Abstract
A single 200-mg dose of clinafloxacin was given orally to each of nine healthy male volunteers, and the concentrations of the drug were measured in plasma, cantharidin-induced inflammatory fluid, and urine over the following 24 h (48 h in the case of urine). The mean maximum concentration in plasma was 1.34 μg/ml at a mean time of 1.8 h postdose. The mean maximum concentration in the inflammatory fluid was 1.3 μg/ml at 3.8 h postdose. The mean elimination half-life of clinafloxacin in plasma was 5.65 h. The overall penetration into the inflammatory fluid was 93.1%, as assessed by determining the ratio of area under the concentration-time curves. Recovery of clinafloxacin in urine was 58.8% by 24 h and 71.8% by 48 h postdose.
Clinafloxacin (PD127,391, CI-960) is a dihalogenated quinolone which has an enhanced antibacterial spectrum and is particularly active against gram-positive cocci (5, 8). In this study, the pharmacokinetics of a single 200-mg oral dose of clinafloxacin were studied in nine volunteers with the following characteristics: mean age, 34 years (range, 22 to 41 years); mean weight, 77.5 kg (range, 64.5 to 88.5 kg); and mean height, 178 cm (range, 171 to 184 cm). The volunteers agreed to participate after informed consent had been obtained. The extent of penetration into a chemically induced inflammatory exudate (7) was investigated.
Approval by the hospital’s Ethical Committee was obtained, and medical histories and physical examinations of all volunteers were normal. Hematological and biochemical profiles of all volunteers were normal, as were urinalyses. On the night before each trial day, three 0.2% cantharidin-impregnated plasters (1 by 1 cm) were applied to the forearm of each volunteer. One additional patch was applied at 12 h for the 24-h sample. After overnight fasting each subject was given a capsule of 200 mg of clinafloxacin with 240 ml of water. All volunteers applied a factor 30 sunblock (Ambre Solaire; Laboratoire Garnier, Paris, France) preparation to light-exposed areas of skin, as phototoxicity has been observed following significant exposure to sunlight (data on file; Parke-Davis). A light meal was served 2 h after antimicrobial dosing, and fluids were allowed ad libitum. Blood samples were taken prior to (0 h) and at 30 min and 1, 2, 3, 4, 6, 8, 12, and 24 h following administration. The blisters were sampled at 0, 0.5, 1, 2, 3, 4, 6, 8, 12, and 24 h after administration by puncture, and approximately 50 μl was removed; the integrity of the blisters was maintained by spraying them with a fast-drying plastic dressing (Opsite; Smith and Nephew Medical Ltd., Hull, United Kingdom). A predose urine sample was collected, as was all urine over 0 to 4, 4 to 8, 8 to 12, 12 to 24, 24 to 48 and 48 to 72 h postdose; the volumes were measured and an aliquot was taken for antibiotic assay. At follow-up, 7 and 14 days later, a further hematological and biochemical profile was performed.
Concentrations of clinafloxacin were determined within 1 h of collection by an agar plate diffusion method, there being no metabolism to bioactive compounds (data on file; Parke-Davis). The assay organism used was Escherichia coli 4004 (Bayer AG, Wuppertal, Germany), which was flooded onto the surfaces of assay plates containing Iso-Sensitest agar (Oxoid, Basingstoke, United Kingdom), after dilution of an overnight broth culture in distilled water to an optical density of 0.004 at 630 nm. Calibrators were prepared in human plasma at concentrations of 0.125, 0.25, 0.5, 1, and 2 mg/liter and 0.015, 0.03, 0.06, 0.12, and 0.25 mg/liter for samples collected up to 8 h and beyond 8 h postdose, respectively. Internal controls of 1.5, 0.2, and 0.02 mg/liter were also included. Blister fluid samples were assayed against calibrators prepared in 70% human plasma in pH 7 phosphate buffer (concentrations, 0.015, 0.03, 0.06, 0.12, and 0.25 mg/liter). Internal controls of 0.2 and 0.02 mg/liter were also assayed. Preliminary studies had shown that the assay of clinafloxacin in urine samples diluted in water or pH 7 phosphate buffer and then tested against calibrations prepared in water or pH 7 phosphate buffer was unreliable. However, the recovery of clinafloxacin from urine spiked with known concentrations of the drug was acceptable (i.e., the coefficient of variation was within that determined for plasma) (see below) if calibrations and urine dilution were performed in human serum (Bradsure Biologicals, Market Harborough, United Kingdom). Internal controls for the assay of concentrations in urine of 1.5 and 0.2 mg/liter were also assayed. Quality assurance samples for plasma, urine, and 70% plasma in pH 7 buffer were also included (ranges, 0.03 to 1.6, 0.022 to 0.21, and 8 to 170 mg/liter, respectively). Samples were applied in a random pattern in triplicate by cutting 5-mm-diameter wells into the agar and then completely filling the wells with sample. After overnight incubation at 30°C, zones of inhibition were measured with an image analyzer (Image Analysing Associates, Thame, United Kingdom), and the calibration curve was constructed by using Bennett’s calculation (1). The mean percent errors plus 2 standard deviations (SDs) for the quality assurance samples prepared in plasma, 70% plasma in pH 7 phosphate buffer, and urine were 18, 19.7, and 20.19, respectively. The coefficients of variation for the internal controls ranged from 8.2 to 10.2%.
Pharmacokinetic analysis of plasma and inflammatory fluid samples was performed with the WinNonLin program (Scientific Consulting Inc., Apex, N.C.) by a noncompartmental procedure. The degree of penetration into the inflammatory fluid was determined by comparison of the area under the concentration-time curve from time zero to infinity (AUC0–∞) for the inflammatory fluid to that for plasma. The AUC was determined by a log linear trapezoidal method. Apparent oral clearance (CL/F) was determined by dividing the dose by the AUC0–∞. The apparent volume of distribution was calculated by dividing the CL/F by the elimination constant.
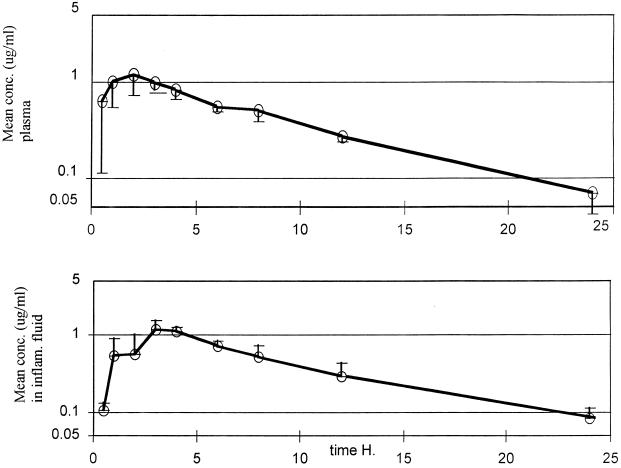
The mean concentrations of clinafloxacin, following the 200-mg oral dose, in plasma and inflammatory fluid are shown in Fig. 1, and the derived pharmacokinetic parameters are provided in Table 1. The correlation coefficient between the time and the log of the concentration, in the plasma elimination (or log linear) phase, was 0.988, and the regression coefficient for the same phase was 0.977.
FIG. 1.
Mean plasma and inflammatory fluid clinafloxacin concentrations following a 200-mg dose of the drug. Vertical bars, SDs.
TABLE 1.
Pharmacokinetic parameters following a 200-mg oral dose of clinafloxacin
| Pharmaco- kinetic parameter | Plasma
|
Inflammatory fluid
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cmax (μg/ml) | Tmax (h) | t1/2 (h) | AUC0–∞ (μg · h/liter) | Apparent vol of distribution (liter) | CL/F (ml/min) | % of drug recovered in urine
|
Cmax (μg/ml) | Tmax (h) | t1/2 (h) | AUC0–∞ (μg · h/liter) | % Pene- tration | ||
| 0–24 h | 0–48 h | ||||||||||||
| Mean | 1.34 | 1.78 | 5.65 | 9.86 | 166.3 | 341.7 | 58.8 | 71.8 | 1.13 | 3.8 | 4.6 | 9.22 | 93.1 |
| SD | 0.32 | 0.83 | 0.72 | 1.13 | 19.4 | 41.7 | 3.16 | 3.3 | 0.31 | 1.3 | 1.5 | 2.21 | 16.1 |
| Minimum | 1.01 | 1 | 4.90 | 7.98 | 138.6 | 301.7 | 55.2 | 66.2 | 0.78 | 3 | 2.2 | 6.80 | 70.5 |
| Maximum | 1.95 | 3 | 7.31 | 11.04 | 198.4 | 418.3 | 63.1 | 75.7 | 1.69 | 6 | 5.2 | 12.63 | 117.2 |
Inspection of the individual graphs of plasma data suggested that distribution was essentially completed during the absorption phase, as no biphasic response was noted. Absorption was rapid, the mean time to maximum concentration of the drug in plasma (Tmax) being 1.78 h and ranging from 1 to 3 h. The mean maximum concentration of the drug (Cmax) in plasma was 1.34 μg/ml, with little individual variation (SD, 0.32 μg/ml).
The mean elimination half-life in plasma (t1/2) was 5.65 h, and the range was 4.9 to 7.31 h. The total clearance of that fraction of the drug absorbed was 341.7 ml/min. Over 24 h, 58.8% of the drug was recovered in the urine, and the recovery increased to 71.8% by 72 h. The mean concentration of the drug in urine in the final sample assayed (24 to 48 h postdose) was 3.0 μg/ml (SD, 1.04 μg/ml).
Clinafloxacin penetrated moderately rapidly into the inflammatory exudate, achieving a maximum at a mean time of 3.8 h postdose (SD, 1.3 h), and the mean maximum concentration was 1.13 μg/ml, 77% of the mean peak concentration in plasma. The mean rate of elimination of clinafloxacin from the inflammatory exudate was apparently higher (4.6 h) than that for plasma but more variable (1.5 h) than that for plasma. The degree of penetration into the inflammatory exudate, calculated from a comparison of the AUC0–∞ in the exudate with that in plasma, was 93.1% (range, 70.5 to 117.2%).
One of the nine volunteers complained of a moderate headache and photophobia which developed 28 h after dosing and lasted approximately 12 h; it was difficult to assess if this was associated with the study drug.
No hematological or biochemical abnormalities were encountered.
There is little published data on the pharmacokinetics of clinafloxacin. A report of a dose range study (2) suggested that the agent was rapidly absorbed, with a Cmax of about 1 μg/ml for each 100-mg dose, and was eliminated from plasma with a t1/2 of 4.6 to 6.1 h; between 44 and 65% of the dose given was recovered in the urine over the 72 h after administration.
The present study confirmed that clinafloxacin is rapidly absorbed. The plasma Cmax achieved (1.34 μg/ml) is lower than that in the earlier report for the same dose. The highest value we noted (1.95 μg/ml) was less than the mean value previously reported (2.5 μg/ml). The mean AUC0–∞s in both studies were similar, 10.2 μg · h/ml in the earlier study and 9.86 μg · h/ml in the present study. The urinary recovery in our study was also greater, with a mean of 71.8% over 48 h.
The rapid and extensive penetration of clinafloxacin into the inflammatory exudate contrasts with that of trovafloxacin, for which the mean percent penetration was reported as 62.6% (9) and possibly reflects the high protein binding of trovafloxacin (87.9%) (3) compared with that of clinafloxacin (2 to 7%) (8). The mean penetration of clinafloxacin, 93.1%, was not significantly different from that described for sparfloxacin (113%) (4) or lomefloxacin (100%) (6).
In conclusion, the concentration in both plasma and inflammatory fluid was greater than the MIC at which 90% of the isolates are inhibited for the majority of the Enterobacteriaceae, Streptococcus pneumoniae, methicillin-susceptible Staphylococcus aureus, and Bacteroides fragilis for approximately 20 h following the single 200-mg oral dose. This suggests that this dose should be efficacious in the treatment of systemic infections caused by such pathogens.
Acknowledgments
We thank B. Cunningham for her clinical support and N. Bron of Parke-Davis for advice.
We also thank N. Bron for financial support.
REFERENCES
- 1.Bennett J V, Brodie J L, Benner E J, Kirby W M M. Simplified, accurate method for antibiotic assay of clinical specimens. Appl Microbiol. 1966;14:170–177. doi: 10.1128/am.14.2.170-177.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bron N J, Dorr M B, Mant T S, Webb C, Vassos A B. The tolerance and pharmacokinetics of clinafloxacin (CI-960) in healthy subjects. J Antimicrob Chemother. 1996;38:1023–1029. doi: 10.1093/jac/38.6.1023. [DOI] [PubMed] [Google Scholar]
- 3.Child J, Boswell F, Brenwald N, Andrews J M, Wise R. In vitro activity of CP 99,219, a new naphthyridone antimicrobial: a comparison with other fluoroquinolone agents. J Antimicrob Chemother. 1995;35:869–876. doi: 10.1093/jac/35.6.869. [DOI] [PubMed] [Google Scholar]
- 4.Johnson J H, Cooper M A, Andrews J M, Wise R. Pharmacokinetics and inflammatory fluid penetration of sparfloxacin. Antimicrob Agents Chemother. 1992;36:2444–2446. doi: 10.1128/aac.36.11.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Norrby S R, Jonsson M. Comparative in vitro activity of PD 127,391, a new fluorinated 4-quinolone derivative. Antimicrob Agents Chemother. 1988;32:1278–1281. doi: 10.1128/aac.32.8.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stone J W, Andrews J M, Ashby J P, Griggs D, Wise R. Pharmacokinetics and tissue penetration of orally administered lomefloxacin. Antimicrob Agents Chemother. 1988;32:1508–1510. doi: 10.1128/aac.32.10.1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wise R, Gillette A P, Cadge B, Durham S R, Baker S. The influence of protein binding upon tissue levels of six beta lactam antibiotics. J Infect Dis. 1980;142:77–82. doi: 10.1093/infdis/142.1.77. [DOI] [PubMed] [Google Scholar]
- 8.Wise R, Ashby J P, Andrews J M. In vitro activity of PD 127,391, an enhanced-spectrum quinolone. Antimicrob Agents Chemother. 1988;32:1251–1256. doi: 10.1128/aac.32.8.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wise R, Mortiboy D, Child J, Andrews J M. Pharmacokinetics and penetration into inflammatory fluid of trovafloxacin (CP-99,219) Antimicrob Agents Chemother. 1996;40:47–49. doi: 10.1128/aac.40.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]