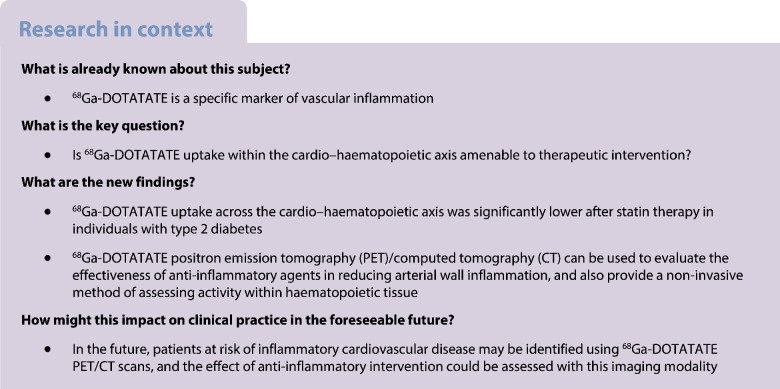
Abstract
Aims/hypothesis
Inflammation is a core component of residual cardiovascular risk in type 2 diabetes. With new anti-inflammatory therapeutics entering the field, accurate markers to evaluate their effectiveness in reducing cardiovascular disease are paramount. Gallium-68-labelled DOTATATE (68Ga-DOTATATE) has recently been proposed as a more specific marker of arterial wall inflammation than 18F-fluorodeoxyglucose (18F-FDG). This study set out to investigate whether 68Ga-DOTATATE uptake is amenable to therapeutic intervention in individuals with type 2 diabetes.
Methods
Individuals aged >50 years with type 2 diabetes underwent 68Ga-DOTATATE positron emission tomography (PET)/computed tomography (CT) at baseline and after 3 months treatment with atorvastatin 40 mg once daily. Primary outcome was the difference in coronary 68Ga-DOTATATE uptake, expressed as target-to-background ratio (TBR). The secondary outcome was difference in bone marrow and splenic uptake, expressed as the standardised uptake value (SUV).
Results
Twenty-two individuals with type 2 diabetes (mean age 63.2±6.4 years, 82% male, LDL-cholesterol 3.42±0.81 mmol/l, HbA1c 55±12 mmol/mol [7.2%±3.2%]) completed both 68Ga-DOTATATE PET/CT scans. The maximum TBR was −31% (95% CI −50, −12) lower in the coronary arteries, and bone marrow and splenic 68Ga-DOTATATE uptake was also significantly lower post statin treatment, with a mean percentage reduction of −15% (95% CI −27, −4) and −17% (95% CI −32, −2), respectively.
Conclusions/interpretation
68Ga-DOTATATE uptake across the cardio–haematopoietic axis was lower after statin therapy in individuals with type 2 diabetes. Therefore, 68Ga-DOTATATE is promising as a metric for vascular and haematopoietic inflammation in intervention studies using anti-inflammatory therapeutics in individuals with type 2 diabetes.
Trial registration
ClinicalTrials.gov NCT05730634
Graphical Abstract
Supplementary Information
The online version contains peer-reviewed but unedited supplementary material available at 10.1007/s00125-023-05990-9.
Keywords: Atherosclerosis, Inflammation, Macrophages, Molecular imaging, Statin
Introduction
Type 2 diabetes is hallmarked by systemic inflammation [1], which is a pivotal process driving atherogenesis [2]. Specifically, diabetes is associated with activation of the cardio–haematopoietic axis [3], wherein inflammatory monocytes produced by the haematopoietic organs migrate to atherosclerotic plaques, accelerating atherosclerotic disease. With new anti-inflammatory therapeutics, such as ziltivekimab [4], entering the clinical stage of testing, accurate surrogate markers of vascular inflammation that reflect activation of the cardio–haematopoietic axis are needed to prevent large scale exposure to ineffective immunosuppressive drugs. A highly promising tool to meet this end is Gallium-68-labelled [1,4,7,10-tetraazacyclododecane-N,N',N'',N'''-tetraacetic acid]-d-Phe1, Tyr3-octreotate (68Ga-DOTATATE), a positron emission tomography (PET) tracer with high affinity for the somatostatin type 2 receptor (SSTR2) that is highly expressed in activated (M1) macrophages within atherosclerotic plaques [5]. A crucial issue for surrogate imaging markers is the amenability of the signal towards therapeutic interventions. In the present study, we sought to investigate whether statin treatment, an established intervention to reduce cardiovascular events and with anti-inflammatory activity [6] is able to reduce 68Ga-DOTATATE uptake in the coronary arteries and aorta, and in the bone marrow and spleen as the key haematopoietic organs.
Methods
Detailed methods are included in the electronic supplementary material (ESM).
In short, individuals with type 2 diabetes from the Amsterdam UMC were eligible, if they were >50 years old, statin-naive for at least 6 weeks, had HbA1c levels <65 mmol/mol (8.1%) and no changes in glucose-lowering medication within 3 months of inclusion. All patients provided written informed consent. Atorvastatin 40 mg once daily was initiated after the first 68Ga-DOTATATE PET/computed tomography (CT) scan, for a period of 3 months. After statin therapy was completed, the patients were subjected to a follow-up 68Ga-DOTATATE PET/CT scan. Blood was collected at baseline and follow-up visits, to determine lipid, metabolic and inflammatory variables. To quantify uptake of 68Ga-DOTATATE in coronary arteries, we used the maximum target-to-background ratio (TBRmax). We reported both the per vessel TBRmax, as well as the overall coronary tree TBRmax. The maximum standardised uptake value (SUVmax) in bone marrow and spleen was assessed by drawing volumes of interest (VOIs) around each respective structure. Methods regarding the measurement of uptake in lung and muscle tissue can be found in the ESM Methods. The study protocol was approved by the local medical ethics committee and performed in accordance with the Declaration of Helsinki
Results
Patient characteristics
Of the 24 patients included, one patient withdrew from the study prior to first scan and another patient discontinued study participation owing to myalgia and did not complete follow-up PET/CT. Accordingly, 22 patients (mean age 63±6 years, 82% male, HbA1c 55 mmol/mol [7.2%]) were included in subsequent analyses. A flowchart of the inclusions can be found in ESM Fig. 1. The baseline and follow-up characteristics after 12 weeks of statin therapy are listed in Table 1. Of note, LDL-cholesterol levels decreased by 58% and C-reactive protein (CRP) by 20%, while the HbA1c did not decrease at follow-up.
Table 1.
Baseline characteristics and changes in laboratory variables and imaging parameters after 12 weeks of atorvastatin treatment
| Study participant characteristics (n=22) | Baseline | Follow-up | Percentage difference at follow-up | p value |
|---|---|---|---|---|
| Age, years | 63.2±6.4 | – | – | – |
| Male sex | 18 (81.8) | – | – | – |
| Smoking | ||||
| Never | 5 (22.7) | – | – | – |
| Former | 16 (72.7) | – | – | – |
| Active | 1 (4.5) | – | – | – |
| BMI, kg/m2 | 29.1±3.8 | – | – | – |
| Systolic blood pressure, mmHg | 140±16 | – | – | – |
| Diastolic blood pressure, mmHg | 86±8 | – | – | – |
| Laboratory variables | ||||
| CRP, mg/l | 1.25 [0.80, 2.28] | 0.90 [0.60, 2.00] | −20 [–33, 0] | 0.077 |
| Fasting glucose, mmol/l | 8.4±2.1 | 8.82±2.92 | −5 (−9, 18) | 0.493 |
| HbA1c, mmol/mol | 55±12 | 58±14 | 6 (−0.9, 12) | 0.087 |
| HbA1c, % | 7.2±3.2 | 7.5±3.4 | 6 (−0.9, 12) | 0.087 |
| Total cholesterol, mmol/l | 5.66±1.01 | 3.36±0.97 | −41 (−44, −37) | <0.001 |
| HDL-cholesterol, mmol/l | 1.20±0.33 | 1.19±0.35 | 1 (−5, 3) | 0.530 |
| LDL-cholesterol, mmol/l | 3.42±0.81 | 1.44±0.62 | −58 (−65, −51) | <0.001 |
| Triglycerides, mmol/l | 1.99 [1.08, 2.65] | 1.16 [0.80, 1.61] | −32 [−48, −19] | <0.001 |
| Apolipoprotein B, g/l | 1.13±0.21 | 0.61±0.22 | −46 (−51, −41) | <0.001 |
| Imaging parameters | ||||
| Coronary artery calcium, AU | 378.70 [56.57, 700.80] | 337.30 [56.85, 737.52] | 2 [−12, 20] | 0.411 |
| Highest TBRmax in coronary arteries | 2.27±0.91 | 1.57±0.39 | −31 (−50, −12) | <0.05 |
| Left anterior descending coronary artery (SUVmax) | 1.17±0.35 | 1.05±0.33 | −7 (−22, 7) | 0.304 |
| Left anterior descending coronary artery (TBRmax) | 1.82±0.66 | 1.44±0.39 | −21 (−38, −3) | <0.05 |
| Left circumflex coronary artery (SUVmax) | 1.07±0.32 | 1.07±0.41 | −0.3 (−14, 14) | 0.955 |
| Left circumflex coronary artery (TBRmax) | 1.82±0.66 | 1.43±0.43 | −21 (−37, −6) | <0.05 |
| Right coronary artery (SUVmax) | 1.20±0.70 | 1.20±0.70 | −23 (−57, 10) | 0.163 |
| Right coronary artery (TBRmax) | 1.89±0.94 | 1.30±0.21 | −31 (−55, −8) | <0.05 |
| Ascending aorta (SUVmax) | 1.84±0.45 | 1.53±0.51 | −15 (−30, 0.3) | 0.054 |
| Ascending aorta (TBRmax) | 2.90±0.99 | 1.89±0.88 | −25 (−45, −6) | <0.05 |
| Bone marrow (SUVmax) | 1.99±0.55 | 1.69±0.45 | −15 (−27, −4) | <0.05 |
| Spleen (SUVmax) | 37.9±14.0 | 31.32± 6.88 | −17 (−32, −2) | <0.05 |
| Background uptake in left atrium (SUVmax) | 0.67±0.22 | 0.74±0.22 | 10 (−6, 28) | 0.194 |
| Lung (SUVmean) | 0.23±0.07 | 0.23±0.06 | −0.6 (−9, 10) | 0.897 |
| Lung (TBRmean) | 0.36±0.09 | 0.33±0.10 | −9 (−25, 7) | 0.230 |
| Muscle (SUVmean) | 0.31±0.10 | 0.34±0.09 | 8 (−3, 20) | 0.150 |
| Muscle (TBRmean) | 0.51±0.17 | 0.50±0.15 | −2 (−22, 18) | 0.829 |
Values are presented as mean ± SD, median [IQR] or number (percentage). Differences are depicted as mean (95% CI) or median [IQR] percentage difference
AU, Agatston units
Changes in 68Ga-DOTATATE uptake in the cardio–haematopoietic axis after atorvastatin treatment
The imaging parameters at baseline and follow-up are shown in Table 1. Overall, we observed a consistent and significant decrease of 68Ga-DOTATATE TBRmax in the coronary arteries (−31%) and ascending aorta (−25%), and SUVmax in bone marrow (−15%) and spleen (−17%) (Fig. 1 and Table 1). The difference in TBRmax within the ascending aorta correlated with the difference in 68Ga-DOTATATE within the coronary arteries (r=0.49, p=0.025). In contrast, no correlations were found between changes in CRP (r=0.24, p=0.289) or LDL-cholesterol levels (r=0.214, p=0.349) with changes in coronary 68Ga-DOTATATE.
Fig. 1.

68Ga-DOTATATE uptake throughout the cardio–haematopoietic axis at baseline and follow-up. The uptake of the 68Ga-DOTATATE within the coronary arteries, ascending aorta, bone marrow and spleen, at baseline and after 12 weeks of atorvastatin treatment in patients with type 2 diabetes. Paired t tests were performed to test for statistical significance: *p<0.05, **p<0.01
Discussion
We provide evidence of therapeutic modulation of vascular 68Ga-DOTATATE uptake in individuals with type 2 diabetes. We discovered significant reductions in 68Ga-DOTATATE uptake in the coronary arteries and ascending aorta after a 12 week regimen of atorvastatin 40 mg daily (Fig. 1). These changes did not correlate with CRP, which currently is the most used surrogate marker of vascular inflammation. Interestingly, 68Ga-DOTATATE uptake in haematopoietic organs was also reduced substantially, suggesting that 68Ga-DOTATATE PET/CT could be used to assess inflammation in other key organs that contribute to atherosclerotic cardiovascular disease. Collectively, these data show that 68Ga-DOTATATE PET/CT holds promise as a surrogate marker to non-invasively evaluate the treatment response of inflammatory activity throughout the cardio–haematopoietic axis in individuals with type 2 diabetes.
We observed a significant change in the coronary TBRmax after statin treatment, indicative of a reduction in inflammatory activity in the coronary arteries [5]. We substantiated this finding by demonstrating a similar effect in the ascending aorta, while the background uptake of 68Ga-DOTATATE measured in the left atrium did not change after statin treatment. Comparing this imaging modality with the currently used 18F-fluorodeoxyglucose (18F-FDG) PET/CT, not only can 68Ga-DOTATATE readily be used to evaluate vascular inflammation in the coronary arteries without being affected by myocardial spillover, but also greater effect sizes are observable. This allows future studies to have smaller study populations when using 68Ga-DOTATATE instead of 18F-FDG. A previous study examining changes in arterial 18F-FDG uptake after 12 weeks of atorvastatin treatment reported a mean reduction of 14.4% in TBRmax [7], whereas our study demonstrated a mean reduction of 31% in 68Ga-DOTATATE TBRmax.
We observed a notably higher uptake of 68Ga-DOTATATE in the bone marrow and spleen in individuals with type 2 diabetes, at a similar background signal as was previously reported as physiological uptake in apparently healthy non-diabetic individuals [8]. Notably, we also identified a lower 68Ga-DOTATATE uptake within the bone marrow and spleen after statin treatment. Future studies, including bone marrow biopsies in tandem with 68Ga-DOTATATE PET/CTs, are required to determine whether a decrease in 68Ga-DOTATATE uptake is caused by a decreased production of M1 macrophages, polarisation towards an M2 phenotype, or a combination of the two. In mice both chronic and transient intermittent hyperglycaemia promote myelopoiesis [3]. The fact that 68Ga-DOTATATE uptake reduction in the haematopoietic organs bears striking resemblance to a reduction in the coronary arteries is in accordance with the hypothesis that haematopoietic activation may be a contributing factor for atherosclerosis in individuals with type 2 diabetes. In support of this, 18F-FDG studies have demonstrated that haematopoietic uptake in apparently healthy individuals is also associated with early atherosclerosis [9].
Limitations of our study include the lack of a placebo group. Since international guidelines recommend statin therapy for all patients with diabetes mellitus of 40 years and older [10], it was not considered ethical to include a placebo group. Nonetheless, the interpretation of our results is unlikely to be affected by lack of a control group, as we do not consider spontaneous resolution of atherosclerotic inflammation to be a likely phenomenon. Second, we may have underestimated the total coronary plaque burden because vascular PET imaging has relatively low spatial resolution. Therefore, to limit the challenges of identifying the coronary arteries, we performed ECG-gated, breathing-corrected PET/CT scans. Accordingly, we drew VOIs along the grooves of the coronary tree, to approximate the TBRmax of the coronary arteries as closely as possible. Despite the spatial limitations inherent to current PET/CT techniques, we were clearly able to detect a strong decrease in coronary uptake of 68Ga-DOTATATE.
In conclusion, we show that 68Ga-DOTATATE PET can be used to identify changes in arterial wall inflammation, as well as activity in haematopoietic organs, providing a rapid readout after just 3 months of drug therapy. Therefore, 68Ga-DOTATATE holds promise as a surrogate marker in upcoming intervention trials that dampen inflammation. However, owing to limitations of the study design, these results will require further confirmation.
Supplementary Information
Below is the link to the electronic supplementary material.
Abbreviations
- CRP
C-reactive protein
- CT
Computed tomography
- FDG
Fluorodeoxyglucose
- PET
Positron emission tomography
- SUV
Standardised uptake value
- TBR
Target-to-background ratio
- VOI
Volume of interest
Acknowledgements
Some of the data were presented as an abstract at the European Atherosclerosis Society conference in Milan in 2022 and at the American College of Cardiology conference in New Orleans in 2023.
Data availability
The data that support the findings of this study are not openly available due to reasons of sensitivity and are available from the corresponding author upon reasonable request. Data are located in controlled access data storage at the Amsterdam UMC, location AMC.
Funding
YK and ESGS were supported by the Netherlands Heart Foundation CVON 2017-20: generating the best evidence-based pharmaceutical targets for atherosclerosis [GENIUS II]). PJS has received funding from the National Institutes of Health. NMJH is supported by a senior clinical scientist grant from the Netherlands Heart Foundation (grant number 2021T055)
Authors’ relationships and activities
PJS has served as a consultant to SYNEKTIK, received research funding from Siemens, has received royalties for software from Cedars-Sinai Medical Center and is associate editor for the Journal of Nuclear Cardiology. NMJH has received honorarium from Novo Nordisk and Boehringer Ingelheim. JK received a preclinical research grant from Oxitope Pharma B.V. NSN is cofounder of Lipid Tools. MRD has served as consultant to Edwards Lifesciences and Medtronic; received research funding from Edwards Lifesciences and Medtronic; and has received speaking fees from NVT. ESGS has received lecturing fees to his institution from Amgen, Sanofi, Esperion, Novartis, NovoNordisk and Ionis Pharmaceuticals. All other authors declare that there are no relationships or activities that might bias, or be perceived to bias, their work.
Contribution statement
RFO was involved in planning of the study, conducted the clinical experiments, assisted in imaging analyses, and was responsible for the statistical analyses, creating the figures and writing the manuscript. YK conceptualised the study, was responsible for the planning of the study, conducted the clinical experiments, and assisted in the statistical analyses and drafting the manuscript. MRS was involved in the interpretation of the imaging data and writing the manuscript. NSN was involved in planning of the study and conducting clinical experiments. ET performed the imaging analyses and assisted in drafting the manuscript and creating the figures. MRD assisted in the imaging analyses and drafting of the manuscript. JK took part in interpretation of data and conceptualising the study. AJM took part in interpretation of the data and revising the article. DD and PJS allowed access to FusionQuant for the analyses of the scans and provided crucial assistance in the imaging analyses. HJV and ESGS were responsible for conceptualising the study and were involved in planning the study, interpretation of data and writing the manuscript. NMJH assisted in interpretation of the data, writing the manuscript, and revised the article critically for important intellectual content. All authors contributed to interpretation of the data, critically reviewed and edited the manuscript and approved the version to be published. NMJH is the guarantor of this work and, as such, had full access to all the data in the study and takes full responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Reindert F. Oostveen and Yannick Kaiser contributed equally.
References
- 1.Einarson TR, Acs A, Ludwig C, Panton UH. Prevalence of cardiovascular disease in type 2 diabetes: a systematic literature review of scientific evidence from across the world in 2007–2017. Cardiovasc Diabetol. 2018;17(1):83. doi: 10.1186/s12933-018-0728-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hansson GK, Libby P, Tabas I. Inflammation and plaque vulnerability. J Intern Med. 2015;278(5):483–493. doi: 10.1111/joim.12406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nagareddy PR, Murphy AJ, Stirzaker RA, et al. Hyperglycemia promotes myelopoiesis and impairs the resolution of atherosclerosis. Cell Metab. 2013;17(5):695–708. doi: 10.1016/j.cmet.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ridker PM, Devalaraja M, Baeres FMM, et al. IL-6 inhibition with ziltivekimab in patients at high atherosclerotic risk (RESCUE): a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet. 2021;397(10289):2060–2069. doi: 10.1016/S0140-6736(21)00520-1. [DOI] [PubMed] [Google Scholar]
- 5.Tarkin JM, Joshi FR, Evans NR, et al. Detection of atherosclerotic inflammation by 68Ga-DOTATATE PET compared to [18F]FDG PET imaging. J Am Coll Cardiol. 2017;69(14):1774–1791. doi: 10.1016/j.jacc.2017.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Satny M, Hubacek JA, Vrablik M. Statins and inflammation. Curr Atheroscler Rep. 2021;23(12):80. doi: 10.1007/s11883-021-00977-6. [DOI] [PubMed] [Google Scholar]
- 7.Tawakol A, Fayad ZA, Mogg R, et al. Intensification of statin therapy results in a rapid reduction in atherosclerotic inflammation. J Am Coll Cardiol. 2013;62(10):909–917. doi: 10.1016/j.jacc.2013.04.066. [DOI] [PubMed] [Google Scholar]
- 8.Moradi F, Jamali M, Barkhodari A, et al. Spectrum of 68Ga-DOTA TATE uptake in patients with neuroendocrine tumors. Clin Nucl Med. 2016;41(6):281–287. doi: 10.1097/rlu.0000000000001100. [DOI] [PubMed] [Google Scholar]
- 9.Devesa A, Lobo-González M, Martínez-Milla J, et al. Bone marrow activation in response to metabolic syndrome and early atherosclerosis. Eur Heart J. 2022;43(19):1809–1828. doi: 10.1093/eurheartj/ehac102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Piepoli MF, Hoes AW, Agewall S, et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR) Eur Heart J. 2016;37(29):2315–2381. doi: 10.1093/eurheartj/ehw106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are not openly available due to reasons of sensitivity and are available from the corresponding author upon reasonable request. Data are located in controlled access data storage at the Amsterdam UMC, location AMC.