Abstract
In Mercurialis annua L. (2n = 16) genes for sex determination are considered as major regulator genes controlling stamen and ovary development and sexual phenotypes. After stamen induction, sterility determinants control sporogenous tissue and pollen formation. Moreover, exogenous auxins are able to induce male flowers on female plants. In order to verify if sex and sterility genes have an effect on indole-3-acetic acid (IAA) contents of these plants, various wild or genetically constructed strains were assayed. The IAA levels of their apices were determined by HPLC followed by gas chromatography, selected ion monitoring, mass spectrometry. Results show that high auxin levels are linked to male phenotypes. The genes inducing maleness and the determinants of restored male fertility appear to control and modulate the IAA content. Close correspondence between the number of these dominant genes and IAA levels was established. A final hypothesis about the control of sexual specialization by phytohormones induced by the presence of these genes is discussed.
Full text
PDF

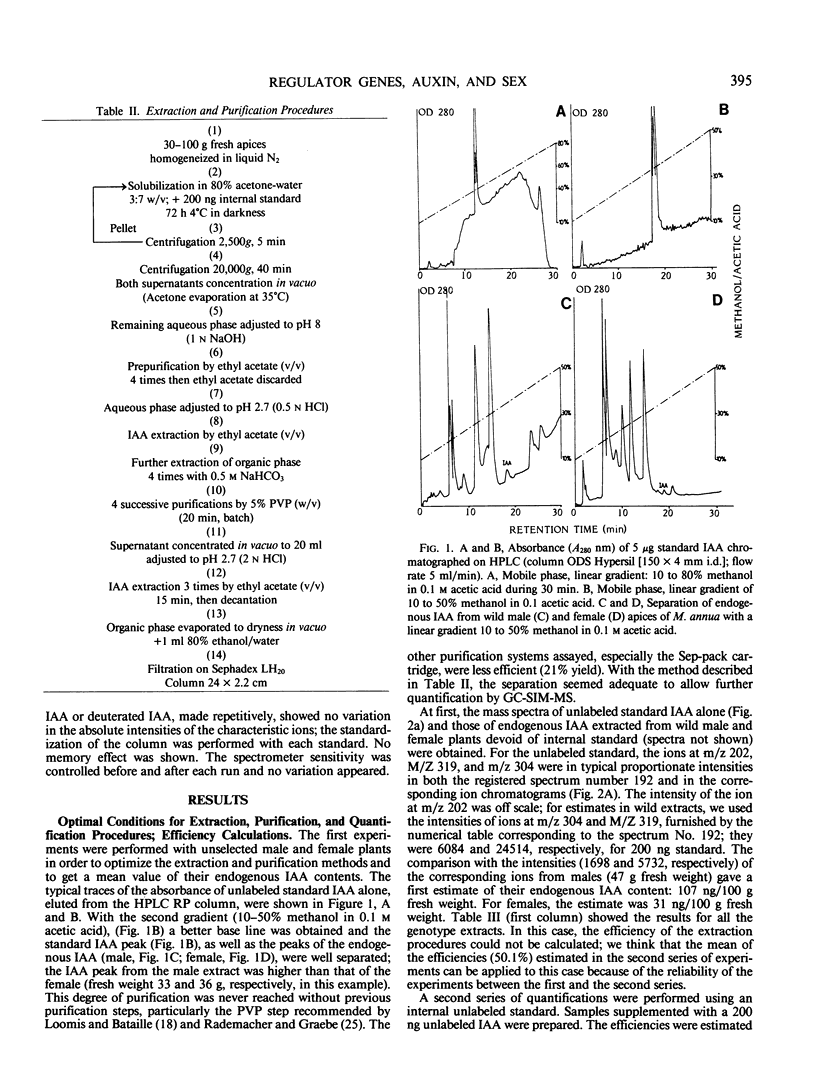
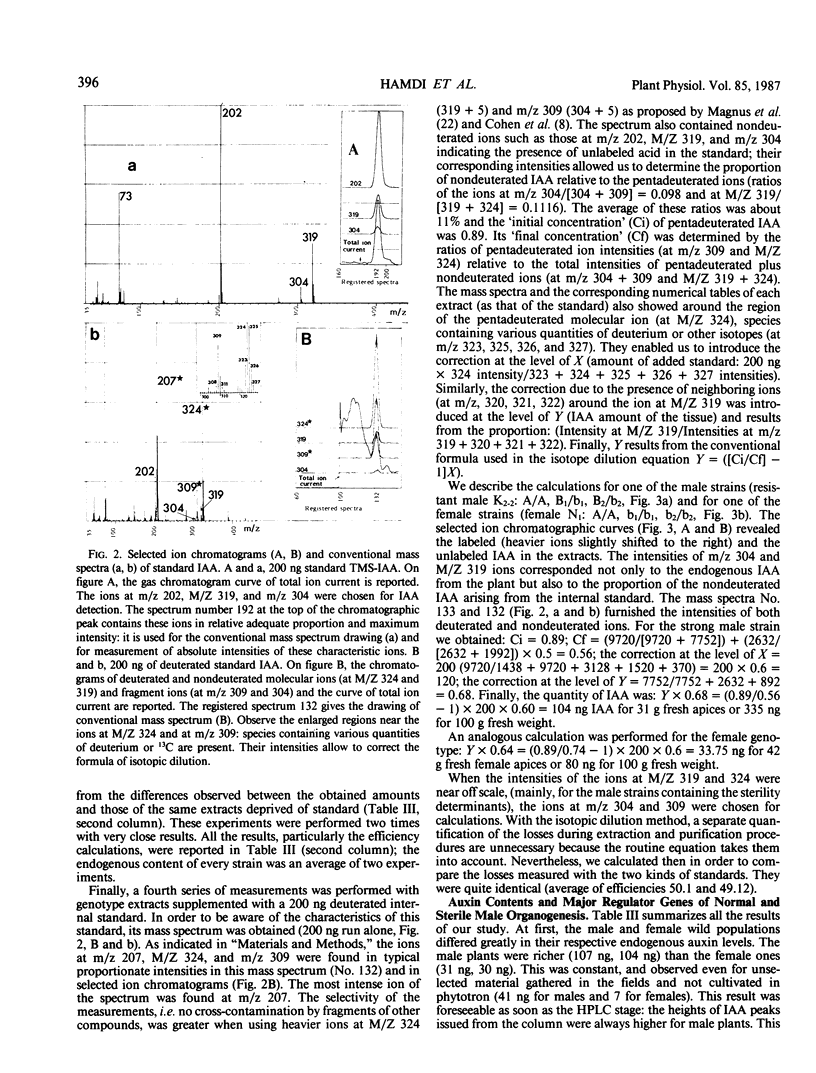
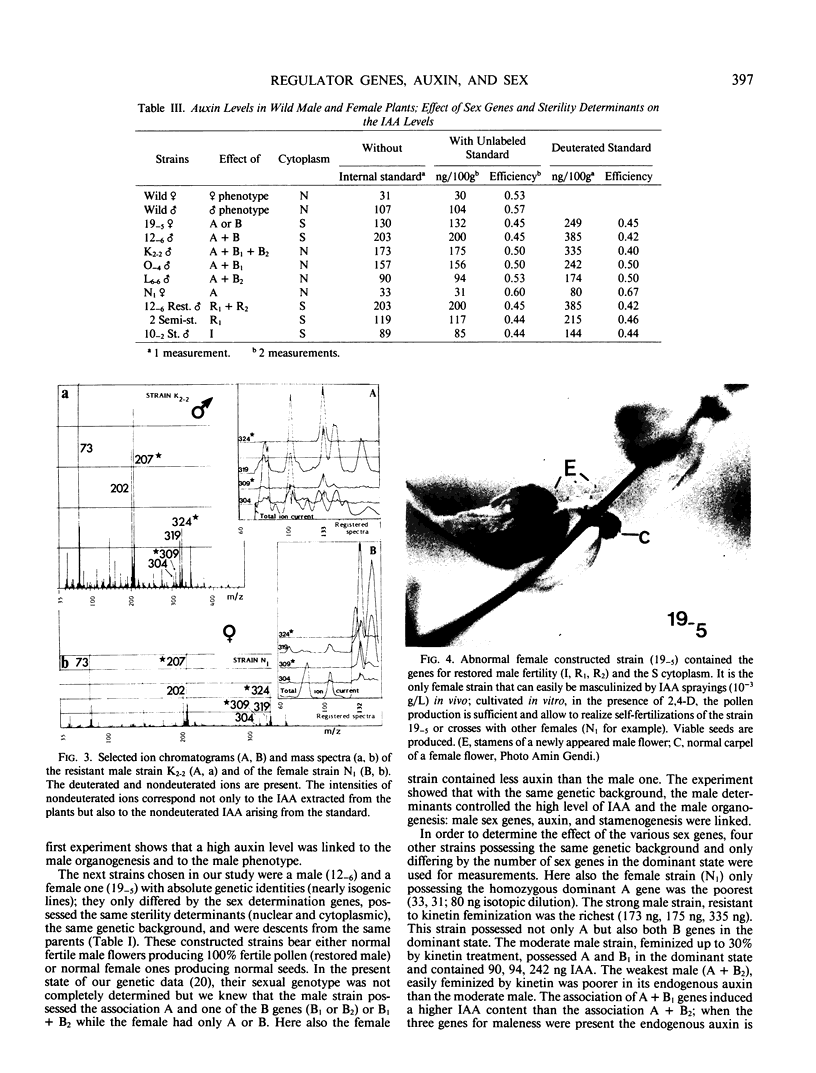


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bandurski R. S., Schulze A. Concentration of Indole-3-acetic Acid and Its Derivatives in Plants. Plant Physiol. 1977 Aug;60(2):211–213. doi: 10.1104/pp.60.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belote J. M., McKeown M. B., Andrew D. J., Scott T. N., Wolfner M. F., Baker B. S. Control of sexual differentiation in Drosophila melanogaster. Cold Spring Harb Symp Quant Biol. 1985;50:605–614. doi: 10.1101/sqb.1985.050.01.073. [DOI] [PubMed] [Google Scholar]
- Caruso J. L., Smith R. G., Smith L. M., Cheng T. Y., Daves G. D. Determination of Indole-3-acetic Acid in Douglas Fir Using a Deuterated Analog and Selected Ion Monitoring: Comparison of Microquantities in Seedling and Adult Tree. Plant Physiol. 1978 Dec;62(6):841–845. doi: 10.1104/pp.62.6.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S. R., Durand R., Durand B. Differential cytokinin binding to dioecious plant ribosomes. FEBS Lett. 1979 Jun 15;102(2):211–215. doi: 10.1016/0014-5793(79)80002-2. [DOI] [PubMed] [Google Scholar]
- Cohen J. D., Baldi B. G., Slovin J. P. C(6)-[benzene ring]-indole-3-acetic Acid: a new internal standard for quantitative mass spectral analysis of indole-3-acetic Acid in plants. Plant Physiol. 1986 Jan;80(1):14–19. doi: 10.1104/pp.80.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herskowitz I. Master regulatory loci in yeast and lambda. Cold Spring Harb Symp Quant Biol. 1985;50:565–574. doi: 10.1101/sqb.1985.050.01.069. [DOI] [PubMed] [Google Scholar]
- Jutley J. K., Stewart A. D. Genetic analysis of the Y-chromosome of the mouse: evidence for two loci affecting androgen metabolism. Genet Res. 1986 Feb;47(1):29–34. doi: 10.1017/s0016672300024472. [DOI] [PubMed] [Google Scholar]
- Kahlem G. Isolation and localization by histoimmunology of isoperoxidases specific for male flowers of the dioecious species Mercurialis annua L. Dev Biol. 1976 May;50(1):58–67. doi: 10.1016/0012-1606(76)90067-1. [DOI] [PubMed] [Google Scholar]
- Magnus V., Bandurski R. S., Schulze A. Synthesis of 4,5,6,7 and 2,4,5,6,7 Deuterium-labeled Indole-3-Acetic Acid for Use in Mass Spectrometric Assays. Plant Physiol. 1980 Oct;66(4):775–781. doi: 10.1104/pp.66.4.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noma M., Koike N., Sano M., Kawashima N. Endogenous indole-3-acetic Acid in the stem of tobacco in relation to flower neoformation as measured by mass spectroscopic assay. Plant Physiol. 1984 May;75(1):257–260. doi: 10.1104/pp.75.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nöthiger R., Steinmann-Zwicky M. A single principle for sex determination in insects. Cold Spring Harb Symp Quant Biol. 1985;50:615–621. doi: 10.1101/sqb.1985.050.01.074. [DOI] [PubMed] [Google Scholar]
- Steen I., Eliasson L. Separation of growth egulatrs from picea abies Karst. on Sephadex LH-20. J Chromatogr. 1969 Sep 23;43(4):558–560. doi: 10.1016/s0021-9673(00)99253-7. [DOI] [PubMed] [Google Scholar]



