Abstract
Definitive radiation therapy is an effective local treatment for several cutaneous malignancies. Patients with diffuse or generalized skin manifestations might require total skin electron beam therapy (TSEBT) as an alternative treatment to the chasing technique. In this short communication, we highlight the evolving role of TSEBT and present its role in various forms of skin malignancies.
Keywords: Radiotherapy, Kaposi sarcoma, Cutaneous lymphoma, Metastasis, Leukemia cutis
Radiotherapy technique
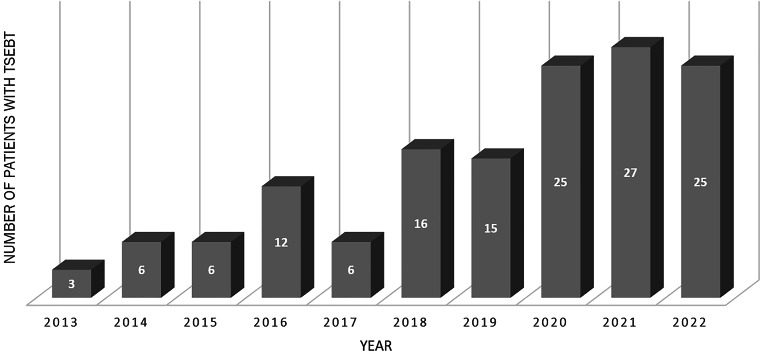
The “six-dual-field” or modified Stanford technique is the most commonly used technique to deliver total skin electron beam therapy (TSEBT; [1, 2]). On the other hand, several institutions have also successfully used the rotational TSEBT technique [3–6]. The radiation treatment typically takes 20 min per fraction. Radiation dose distribution on the skin surface is usually assessed by thermoluminescent dosimeter (TLD) measurements [7, 8]. Supplementary local radiation to the underdosed areas, tumorous skin lesions, or pathologically enlarged lymph nodes may be applied to compensate for underdosing if clinically necessary. Based on the encouraging national and international data on reduced-dose TSEBT, dermatologists have had an increasing interest during the past decade in referring patients to radiotherapy (Fig. 1). Our technique has been previously described [7]. Indications for TSEBT usually include primary cutaneous T‑cell lymphoma (CTCL), Kaposi sarcoma, leukemia cutis, skin metastasis, and primary cutaneous B‑cell lymphoma with generalized skin involvement (Fig. 2).
Fig. 1.
Number of patients undergoing total skin electron beam therapy (TSEBT) at Münster University Hospital
Fig. 2.
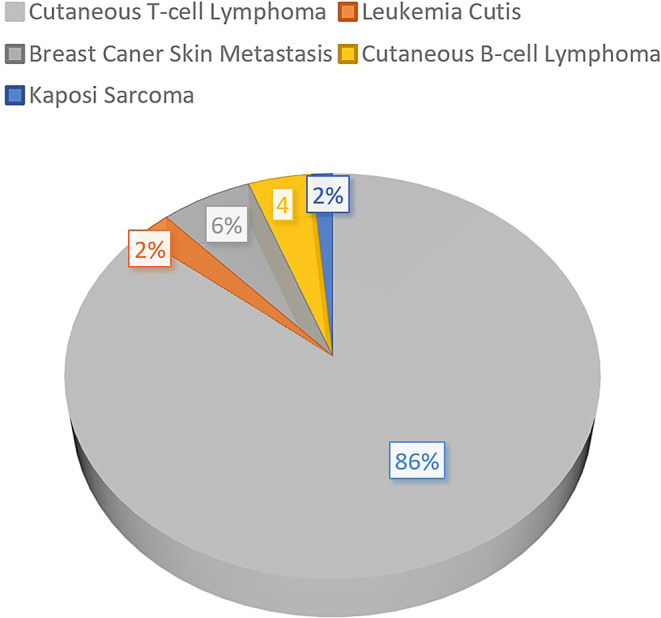
Indications for total (or partial) skin electron beam therapy at Münster University Hospital between 1988 and 2022 (N = 214)
Role of TSEBT in the management of cutaneous T-cell lymphoma
The treatment algorithm for cutaneous T‑cell lymphoma (CTCL) is very complex and requires interdisciplinary decision-making [9, 10]. Radiotherapy (RT) is one of the most efficacious therapies for patients with primary CTCL due to its radiosensitivity [9]. Scholtz first employed RT to treat MF in the early 1900s [11]. However, the finding that most patients experience relapse outside of the radiation field argues in favor of systemic therapy to prevent relapse. Therefore since 1951, TSEBT has been used to treat diffuse CTLC involving more than 10% of the body surface area, although it remains a very specialized treatment that is not widely available [12]. The conventional 30–36-Gy regimen is time-consuming and is associated with significant treatment-related skin toxicities and late relapses [13–15]. Thus reduced-dose TSEBT regimens have been gaining interest recently with the hope of minimizing the risk of adverse events and the possibility for repetition in the event of relapse [14, 16–18]. In order to shorten hospital visits, a technique of once-weekly TSEBT (with a 4-Gy fraction) was employed at Memorial Hospital to a total dose of 32 Gy [19, 20]. However, low-dose RT (≤ 12 Gy) has yielded favorable results with a comparable overall response rate [14, 21–23]. In a toxicity analysis, the rate of RT-related adverse events was lower following low-dose regimens [24].
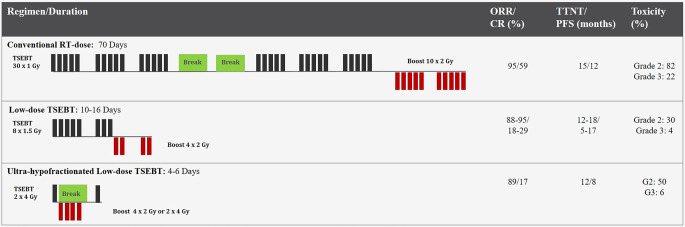
Following a total dose of 12 Gy, clinical response is achieved in almost all patients [18, 25]. However, the response duration is usually short [8, 17, 18]. In combination with maintenance therapy, TSEBT has been associated with improved outcomes [26, 27]. In a prospective German trial, ultra-hypofractionated TSEBT with 8 Gy in two fractions achieved reasonable disease control and symptom palliation with acceptable toxicity, greater comfort, and fewer hospital visits (Fig. 3; [28]). Concurrently and following TSEBT, immunotherapy has shown efficacy with a favorable safety profile [29–31]. Moreover, TSEBT improves patients’ symptoms and health-related quality of life within 2–4 weeks [27, 32, 33]. Furthermore, recent research indicates that TSEBT may improve peripheral blood involvement in patients with Sezary syndrome (SS) [34, 35]. Refractory skin manifestations from primary nodal non-Hodgkin lymphomas can also be treated with RT. However, careful consideration should be given to the skin toxicities associated with concurrent systemic therapies to TSEBT.
Fig. 3.
Course of total skin electron beam therapy (TSEBT) dose/fractionation and the clinical outcome of each regimen in cutaneous T‑cell lymphoma. ORR overall response rate, CR complete response, TTNT time to next treatment, PFS progression-free survival, RT radiotherapy
Role of radiotherapy in the management of cutaneous B-cell lymphoma
In a long-term analysis of patients with cutaneous B‑cell lymphoma (CBCL; [36]), the 5‑year local control rate following low-dose focal RT is similar to conventional doses (86% vs. 90%, p = 0.4). Based on our experience, reduced-dose RT (4 Gy) might be applied in primary indolent CBCL, with the possibility of its repetition if required or dose-escalation (up to 24–30 Gy) in the case of refractory disease. Therefore, patients with CBCL and diffuse skin manifestations might require TSEBT instead of multiple local RT fields. Patients with primary indolent CBCL treated with TSEBT at Münster University Hospital with ≥ 12 Gy demonstrated an overall response rate of 100% [7].
Role of radiotherapy in the management of leukemia cutis
The skin represents one of the sanctuary sites for residual leukemic cells after aggressive therapies. Leukemia cutis is a rare clinical leukemia presentation associated with a poor prognosis [37–40]. Therefore, patients are usually referred to RT after exhibiting progressive disease following different systemic treatment or stem cell transplantation. Reduced-dose TSEBT with 26 Gy is an effective treatment for controlling leukemia cutis progression. Lower TSEBT doses (12 Gy) might also be applied in palliative cases or in the case of concurrent systemic therapy.
Role of radiotherapy in the management of Kaposi sarcoma
Owing to Kaposi sarcoma radiosensitivity, local RT is very effective for this type of disease [41]. In a randomized prospective trial, the conventional local RT dose of 24 Gy in 12 fractions was found to be safe as a hypofractionated regimen with 20 Gy in five fractions [42]. In patients with diffuse or generalized skin involvement, TSEBT can be applied to avoid the chasing technique [19, 43]. Furthermore, TSEBT in 4‑Gy fractions weekly to a cumulative dose of up to 32 Gy was very effective compared to the chasing technique using multiple local RT fields [19]. The efficacy of lower radiation doses in Kaposi sarcoma (< 20 Gy) remains questionable and warrants further investigation.
Role of radiotherapy in the management of cutaneous metastases
In a meta-analysis of cutaneous metastases, the most common cutaneous metastases originate from advanced breast cancer and melanoma. Palliative local RT (normal fractionated or hypofractionated) for skin metastases is an effective and safe treatment with symptom reduction (i.e., fetor, secretions, or bleeding) and improvement in health-related quality of life [44]. Therefore, TSEBT, or partial skin electron beam therapy, can be indicated for patients with widespread skin metastases. The analgesic effect of RT is often observed at lower doses and achieves its maximum within a few weeks. In concomitant visceral metastases, systemic therapies are usually necessary and should be administered sequentially to avoid additional skin toxicity [45]. Consensus-based guidelines on integration of RT into targeted treatments for breast cancer are warranted.
Conclusion
To sum up, TSEBT for skin manifestations of lymphoma, leukemia, Kaposi sarcoma, and metastases from solid tumors is a very effective treatment modality achieving a rapid reduction of disease burden and symptoms and improving health-related quality of life.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Conflict of interest
K. Elsayad and H.T. Eich declare that they have no competing interests.
References
- 1.Page V, Gardner A, Karzmark CJ. Patient dosimetry in the electron treatment of large superficial lesions. Radiology. 1970;94:635–641. doi: 10.1148/94.3.635. [DOI] [PubMed] [Google Scholar]
- 2.Karzmark CJ, Loevinger R, Steele RE, et al. A technique for large-field, superficial electron therapy. Radiology. 1960;74:633–644. doi: 10.1148/74.4.633. [DOI] [PubMed] [Google Scholar]
- 3.Heumann TR, Esiashvili N, Parker S, et al. Total skin electron therapy for cutaneous T-cell lymphoma using a modern dual-field rotational technique. Int J Radiat Oncol Biol Phys. 2015;92:183–191. doi: 10.1016/j.ijrobp.2014.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kumar PP, Henschke UK, Nibhanupudy JR. Problems and solutions in achieving uniform dose distribution in superficial total body electron therapy. J Natl Med Assoc. 1977;69:645–647. [PMC free article] [PubMed] [Google Scholar]
- 5.Schüttrumpf L, Neumaier K, Maihoefer C, et al. Dosisoptimierung bei Ganzhaut- und Teilhautelektronenbestrahlung mittels Thermolumineszenzdosimetrie. Strahlenther Onkol. 2018;194:444–453. doi: 10.1007/s00066-018-1263-9. [DOI] [PubMed] [Google Scholar]
- 6.Newman NB, Patel CG, Ding GX, et al. Prospective observational trial of low-dose skin electron beam therapy in mycosis fungoides using a rotational technique. J Am Acad Dermatol. 2021;85:121–127. doi: 10.1016/j.jaad.2020.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elsayad K, Moustakis C, Simonsen M, et al. In-vivo dosimetric analysis in total skin electron beam therapy. Phys Imaging Radiat Oncol. 2018;6:61–65. doi: 10.1016/j.phro.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Specht L, Dabaja B, Illidge T, et al. Modern radiation therapy for primary cutaneous lymphomas: field and dose guidelines from the International Lymphoma Radiation Oncology Group. Int J Radiat Oncol Biol Phys. 2015;92:32–39. doi: 10.1016/j.ijrobp.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 9.Elsayad K, Susek KH, Eich HT. Total skin electron beam therapy as part of multimodal treatment strategies for primary cutaneous T-cell lymphoma. Oncol Res Treat. 2017;40:244–252. doi: 10.1159/000475634. [DOI] [PubMed] [Google Scholar]
- 10.Dippel E, Assaf C, Becker JC, et al. S2k guidelines—Cutaneous lymphomas update 2016—Part 2: treatment and follow-up (ICD10 C82–C86) JDDG. 2018;16:112–122. doi: 10.1111/ddg.13401. [DOI] [PubMed] [Google Scholar]
- 11.Scholtz W. Ueber den Einfluss der Röntgenstrahlen auf die Haut in gesundem und krankem Zustande. Arch Dermatol Syph. 1902;59:421–446. [Google Scholar]
- 12.Trump JG, Wright KA, Evans WW, et al. High energy electrons for the treatment of extensive superficial malignant lesions. Am J Roentgenol Radium Ther Nucl Med. 1953;69:623–629. [PubMed] [Google Scholar]
- 13.Hoppe RT, Fuks Z, Bagshaw MA. Radiation therapy in the management of cutaneous T-cell lymphomas. Cancer Treat Rep. 1979;63:625–632. [PubMed] [Google Scholar]
- 14.Harrison C, Young J, Navi D, et al. Revisiting low-dose total skin electron beam therapy in mycosis fungoides. Int J Radiat Oncol Biol Phys. 2011;81:e651–e657. doi: 10.1016/j.ijrobp.2011.01.023. [DOI] [PubMed] [Google Scholar]
- 15.Morris SL, McGovern M, Bayne S, et al. Results of a 5-week schedule of modern total skin electron beam radiation therapy. Int J Radiat Oncol Biol Phys. 2013;86:936–941. doi: 10.1016/j.ijrobp.2013.04.042. [DOI] [PubMed] [Google Scholar]
- 16.Elsayad K, Kriz J, Moustakis C, et al. Total skin electron beam for primary cutaneous T-cell lymphoma. Int J Radiat Oncol Biol Phys. 2015;93:1077–1086. doi: 10.1016/j.ijrobp.2015.08.041. [DOI] [PubMed] [Google Scholar]
- 17.Kamstrup MR, Gniadecki R, Iversen L, et al. Low-dose (10-Gy) total skin electron beam therapy for cutaneous T-cell lymphoma: an open clinical study and pooled data analysis. Int J Radiat Oncol Biol Phys. 2015;92:138–143. doi: 10.1016/j.ijrobp.2015.01.047. [DOI] [PubMed] [Google Scholar]
- 18.Hoppe RT, Harrison C, Tavallaee M, et al. Low-dose total skin electron beam therapy as an effective modality to reduce disease burden in patients with mycosis fungoides: results of a pooled analysis from 3 phase-II clinical trials. J Am Acad Dermatol. 2015;72:286–292. doi: 10.1016/j.jaad.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 19.Nisce LZ, Safai B, Poussin-rosillo H. Once weekly total and subtotal skin electron beam therapy for kaposi’s sarcoma. Cancer. 1981;47:640–644. doi: 10.1002/1097-0142(19810215)47:4<640::aid-cncr2820470403>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 20.Nisce LZ, Safai B, Kim JH. Effectiveness of once weekly total skin electron beam therapy in mycosis fungoides and Sezary syndrome. Cancer. 1981;47:870–876. doi: 10.1002/1097-0142(19810301)47:5<870::aid-cncr2820470510>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 21.Lo TC, Salzman FA, Moschella SL, et al. Whole body surface electron irradiation in the treatment of mycosis fungoides. An evaluation of 200 patients. Radiology. 1979;130:453–457. doi: 10.1148/130.2.453. [DOI] [PubMed] [Google Scholar]
- 22.Braverman IM, Yager NB, Chen M, et al. Combined total body electron beam irradiation and chemotherapy for mycosis fungoides. J Am Acad Dermatol. 1987;16:45–60. doi: 10.1016/s0190-9622(87)70004-8. [DOI] [PubMed] [Google Scholar]
- 23.Rübe C, Busch M, Willich N, et al. Ganzhautelektronenbestrahlung des kutanen T-Zell Lymphoms. Strahlenther Onkol. 1996;172:74–80. [PubMed] [Google Scholar]
- 24.Kroeger K, Elsayad K, Moustakis C, et al. Low-dose total skin electron beam therapy for cutaneous lymphoma. Strahlenther Onkol. 2017;193:1024–1030. doi: 10.1007/s00066-017-1188-8. [DOI] [PubMed] [Google Scholar]
- 25.Morris S, Scarisbrick J, Frew J, et al. The results of low-dose total skin electron beam radiation therapy (TSEB) in patients with mycosis fungoides from the UK cutaneous lymphoma group. Int J Radiat Oncol Biol Phys. 2017;99:627–633. doi: 10.1016/j.ijrobp.2017.05.052. [DOI] [PubMed] [Google Scholar]
- 26.Kudelka MR, Switchenko JM, Lechowicz MJ, et al. Maintenance therapy for cutaneous T-cell lymphoma after total skin electron irradiation: evidence for improved overall survival with ultraviolet therapy. Clin Lymphoma Myeloma Leuk. 2020;20:757–767.e3. doi: 10.1016/j.clml.2020.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elsayad K, Rolf D, Sunderkötter C, et al. Low-dose total skin electron beam therapy plus oral bexarotene maintenance therapy for cutaneous T-cell lymphoma. JDDG. 2022;20:279–285. doi: 10.1111/ddg.14657. [DOI] [PubMed] [Google Scholar]
- 28.Elsayad K, Weishaupt C, Moustakis C, et al. Int J Radiat Oncol Biol Phys. 2023 doi: 10.1016/j.ijrobp.2023.02.052. [DOI] [PubMed] [Google Scholar]
- 29.Oymanns M, Daum-Marzian M, Bellm A, et al. Near complete responses to concurrent brentuximab vedotin and ultra-hypofractionated low-dose total skin electron beam radiation in advanced cutaneous T-cell lymphoma. Br J Dermatol. 2023;188:145–146. doi: 10.1093/bjd/ljac012. [DOI] [PubMed] [Google Scholar]
- 30.Fong S, Hong EK, Khodadoust MS, et al. Low-dose total skin electron beam therapy combined with mogamulizumab for refractory mycosis fungoides and Sézary syndrome. Adv Radiat Oncol. 2020 doi: 10.1016/j.adro.2020.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beygi S, Fernandez-Pol S, Duran G, et al. Pembrolizumab in mycosis fungoides with PD-L1 structural variants. Blood Adv. 2021;5:771–774. doi: 10.1182/bloodadvances.2020002371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Song A, Gochoco A, Zhan T, et al. A prospective cohort study of condensed low-dose total skin electron beam therapy for mycosis fungoides: Reduction of disease burden and improvement in quality of life. J Am Acad Dermatol. 2020;83:78–85. doi: 10.1016/j.jaad.2020.01.046. [DOI] [PubMed] [Google Scholar]
- 33.Elsayad K, Kroeger K, Greve B, et al. Low-dose total skin electron beam therapy: Quality of life improvement and clinical impact of maintenance and adjuvant treatment in patients with mycosis fungoides or Sezary syndrome. Strahlenther Onkol. 2020;196:77–84. doi: 10.1007/s00066-019-01517-7. [DOI] [PubMed] [Google Scholar]
- 34.Klein RS, Dunlop JD, Samimi SS, et al. Improvement in peripheral blood disease burden in patients with Sezary syndrome and leukemic mycosis fungoides after total skin electron beam therapy. J Am Acad Dermatol. 2013;68:972–977. doi: 10.1016/j.jaad.2012.09.056. [DOI] [PubMed] [Google Scholar]
- 35.Durgin JS, Jariwala NN, Wysocka M, et al. Low-dose total skin electron beam therapy as part of a multimodality regimen for treatment of Sézary syndrome: clinical, immunologic, and molecular analysis. JAMA Dermatol. 2020 doi: 10.1001/jamadermatol.2020.3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oertel M, Elsayad K, Weishaupt C, et al. Deeskalierte Strahlentherapie beim indolenten primär kutanen B-Zell-Lymphom. Strahlenther Onkol. 2020;196:126–131. doi: 10.1007/s00066-019-01541-7. [DOI] [PubMed] [Google Scholar]
- 37.Elsayad K, Oertel M, Haverkamp U, et al. The effectiveness of radiotherapy for leukemia cutis. J Cancer Res Clin Oncol. 2017;143:851–859. doi: 10.1007/s00432-016-2338-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hauswald H, Zwicker F, Rochet N, et al. Total skin electron beam therapy as palliative treatment for cutaneous manifestations of advanced, therapy-refractory cutaneous lymphoma and leukemia. Radiat Oncol. 2012;7:118. doi: 10.1186/1748-717X-7-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bakst R, Yahalom J. Radiation therapy for leukemia cutis. Pract Radiat Oncol. 2011;1:182–187. doi: 10.1016/j.prro.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 40.Nisce LZ, Chu FC, Lee HS, et al. Total skin electron beam therapy for cutaneous lymphomas and leukemias. Int J Radiat Oncol Biol Phys. 1982;8:1587–1592. doi: 10.1016/0360-3016(82)90621-6. [DOI] [PubMed] [Google Scholar]
- 41.Tsao MN, Sinclair E, Assaad D, et al. Radiation therapy for the treatment of skin Kaposi sarcoma. Ann Palliat Med. 2016;5:298–302. doi: 10.21037/apm.2016.08.03. [DOI] [PubMed] [Google Scholar]
- 42.Singh NB, Lakier RH, Donde B. Hypofractionated radiation therapy in the treatment of epidemic Kaposi sarcoma—A prospective randomized trial. Radiother Oncol. 2008;88:211–216. doi: 10.1016/j.radonc.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 43.DEGRO . Abstractbuch. 2023. pp. 1–192. [DOI] [PubMed] [Google Scholar]
- 44.Spratt DE, Gordon Spratt EA, Wu S, et al. Efficacy of skin-directed therapy for cutaneous metastases from advanced cancer: a meta-analysis. J Clin Oncol. 2014;32:3144–3155. doi: 10.1200/JCO.2014.55.4634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kähler KC, Egberts F, Gutzmer R. Palliative treatment of skin metastases in dermatooncology. JDDG. 2013;11:1041–1045. doi: 10.1111/ddg.12197. [DOI] [PubMed] [Google Scholar]