramping and abdominal discomfort |
No clear maximum dose. Often recommended maximum is 4 tablets twice per day | <$50 | Also present in many laxative teas, where dose may be difficult to calculate Long-term safety and efficacy unknown |
| Lubiprostone | Intestinal secretagogue acting on chloride channel type 2 in the gut that increases chloride secretion | 24 μg bid | Per symptom response Diarrhea may occur in a subset of patients, leading to discontinuation |
24 μg BID | $374 | May have benefit for abdominal pain. Also approved for the treatment of IBS-C at a dose of 8 μg BID |
| Linaclotide | Intestinal secretagogue acting on guanylate cyclase-C, which activates CFTR in the gut to increase chloride secretion | 72–145 μg daily | Per symptom response Diarrhea may occur in a subset of patients, leading to discontinuation |
290 μg daily | $523 | May have benefit for abdominal pain. Also approved for the treatment of IBS-C |
| Plecanatide | Intestinal secretagogue acting on guanylate cyclase-C, which activates CFTR in the gut to increase chloride secretion | 3 mg daily | Per symptom response Diarrhea may occur in a subset of patients, leading to discontinuation |
3 mg daily | $526 | Also approved for the treatment of IBS-C |
| Prucalopride | 5-HT4 agonist | 1–2 mg daily | Per symptom response Headaches and diarrhea may occur in a subset of patients, leading to discontinuation |
2 mg daily | $563 | May have additional benefit for abdominal pain |
5-HT4, serotonin type 4; BID, twice a day; IBS-C, irritable bowel syndrome with constipation; PEG, polyethylene glycol; USD, US dollar.
The given cost accommodates the extent of generic and prescription medications and may not be the exact cost. In addition, the given cost is not the cost-effectiveness of the medication, but a probable cost per month.
Fiber
| Recommendation 1: In adults with CIC, the panel suggests the use of fiber supplementation over management without fiber supplements (conditional recommendation, low certainty of evidence). |
Implementation considerations
|
We evaluated evidence for fiber supplementation in the form of bran, inulin, psyllium, and methylcellulose. The overall certainty of evidence for the use of fiber in the management of CIC compared with management without fiber was low. No studies were found on the use of methylcellulose, but data on bran, inulin, and psyllium are out-lined below.
Bran
Summary of evidence
One randomized study conducted in Italy with a cross-over design evaluated the efficacy and safety of bran in 29 patients with chronic nonorganic constipation.26 Participants received either a ground bran (6.6 g) or an identical-looking preparation of placebo 3 times a day for two 4-week periods.26
Benefits and harms
Based on this 1 small study, bran may lead to an increase in SBMs per week; however, the CI was wide and included a possible null effect (MD 1.30, CI −0.98 to 3.58).26 Only 1 adverse event was noted in the treatment group compared with no events in the placebo arm (RR 2.79, CI 0.12–62.48).26 There were no data on CSBMs per week, responder rates, diarrhea, global relief, quality of life, or stool form.
Certainty in evidence of effects
We are very uncertain about the effects of bran. The overall certainty of evidence for bran is very low because of concerns about the adequacy of randomization and allocation concealment as well as very serious imprecision.26
Inulin
Summary of evidence
Two studies assessed the effect of inulin for the treatment of CIC.27,28 The first study was a randomized placebo-controlled study conducted in Brazil involving 60 female participants aged 18–65 years with at least 3 months of constipation and <3 bowel movements per day.27 Twenty-eight patients were in the fiber group and 32 in the placebo group.27 Participants were given 4 days to adapt to the mixture of inulin and partially hydrolyzed guar gum or placebo before beginning a 3-week treatment with either 15g/d of inulin or 15 g/d of malto-dextrin (placebo) that was divided into 3 sachets of 5 g each.27 The second study was conducted in Belgium on participants aged 50–70 years who were randomized to 7.5 g of inulin sachet and placebo for 28 days.28 The inulin and placebo groups included 25 participants each. The duration of follow-up was 28 days.
Benefits and harms
All included studies did not contribute data for our outcomes of interest. Based on one small study, treatment with inulin had little to no effect on SBMs per week (MD −0.75, CI −2.60 to 1.10) and responder rate, defined as >3 CSBMs per week (RR 1.21, CI 0.83–1.74).27 Regarding side effects, no serious adverse events were reported, although a minor side effect of flatulence occurred more frequently in the inulin group, according to one study.28 There were no data on CSBMs per week, diarrhea, serious adverse effects, quality of life, or stool form.
Certainty in evidence of effects
We are very uncertain about the effects of inulin. The certainty of evidence for the effect of inulin on SBMs and the responder rate was very low and low, respectively, because of concerns about risk of bias and imprecision because of the small number of included participants in the study.
Psyllium
Summary of evidence
Three RCTs conducted between 1986 and 1995 studied psyllium vs placebo.29-31 Psyllium was evaluated in a small study from the United States that included 22 adult participants with CIC confirmed by prospectively administered stool diaries.29 Constipation was defined as <3 bowel movements per week. A 4-week baseline phase was followed by 8 weeks of double-blinded treatment, during which patients received either 5 g twice daily of psyllium or placebo, followed by a 4-week washout period.29 The second study included 201 participants with functional constipation between 18 and 70 years.30 Participants were randomly allocated to either Regulan (a refined hydrophilic mucilloid derived from psyllium seed husks) or matching placebo for 14 days.30 Participants received one sachet (containing 3.6 g of active ingredient) of either psyllium or placebo 3 times daily.30 A third study included 35 participants with constipation who were randomized to receive either celandine-aloe vera-psyllium or placebo.31 Capsules of 500 mg contained the active ingredients celandine, aloe vera, and psyllium in the ratio of 6:3:1. The initial dose was 1 capsule per day, taken with water at bedtime, and increased to 3 capsules per day depending on the response.31
Benefits and harms
Based on the meta-analysis of data from 3 studies,29-31 the use of psyllium may lead to an increase in SBMs per week (MD 2.32, CI 0.86–3.79). Combined data from 2 studies showed that the use of psyllium may increase global relief symptoms (RR 1.86, CI 1.49–2.30), but there was little to no difference in stool consistency (MD −1.08, CI −1.33 to 0.83).30,31 In absolute terms, psyllium was associated with 391 per 1,000 more individuals with global relief (from 223 more to 591 more). One study30 examined withdrawal from the study because of diarrhea but with only 3 events; the summary estimate was too imprecise to make any conclusive statement (RR 0.47, CI 0.04–5.06); and no serious adverse events were reported in either arm. There were no data on CSBMs per week, responder rate, diarrhea, quality of life, or stool form.
Certainty in evidence of effects
The certainty of evidence for SBM, serious adverse events, and global relief was low and for diarrhea, was very low. We rated down certainty of evidence because of high risk of bias (concerns about methods of randomization and allocation concealment in 2 studies and high attrition in another study), indirectness, and imprecision.
Discussion
Fiber can be divided into soluble and insoluble fiber. Wheat bran, an insoluble fiber, is produced when the hard outer fiber of the wheat kernel is removed during the refining process. Inulin is a naturally occurring polysaccharide present in many plants and most often extracted from chicory. Inulin is a fructan and is considered both a soluble fiber and a prebiotic, meaning that it can stimulate the growth or activity of intestinal bacteria32 that are believed to promote good gut health. Psyllium is also considered to be a soluble fiber and may also have prebiotic potential.33 Fiber has been recognized as important for normal laxation, primarily because it increases stool weight, and this has the secondary effect of reducing transit time. Fiber increases stool weight by its presence but also by increasing the water held by the fiber, as well as increasing bacterial mass from fermentation. However, inulin does not increase stool weight to the extent that wheat bran and psyllium do, but does undergo extensive fermentation.32
Fiber is often recommended as a therapy to supplement dietary intake of fiber. However, as the studies above indicate, there are different formulations and types of fibers that have been evaluated and the included studies did not quantify the intake of dietary fiber. At least 2 studies were conducted with fiber that contained other ingredients such as milk or aloe vera that may additionally influence laxation. All studies are 30–40 years old, and the number of participants in the studies has been small and most are primarily conducted in women. Most of the included studies on fiber supplementation did not report on relevant patient important outcomes. The best data exist for psyllium, but even those are of low quality. In addition, wheat bran can exist as a finely ground powder that can decrease stool water content and harden stool.34 The chief side effect of fiber supplementation seems to be flatulence. In individuals with mild-to-moderate symptoms of constipation, especially who consume diets deficient in fiber, a trial of fiber supplementation is warranted because it is low-risk, low-cost, and easily accessible. In general, chronically constipated patients and nonconstipated persons drink similar amounts of fluid on a daily basis. However, when individuals are placed in quartiles based on daily fluid intake, those in the lowest quartile for fluid intake are more likely to be constipated. Thus, efforts to increase fluid intake should be focused on those with low levels of fluid intake.35 Standard doses of fiber supplements are typically taken with 8–10 ounces of fluid.36
Osmotic Laxatives
| Recommendation 2: In adults with CIC, the panel recommends the use of PEG compared with management without PEG (strong recommendation, moderate certainty of evidence). |
Implementation considerations
|
Polyethylene Glycol
Summary of evidence
Three randomized, placebo-controlled trials studied the effect of PEG on constipation.37-39 Two of these studies were multicenter trials conducted in Italy and enrolled participants aged 18–70 years with chronic constipation defined in accordance with the Rome criteria, that is, less than 2 bowel movements per week for at least 12 months or the presence of 2 or more of the following symptoms: <3 bowel movements per week, straining at defecation, sense of incomplete evacuation, and hard stools on at least 25% of occasions.37,38 In both trials, the treatment consisted of 17.5 g of PEG with electrolytes as a granular preparation or placebo dissolved in 250 mL of water taken twice daily. One study included 55 participants, and the treatment period lasted 8 weeks.38 The second study37 included 70 participants and had 2 consecutive periods in which all participants received the active treatment, PEG for 4 weeks, and then those who responded were randomized to receive either PEG or placebo for 20 weeks. The third study was conducted in the United States and included 304 participants with chronic constipation based on modified Rome criteria, where participants reported <3 bowel movements per week for at least 3 months and one or more of the following: straining, lumpy or hard stools, and sensation of incomplete evacuation in >25% of defecations.39 Participants were randomized in a 2:1 ratio to PEG 3350 (n = 204) at a dose of 17 g or placebo (n = 100) mixed in 8 ounces of liquid once daily for 6 months.
Benefits and harms
PEG likely results in an increase in CSBMs per week compared with placebo (MD 2.90, CI 2.12–3.68), based on one study,39 and SBMs per week (MD 2.30, CI 1.55–3.06), based on meta-analyzed data from 3 studies.37-39 Across 2 studies, a higher rate of individuals met the responder definition, one study defined responder as normalization of bowel moments37,38 and other defined based on the US Food and Drug Administration (FDA) end points,40 compared with placebo (RR 3.13, CI 2.00–4.89); PEG was associated with 312 more per 1,000 (from 146 more to 569 more). Diarrhea was noted more commonly in the treatment arm (158 more per 1,000, from 6 fewer to 896 more). A higher proportion of participants had global relief of symptoms with PEG compared with placebo with 454 per 1,000 in the PEG group (from 159 more to 948 more). There were no data for the following outcomes: stool form and quality of life. Two studies examined serious adverse events, but the number of events was very small, and no conclusive statements could be made about risk of serious adverse events with the use of PEG (RR 0.47, CI 0.16–1.33).
Certainty in evidence of effects
The certainty of evidence for CSBMs, SBMs, responder rate, and global relief was moderate (because of imprecision). The certainty of evidence for serious adverse events was low because the CI includes both low and high risk of serious adverse events. The overall certainty of evidence for PEG was moderate.
Discussion
PEG is a long-chain polymer of ethylene oxide, which acts as an osmotic laxative. PEG is approved at a dose of 17 g daily for the treatment of occasional constipation by the FDA in the United States and is widely available OTC. Two of the studies used PEG with electrolytes given twice daily37,38 while the other larger study evaluated the efficacy of PEG 3350 without electrolytes administered once daily.39 The 2 studies of PEG 3350 electrolytes measured the frequency of SBMs per week, but not CSBMs per week,37,38 while the PEG 3350-only study measured CSBM and SBM frequency along with other outcomes.39 Despite the differences in the PEG preparations, doses, and treatment durations, the studies all demonstrated that PEG was associated with a greater efficacy in increasing CSBMs, SBMs, responder rate, improvements in stool form, straining, and global relief compared with placebo, but not abdominal pain. Although PEG is approved by the FDA for the treatment of occasional constipation and not CIC, it has been shown to be efficacious in individuals with CIC for up to 6 months.39 There are additional treatment trials comparing the efficacy of PEG with tegaserod, prucalopride, and lactulose, in which PEG demonstrated a similar or greater efficacy in individuals with CIC than these other medications,41,42 although these trials used different primary end points.
There were no differences in side effect profiles observed between PEG and placebo, although data are limited. Individuals treated with PEG may experience bloating, flatulence, and diarrhea. These effects are consistent with and expected from laxative therapy, and most of these events were mild or moderate. However, PEG is widely available without the need for a prescription and is relatively inexpensive. It is, therefore, reasonable to use PEG earlier in the algorithm for the management of CIC, either after a trial of fiber supplementation or in combination with fiber supplementation.
| Recommendation 3: In adults with CIC, the panel suggests the use of MgO over management without MgO (conditional recommendation, very low certainty of evidence certainty). |
Implementation considerations
|
Magnesium Oxide
Summary of evidence
Two randomized, placebo-controlled trials evaluated the use of MgO for the management of CIC.43,44 Both trials were completed in Japan. The dose of MgO studied was 1.5 g/d for 4 weeks. The 2 trials randomized a total of 47 participants to MgO and 47 participants to the placebo arm, and 93% of the participants were females. At baseline, participants randomized to the placebo group had 4.6 SBMs per week. Those randomized to MgO had 4 SBMs per week at baseline.
Benefits and harms
Compared with placebo, treatment with MgO may increase the number of CSBMs per week (MD 4.29, 95% CI 2.93–5.65) and SBMs per week (MD 3.59, 95% CI 2.64–4.54). Participants treated with MgO achieved a higher treatment response compared with placebo (RR 3.93, 95% CI 2.04–7.56). In absolute terms, 499 more participants per 1,000 might respond to MgO (from 177 to 1,000 more). There was little to no difference in the degree of diarrhea leading to treatment dose change or discontinuation between the 2 study groups (RR 1.07, 95% CI 0.65–1.74). Participants treated with MgO may have better quality-of-life scores as measured by PAC-QOL (MD 16.23, 95% CI 11.44–21.01) and better stool consistency based on the Bristol Stool Form Scale (MD 1.89, 95% CI 1.44–2.33).
Certainty in evidence of effects
The certainty of evidence was very low for the outcomes of CSBMs per week and SBMs per week because of concerns related to inconsistency, indirectness, and imprecision. The certainty of evidence was moderate for the outcomes of responder rate and adverse events due to diarrhea because of imprecision and inconsistency, respectively. The outcomes of quality of life and stool form were rated as low certainty because of indirectness and imprecision. The overall certainty of evidence for MgO was very low.
Discussion
Magnesium is a naturally occurring element that plays an important role in a wide range of biological and biochemical processes.45 Within the lumen of the GI tract, nonabsorbed magnesium creates an osmotic gradient, which leads to net secretion of water and electrolytes, which can exert a beneficial effect on constipation-related symptoms. MgO dosing in the available RCTs was 1.5 g/d. Although not studied in RCTs, lower MgO doses of 500 mg/d to 1 g/d are often used in clinical practice. Only MgO has been evaluated in RCTs; the bioavailability and clinical efficacy of other formulations of magnesium (eg, citrate, glycinate, lactate, malate, sulfate) for CIC are unknown. Data on adverse effects of MgO from the available trials are limited. The available data suggest no increased reports of diarrhea with MgO compared with placebo.43 Systemic regulation of magnesium levels is maintained by renal excretion.46 Therefore, hypermagnesemia is more likely to occur in individuals with significant renal impairment and magnesium supplements should be avoided in those with a creatinine clearance of <20 mg/dL47
The combination of efficacy, tolerability, availability of OTC, and low cost make MgO an attractive first-line option for individuals with CIC. Limitations to consider include the small number of clinical trials and included participants with CIC, all trials being conducted in Japan, formulations other than MgO not being evaluated, the dose of MgO used in trials being higher than that typically used in clinical practice, and no long-term effectiveness or harms data being available.
| Recommendation 4: In adults with CIC who fail or are intolerant to OTC therapies, the panel suggests the use of lactulose over management without lactulose (conditional recommendation, very low certainty of evidence). |
Implementation considerations
|
Lactulose
Summary of evidence
Two RCTs studied the efficacy of lactulose syrup for the treatment of CIC in elderly participants.48,49 One multicenter study performed in the Netherlands included 103 participants who were regularly taking laxatives for the treatment of chronic constipation.49 The initial dose of 15 mL of either 50% lactulose syrup or 50% glucose syrup was administered daily for a total of 3 weeks. The daily dose was reduced by half after 3 consecutive days with defecation, but if no defecation occurred for more than 48 hours, the dose was doubled. If defecation occurred on 3 consecutive days with the doubled dose, the dose was reduced back to 15 mL.49 The second study, conducted in the United States, included 55 constipated participants.48 Participants received 30 mL daily of either 50% lactulose syrup or 50% glucose syrup, taken at bedtime for 12 weeks.48
Benefits and harms
Based on one study, lactulose may have little to no effect on SBMs per week (MD 0.35, CI −0.91 to 1.61). A second study, however, showed that lactulose may be associated with a large increase in global relief (RR 2.42, CI 1.29–4.54, in absolute terms, 473 more per 1,000, CI 97 more to 1,000 more). Across the 2 studies, there was a higher rate of individuals taking lactulose who met the responder definition (defined as >1 SBM from baseline in 1 study and lack of need of other laxatives in other study) compared with placebo. Lactulose was associated with 267 more per 1,000 (from 108 more to 471 more) responders. The studies did not report on CSBMs per week, diarrhea, serious adverse events, quality of life, or stool form.
Certainty in evidence of effects
The certainty of evidence for SBMs was very low because of risk of bias (unclear methods of randomization and blinding) and imprecision. The certainty of evidence for global relief and responder rate was low because of concerns for risk of bias and imprecision. The overall certainty of evidence for lactulose was very low.
Discussion
Lactulose is β-galactosido-fructose, a synthetic disaccharide not digested in the small intestine that exerts an osmotic laxative effect in the colon to promote peristalsis. It is approved by the FDA in the United States for the treatment of constipation at a dose of 10–20 g (15–30 mL or 1–2 packets) daily and is widely available in other countries. The dose may be increased to 40 g (60 mL or 2–4 packets) daily if needed. There were significant limitations in the 2 RCTs of lactulose; both trials were conducted over 40 years ago, included relatively small numbers of elderly participants, and did not report the diagnostic criteria for constipation.48,49 In the US study, most participants were women living in a nursing home and medical facility and the mean age was in the mid-80s.48 Bowel movement frequency and the severity of symptoms improved to a greater degree in the lactulose group compared with the glucose group. Interestingly, the most dramatic finding was the decrease in impactions and need for enemas in individuals receiving lactulose. The other study from the Netherlands did not report demographics of the patient population.
There were minimal data on adverse events from the 2 published studies;48,49 however, bloating and flatulence (which are dose-dependent) are considered very common side effects of lactulose in clinical practice, which limit its use. Some brands of lactulose may be expensive, although generic lactulose is generally low cost. Lactulose can be considered if symptoms of CIC have failed to improve with fiber and OTC laxatives, and individuals do not experience significant bloating or abdominal pain with lactulose use. The use of lactulose in mildly constipated, noninsulin-dependent patients with diabetes mellitus type 2 may not lead to increase in blood sugar levels.50
Stimulant Laxatives
| Recommendation 5: In adults with CIC, the panel recommends the use of bisacodyl or sodium picosulfate (SPS) short term or as rescue therapy over management without bisacodyl or SPS (strong recommendation, moderate certainty of evidence). |
Implementation considerations
|
Summary of evidence
The panel considered studies that evaluated bisacodyl and SPS, which are mechanistically related, for the management of CIC. Of note, SPS tablets/drops are not available for use in the United States; however, they are approved for use in Europe. In the United States, SPS is available in combination with other laxatives and used for bowel preparation before colonoscopy. Given their common mechanism of action and limited number of trials on these drugs in treatment of CIC, the data from available trials were pooled in calculations of estimates of effect. A total of 2 studies were included. One of these studies was a multicenter randomized double-blinded placebo-controlled trial in the United Kingdom where recruited participants were assigned in a 2:1 ratio to bisacodyl (n = 247) vs placebo (n = 121) and treated for 4 weeks.51 The other study examined effects of SPS in a multicenter double-blinded placebo-controlled RCT conducted in Germany, and participants were randomized in a 2:1 ratio to SPS (n = 229) or placebo (n = 133) and treated for 4 weeks.52
Benefits and harms
Based on meta-analyzed data from 2 studies, SPS likely leads to a large increase in CSBMs per week (MD 2.54, 95% CI 1.07–4.01) and SBMs per week (MD 4.04, 95% CI 2.37–5.71) and improved the consistency of stool on the Bristol Stool Form Scale53 (MD 2.4 points higher, 95% CI 2.07–2.73) and PAC-QOL scores (MD 0.65, 95% CI 0.50–0.80) compared with placebo. Furthermore, the use of SPS leads to higher responder rates (RR 2.60, 95% CI 2.05–3.30) and an increased proportion of individuals with global relief (RR 1.75, 95% CI 1.48–2.07). In absolute terms, of 1,000 individuals treated with SPS, there would be 359 more responders (236 more to 516 more) and 357 more with global relief (228 more to 509 more). Use of SPS may increase the proportion of individuals who experience diarrhea compared with placebo (RR 8.76, 95% CI 4.99–15.39). One study reported serious adverse events but with only 3 events, results were very imprecise (RR 0.24, 95% CI 0.02–2.67). The rate of diarrhea leading to discontinuation of treatment was higher in the SPS group compared with placebo (RR 8.76, 95% CI 4.99–15.39).
Certainty in evidence of effects
The certainty of evidence was rated as moderate for the following outcomes: CSBM and SBM frequency, responder rate, global relief, and stool consistency (because of risk of bias). The certainty of evidence was very low for the outcomes of diarrhea and serious adverse events (because of risk of bias, indirectness, and imprecision) and low for quality of life (because of risk of bias and indirectness). The overall certainty of evidence for bisacodyl was moderate.
Discussion
Bisacodyl and SPS are converted in the gut into the same active metabolite, bis-(phydroxyphenyl)-pyridyl-2-methane (BHPM). Bisacodyl is converted into BHPM by small bowel and colonic mucosal deacetylase enzymes while SPS is converted into BHPM by colonic bacteria desulfate enzymes. BHPM acts directly on the colonic mucosa to stimulate colonic peristalsis and secretion. Similar to other stimulant laxatives (eg, senna), use of antibiotics can potentially decrease the efficacy of SPS because they may affect colonic bacteria that produce the active metabolite of the drug.54
Initial dosing in the available RCTs was 10 mg orally for bisacodyl and SPS, although dose reduction was permitted. At this dose, adverse effects were common (see below), and therefore in clinical practice, 5 mg orally is often used initially. Although not studied in RCTs, bisacodyl is also available as a rectal suppository (10 mg). The onset of action is typically 6–12 hours for the oral tablet while the suppository works within 30–60 minutes.
The most common adverse effects for bisacodyl and SPS were diarrhea and abdominal pain. For bisacodyl at the initial starting dose of 10 mg compared with placebo, diarrhea occurred in 53.4% vs 1.7%, respectively, while abdominal pain occurred in 24.7% vs 2.5%, respectively.51 Most adverse events occurred in the first week of treatment. For SPS at the initial starting dose of 10 mg compared with placebo, diarrhea was reported by 31.8% vs 4.5%, respectively, while for abdominal pain, it was reported by 5.6% vs 2.2%, respectively.52 Bisacodyl and SPS are contraindicated in individuals with ileus, intestinal obstruction, severe dehydration, or acute inflammatory conditions in the bowel.
Although effective, side effects are common, and the panel recommended the use of bisacodyl and SPS for a short term or rescue therapy. The long-term effectiveness of these agents has not been studied.
| Recommendation 6: In adults with CIC, the panel suggests the use of senna over management without senna (conditional recommendation, low certainty of evidence). |
Implementation considerations
|
Senna
Summary of evidence
One placebo-controlled RCT examined the safety and efficacy of senna in the management of CIC.44 Participants were randomly assigned to 1 g of senna (n = 30) or placebo (n = 30) and treated for 28 days.
Benefits and harms
Participants treated with senna may have higher CSBMs per week (MD 7.60, 95% CI 5.90–9.30) and SBMs per week (MD 7.6, 95% CI 6.42–8.78) compared with the placebo group. The response rate might be higher in the senna-treated group compared with placebo (RR 5.25, 95% CI 2.05–13.47), 567 more per 1,000 in the senna group (from 140 to 1,000 more). The quality-of-life scores may be higher in the senna group compared with placebo (MD 7.80, 95% CI 1.40–14.20). Participants taking senna might have higher rates of diarrhea, 175 more per 1,000 (from 100 fewer to 1,000 more). No participants in the senna and placebo arms experienced a severe treatment-related adverse event.
Certainty in evidence of effects
The certainty of evidence was low for the outcomes of CSBMs per week, SBMs per week, and quality of life as the panel rated down because of concerns for indirectness and imprecision. The certainty of evidence for responder rate was moderate because of imprecision. The overall certainty of evidence for senna was low.
Discussion
Senna is a natural derivative of the senna plant. Sennosides A and B are sequentially metabolized by the gut microbiota to the active metabolites, rheinanthrone and rhein, which stimulate the production of prostaglandin E2 and secretion of chloride ions leading to attendant changes in colonic peristalsis and luminal water content.55,56 Over 90% of sennosides and their metabolites are excreted in the feces.56 Dosing in the single RCT published to date was 1 g by mouth daily for 4 weeks, which is higher than that typically used in clinical practice. It is notable that while no details were provided, 83% of participants randomized to senna reduced their daily dose during the trial.44 Most commercially available senna products contain 8–9 mg per tablet. Rigorous dose ranging data with senna are currently not available. In the clinical trial by Moshita et al.,44 no participants experienced a severe treatment-related adverse event. However, as Moshita et al.44 did not provide rates for the mild treatment-related adverse events, the fact that 83% of participants with CIC randomized to senna engaged in dose reduction raises concerns about potential adverse events such as abdominal pain, cramping, or diarrhea with the higher dose of senna. There are no long-term safety studies with senna in humans. Sennosides are not recommended in pregnant women because chemically similar substances have been found to exert weak genotoxic effects in animals, although the supporting evidence is controversial.57
The combination of efficacy, impact on quality of life, availability OTC, and low cost makes senna an attractive first-line option for individuals with CIC. Limitations to consider include the following: only a single, small RCT from Japan supports its efficacy; the dose of senna used in the trial is higher than that typically used in clinical practice; there are no long-term effectiveness data; and very limited short-term and no long-term harms data are available.
Secretagogues
| Recommendation 7: In adults with CIC who do not respond to OTC agents, the panel suggests the use of lubiprostone over management without lubiprostone (conditional recommendation, low certainty of evidence). |
Implementation considerations
|
Lubiprostone
Summary of evidence
Three 4-week randomized double-blinded placebo-controlled trials evaluated the use of lubiprostone for the management of CIC.58-60 The studied dose of lubiprostone was 24 μg twice daily, and studies were conducted in the United States and Japan. Lubiprostone is a chloride channel activator, resulting in increased intestinal fluid and accelerated GI transit.
Benefits and harms
The pooled data showed that lubiprostone resulted in an increased number of SBMs per week compared with placebo (MD 1.98, 95% CI 1.17–2.79). The data for the outcome of CSBMs per week were not available. Use of lubiprostone in adults with CIC may increase responder rates (RR 1.67, 95% CI 1.36–2.06), 226 more per 1,000 (from 122 to 358 more). Individuals may also be at increased risk of diarrhea leading to discontinuation of the treatment compared with placebo (RR 5.30, 95% CI 1.53–18.44), 28 more per 1,000 (from 4 more to 115 more). There was little to no difference in serious adverse events; however, the CI around the summary estimate was wide, and increased risk of serious adverse events could not be ruled out (RR 1.22, 95% CI 0.62–2.42). The data on quality of life from the available studies were not reported. Stool form, using a 0- to 4-point scale (very loose to very hard, where a lower score is better), was evaluated in 2 studies and improved in the lubiprostone group (MD 1.09 lower, 95% CI 0.16–2.03 lower). Finally, the rate of global relief, evaluated in one study using a 0- to 4-point scale (not effective to very effective, where higher is better), was higher in the lubiprostone group (MD 0.75, 95% CI 0.42–1.08 higher).
Certainty in evidence of effects
The certainty of evidence was moderate for the outcome of SBMs per week (because of imprecision) and low for the remainder of the outcomes (because of very serious imprecision). The overall certainty in evidence for lubiprostone was low.
Discussion
Lubiprostone, a bicyclic fatty acid derived from prostaglandin E1 that increases intestinal chloride secretion by activating type 2 chloride channels on epithelial cells, is approved by FDA for treating CIC at a dose of 24 μg 2 times daily. For IBS-C, the approved dose is 8 μg 2 times daily. Lubiprostone improved stool frequency and consistency as well as abdominal discomfort and bloating, which is a both-ersome symptom in some individuals with CIC.60,61 Among individuals who respond, these effects generally manifest within 2 days. The efficacy in persons 65 years and older is comparable with the overall study population. Lubiprostone accelerates small intestinal and colonic transit in healthy people,62 should be taken with meals, and is contraindicated in individuals with known or suspected mechanical GI obstruction. Observed in 35% of individuals, nausea was the most common adverse event and typically mild or moderate, but led to discontinuation of therapy in only 5% of individuals.60 The risk of nausea is dose-dependent and seems to be lower when taken with food and water.63
Systemic absorption of oral lubiprostone is negligible. Individuals with moderate or severe hepatic insufficiency should receive a lower dose (ie, 8 μg twice daily).
| Recommendation 8: In adults with CIC who do not respond to OTC agents, the panel recommends the use of linaclotide over management without linaclotide (strong recommendation, moderate certainty of evidence). |
Implementation considerations
|
Summary of evidence
Three 12-week randomized double-blinded placebo-controlled trials evaluated the use of linaclotide for the management of CIC.10,64 The studied doses of linaclotide were 145 and 290 μg daily, and all studies were conducted in the United States and Canada. The following dose has also been studied (72 μg).65 Linaclotide is a guanylate cyclase-C agonist, which increases cyclic guanosine monophosphate concentrations resulting in luminal chloride and bicarbonate secretion, thereby increasing intestinal fluid and accelerating GI transit.
Benefits and harms
The use of linaclotide leads to increases in the number of CSBMs per week (MD 1.37, 95% CI 1.07–1.95) and SBMs per week (MD 1.97, 95% CI 1.59–2.36), improves stool consistency (MD 1.25, 95% CI 1.1–1.39 higher), and increases the rates of global relief (RR 1.96, 95% CI 1.63–2.35). The use of linaclotide might lead to a large increase in responder rates compared with placebo (RR 3.14, 95% CI 1.68–5.88), 119 more per 1,000 (from 38 to 271 more). However, participants treated with linaclotide might be 3 times more likely to have diarrhea leading to treatment discontinuation compared with placebo (RR 3.35, 2.09–5.36), 83 more per 1,000 (from 38 to 154 more). The use of linaclotide might improve the PAC-QOL scores compared with placebo;64 however, data could not be pooled.
Certainty in evidence of effects
We rated the certainty of evidence as high for outcomes of CSBMs per week, SBMs per week, stool form, and global relief and moderate for the responder outcome and diarrhea (rating down for imprecision). The outcome of serious adverse events was rated down to low because of very serious imprecision. The overall certainty of evidence for linaclotide was moderate.
Discussion
Linaclotide is a guanylate cyclase-C agonist FDA-approved for the treatment of CIC at a dose of 72 μg or 145 μg daily. The 290 μg daily dose is approved for IBS-C, recognizing that CIC and IBS-C overlap and are often indistinguishable in practice.66 Linaclotide is also approved in many other countries. Linaclotide has been demonstrated to improve abdominal symptoms of bloating, discomfort, and pain in IBS-C trials.67 Because of its effect on abdominal discomfort, pain, and bloating, it may be useful in individuals with these coexisting symptoms. Patients should be instructed to take linaclotide without food, at least 30 minutes before the first meal of the day. Linaclotide is contraindicated in individuals with known or suspected mechanical GI obstruction.
The use of linaclotide might be associated with diarrhea leading to discontinuation or dose reduction; however, this was not very common (in one study, 4.7% discontinued the medication because of diarrhea).68,69 The most common reasons for discontinuation over the first year of treatment were loss of efficacy and insurance coverage barriers related to obtaining prescription refills and not discontinuations because of adverse events, in a retrospective analysis at a large health system.70 Descriptively, there were no clear differences in outcomes among individuals older than 65 years in clinical trials, although the sample size was too small to support formal analysis on differences in outcomes related to age.
| Recommendation 9: In adults with CIC who do not respond to OTC agents, the panel recommends the use of plecanatide over management without plecanatide (strong recommendation, moderate certainty of evidence). |
Implementation considerations
|
Plecanatide
Summary of evidence
Three 12-week randomized double-blinded placebo-controlled trials evaluated the use of plecanatide for the management of CIC.71-73 The studied dose of plecanatide was 3 mg/6 mg daily, and all studies were conducted in the United States and Canada. Plecanatide is a guanylate cyclase-C agonist, which increases cyclic guanosine monophosphate concentrations resulting in luminal chloride and bicarbonate secretion, thereby increasing intestinal fluid and accelerating GI transit.
Benefits and harms
The pooled data showed that the use of plecanatide in adults with CIC leads to an increase in the number of CSBMs per week (MD 1.1, 95% CI 85–1.35) and SBMs per week (MD 1.66, 95% CI 1.37–1.94) and improves the quality-of-life scores. The intervention group had increased responder rates, defined as ≥3 CSBMs per week and ≥1 CBSM over baseline for ≥9 of 12 weeks including ≥3 of the last 4 weeks, compared with placebo (RR 1.78, 95% CI 1.46–2.18), 88 more per 1,000 (from 52 to 134 more). Participants treated with plecanatide might have higher rates of reported diarrhea leading to treatment discontinuation (RR 5.39, 95% CI 2.40–12.11), 27 more per 1,000 (from 9 to 69 more). The use of plecanatide might improve stool consistency based on the Bristol Stool Form Scale compared with placebo (MD 0.83, 95% CI 0.6–1.05).
Certainty in evidence of effects
The certainty of evidence was high for outcomes of CSBM and SBM frequency and QOL and moderate for diarrhea, leading to treatment discontinuation, serious adverse events, and stool form. The panel rated down certainty of these outcomes because of imprecision. The overall certainty in evidence for plecanatide was moderate.
Discussion
Plecanatide is a pH-dependent guanylate cyclase-C agonist approved by the FDA for CIC at a dose of 3 mg daily taken with or without food. Plecanatide is also approved at the same dose for IBS-C. Plecanatide may have beneficial concurrent effects with relief in abdominal pain based on indirect evidence from IBS-C trials.74 Individuals using plecanatide might be at higher risk of diarrhea leading to discontinuation of medication; however, the absolute risk seems small.68 Descriptively, there were no clear differences in outcomes among individuals older than 65 years in clinical trials, although the sample size was too small to support formal analysis on differences in outcomes related to age.
5-HT4 Agonist
| Recommendation 10: In adults with CIC who do not respond to OTC agents, the panel recommends the use of prucalopride over management without prucalopride (strong recommendation, moderate certainty of evidence). |
Implementation considerations
|
Prucalopride
Summary of evidence
Five 12-week randomized double-blinded placebo-controlled trials evaluated the use of prucalopride (2 mg daily) for the management of CIC.75-79 The studies were conducted in the United States, Europe, and the Asia-Pacific region. The 4 mg dose has also been studied.75 Prucalopride is a selective, high-affinity 5-HT4 agonist that promotes neurotransmission by the enteric neurons resulting in stimulation of the peristaltic reflex, intestinal secretions, and GI motility.
Benefits and harms
Compared with placebo, prucalopride resulted in an increased number of CSBMs per week (MD 0.96, 95% CI 0.64–1.29). SBMs per week was not studied in any of the included study. Responder rates, defined as ≥3 CSBMs per week, were higher in the prucalopride group (RR 2.37, 95% CI 1.97–2.85) with 165 more responders per 1,000 (range 117–222 more). An alternative responder end point, deemed alternative end point A, defined as ≥3 CSBM per week and ≥1 CBSM over baseline for ≥75% of study weeks, was higher in the prucalopride group (RR 2.51, 95% CI 1.97–3.21) with 109 more responders per 1,000 (range 70–160 more). The rates of diarrhea leading to treatment discontinuation might be higher in the prucalopride group compared with placebo (RR 3.00, 95% CI 1.89–4.78). The occurrence of serious adverse events was low; however, the CI around the summary estimate was imprecise and included a possible increased risk. PAC-QOL, where lower scores are better, improved in 4 studies in the prucalopride group compared with placebo (MD 0.32 lower, 95% CI 0.41–0.23 lower). Definitions and scales used to assess stool form varied widely across studies and could not be pooled. Global relief was reported in 4 studies and defined as those who felt that treatment was extremely or quite a bit effective, and the responder rates were higher in the prucalopride group compared with placebo (RR 2.09, 95% CI 0.15–3.0).
Certainty in evidence of effects
We rated the certainty of evidence as high for outcomes of CSBM frequency, responder rate, and alternative end point A and moderate for diarrhea, leading to treatment discontinuation, serious adverse events, quality of life, and global relief (because of small event rates and wide CIs around the summary estimates). The overall certainty in evidence for prucalopride was moderate.
Discussion
For prucalopride, a selective agonist of serotonin 5-HT4 receptors, the recommended dose is 2 mg once daily in adults and 1 mg daily in individuals with severe renal impairment (ie, creatinine clearance <30 mL/min).80 The efficacy in persons 65 years and older is comparable with the overall study population. Besides increasing bowel frequency, prucalopride also improved constipation symptoms, abdominal symptoms, quality of life, and satisfaction with treatment vs placebo assessed with the PAC instrument.81 Arguably, these effects are at least partly explained by the ability of prucalopride to induce and increase the amplitude of colonic high-amplitude propagated contractions.73,82 Such high-amplitude propagated contractions propagate colonic contents.83
The most frequent, generally transient, side effects are headache, abdominal pain, nausea, and diarrhea.84 In most of the individuals who reported headache and diarrhea, this side effect occurred within the first week of treatment and typically resolved within a few days. Five percent of individuals discontinued prucalopride because of side effects. Cardiovascular adverse events were not more common than placebo. In a safety database of 4,476 subjects, 4 individuals attempted suicides and 2 completed suicides, both of whom had discontinued prucalopride more than 1 month before the event. The label cautions patients and clinicians to be alert to unusual changes in mood and behavior and suicidal ideation. It is, however, unclear what the mechanism of action is or whether there is a causal association between the use of prucalopride and risk of suicide.85 No drug-associated risks of miscarriage, major birth defects, or adverse maternal or fetal outcomes have been identified. Prucalopride is contraindicated in patients with intestinal perforation or obstruction, Crohn’s disease, ulcerative colitis, and toxic megacolon/megarectum.
Limitations and Evidence Gap
An important limitation of this body of evidence was that clinical trials did not uniformly evaluate interventions for patient important outcomes on efficacy, adverse effects, and tolerability. Importantly, there was a paucity of data for the most commonly used treatments of CIC such as fiber, lactulose, senna, and docusate. There was also variability in the definition of inclusion criteria, efficacy, and tolerability outcomes, as well as variance in acceptable clinical trial length by regulators over time. Most of the included studies followed the patients for the short term, and the safety and tolerance of these medications in the long term is not well studied. Future research is needed to assess the long-term safety of these medications and to assess whether the patients develop tolerance to these medications over time. In our systematic review, we compared individual drugs against placebo arms and did not aim to inform the relative efficacy of pharmacological agents. Network meta-analysis is an appropriate statistical method to facilitate indirect comparison against a common comparator such as placebo or other active treatment.8 However, for the reasons stated, readers should be cautioned on the limitation of indirect comparisons to support substantive claims on superiority or inferiority to inform care or policy decisions.
This guideline is limited to covering pharmacological interventions for the treatment of CIC in otherwise healthy adults and does not apply to pediatric populations or to individuals who are pregnant or with opioid-induced constipation or malignancy. The evidence on the management of constipation during pregnancy has been reviewed in a recent publication that discusses the safety of almost all the pharmacological agents assessed in this guideline.86 This guideline does not review anorectal evacuation disorders that were evaluated in a recent ACG guideline87 and an AGA review.80 We also did not assess the efficacy of dietary fiber including fruit-based laxatives in CIC, which was evaluated in a recent systematic review.8 Other interventions not included in this review include lifestyle modifications, such as increasing water and physical activity, and other pharmacological agents, such as elobixibat, mizagliflozin, naronapride, tegaserod, tenapanor, or velusetrag, or the efficacy of surgical interventions for the management of CIC. We did not assess the evidence on the use of probiotics for the treatment of CIC, but it has been synthesized elsewhere in a recent systematic review.88 Although we considered the cost of pharmacological agents evaluated in this guideline during the evidence to decision-making process, we did not perform formal cost-effectiveness analyses and refer the audience to recently published evidence that addresses this topic.89 There was no patient representative in the guideline development panel, which is a limitation for this study.
Implementation, Cost, and Health Equity Considerations
This document provides a comprehensive outline of the various OTC and prescription pharmacological agents available for the treatment of CIC. The guidelines are meant to provide a template for approach to management and practitioners should engage in shared decision making based on the preference of patients and cost and availability of the medications. Although the recommendations in this guideline were based on available evidence, the implementation considerations included suggestions from the collective experience of the expert panel and may not be based on evidence. Most of the medications assessed in this guideline document are readily available; however, some of them are still available only in brand name formulations because generic formulations do not exist. As a result, it is important to consider the out-of-pocket expenses for patients that may depend on prescription coverage with various insurance plans.89 Prior authorization might be required for some of the medications.90 The clinical decision support tool is available from the website of AGA.
Plans for Updating
Considerable resources are expended for the development of guidelines, and keeping guidelines up to date is a challenging process. Future update of this guideline will depend on the availability of new evidence on the existing interventions and new intervention. We hope to incorporate the advances in the technological platforms and models of guideline development in the future updates without duplication or reproduction of the current guideline document.
Supplementary Material
Acknowledgments
We thank Claire Neumann, Stephanie M. Demian, Jenny Castano, and Nelly Ahmet for their help for coordination of work for this joint guideline.
Footnotes
This article has an accompanying continuing medical education activity, also eligible for MOC credit, on page e25. Learning Objective: Upon completion of this CME activity, successful learners will be able to identify pharmacologic treatment options for the management of individuals with chronic idiopathic constipation.
Supplementary Material
Note: To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org, and at https://doi.org/10.1053/j.gastro.2023.03.214.
Conflicts of interest
Potential competing interests: These authors disclose the following: L.C. has served as a member of the scientific advisory boards for Ardelyx, Atmo, Immunic, Ironwood, and Mauna Kea Technologies; has served as a consultant for Bausch Health and Trellus Health and a speaker for Ironwood; has received research support from the National Institute of Health, Arena, AnX Robotica, Ironwood, and Vanda Pharmaceuticals; and has stock options with ModifyHealth and Trellus Health. W.D.C. has served as a consultant for AbbVie, Ardelyx, Biomerica, Ironwood, Isothrive, QOL Medical, Nestle, Phathom Pharmaceuticals, Redhill, Salix/Valeant, Takeda, and Vibrant; has received research grants from FDA, NIH, Commonwealth Diagnostics, QOL Medical, and Salix; has board membership at ACG, GI on Demand, International Foundation for Functional Gastrointestinal Disorders (IFFGD), and the Rome Foundation; holds patents for Digital Manometry and the Rectal Expulsion Device; and has stock options in Coprata, Dieta, Kiwi Bioscience, ISOThrive, and Modify Health. C.V.A. has served as a consultant for Owlstone Medical; and has stock options in My Total Health and Owlstone Medical. A.E.B. receives royalties from a patent created for Medispira Medical Technologies. L.A.H. has consulted for AbbVie/Ironwood, Alyman, and Takeda. E.D.S has served as a consultant for Salix, Mahana, Ardelyx, Takeda, GI Supply and Neuraxis outside the scope of the current work. He holds a patent on the Rectal Expulsion Device. A.J.L. has consulted for Vibrant and Mylan; owns equity in Johnson & Johnson and Bristol Meier & Squibb; and has received research support from Takeda, Vanda, Shire, and Biomerica. The remaining authors disclose no conflicts.
References
- 1.Palsson OS, Whitehead W, Tornblom H, et al. Prevalence of Rome IV functional bowel disorders among adults in the United States, Canada, and the United Kingdom. Gastroenterology 2020;158:1262–1273.e3. [DOI] [PubMed] [Google Scholar]
- 2.Vriesman MH, Koppen IJN, Camilleri M, et al. Management of functional constipation in children and adults. Nat Rev Gastroenterol Hepatol 2020;17:21–39. [DOI] [PubMed] [Google Scholar]
- 3.Lacy BE, Mearin F, Chang L, et al. Bowel disorders. Gastroenterology 2016;150:1393–1407.e5. [DOI] [PubMed] [Google Scholar]
- 4.Herrick LM, Spalding WM, Saito YA, et al. A case-control comparison of direct healthcare-provider medical costs of chronic idiopathic constipation and irritable bowel syndrome with constipation in a community-based cohort. J Med Econ 2017;20:273–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCormick D. Managing costs and care for chronic idiopathic constipation. Am J Manag Care 2019;25:S63–S69. [PubMed] [Google Scholar]
- 6.Lembo A, Bharucha AE, Dorn SD, et al. American Gastroenterological Association medical position statement on constipation. Gastroenterology 2013;144:211–217. [DOI] [PubMed] [Google Scholar]
- 7.Luthra P, Camilleri M, Burr NE, et al. Efficacy of drugs in chronic idiopathic constipation: A systematic review and network meta-analysis. Lancet Gastroenterol Hepatol 2019;4:831–844. [DOI] [PubMed] [Google Scholar]
- 8.Rao SSC, Brenner DM. Efficacy and safety of over-the-counter therapies for chronic constipation: An updated systematic review. Am J Gastroenterol 2021;116:1156–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harris LA, Horn J, Kissous-Hunt M, et al. The Better Understanding and Recognition of the Disconnects, Experiences, and Needs of Patients with Chronic Idiopathic Constipation (BURDEN-CIC) study: Results of an online questionnaire. Adv Ther 2017;34:2661–2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lacy BE, Shea EP, Manuel M, et al. Lessons learned: Chronic idiopathic constipation patient experiences with over-the-counter medications. PLoS One 2021;16:e0243318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Institute of Medicine (US). Committee on Standards for Developing Trustworthy Clinical Practice Guidelines. Clinical Practice Guidelines We Can Trust. Washington, DC: National Academies Press, 2011. [Google Scholar]
- 12.Schunemann HJ, Al-Ansary LA, Forland F, et al. Guidelines international network: Principles for disclosure of interests and management of conflicts in guidelines. Ann Intern Med 2015;163:548–553. [DOI] [PubMed] [Google Scholar]
- 13.Schunemann HJ, Wiercioch W, Etxeandia I, et al. Guidelines 2.0: Systematic development of a comprehensive checklist for a successful guideline enterprise. CMAJ 2014;186:E123–E142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang L, Sultan S, Lembo A, et al. AGA clinical practice guideline on the pharmacological management of irritable bowel syndrome with constipation. Gastroenterology 2022;163:118–136. [DOI] [PubMed] [Google Scholar]
- 15.Lacy BE, Pimentel M, Brenner DM, et al. ACG clinical guideline: Management of irritable bowel syndrome. Am J Gastroenterol 2021;116:17–44. [DOI] [PubMed] [Google Scholar]
- 16.Christodoulides S, Dimidi E, Fragkos KC, et al. Systematic review with meta-analysis: Effect of fibre supplementation on chronic idiopathic constipation in adults. Aliment Pharmacol Ther 2016;44:103–116. [DOI] [PubMed] [Google Scholar]
- 17.Nelson AD, Camilleri M, Chirapongsathorn S, et al. Comparison of efficacy of pharmacological treatments for chronic idiopathic constipation: A systematic review and network meta-analysis. Gut 2017;66:1611–1622. [DOI] [PubMed] [Google Scholar]
- 18.The EndNote Team. EndNote 20. ed. Philadelphia, PA: EndNote, 2013. [Google Scholar]
- 19.Covidence Systematic Review Software. Veritas Health Innovation: Melbourne, Australia. Available at: www.covidence.org. Accessed December 31, 2022. [Google Scholar]
- 20.Murad MH, Wang Z, Chu H, et al. When continuous outcomes are measured using different scales: Guide for meta-analysis and interpretation. BMJ 2019;364:k4817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Review Manager (RevMan) [Computer program]. Version 5.3. The Nordic Cochrane Centre. Copenhagen, Denmark: The Cochrane Collaboration, 2014. [Google Scholar]
- 22.Higgins JPT, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McMaster University. GRADEpro GDT: GRADEpro Guideline Development Tool [Software]. Evidence Prime: Kraków, Poland, 2020. Available at: gradepro.org. Accessed January 31, 2023. [Google Scholar]
- 24.Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 2011;64:383–394. [DOI] [PubMed] [Google Scholar]
- 25.Alonso-Coello P, Schunemann HJ, Moberg J, et al. GRADE Evidence to Decision (EtD) frameworks: A systematic and transparent approach to making well informed healthcare choices. 1: Introduction. BMJ 2016;353:i2016. [DOI] [PubMed] [Google Scholar]
- 26.Badiali D, Corazziari E, Habib FI, et al. Effect of wheat bran in treatment of chronic nonorganic constipation. A double-blind controlled trial. Dig Dis Sci 1995;40:349–356. [DOI] [PubMed] [Google Scholar]
- 27.Linetzky Waitzberg D, Alves Pereira CC, Logullo L, et al. Microbiota benefits after inulin and partially hydrolyzed guar gum supplementation: A randomized clinical trial in constipated women. Nutr Hosp 2012;27:123–129. [DOI] [PubMed] [Google Scholar]
- 28.Lopez Roman J, Martinez Gonzalvez AB, Luque A, et al. The effect of a fibre enriched dietary milk product in chronic primary idiopathic constipation [in Spanish]. Nutr Hosp 2008;23:12–19. [PubMed] [Google Scholar]
- 29.Ashraf W, Park F, Lof J, et al. Effects of psyllium therapy on stool characteristics, colon transit and anorectal function in chronic idiopathic constipation. Aliment Pharmacol Ther 1995;9:639–647. [DOI] [PubMed] [Google Scholar]
- 30.Fenn GC, Wilkinson PD, Lee CE, et al. A general practice study of the efficacy of Regulan in functional constipation. Br J Clin Pract 1986;40:192–197. [PubMed] [Google Scholar]
- 31.Odes HS, Madar Z. A double-blind trial of a celandin, aloevera and psyllium laxative preparation in adult patients with constipation. Digestion 1991;49:65–71. [DOI] [PubMed] [Google Scholar]
- 32.Gibson GR, Roberfroid MB. Dietary modulation of the human colonic microbiota: Introducing the concept of prebiotics. J Nutr 1995;125:1401–1412. [DOI] [PubMed] [Google Scholar]
- 33.Slavin J. Fiber and prebiotics: Mechanisms and health benefits. Nutrients 2013;5:1417–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McRorie JW Jr, Fahey GC Jr, Gibb RD, et al. Laxative effects of wheat bran and psyllium: Resolving enduring misconceptions about fiber in treatment guidelines for chronic idiopathic constipation. J Am Assoc Nurse Pract 2020;32:15–23. [DOI] [PubMed] [Google Scholar]
- 35.Markland AD, Palsson O, Goode PS, et al. Association of low dietary intake of fiber and liquids with constipation: Evidence from the National Health and Nutrition Examination Survey. Am J Gastroenterol 2013;108:796–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anti M, Pignataro G, Armuzzi A, et al. Water supplementation enhances the effect of high-fiber diet on stool frequency and laxative consumption in adult patients with functional constipation. Hepatogastroenterology 1998;45:727–732. [PubMed] [Google Scholar]
- 37.Corazziari E, Badiali D, Bazzocchi G, et al. Long term efficacy, safety, and tolerability of low daily doses of isosmotic polyethylene glycol electrolyte balanced solution (PMF-100) in the treatment of functional chronic constipation. Gut 2000;46:522–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Corazziari E, Badiali D, Habib FI, et al. Small volume isosmotic polyethylene glycol electrolyte balanced solution (PMF-100) in treatment of chronic nonorganic constipation. Dig Dis Sci 1996;41:1636–1642. [DOI] [PubMed] [Google Scholar]
- 39.Dipalma JA, Cleveland MV, McGowan J, et al. A randomized, multicenter, placebo-controlled trial of polyethylene glycol laxative for chronic treatment of chronic constipation. Am J Gastroenterol 2007;102:1436–1441. [DOI] [PubMed] [Google Scholar]
- 40.Menees SB, Lembo AJ, Chey WD. Polyethylene glycol 3350 in the treatment of chronic idiopathic constipation: Post hoc analysis using FDA endpoints. Can J Gastroenterol Hepatol 2022;2022:3533504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cinca R, Chera D, Gruss HJ, et al. Randomised clinical trial: macrogol/PEG 3350+electrolytes versus prucalopride in the treatment of chronic constipation: A comparison in a controlled environment. Aliment Pharmacol Ther 2013;37:876–886. [DOI] [PubMed] [Google Scholar]
- 42.Di Palma JA, Cleveland MV, McGowan J, et al. A randomized, multicenter comparison of polyethylene glycol laxative and tegaserod in treatment of patients with chronic constipation. Am J Gastroenterol 2007;102:1964–1971. [DOI] [PubMed] [Google Scholar]
- 43.Mori S, Tomita T, Fujimura K, et al. A randomized double-blind placebo-controlled trial on the effect of magnesium oxide in patients with chronic constipation. J Neurogastroenterol Motil 2019;25:563–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morishita D, Tomita T, Mori S, et al. Senna versus magnesium oxide for the treatment of chronic constipation: A randomized, placebo-controlled trial. Am J Gastroenterol 2021;116:152–161. [DOI] [PubMed] [Google Scholar]
- 45.Altura BM. Basic biochemistry and physiology of magnesium: A brief review. Magnes Trace Elem 1991;10:167–171. [PubMed] [Google Scholar]
- 46.Felsenfeld AJ, Levine BS, Rodriguez M. Pathophysiology of calcium, phosphorus, and magnesium dysregulation in chronic kidney disease. Semin Dial 2015;28:564–577. [DOI] [PubMed] [Google Scholar]
- 47.Cascella M, Vaqar S. Hypermagnesemia. In StatPearls [Internet]. StatPearls Publishing: Treasure Island, FL, 2022. Available at: https://www.ncbi.nlm.nih.gov/books/NBK549811/. Accessed January 31, 2023. [Google Scholar]
- 48.Sanders JF. Lactulose syrup assessed in a double-blind study of elderly constipated patients. J Am Geriatr Soc 1978;26:236–239. [DOI] [PubMed] [Google Scholar]
- 49.Wesselius-De Casparis A, Braadbaart S, Bergh-Bohlken GE, et al. Treatment of chronic constipation with lactulose syrup: Results of a double-blind study. Gut 1968;9:84–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pieber TR, Svehlikova E, Mursic I, et al. Blood glucose response after oral lactulose intake in type 2 diabetic individuals. World J Diabetes 2021;12:893–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kamm MA, Mueller-Lissner S, Wald A, et al. Oral bisacodyl is effective and well-tolerated in patients with chronic constipation. Clin Gastroenterol Hepatol 2011;9:577–583. [DOI] [PubMed] [Google Scholar]
- 52.Mueller-Lissner S, Kamm MA, Wald A, et al. Multicenter, 4-week, double-blind, randomized, placebo-controlled trial of sodium picosulfate in patients with chronic constipation. Am J Gastroenterol 2010;105:897–903. [DOI] [PubMed] [Google Scholar]
- 53.Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol 1997;32:920–924. [DOI] [PubMed] [Google Scholar]
- 54.Krueger D, Demir IE, Ceyhan GO, et al. bis-(phydroxyphenyl)-pyridyl-2-methane (BHPM)-the active metabolite of the laxatives bisacodyl and sodium picosulfate-enhances contractility and secretion in human intestine in vitro. Neurogastroenterol Motil 2018;30:e13311. [DOI] [PubMed] [Google Scholar]
- 55.Mascolo N, Capasso R, Senna Capasso F. A safe and effective drug. Phytotherapy Res 1998;12:S143–S145. [Google Scholar]
- 56.Drugbank Online. Sennosides. Available at: https://go.drugbank.com/drugs/DB11365. Accessed January 15, 2023.
- 57.Morales MA, Hernandez D, Bustamante S, et al. Is senna laxative use associated to cathartic colon, genotoxicity, or carcinogenicity? J Toxicol 2009;2009:287247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Barish CF, Drossman D, Johanson JF, et al. Efficacy and safety of lubiprostone in patients with chronic constipation. Dig Dis Sci 2010;55:1090–1097. [DOI] [PubMed] [Google Scholar]
- 59.Fukudo S, Hongo M, Kaneko H, et al. Lubiprostone increases spontaneous bowel movement frequency and quality of life in patients with chronic idiopathic constipation. Clin Gastroenterol Hepatol 2015;13:294–301.e5. [DOI] [PubMed] [Google Scholar]
- 60.Johanson JF, Morton D, Geenen J, et al. Multicenter, 4-week, double-blind, randomized, placebo-controlled trial of lubiprostone, a locally-acting type-2 chloride channel activator, in patients with chronic constipation. Am J Gastroenterol 2008;103:170–177. [DOI] [PubMed] [Google Scholar]
- 61.Bharucha AE, Sharma M. Painful and painless constipation: All roads lead to (A change in) Rome. Dig Dis Sci 2018;63:1671–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Camilleri M, Bharucha AE, Ueno R, et al. Effect of a selective chloride channel activator, lubiprostone, on gastrointestinal transit, gastric sensory, and motor functions in healthy volunteers. Am J Physiol Gastrointest Liver Physiol 2006;290:G942–G947. [DOI] [PubMed] [Google Scholar]
- 63.Cryer B, Drossman DA, Chey WD, et al. Analysis of nausea in clinical studies of lubiprostone for the treatment of constipation disorders. Dig Dis Sci 2017;62:3568–3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lembo AJ, Schneier HA, Shiff SJ, et al. Two randomized trials of linaclotide for chronic constipation. N Engl J Med 2011;365:527–536. [DOI] [PubMed] [Google Scholar]
- 65.Schoenfeld P, Lacy BE, Chey WD, et al. Low-dose linaclotide (72 μg) for chronic idiopathic constipation: A 12-week, randomized, double-blind, placebo-controlled trial. Am J Gastroenterol 2018;113:105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shah ED, Almario CV, Spiegel BMR, et al. Lower and upper gastrointestinal symptoms differ between individuals with irritable bowel syndrome with constipation or chronic idiopathic constipation. J Neurogastroenterol Motil 2018;24:299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chang L, Lacy BE, Moshiree B, et al. Efficacy of linaclotide in reducing abdominal symptoms of bloating, discomfort, and pain: A phase 3B trial using a novel abdominal scoring system. Am J Gastroenterol 2021;116:1929–1937. [DOI] [PubMed] [Google Scholar]
- 68.Shah ED, Kim HM, Schoenfeld P. Efficacy and tolerability of guanylate cyclase-C agonists for irritable bowel syndrome with constipation and chronic idiopathic constipation: A systematic review and meta-analysis. Am J Gastroenterol 2018;113:329–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.U.S. Food and Drug Administration. Clinical review for linaclotide. 2017. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/202811Orig1s010.pdf. Accessed January 31, 2023.
- 70.Shah ED, Suresh S, Jou J, et al. Evaluating when and why patients discontinue chronic therapy for irritable bowel syndrome with constipation and chronic idiopathic constipation. Am J Gastroenterol 2020;115:596–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Barish C, Dorn S, Fogel RP, et al. Plecanatide is effective and safe in the treatment for chronic idiopathic constipation: Results of a phase II trial. Dig Dis Sci 2021;66:537–540. [DOI] [PubMed] [Google Scholar]
- 72.DeMicco M, Barrow L, Hickey B, et al. Randomized clinical trial: Efficacy and safety of plecanatide in the treatment of chronic idiopathic constipation. Therap Adv Gastroenterol 2017;10:837–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Miner PB Jr, Koltun WD, Wiener GJ, et al. A randomized phase III clinical trial of plecanatide, a uroguanylin analog, in patients with chronic idiopathic constipation. Am J Gastroenterol 2017;112:613–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brenner DM, Fogel R, Dorn SD, et al. Efficacy, safety, and tolerability of plecanatide in patients with irritable bowel syndrome with constipation: Results of two phase 3 randomized clinical trials. Am J Gastroenterol 2018;113:735–745. [DOI] [PubMed] [Google Scholar]
- 75.Camilleri M, Kerstens R, Rykx A, et al. A placebo-controlled trial of prucalopride for severe chronic constipation. N Engl J Med 2008;358:2344–2354. [DOI] [PubMed] [Google Scholar]
- 76.Ke M, Zou D, Yuan Y, et al. Prucalopride in the treatment of chronic constipation in patients from the Asia-pacific region: A randomized, double-blind, placebo-controlled study. Neurogastroenterol Motil 2012;24. 999–e541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Quigley EMM, Vandeplassche L, Kerstens R, et al. Clinical trial: The efficacy, impact on quality of life, and safety and tolerability of prucalopride in severe chronic constipation: A 12-week, randomized, double-blind, placebo-controlled study. Aliment Pharmacol Ther 2009;29:315–328. [DOI] [PubMed] [Google Scholar]
- 78.Tack J, van Outryve M, Beyens G, et al. Prucalopride (Resolor) in the treatment of severe chronic constipation in patients dissatisfied with laxatives. Gut 2009;58:357–365. [DOI] [PubMed] [Google Scholar]
- 79.Yiannakou Y, Piessevaux H, Bouchoucha M, et al. A randomized, double-blind, placebo-controlled, phase 3 trial to evaluate the efficacy, safety, and tolerability of prucalopride in men with chronic constipation. Am J Gastroenterol 2015;110:741–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bharucha AE, Lacy BE. Mechanisms, evaluation, and management of chronic constipation. Gastroenterology 2020;158:1232–1249.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tack J, Camilleri M, Dubois D, et al. Association between health-related quality of life and symptoms in patients with chronic constipation: An integrated analysis of three phase 3 trials of prucalopride. Neurogastroenterol Motil 2015;27:397–405. [DOI] [PubMed] [Google Scholar]
- 82.Corsetti M, Thys A, Harris A, et al. High-resolution manometry reveals different effect of polyethylene glycol, bisacodyl, and prucalopride on colonic motility in healthy subjects: An acute, open label, randomized, crossover, reader-blinded study with potential clinical implications. Neurogastroenterol Motil 2021;33:e14040. [DOI] [PubMed] [Google Scholar]
- 83.Bharucha AE. High amplitude propagated contractions. Neurogastroenterol Motil 2012;24:977–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.FDA. Prucalopride. Available at:. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/210166Orig1s000RiskR.pdf. Accessed January 31, 2023.
- 85.SHIRE. Integrated summary of safety for NDA 210166, 2017. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/210166Orig1s000RiskR.pdf. Accessed January 31, 2023.
- 86.Rao SSC, Qureshi WA, Yan Y, et al. Constipation, hemorrhoids, and anorectal disorders in pregnancy. Am J Gastroenterol 2022;117:16–25. [DOI] [PubMed] [Google Scholar]
- 87.Wald A, Bharucha AE, Limketkai B, et al. ACG clinical guidelines: Management of benign anorectal disorders. Am J Gastroenterol 2021;116:1987–2008. [DOI] [PubMed] [Google Scholar]
- 88.van der Schoot A, Helander C, Whelan K, et al. Probiotics and synbiotics in chronic constipation in adults: A systematic review and meta-analysis of randomized controlled trials. Clin Nutr 2022;41:2759–2777. [DOI] [PubMed] [Google Scholar]
- 89.Shah ED, Staller K, Nee J, et al. Evaluating the impact of cost on the treatment algorithm for chronic idiopathic constipation: Cost-effectiveness analysis. Am J Gastroenterol 2021;116:2118–2127. [DOI] [PubMed] [Google Scholar]
- 90.Shah ED, Amann ST, Hobley J, et al. 2021 national survey on prior authorization burden and its impact on gastroenterology practice. Am J Gastroenterol 2022;117:802–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.