Abstract
BACKGROUND & AIMS:
Anorectal manometry (ARM) is a comprehensive diagnostic tool for evaluating patients with constipation, fecal incontinence, or anorectal pain; however, it is not widely utilized for reasons that remain unclear. The aim of this round table discussion was to critically examine the current clinical practices of ARM and biofeedback therapy by physicians and surgeons in both academic and community settings.
METHODS:
Leaders in medical and surgical gastroenterology and physical therapy with interest in anorectal disorders were surveyed regarding practice patterns and utilization of these technologies. Subsequently, a roundtable was held to discuss survey results, explore current diagnostic and therapeutic challenges with these technologies, review the literature, and generate consensus-based recommendations.
RESULTS:
ARM identifies key pathophysiological abnormalities such as dyssynergic defecation, anal sphincter weakness, or rectal sensory dysfunction, and is a critical component of biofeedback therapy, an evidence-based treatment for patients with dyssynergic defecation and fecal incontinence. Additionally, ARM has the potential to enhance health-related quality of life and reduce healthcare costs. However, it has significant barriers that include a lack of education and training of healthcare providers regarding the utility and availability of ARM and biofeedback procedures, as well as challenges with condition-specific testing protocols and interpretation. Additional barriers include understanding when to perform, where to refer, and how to use these technologies, and confusion over billing practices.
CONCLUSIONS:
Overcoming these challenges with appropriate education, training, collaborative research, and evidence-based guidelines for ARM testing and biofeedback therapy could significantly enhance patient care of anorectal disorders.
Keywords: Anorectal Manometry, Biofeedback Therapy, Fecal Incontinence, Dyssynergic Defecation
Anorectal manometry (ARM), a diagnostic tool for identifying pathophysiological mechanisms, helps explain symptoms of fecal incontinence (FI), constipation, irritable bowel syndrome (IBS), rectal prolapse, and anorectal pain.1–6 The prevalence of these conditions varies widely, but they are commonly encountered in clinical practice (Table 1).7–17 Symptoms of defecatory disorders can overlap with urinary dysfunction, suggesting a shared underlying pathophysiology.18,19 ARM is also a therapeutic component of biofeedback therapy (BT), used to treat pelvic floor dysfunction and improve symptoms.20,21 While important in the diagnostic workup of refractory constipation,22 ARM may also be useful when evaluating surgical options for rectal cancer and inflammatory bowel disease23 and managing postsurgical pelvic floor issues.24 ARM provides objective, evidence-based assessments that help identify patient subgroups likely to experience symptom and quality-of-life improvements after BT.25
Table 1.
Indications for ARM as a Diagnostic Test, Prevalence of These Disorders, and Sensorimotor Abnormalities That May Be Detected in Each Condition
| Condition | Prevalence Estimate | Findings of ARM and Anorectal Function Tests5,12,15,61 |
|---|---|---|
| Fecal incontinence | Fecal incontinence is experienced by 14.4% of Americans and 18.8% of women.7,8 | • Weak resting pressure suggests sphincter weakness • Weak squeeze pressure suggests sphincter weakness • Abnormal cough reflex suggests neuropathy or spinal cord injury • Rectal hyposensitivity or rectal hypersensitivity • Impaired rectal compliance • Lumbar and sacral plexus neuropathy |
| Constipation | In In a large-scale multinational study using Rome IV diagnostic criteria, functional bowel disorders were most common and functional constipation was the most prevalent bowel disorder, affecting nearly 9% of the U.S. population.9 | • Hypertonic resting sphincter pressure • Abnormal rectoanal inhibitory reflex suggests Hirshsprungs disease • Rectal hyposensitivity or rectal hypersensitivity • Prolonged balloon expulsion time |
| Rectal sensory disorder | Rectal sensory disorders can affect 4%— 19% of fecal incontinence patients (rates vary by sex).10 | • Rectal hyposensitivity • Rectal hypersensitivity |
| Dyssynergic defecation | Prevalence of dyssynergic defecation in the general population is unknown.11 Dyssynergic defecation affects between one-third to one-half of constipated patients referred for anorectal testing.12–14 | • Hypertonic resting pressure • Abnormal defecation patterns, type I—IV dyssynergia • Rectal hyposensitivity or hypersensitivity • Prolonged balloon expulsion time |
| Descending perineum syndrome | Descending perineum syndrome is seen in 7.7% of constipated patients.15 | • Excessive perineal descent • Rectal mucosal intussusception • Weak resting pressure • Weak squeeze pressure • Dyssynergic defecation • Prolonged balloon expulsion time |
| Anorectal pain | The prevalence of functional anorectal pain, levator ani syndrome, and proctalgia fugax is estimated to be 11.3%, 6.0%, and 7.9%, respectively.16 | • Weak resting pressure • Weak squeeze pressure • Rectal sensation abnormality • Hypertonic anal sphincter • Lumbar and sacral plexus neuropathy |
| Rectal prolapse | Estimates of rectal prolapse are low (<0.5%).17 | • Rectal mucosal intussusception or prolapse • Rectal hypersensitivity • Weak resting pressure |
ARM, anorectal manometry.
However, diagnostic and therapeutic ARM procedure utilization varies considerably in standard practice and among experts.26 ARM technology has evolved from the Schuster balloon, to water-perfused systems utilizing a pneumohydraulic pump, to more advanced high-resolution solid-state systems with strain-gauge microtransducers.22,27–30 Despite advances in technology and software refinements, ARM is not widely utilized across the community and academic medical centers, for reasons unknown. This roundtable discussion critically reviews current ARM and BT clinical practices by physicians, surgeons, and a physical therapist in various settings; explores diagnostic and therapeutic challenges; and recommends creative solutions.
Materials And Methods
Key opinion leaders (KOLs) with specialties in medical gastroenterology (n = 14), colorectal surgery (n = 2), clinical research (n = 1), and pelvic floor physical therapy (n = 1) in the United States with significant ARM experience and interest in anorectal disorders were surveyed regarding practice patterns and utilization of ARM and BT. A roundtable discussion was held in April 2021 to discuss survey results, discuss current ARM practices, critically examine the challenges with the current use of these technologies, and explore practical solutions to enhance and expand ARM use and generate consensus recommendations. Consensus was reached through exchange of ideas and comments during the roundtable, as well as during numerous reviews and revisions of the manuscript. A literature search was also conducted in PubMed (latest search date May 20, 2022) to further inform KOLs of current ARM practices. Search terms included “anorectal,” “manometry,” “biofeedback therapy/training,” “dyssynergia defecation,” “fecal incontinence,” and “pelvic floor disorder/dysfunction,” among others.
Results
Our findings are grouped under the broad headings of diagnostic and therapeutic use. Within each modality, we discuss the strengths and limitations of each technology and potential solutions to overcome current challenges.
Diagnostic Use
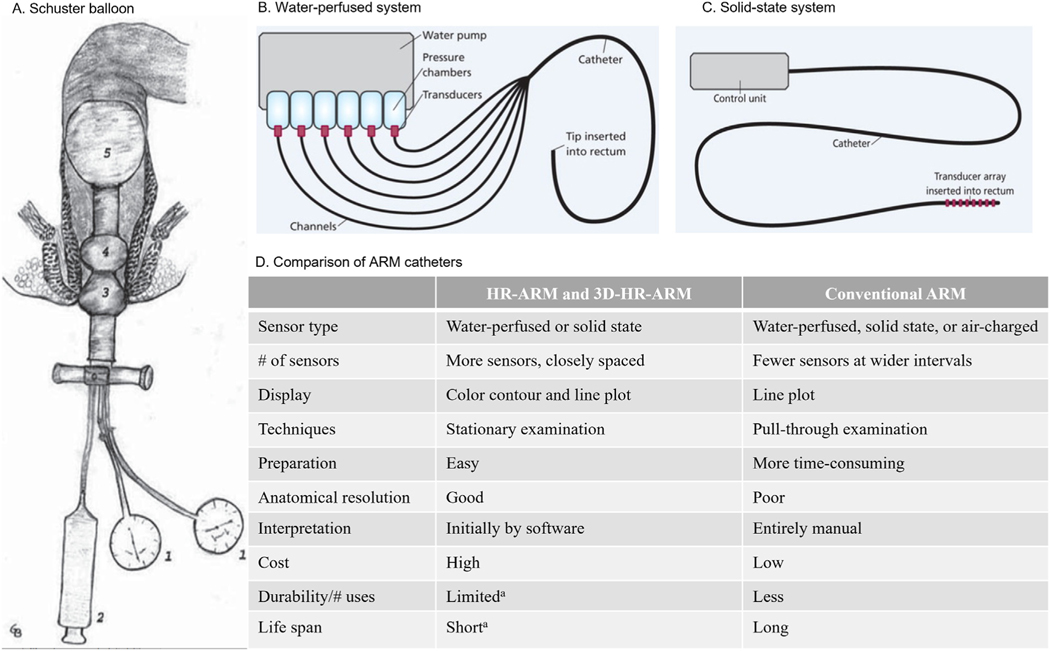
Several catheter-based manometry systems with varying technological sophistication are available in clinical practice (Figure 1),22,26–31 including solid-state circumferential transducers mounted on flexible probes, air-charged catheters, and rigid probes with 256 pressure sensors for 3-dimensional high-definition manometry systems.22,31,32 Portable air-charged catheters provide less spatial resolution than high-resolution catheters31 but are less expensive and can also be used to provide BT.33 Overall, solid-state catheters have a shorter lifespan compared with water-perfused catheters.31Visual technology to display isobaric contour plots and 3-dimensional display capabilities have also been introduced.29,31,32
Figure 1.
ARM system descriptions. (A). Air-filled balloon of Schuster. 1, aneroid manometer; 2, syringe for air insufflations; 3, pear-shaped balloon (for the external anal sphincter); 4, doughnut-shaped balloon (for the internal anal sphincter); 5, rectal balloon for eliciting the rectoanal inhibitory reflex. Reprinted with permission from Pfeifer and Oliveira.28 (B). Water-perfused system. Reprinted with permission from Solanki D, Hibberts F, Williams AB. Pelvic floor investigations for bowel dysfunction (part 2): anorectal physiology (manometry). Gastrointest Nurs. 2019;17:24. (C). Solid-state system. Reprinted with permission from Solanki D, Hibberts F, Williams AB. Pelvic floor investigations for bowel dysfunction (part 2): anorectal physiology (manometry). Gastrointest Nurs. 2019;17:24. (D). Comparison of ARM catheters. Reprinted with permission from Bharucha et al.31 aFor high-resolution ARM (HR-ARM) catheters that use solid-state sensors. 3D, 3-dimensional.
Among the authors, 33% perform or interpret an estimated <20 ARMs per month, 40% perform or interpret 20–40 per month, and the remainder perform or interpret >40 ARMs per month. Frequency may be limited by lack of staff, technician, or clinician training; time constraints; equipment availability; and costs. Most authors (81%) currently use solid-state systems only, while 6% use water-perfused systems only (13% use both). For practices lacking ARM access, balloon expulsion tests can be an acceptable alternative to evaluate for dyssynergic defection, albeit with a lack of standardization.31,34 Generally, a balloon expulsion test is performed using a nonlatex balloon filled with 50 ml of warm water, with patients in the seated position; in patients who cannot spontaneously expel the balloon, weighted measures have been used.31 While Foley catheters are also utilized, they are often challenging even for many healthy individuals to evacuate within the 2-minute cutoff, and therefore are less preferred.31,34
Normative data have been published with some but not all maneuvers and manometric systems.31 These studies were often performed from single centers or in small geographically limited sample sizes.35–38 There has been little effort to develop a broad range of multicenter normative data that considers ethnicity, age, sex, and nulliparous or multiparous status of women. Importantly, data are lacking using a standardized approach for each available manometric system. Additional barriers to ARM use and proposed solutions are discussed (Table 2).
Table 2.
ARM: Barriers for Its Use and Proposed Solutions and Graphic Representations
| Barriers | Solution | Graphic Figure |
|---|---|---|
| Education and Training: • Lack of understanding of the indications and clinical utility of ARM • Uncertainty regarding how to perform the procedure |
• Collective efforts from societies, foundations, industry, and practicing physicians to develop workshops and training modules (including hands-on training). • Incorporate ARM education in gastroenterology fellowship training; especially on how to perform and interpret ARM (both anatomical and technical aspects). • ARM should be discussed as a screening tool for other pelvic floor disorders (rectal intussusception, descending perineum syndrome, anorectal pain, and dysfunctional urinary symptoms with overlapping defecatory disorders), in addition to fecal incontinence and constipation. • Utilize the ANMS Clinical Training Program for gastroenterology fellows to obtain first-hand knowledge of these techniques and develop careers in neurogastroenterology/motility. • Support research in improving ARM diagnostic techniques and expanded indications, and development of newer tools. |
|
| Methods: evidence-based protocols/deviations are neededa | The IAPWG protocol could be improved upon by considering the following: • Provide guidance on addition of provocative testing to complement the findings depending on symptom profiles of constipation vs fecal incontinence. • Provide technique-specific and equipment-specific normal values where available, minor variations in the SOP are acceptable. The specific SOP is dictated by the equipment. • Implement seated HR-ARM if feasible for the assessment of defecation disorders. • Improve measurements for puborectalis pressure and coordination during simulated defecation. • Improve which cutoffs to use, as there are many sources with varying degrees of age-matched, sex-matched, etc. normative data. • Use of “push” with a standard rectal balloon volume as a better way to detect dyssynergia. For evacuation disorder, consider defecography to confirm or refute ARM findings suggesting dyssynergia. • Provide further protocol iterations that include normative parameters for quantifying disorders of rectoanal coordination, squeeze, rectal compliance, and sensory thresholds, including defecation index, rectoanal gradient, and integrated pressurized volume. |
|
| Interpretation: guidelines are lackinga | • Need additional normal values: • For subpopulations defined by age, sex, parity, BMI, and ethnicity • For all ARM systems (technique-specific values; HR- ARM vs HD-ARM) • Day-to-day reproducibility • Standardize description of findings and provide more conclusive interpretations. • Improve the definition for poor propulsion. • Determine which elements of the protocol predict interventional success (ie, likelihood of BT response based on abnormalities as defined by the London Classification). • Determine treatment recommendations based on 4 categorizations of dyssynergic defecation. • Provide evidence-based rationale for major and minor disorder classifications. • Define abnormalities identified via ARM that may warrant additional assessment. • Consensus guidelines are needed on how to define sensory abnormalities (rectal hyposensitivity, rectal hypersensitivity), and sensory biofeedback therapy. • Interpretation of ARM using the IAPWG protocol and its flow is cumbersome and less user-friendly and could be improved, including terminology. |
|
| Miscellaneous | • Teach proper rectal exam skills using video modules, demonstrations, instruction, and guidance on how to find prolapse and rectoceles, including positioning of patient. • Emphasize the utility of balloon expulsion testing. •Equipment manufacturers should provide standard protocols with flexible options to address specific patient needs. • Improve hands on practical training using small group workshops, seminars, and live demonstrations. • Impact of ARM on diagnosis and treatment outcomes are needed. |

|
ANMS, American Neurogastroenterology and Motility Society; ARM, anorectal manometry; BMI, body mass index; BT, biofeedback therapy; EMG, electromyography; HCP, healthcare provider; HD-ARM, high-definition anorectal manometry; HR-ARM, high-resolution anorectal manometry; IAPWG, International Anorectal Physiology Working Group; SOP, standard operating procedure.
Prior to the roundtable meeting, key opinion leaders in the gastroenterology field completed a brief survey. The survey question asked, “In the ideal London Classification, what would you improve? Please prioritize.”
Education: When and Why to Use ARM.
KOLs felt that although healthcare providers (HCPs) may be aware of ARM as a procedure, they may lack an understanding of the clinical utility of ARM, reducing enthusiasm for ordering ARM in clinical practices. For institutions lacking equipment, the ability to perform ARM is further hindered by the perceived economic risks of investing in the requisite equipment. Infrastructure considerations, including personnel time and equipment, also appear to influence the number of procedures that can be performed. High-level disinfection requirements may present additional financial and logistical burdens.39
ARM publications have focused predominantly on common clinical problems, including constipation and FI,31 but the utility of ARM as a screening tool for other pelvic floor disorders, including rectal intussusception, descending perineum syndrome, anorectal pain, and dysfunctional urinary symptoms with overlapping defecatory disorders, is seldom addressed.18,19 Moreover, ARM has a role in the preoperative evaluation of patients in whom a resection or distal anastomosis is contemplated, including patients with rectal cancer and ulcerative colitis.23,40,41 These patients may experience postoperative functional compromise in evacuation or continence warranting manometric assessment, and although robust data are lacking, ARM has been found to be particularly useful in identifying anal sphincter weakness, poor rectal compliance, or dyssynergic defecation.24,40,42–44 Constipation symptoms that seem to map to the anorectum (eg, straining, incomplete evacuation) are poor predictors of underlying pathophysiology.45 In contrast, ARM provides information regarding underlying mechanisms in anorectal sensorimotor functions that can lead to a more precise diagnosis, selection of the most appropriate operative procedure, and better quality of life. These findings are particularly useful when planning reanastomosis after colonic diversion. For example, for a patient with weak sphincter muscles, restoring colorectal anatomy would predispose them to FI. Likewise, if a patient has features of dyssynergic defecation, correction with BT prior to revision surgery is advisable. However, more prospective studies are needed to better understand the role of ARM.
While ARM is an accurate test for diagnosing FI with intrapatient repeatability,46,47 there is some day-to-day variability in anorectal pressures, especially during simulated evacuation.48,49 Approaches to reverse engineer intrapatient data have been proposed.50 As the summary variables derived from standard software include only a fraction of the actual data captured during ARM, the reverse-engineering approach offers a way to extract and analyze raw data, providing a potentially more useful summary of rectoanal pressure variables, but this merits further study. Several types of dyssynergia have been identified by ARM (Supplementary Figure 1)12 and shown to be reproducible.45 Although dyssynergia type does not affect diagnosis or outcomes, it informs the biofeedback therapist and patient which aspects of BT will help achieve normal bowel function and how to tailor the treatment based on the patho-physiological dysfunction.51
Overcoming these challenges requires the engagement of researchers, academicians, community physicians, and advanced practice providers. HCPs need to understand the importance of ARM, the indications or contraindications, and when or where to refer. Likewise, community physicians and institutions should consider adding this technology to improve the care of patients with anorectal disorders. Data demonstrating the impact of ARM on management decisions and treatment outcomes are needed. These limitations can be overcome through increased knowledge and the perceived value of ARM.
Training: How to Perform ARM and Interpret Results.
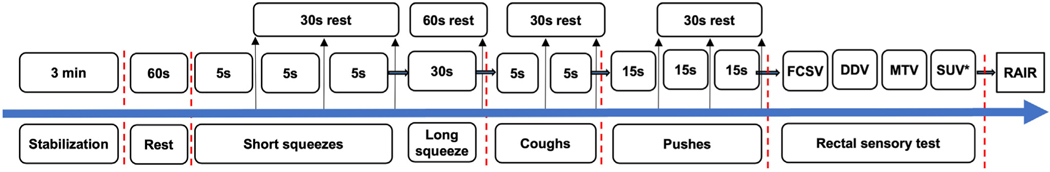
Gastroenterology and colorectal surgery trainees should learn how to perform and interpret ARM (both anatomical and technical aspects). However, motility training opportunities are relatively sparse. Even in ARM-performing centers, trainees are not always exposed to this procedure. Avenues are available for training nurses and technicians on the performance of ARM and BT,52 although no formal procedure-specific certifications exist. Academic centers and equipment manufacturers offer small group workshops, which is not widely known. Also, the American Neurogastroenterology and Motility Society (ANMS) sponsors a 1-month apprenticeship training for gastroenterology fellows that trainees highly rate.53,54 ANMS and the European Society of Neurogastroenterology and Motility have published ARM guidance based on the use of solidstate or water-perfused manometry systems in the hope of standardizing the procedure.5 The advent of highresolution topographic ARM and the creation and adoption of the Chicago Classification for esophageal manometry has led to a renewed interest in developing a more formal ARM consensus protocol.55,56 The International Anorectal Physiology Working Group (IAPWG) protocol (Figure 2), the London Classification, and other studies provide the foundation for standardizing the conduct and interpretation of ARM.31,56–58
Figure 2.
IAPWG protocol. *Optional threshold. DDV, desire to defecate volume; FCSV, first constant sensation volume; MTV, maximum tolerated volume; RAIR, rectoanal inhibitory reflex; SUV, sustained urgency volume.
Among the KOLs surveyed, 60% routinely use the IAPWG protocol. Although all KOLs perform assessments of resting and squeeze anal pressure, rectoanal inhibitory reflex, push maneuvers, rectal sensory threshold, cough reflex, and rectal compliance, there were variations in the length of the resting interval(s) and use of an empty vs inflated rectal balloon for squeeze and push maneuvers. Following the exact IAPWG sequence was not as high a priority as was ensuring that all manometric and sensory assessments were completed. Several limitations to the IAPWG protocol were identified (Table 2), including a lack of validated normal values stratified by demographic factors (eg, age, sex, geographic location, ethnicity) for all catheter systems.31 Other challenges include a lack of standardized rectal sensory testing methodology, vagaries involving rectoanal inhibitory reflex testing,56 and a lack of positional requirements (seated ARM is preferred).59
A collective effort from experts, societies, and industry participants is needed to develop small group workshops and training modules (including hands-on training). The IAPWG protocol and London Classification were discussed as potential training tools to educate future gastroenterologists; however, more clinical evidence is necessary to identify the ideal ARM protocol, which will likely vary by equipment and indication. The IAPWG protocol (measuring anal relaxation of 3 defecation attempts) effectively rules out dyssynergic defecation, but the balloon expulsion test is more relevant at “ruling in” dyssynergic defecation.34,60 Considering other evacuation disorders, apart from dyssynergic defecation, the impact of minor protocol differences (eg, 30-second vs 60-second rest intervals, 2 vs 3 squeezes) remains debatable.58 Rectal sensation testing methodology guidance and standardization warrant additional discussion (eg, intermittent vs continuous balloon distension). Understanding which protocol elements predict interventional success is important for clinical decision making. Additionally, research is needed to link ARM results to treatment outcomes to understand better when to refer patients for BT.61,62 Other ARM-identified abnormalities may warrant additional assessment (rectal hyposensitivity and rectal hypersensitivity, anorectal tone, and other London Classification categories).56 Consensus guidelines on how to define sensory abnormalities are also needed. For example, should one sensation outside the normal range be categorized as abnormal, or should 2 or more be required? Given the subjective nature of these assessments, recent evidence supports using more than 1 sensory assessment to define these conditions.63,64
Therapeutic Use
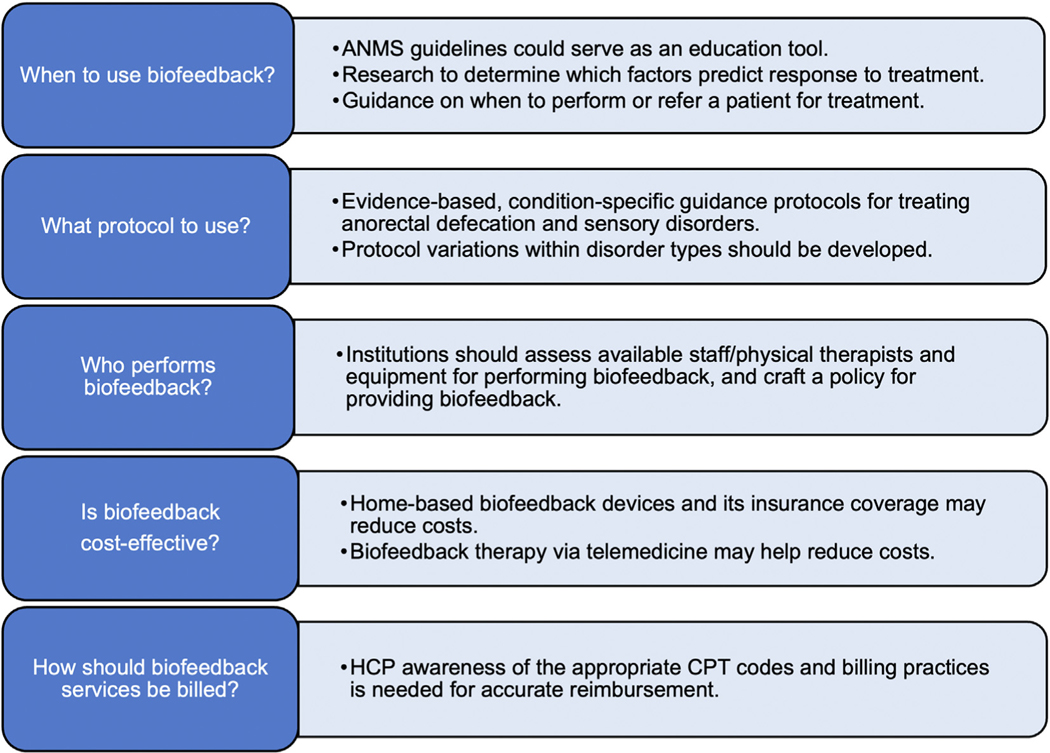
In addition to diagnostics, ARM is used for BT,21 an instrument-based treatment modality for the treatment of dyssynergic defecation and FI and is used in pelvic floor physical therapy and anorectal labs. BT utilizes visual (computer monitor) or audible or verbal (therapist) feedback techniques to inform the patient and therapist of the strength of muscle contraction or coordinated changes in rectal and anal sphincter pressures during attempted defecation.65 Based on the principles of operant conditioning, BT improves constipation symptoms in patients with dyssynergic defecation and facilitates more normal evacuation and was afforded a Grade A recommendation by ANMS and European Society of Neurogastroenterology and Motility.21 BT also enhances the rectal sensory perception and increases anal sphincter tone in individuals with FI, thereby restoring bowel function.21 BT also improves FI- and constipation-related symptoms in patients with anatomical abnormalities such as rectocele or rectal intussusception66–68 and is useful for rectal hyposensitivity training in patients with FI and constipation.21,63,69–71 Sensory adaptation training can also treat rectal hypersensitivity.64 Likewise, rectal sensorimotor coordination training improves rectal urgency in patients with FI.72 We identified several barriers to using BT and offer creative solutions summarized in Figure 3.
Figure 3.
BT: barriers to use and solutions. ANMS, American Neurogastroenterology and Motility Society; CPT, Current Procedural Terminology; HCP, healthcare provider.
When Is BT Useful?
The clinical utility of BT is not universally understood. Guidance is lacking on what constitutes the phenotypical patient who would benefit from BT. Among patients with clinical features of difficult evacuation, a dyssynergic defecation pattern identified by ARM together with an abnormal balloon expulsion test justifies consideration of BT (Table 3).21,73,74 In the case of discordant ARM and balloon expulsion test results, perhaps a third follow-up test is needed (eg, fluoroscopic or magnetic resonance defecography) to confirm pelvic floor dysfunction or rule out structural pathologies.12 When imaging is unavailable, experts used excessive colonic retention of markers and demonstrated significant improvement following BT.51,75–77 Regardless of follow-up test results, most providers recommend BT for discordant cases.
Table 3.
Indications for Biofeedback Therapy
| Indications for Biofeedback | Contraindications for Biofeedback Therapy |
|---|---|
| • Constipation with dyssynergic defecation20,21,74,76,77,80
• Fecal incontinence (unresponsive to conservative treatment)21,80 • Levator ani syndrome with dyssynergic defecation (unresponsive to standard in absence of structural or inflammatory etiology of pain)21 • Solitary rectal ulcer syndrome with dyssynergic defecation (unresponsive to behavioral measures, laxative, and topical therapy)21 |
• Severe neurological disorders21
• Dementia21 •Inability to sit on a commode21 •Developmental disability21 •Visual impairment21 •Pediatric functional constipation, with or without overflow fecal incontinence92–94 •Anal fissure |
| Additional conditions that may benefit from biofeedback | Additional conditions where biofeedback may be used with caution, but not as standalone treatment |
| • Rectal prolapse95 • Impaired defecation and rectoceles66,96 • Sphincter thinning 97 |
• History of sexual abuse without psychologic assessment98 |
In clinical trials, BT effectively treats 70% to 80% of patients with dyssynergic defecation.12 Similarly, 76% of patients with refractory FI reported adequate relief with BT.78 The likelihood and magnitude of response to BT may vary based on treatment length, diagnosis or symptoms, disease severity, or comorbid illnesses.79,80 Patients with dyssynergic defecation and lower or more normal baseline thresholds for first rectal sensation and urge were more likely to respond to BT, while depression and elevated first rectal sensory threshold volume were independent predictors of poor BT efficacy.71,81 Identifying patients with FI who may respond better to BT has been challenging.79 The presence of IBS or chronic constipation in patients with dyssynergic defecation does not affect BT response.51,82 Anorectal physiology testing is recommended for refractory IBS to identify abnormalities that respond more favorably to BT (eg, lower rectal sensory thresholds); in certain IBS patients, abdominal pain and bloating improved with BT.4,83 In patients with refractory slow transit constipation, increased frequency of abdominal pain predicted a poor response to BT.74 Lower baseline constipation scores, shorter colonic transit times, and lower intolerable urgency thresholds can predict treatment outcomes in patients with dyssynergic defecation regardless of IBS.82 In patients with chronic constipation and dyssynergic defecation, lower bowel satisfaction scores at baseline and use of digital maneuvers were associated with BT success, suggesting the appropriateness of offering BT to patients with dyssynergic defecation.51 BT is an option for patients who develop evacuatory compromise following surgery with a distal colorectal, coloanal, or ileoanal anastomosis.40
Because the goal of BT is to restore normal function, understanding the dyssynergia type (Supplementary Figure 1) helps to personalize and tailor the biofeedback maneuvers to optimally benefit a patient.12 Additionally, rectal desensitization training or sensory adaptation training can be performed using serial balloon inflation; computerized barostat-assisted balloon distension systems may also be used.64 Studies are needed to anticipate patient response to BT based on symptoms and diagnosis. Suggested BT protocols in patients with anorectal disorders exist,21 but evidencebased disorder-specific protocols are needed.
Who Performs BT?
Among the KOLs surveyed, 40% did not perform BT, 13% performed fewer than 20 monthly procedures, 33% performed between 20‒40 monthly procedures, and 13% between 40‒60 monthly procedures. Overall, KOLs performed fewer BT procedures compared with diagnostic ARM procedures. This discrepancy is partly due to institutional requirements regarding who can perform BT, how therapists are supervised, or billing constraints. There was no consensus on the optimal BT provider: most KOLs use a pelvic floor physical therapist to perform BT; however, some utilize advanced practice registered nurses or trained registered nurse therapists or refer patients to external providers. Determining where to refer a patient for therapy can be challenging in less specialized settings. Further, some KOLs felt that physical therapists are generally well equipped and trained to perform BT for patients with FI but less so for patients with dyssynergic defecation, which in part is due to a lack of appropriate equipment that can provide feedback regarding changes in abdominal and rectal push effort simultaneously with changes in anal and pelvic floor relaxation.
Confusion over recent reimbursement changes was addressed as a deterrent to providing BT. Prior to 2020, Current Procedural Terminology code 90911 was used for BT.84 In 2020, new codes were implemented to allow for time-based therapy; however, they stipulate that BT can only be performed by physicians, nurse practitioners, physician assistants, or certified nurse specialists, thus disallowing registered nurses and other trained personnel from providing BT under physician supervision (Supplementary Figure 2).84 BT performed by a physical therapist uses separate codes.85,86 Confusion over billing practices and which providers can perform BT may lead gastroenterologists and surgeons to outsource BT to avoid reimbursement errors, further limiting patient access and care.
Recommending a certain specialist or even a universal BT protocol may be overly strict, but participants agreed that to provide patient feedback regarding the dynamic changes taking place in the anorectum during attempted defecation or volitional control of evacuation, BT should include anorectal probe placement with a rectal balloon providing simulated stooling, especially during rectal sensory training. The success of BT likely depends on the provider’s competency and patient’s willingness to complete the sessions. When used appropriately, billing codes may be instrumental in determining whether institutions provide BT. While tracking a provider’s qualifications or expertise is important, determining which providers administer appropriate BT for anorectal disorders remains challenging because many therapists may be trained for urinary but not for anorectal problems.
BT Costs.
Most KOLs felt insurance coverage to be a major barrier for BT. Although it is a covered benefit under Medicare, coverage may vary geographically, and some private insurance agencies in the United States may not cover BT.75,87 Institute of Certified Professional Managers programs in states such as Pennsylvania routinely cover BT; however, it is not reimbursed in other states (eg, Washington and Georgia) by third-party payers, limiting utilization of BT in these regions.88 Insurance may also restrict the number of covered sessions per year or for specific indications (eg, some insurance carriers pay for BT for patients with FI but not for patients with dyssynergic defecation). Recent changes in CPT codes have worsened this issue.
Uncertainty over the cost-effectiveness of BT may limit its broader clinical use. While BT is standardly an office-based outpatient procedure, studies have shown that both office- and home-based procedures are inexpensive.89 Home-based BT is significantly more cost-effective and has similar efficacy compared with office-based therapy.89 Devices for home-based BT for FI are commercially available, while home-based devices for constipation are lacking.89 With telemedicine expanding during the COVID-19 pandemic,90 homebased BT may become preferred. Providers must consider licensing requirements, malpractice coverage, platform choices, and reimbursement to integrate home-based therapy sustainably.90 Dedicated BT instruments, user-friendly software systems that help patients to better connect with their bowel dysfunctions, and the ability to administer home or office BT are urgently needed to improve access and provide optimal care.
Limitations
Although this study offers a consensus among experts, it does have limitations. Specifically, additional or different experts’ opinions could have been solicited. Unfortunately, at this point it is not possible to redo the study, but we do realize that the inclusion of additional experts, especially in colorectal surgery and pelvic floor therapy, might have been desirable. Additional limitations are inherent in the heterogeneity of techniques and diagnostic and therapeutic indications. Last, although the study identifies these limitations and challenges that allow general conclusions, the data are insufficient to allow specific recommendations to overcome the identified obstacles to ARM.
Conclusions
ARM is a beneficial tool for diagnosing anorectal disorders and performing BT. Multiple barriers for its use still exist but can be overcome with additional research, education or training, and evidence-based guidance. Studies identifying normative values within demographic subclasses and comparing different protocols for both the performance and interpretation of ARM are necessary.31,56–58,91 ARM protocols should be tested independently in academic and community settings to vet all maneuvers and proposed deviations. Uncertainty over when to use BT, where to refer patients, and how to bill for services limits BT use. Institutions should carefully assess equipment, provider availability and expertise (or appropriate referral location), and reimbursement and billing practices to develop an appropriate policy for providing BT to their patients.
Lack of training using a uniform standardized approach was unanimously felt as a significant barrier to adopting ARM for diagnostic and therapeutic use. A formal collaboration between experts and industry is needed to develop training modules and guidelines addressing the current education gaps. An ideal scenario may entail collaboration between motility societies and device companies to develop an extended educational training program in which, upon completion, participants return to their institution to train others. For example, ANMS has developed an annual Allied Health Training program as a clinical course; ARM training could be incorporated into a similar program. The industry can provide additional equipment-specific training videos. Whether training acquired with one type of equipment or at one center can apply to broader clinical use is unclear. Also, dedicated BT instruments and software are needed. By improving the understanding and availability of ARM and BT and providing guidelines and education around its utility and benefits across the spectrum of HCPs, the standard of care for patients with anorectal and pelvic floor disorders will be greatly enhanced.
Supplementary Material
Supplementary Figure 2. Biofeedback Current Procedural Terminology (CPT) codes. aAllows for flexible time-based sessions; must be billed with 90912. bApplication of 9% reduction reimbursement started in 2021. GP modifier, services delivered under an outpatient physical therapy plan of care; HCP, healthcare provider.
Supplementary Figure 1. High-resolution manometry and conventional manometry patterns commonly seen in a healthy individual (normal pattern) and in patients with different types of dyssynergic defecation. Reprinted with permission from Rao SS, Patcharatrakul T. Diagnosis and treatment of dyssynergic defecation.
Acknowledgments
The authors thank Sarah Vogler, MD, MBA, of the Cleveland Clinic (Port St. Lucie, FL) for participating in the survey and roundtable discussion. Medical writing and editorial support was provided by Daniel Turkewitz, PhD, and Julie O’Grady, BA, of The Medicine Group, LLC (New Hope, PA), in accordance with Good Publication Practice guidelines. Funding for medical writing and editorial support was provided by GI Supply (Mechanicsburg, PA), which was acquired by Laborie Medical Technologies (Portsmouth, NH) in April 2022.
Funding
Funding for the roundtable meeting and medical writing and editorial support was provided by GI Supply (Mechanicsburg, PA), which was acquired by Laborie Medical Technologies (Portsmouth, NH) in April 2022.
Abbreviations used in this paper:
- ANMS
American Neurogastroenterology and Motility Society
- ARM
anorectal manometry
- BT
biofeedback therapy
- FI
fecal incontinence
- HCP
healthcare provider
- IAPWG
International Anorectal Physiology Working Group
- IBS
irritable bowel syndrome
- KOL
key opinion leader
Footnotes
Conflicts of interest
The authors disclose the following: Satish S.C. Rao has served on the advisory boards for Medtronic, Takeda Pharmaceuticals, Ironwood Pharmaceuticals, Sanofi Pharmaceuticals, Vibrant Ltd, and Salix Pharmaceuticals. Nitin K. Ahuja has served on a medical advisory board for GI Supply and Takeda; received research support from Vanda Pharmaceuticals and Nestlé; served a consultant for GlaxoSmithKline Consumer Healthcare; and received payment for educational events from Medtronic. Adil E. Bharucha has received funding from Minnesota Medical Technologies; has royalties/licenses from Medspira and Minnesota Medical Technologies; has served as a consultant for GI Supply and Medical Insights Group; has patents issued or pending with Medtronic, Minnesota Medical Technologies, and Medspira; and has received equipment from Cairn Diagnostics. Darren M. Brenner has served a consultant for AbbVie (Allergan), Ironwood, Takeda, RedHill Biopharma, Alnylam, AlphaSigma, Ardelyx, Bayer, Gemelli, Arena Pharmaceuticals, Vibrant, GI Health Foundation, IFFGD, and the Rome Foundation; and is sponsored by an unrestricted grant from the Irene D. Pritzker Foundation. William D. Chey has served a consultant for AbbVie, Ardelyx, Biomerica, Gemelli, IM Health, Ironwood, Nestlé, QOL Medical, Phathom, Progenity, RedHill BioPharma, Salix/Valeant, Takeda, Urovant, and Vibrant; has patents issued from My Nutrition Health, Digital Manometry, Rectal Expulsion Device; has served in a leadership or fiduciary role for the American College of Gastroenterology, Rome Foundation, and International Foundation for Gastrointestinal Disorders; has stock options in Isothrive, Kiwi Bioscience, and Modify Health; and has received research funding from Commonwealth Diagnostics International, the Food and Drug Administration, the National Institutes of Health, QOL Medical, and Salix/ Valeant. Jill K. Deutsch has received honorarium from GI Supply. David C. Kunkel has served a consultant for Palette Life Sciences, Allergan, Salix Pharmaceuticals, QOL Medical, RedHill Biopharma, Shire (Takeda), Arena Pharmaceuticals, Pfizer, and Portola Pharmaceuticals. Baharak Moshiree has received research support from reStalsis, Salix/Bausch Pharmaceuticals, Allergan/Ironwood, Takeda, Cairn Diagnostics, Alnylam, Atome, Nestlé, and Medtronic; has served on an advisory board and received honorarium from Allergan, Takeda, AbbVie, Salix/Bausch Pharmaceuticals, and Alnylam; has received payment for expert testimony; has a patent pending; and has served on the ACG Research Committee, on the ACG Minority Affairs Committee, as the ACG Governor of NC State, and as an American Neurogastroenterology and Motility Society council member. Leila Neshatian has received funding from the Stanford GI Department and Stanford Surgery Department; and served on the Rome Committee and International Continence Society. Robert M. Reveille has received honorarium from GI Supply and travel support from the American Foregut Society. Gregory S. Sayuk has served as a consultant and speaker for Salix Pharmaceuticals, AbbVie/Ironwood Pharmaceuticals, Alnylam, and the GI Health Foundation. Jordan M. Shapiro has received honorarium from GI Supply. Eric D. Shah has served a consultant for Salix Pharmaceuticals. Kyle Staller has received funding from Ironwood and Urovant; served a consultant for Ardelyx, Anji, Gelesis, Sanofi, Shire (Takeda), and GI Supply; and served on an advisory board for Arena Pharmaceuticals. Steven D. Wexner has royalties from Medtronic, Intuitive Surgical, Karl Storz Endoscopy America, and Unique Surgical Innovations LLC; has served a consultant for ICON Language Services, Intuitive Surgical, Stryker, Medtronic, Takeda, ARC Medical/Corvus, Astellas, Baxter, Olympus, AIS Channel, LivsMed, GI Supply, and Leading BioSciences; and has stock in Pragma/GibLib, Renew Medical, CRH Medical, and Intuitive Surgical. Jason R. Baker has served a consultant for GI Supply.
CRediT Authorship Contributions
Roundtable discussion moderators: Satish S.C. Rao and Jason R. Baker. Roundtable discussion: Nitin K. Ahuja, Adil E. Bharucha, Darren M. Brenner, William D. Chey, Jill K. Deutsch, David C. Kunkel, Baharak Moshiree, Leila Neshatian, Robert M. Reveille, Gregory S. Sayuk, Jordan M. Shapiro, Eric D. Shah, Kyle Staller, and Steven D. Wexner participated. Drafting, critical revision, and final approval of the manuscript: Satish S.C. Rao, Nitin K. Ahuja, Adil E. Bharucha, Darren M. Brenner, William D. Chey, Jill K. Deutsch, David C. Kunkel, Baharak Moshiree, Leila Neshatian, Robert M. Reveille, Gregory S. Sayuk, Jordan M. Shapiro, Eric D. Shah, Kyle Staller, Steven D. Wexner, and Jason R. Baker.
Supplementary Material
To access the supplementary material accompanying this article, visit the online version of Clinical Gastroenterology and Hepatology at www.cghjournal.org, and at https://doi.org/10.1016/j.cgh.2023.05.025.
References
- 1.Diamant NE, Kamm MA, Wald A, et al. AGA technical review on anorectal testing techniques. Gastroenterology 1999; 116:735–760. [DOI] [PubMed] [Google Scholar]
- 2.Patcharatrakul T, Rao SSC. Update on the pathophysiology and management of anorectal disorders. Gut Liver 2018; 12:375–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carrington E, Scott SM, Bharucha A, et al. Advances in the evaluation of anorectal function. Nat Rev Gastroenterol Hepatol 2018;15:309–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lacy BE, Pimentel M, Brenner DM, et al. ACG clinical guideline: management of irritable bowel syndrome. Am J Gastroenterol 2021;116:17–44. [DOI] [PubMed] [Google Scholar]
- 5.Rao SSC, Azpiroz F, Diamant N, et al. Minimum standards of anorectal manometry. Neurogastroenterol Motil 2002; 14:553–559. [DOI] [PubMed] [Google Scholar]
- 6.Bharucha AE, Knowles CH, Mack I, et al. Faecal incontinence in adults. Nat Rev Dis Primers 2022;8:53. [DOI] [PubMed] [Google Scholar]
- 7.Menees SB, Almario C, Spiegel BMR, et al. Prevalence of and factors associated with fecal incontinence: results from a population-based survey. Gastroenterology 2018; 154:1672–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown HW, Wexner SD, Segall MM, et al. Accidental bowel leakage in the mature women’s health study: prevalence and predictors. Int J Clin Pract 2012;66:1101–1108. [DOI] [PubMed] [Google Scholar]
- 9.Sperber AD, Bangdiwala SI, Drossman DA, et al. Worldwide prevalence and burden of functional gastrointestinal disorders, results of Rome Foundation global study. Gastroenterology 2021;160:99–114.e3. [DOI] [PubMed] [Google Scholar]
- 10.Rasijeff AMP, García-Zermeño K, DiTanna G-L, et al. Systematic review and meta-analysis of anal motor and rectal sensory dysfunction in male and female patients undergoing anorectal manometry for symptoms of faecal incontinence. Colorectal Disease 2022;24:562–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rao SSC, Bharucha AE, Chiarioni G, et al. Anorectal disorders. Gastroenterology 2016;150:1430–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rao SSC, Patcharatrakul T. Diagnosis and treatment of dyssynergic defecation. J Neurogastroenterol Motil 2016;22:423–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mertz H, Naliboff B, Mayer E. Physiology of refractory chronic constipation. Am J Gastroenterol 1999;94:609–615. [DOI] [PubMed] [Google Scholar]
- 14.Nyam DCNK, Pemberton JH, Ilstrup DM, et al. Long-term results of surgery for chronic constipation. Dis Colon Rectum 1997; 40:273–279. [DOI] [PubMed] [Google Scholar]
- 15.Wang XJ, Chedid V, Vijayvargiya P, et al. Clinical features and associations of descending perineum syndrome in 300 adults with constipation in gastroenterology referral practice. Dig Dis Sci 2020;65:3688–3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drossman DA, Li Z, Andruzzi E, et al. US Householder survey of functional gastrointestinal disorders. Prevalence, socio-demography, and health impact. Dig Dis Sci 1993;38:1569–1580. [DOI] [PubMed] [Google Scholar]
- 17.Kairaluoma MV, Kellokumpu IH. Epidemiologic aspects of complete rectal prolapse. Scand J Surg 2005;94:207–210. [DOI] [PubMed] [Google Scholar]
- 18.Coyne KS, Cash B, Kopp Z, et al. The prevalence of chronic constipation and faecal incontinence among men and women with symptoms of overactive bladder. BJU Int 2011;107:254–261. [DOI] [PubMed] [Google Scholar]
- 19.Klingele CJ, Lightner DJ, Fletcher JG, et al. Dysfunctional urinary voiding in women with functional defecatory disorders. Neuro-gastroenterol Motil 2010;22:1094–e284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chiarioni G, Whitehead WE, Pezza V, et al. Biofeedback is superior to laxatives for normal transit constipation due to pelvic floor dyssynergia. Gastroenterology 2006;130:657–664. [DOI] [PubMed] [Google Scholar]
- 21.Rao SSC, Benninga MA, Bharucha AE, et al. ANMS-ESNM position paper and consensus guidelines on biofeedback therapy for anorectal disorders. Neurogastroenterol Motil 2015; 27:594–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rao SSC, Singh S. Clinical utility of colonic and anorectal manometry in chronic constipation. J Clin Gastroenterol 2010; 44:597–609. [DOI] [PubMed] [Google Scholar]
- 23.Church JM, Saad R, Schroeder T, et al. Predicting the functional result of anastomoses to the anus: the paradox of preoperative anal resting pressure. Dis Colon Rectum 1993; 36:895–900. [DOI] [PubMed] [Google Scholar]
- 24.Dulskas A, Samalavicius NE. Usefulness of anorectal manometry for diagnosing continence problems after a low anterior resection. Ann Coloproctol 2016;32:101–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Öztürk Ö, Özin Y, Bacaksız F, et al. The efficacy of biofeedback treatment in patients with fecal incontinence. Turk J Gastroenterol 2021;32:567–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carrington EV, Heinrich H, Knowles CH, et al. Methods of anorectal manometry vary widely in clinical practice: results from an international survey. Neurogastroenterol Motil 2017;29:e13016. [DOI] [PubMed] [Google Scholar]
- 27.Schuster MM. Biofeedback treatment of gastrointestinal disorders. Med Clin North Am 1977;61:907–912. [DOI] [PubMed] [Google Scholar]
- 28.Pfeifer J, Oliveira L. Anorectal manometry and the rectoanal inhibitory reflex. In: Wexner SD, Duthie GS, eds. Constipation: Etiology, Evaluation, and Management. Berlin, Germany: Springer Nature;, 2006:71–83. [Google Scholar]
- 29.Jones MP, Post J, Crowell MD. High-resolution manometry in the evaluation of anorectal disorders: a simultaneous comparison with water-perfusedmanometry.AmJGastroenterol 2007;102:850–855. [DOI] [PubMed] [Google Scholar]
- 30.Rao SSC. Advances in diagnostic assessment of fecal incontinence and dyssynergic defecation. Clin Gastroenterol Hepatol 2010;8:910–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bharucha AE, Basilisco G, Malcolm A, et al. Review of the indications, methods, and clinical utility of anorectal manometry and the rectal balloon expulsion test. Neurogastroenterol Motil 2022;34:e14335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tantiphlachiva K, Paulson J, Attaluri A, et al. Is high-definition manometry a comprehensive test of anal sphincter function: comparative study with manometry and ultrasound: 1217. Am J Gastroenterol 2008;103:S476. [Google Scholar]
- 33.Sharma M, Lowry AC, Rao SS, et al. A multicenter study of anorectal pressures and rectal sensation measured with portable manometry in healthy women and men. Neuro-gastroenterol Motil 2021;33:e14067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shah ED, Farida JD, Menees S, et al. Examining balloon expulsion testing as an office-based, screening test for dyssynergic defecation: a systematic review and meta-analysis. Am J Gastroenterol 2018;113:1613–1620. [DOI] [PubMed] [Google Scholar]
- 35.Oblizajek NR, Gandhi S, Sharma M, et al. Anorectal pressures measured with high-resolution manometry in healthy people—Normal values and asymptomatic pelvic floor dysfunction. Neurogastroenterol Motil 2019;31:e13597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Coss-Adame E, Rao SSC, Valestin J, et al. Accuracy and reproducibility of high-definition anorectal manometry and pressure topography analyses in healthy subjects. Clin Gastroenterol Hepatol 2015;13:1143–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee HJ, Jung KW, Han S, et al. Normal values for high-resolution anorectal manometry/topography in a healthy Korean population and the effects of gender and body mass index. Neuro-gastroenterol Motil 2014;26:529–537. [DOI] [PubMed] [Google Scholar]
- 38.Li Y, Yang X, Xu C, et al. Normal values and pressure morphology for three-dimensional high-resolution anorectal manometry of asymptomatic adults: a study in 110 subjects. Int J Colorectal Dis 2013;28:1161–1168. [DOI] [PubMed] [Google Scholar]
- 39.Rutala WA, Weber DJ. Disinfection and sterilization: an overview. Am J Infect Control 2013;41:S2–S5. [DOI] [PubMed] [Google Scholar]
- 40.Kochi M, Egi H, Adachi T, et al. Preoperative incremental maximum squeeze pressure as a predictor of fecal incontinence after very low anterior resection for low rectal cancer. Surg Today 2020;50:516–524. [DOI] [PubMed] [Google Scholar]
- 41.Halverson AL, Hull TL, Remzi F, et al. Perioperative resting pressure predicts long-term postoperative function after ileal pouch-anal anastomosis. J Gastrointest Surg 2002;6:316–321. [DOI] [PubMed] [Google Scholar]
- 42.Batignani G, Monaci I, Ficari F, et al. What affects continence after anteriorresectionoftherectum? DisColonRectum 1991;34:329–335. [DOI] [PubMed] [Google Scholar]
- 43.Ihnát P, Vávra P, Prokop J, et al. Functional outcome of low rectal resection evaluated by anorectal manometry. ANZ J Surg 2018;88:E512–E516. [DOI] [PubMed] [Google Scholar]
- 44.Cura Pales CG, An S, Cruz JP, et al. Postoperative bowel function after anal sphincter-preserving rectal cancer surgery: risks factors, diagnostic modalities, and management. Ann Coloproctol 2019;35:160–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rao SSC, Mudipalli RS, Stessman M, et al. Investigation of the utility of colorectal function tests and Rome II criteria in dyssynergic defecation (Anismus). Neurogastroenterol Motil 2004;16:589–596. [DOI] [PubMed] [Google Scholar]
- 46.Yeap ZH, Simillis C, Qiu S, et al. Diagnostic accuracy of anorectal manometry for fecal incontinence: a meta-analysis. Acta Chir Belg 2017;117:347–355. [DOI] [PubMed] [Google Scholar]
- 47.Rogers J, Laurberg S, Misiewicz JJ, et al. Anorectal physiology validated: a repeatability study of the motor and sensory tests of anorectal function. Br J Surg 1989;76:607–609. [DOI] [PubMed] [Google Scholar]
- 48.Triadafilopoulos G, Clarke JO, Kamal A, et al. Intra-subject variability in high resolution anorectal manometry using the London Classification: diagnostic and therapeutic implications. Dig Dis Sci 2022;67:5014–5018. [DOI] [PubMed] [Google Scholar]
- 49.Mishra R, Gautam M, Oblizajek NR, et al. Reproducibility of high-resolution manometry among healthy and constipated persons. Neurogastroenterol Motil 2022;34:e14438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.ElWazir M, Gautam M, Mishra R, et al. Automated extraction of anorectal pressures from high-resolution manometry reports. Neurogastroenterol Motil 2022;34:e14411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Patcharatrakul T, Valestin J, Schmeltz A, et al. Factors associated with response to biofeedback therapy for dyssynergic defecation. Clin Gastroenterol Hepatol 2018;16:715–721. [DOI] [PubMed] [Google Scholar]
- 52.Biofeedback Certification International Alliance (BCIA). PMDB Technician. Available at: https://www.bcia.org/pmdbtechnician. Accessed April 26, 2022.
- 53.Rao SSC, Parkman HP. Advanced training in neurogastroenterology and gastrointestinal motility. Gastroenterology 2015;148:881–885. [DOI] [PubMed] [Google Scholar]
- 54.Vasant DH, Sharma A, Bhagatwala J, et al. Apprenticeship-based training in neurogastroenterology and motility. Expert Rev Gastroenterol Hepatol 2018;12:215–222. [DOI] [PubMed] [Google Scholar]
- 55.Kahrilas PJ, Bredenoord AJ, Fox M, et al. The Chicago Classification of esophageal motility disorders, v3.0. Neurogastroenterol Motil 2015;27:160–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Carrington EV, Heinrich H, Knowles CH, et al. The International Anorectal Physiology Working Group (IAPWG) recommendations: standardized testing protocol and the London classification for disorders of anorectal function. Neurogastroenterol Motil 2020;32:e13679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Oblizajek NR, Deb B, Kathavarayan Ramu S, et al. Optimizing techniques for measuring anal resting and squeeze pressures with high-resolution manometry. Neurogastroenterol Motil 2022; 34:e14383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ang D, Vollebregt P, Carrington EV, et al. Redundancy in the international anorectal physiology working group manometry protocol: a diagnostic accuracy study in fecal incontinence. Dig Dis Sci 2021;67:964–970. [DOI] [PubMed] [Google Scholar]
- 59.Sharma M, Muthyala A, Feuerhak K, et al. Improving the utility of high-resolution manometry for the diagnosis of defecatory disorders in women with chronic constipation. Neurogastroenterol Motil 2020;32:e13910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ortengren AR, Ramkissoon RA, Chey WD, et al. Anorectal manometry to diagnose dyssynergic defecation: systematic review and meta-analysis of diagnostic test accuracy. Neurogastroenterol Motil 2021;33:e14137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhao Y, Ren X, Qiao W, et al. High-resolution anorectal manometry in the diagnosis of functional defecation disorder in patients with functional constipation: a retrospective cohort study. J Neurogastroenterol Motil 2019;25:250–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shah ED, Pelletier EA, Greeley C, et al. An office-based, point-of-care test predicts treatment outcomes with community-based pelvic floor physical therapy in patients with chronic constipation. Clin Gastroenterol Hepatol 2022;21:1082–1090. [DOI] [PubMed] [Google Scholar]
- 63.Rao SSC, Yan Y, Erdogan A, et al. Barostat or syringe-assisted sensory biofeedback training for constipation with rectal hyposensitivity: a randomized controlled trial. Neurogastroenterol Motil 2022;34:e14226. [DOI] [PubMed] [Google Scholar]
- 64.Rao SSC, Coss-Adame E, Yan Y, et al. Sensory adaptation training or escitalopram for IBS with constipation and rectal hypersensitivity: A randomized controlled trial. Clin Transl Gastroenterol 2021;12:e00381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wallace SL, Miller LD, Mishra K. Pelvic floor physical therapy in the treatment of pelvic floor dysfunction in women. Curr Opin Obstet Gynecol 2019;31:485–493. [DOI] [PubMed] [Google Scholar]
- 66.Hicks CW, Weinstein M, Wakamatsu M, et al. In patients with rectoceles and obstructed defecation syndrome, surgery should be the option of last resort. Surgery 2014;155:659–667. [DOI] [PubMed] [Google Scholar]
- 67.Hwang YH, Person B, Choi JS, et al. Biofeedback therapy for rectal intussusception. Tech Coloproctol 2006;10:11–16. [DOI] [PubMed] [Google Scholar]
- 68.Choi JS, Hwang YH, Salum MR, et al. Outcome and management of patients with large rectoanal intussusception. Am J Gastroenterol 2001;96:740–744. [DOI] [PubMed] [Google Scholar]
- 69.Wald A, Bharucha AE, Limketkai B, et al. ACG clinical guidelines: management of benign anorectal disorders. Am J Gastroenterol 2021;116:1987–2008. [DOI] [PubMed] [Google Scholar]
- 70.Wald A, Tunuguntla AK. Anorectal sensorimotor dysfunction in fecal incontinence and diabetes mellitus: modification with biofeedback therapy. N Engl J Med 1984;310:1282–1287. [DOI] [PubMed] [Google Scholar]
- 71.Chiarioni G, Bassotti G, Stegagnini S, et al. Sensory retraining is key to biofeedback therapy for formed stool fecal incontinence. Am J Gastroenterol 2002;97:109–117. [DOI] [PubMed] [Google Scholar]
- 72.Chiarioni G, Scattolini C, Bonfante F, et al. Liquid stool incontinence with severe urgency: anorectal function and effective biofeedback treatment. Gut 1993;34:1576–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Blackett JW, Ramu SK, Gautam M, et al. 957: Anorectal manometry predictors of abnormal balloon expulsion test and reduced evacuation on defecography. Gastroenterology 2022; 162:S–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chiarioni G, Salandini L, Whitehead WE. Biofeedback benefits only patients with outlet dysfunction, not patients with isolated slow transit constipation. Gastroenterology 2005;129:86–97. [DOI] [PubMed] [Google Scholar]
- 75.Rao SSC, Valestin JJ, Xiang X, et al. Home versus office biofeedback therapy for dyssynergic defecation: parallel arm randomized controlled trial. Lancet Gastroenterol Hepatol 2018; 3:768–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rao SSC, Valestin J, Brown CK, et al. Long-term efficacy of biofeedback therapy for dyssynergic defecation: randomized controlled trial. Am J Gastroenterol 2010;105:890–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rao SSC, Seaton K, Miller M, et al. Randomized controlled trial of biofeedback, sham feedback, and standard therapy for dyssynergic defecation. Clin Gastroenterol Hepatol 2007; 5:331–338. [DOI] [PubMed] [Google Scholar]
- 78.Whitehead W, Rao SSC, Lowry A, et al. Treatment of fecal incontinence: state-of-the-science summary for the National Institute of Diabetes and Digestive and Kidney Disease Workshop. Am J Gastroenterol 2015;110:138–146. [DOI] [PubMed] [Google Scholar]
- 79.Mazor Y, Prott G, Jones M, et al. Factors associated with response to anorectal biofeedback therapy in patients with fecal incontinence. Clin Gastroenterol Hepatol 2021;19:492–502. [DOI] [PubMed] [Google Scholar]
- 80.Parker CH, Henry S, Liu LWC. Efficacy of biofeedback therapy in clinical practice for the management of chronic constipation and fecal incontinence. J Can Assoc Gastroenterol 2019;2:126–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yu T, Shen X, Li M, et al. Efficacy and predictors for biofeedback therapeutic outcome in patients with dyssynergic defecation. Gastroenterol Res Pract 2017;2017:1019652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Patcharatrakul T, Gonlachanvit S. Outcome of biofeedback therapy in dyssynergic defecation patients with and without irritable bowel syndrome. J Clin Gastroenterol 2011; 45:593–598. [DOI] [PubMed] [Google Scholar]
- 83.Vasant DH, Paine PA, Black CJ, et al. British Society of Gastroenterology guidelines on the management of irritable bowel syndrome. Gut 2021;70:1214–1240. [DOI] [PubMed] [Google Scholar]
- 84.Centers for Medicare & Medicaid Services. CMS Manual System Publication: 100–04: Medicare Claims Processing Transmittal 4501. Available at: https://www.cms.gov/files/document/r4501cp.pdf. Accessed April 20, 2022.
- 85.Centers for Medicare & Medicaid Services. Billing and Coding: Therapy Evaluation, Re-Evaluation and Formal Testing (A53309). Available at: https://www.cms.gov/medicare-coverage-database/view/article.aspx?articleid=53309. Accessed April 20, 2022.
- 86.Centers for Medicare & Medicaid Services. CY 2021 Revisions to Payment Policies Under the Physician Fee Schedule and Other Changes to Part B Medicare Policies: 42 CFR parts 410, 414, 415, 423, 424, and 425 [CMS-1734-P]. Available at: https://www.cms.gov/files/document/cms-1734-p-pdf.pdf. Accessed April 20, 2022.
- 87.Centers for Medicare & Medicaid Services. NCD – Biofeedback Therapy (30.1). Available at: https://www.cms.gov/medicarecoverage-database/view/ncd.aspx?ncdid=41. Accessed April 26, 2022.
- 88.Fishman SM. Bonica’s Management of Pain. 4th ed. Philadelphia, PA: Lippincott Williams & Wilkins, 2012. [Google Scholar]
- 89.Rao SSC, Go JT, Valestin J, et al. Home biofeedback for the treatment of dyssynergic defecation (DD): does it improve quality of life (QOL) and is it cost-effective? Am J Gastroenterol 2019;114:938–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shah ED, Amann ST, Karlitz JJ. The time is now: a guide to sustainable telemedicine during COVID-19 and beyond. Am J Gastroenterol 2020;115:1371–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wickramasinghe DP, Jayarajah U, Samarasekera DN. Duration taken for the anal sphincter pressures to stabilize prior to anorectal manometry. BMC Res Notes 2018;11:354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tabbers MM, DiLorenzo C, Berger MY, et al. Evaluation and treatment of functional constipation in infants and children: evidence-based recommendations from ESPGHAN and NASPGHAN. J Pediatr Gastroenterol Nutr 2014;58:258–274. [DOI] [PubMed] [Google Scholar]
- 93.Van der Plas RN, Benninga MA, Büller HA, et al. Biofeedback training in treatment of childhood constipation: a randomized controlled study. Lancet 1996;348:776–780. [DOI] [PubMed] [Google Scholar]
- 94.Chiarioni G, Whitehead WE. The role of biofeedback in the treatment of gastrointestinal disorders. Nat Clin Pract Gastroenterol Hepatol 2008;5:371–382. [DOI] [PubMed] [Google Scholar]
- 95.Hämäläinen KPJ, Raivio P, Antila S, et al. Biofeedback therapy in rectal prolapse patients. Dis Colon Rectum 1996; 39:262–265. [DOI] [PubMed] [Google Scholar]
- 96.Mimura T, Roy AJ, Storrie JB, et al. Treatment of impaired defecation associated with rectocele by behavioral retraining (biofeedback). Dis Colon Rectum 2000;43:1267–1272. [DOI] [PubMed] [Google Scholar]
- 97.Lee BH, Kim N, Kang S-B, et al. The long-term clinical efficacy of biofeedback therapy for patients with constipation or fecal incontinence. J Neurogastroenterol Motil 2010;16:177–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Leroi AM, Duval V, Roussignol C, et al. Biofeedback for anismus in 15 sexually abused women. Int J Colorectal Dis 1996; 11:187–190. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 2. Biofeedback Current Procedural Terminology (CPT) codes. aAllows for flexible time-based sessions; must be billed with 90912. bApplication of 9% reduction reimbursement started in 2021. GP modifier, services delivered under an outpatient physical therapy plan of care; HCP, healthcare provider.
Supplementary Figure 1. High-resolution manometry and conventional manometry patterns commonly seen in a healthy individual (normal pattern) and in patients with different types of dyssynergic defecation. Reprinted with permission from Rao SS, Patcharatrakul T. Diagnosis and treatment of dyssynergic defecation.