Abstract
BACKGROUND
Neonatal hypoxic–ischemic encephalopathy is an important cause of death as well as long-term disability in survivors. Erythropoietin has been hypothesized to have neuroprotective effects in infants with hypoxic–ischemic encephalopathy, but its effects on neurodevelopmental outcomes when given in conjunction with therapeutic hypothermia are unknown.
METHODS
In a multicenter, double-blind, randomized, placebo-controlled trial, we assigned 501 infants born at 36 weeks or more of gestation with moderate or severe hypoxic–ischemic encephalopathy to receive erythropoietin or placebo, in conjunction with standard therapeutic hypothermia. Erythropoietin (1000 U per kilogram of body weight) or saline placebo was administered intravenously within 26 hours after birth, as well as at 2, 3, 4, and 7 days of age. The primary outcome was death or neurodevelopmental impairment at 22 to 36 months of age. Neurodevelopmental impairment was defined as cerebral palsy, a Gross Motor Function Classification System level of at least 1 (on a scale of 0 [normal] to 5 [most impaired]), or a cognitive score of less than 90 (which corresponds to 0.67 SD below the mean, with higher scores indicating better performance) on the Bayley Scales of Infant and Toddler Development, third edition.
RESULTS
Of 500 infants in the modified intention-to-treat analysis, 257 received erythropoietin and 243 received placebo. The incidence of death or neurodevelopmental impairment was 52.5% in the erythropoietin group and 49.5% in the placebo group (relative risk, 1.03; 95% confidence interval [CI], 0.86 to 1.24; P = 0.74). The mean number of serious adverse events per child was higher in the erythropoietin group than in the placebo group (0.86 vs. 0.67; relative risk, 1.26; 95% CI, 1.01 to 1.57).
CONCLUSIONS
The administration of erythropoietin to newborns undergoing therapeutic hypothermia for hypoxic–ischemic encephalopathy did not result in a lower risk of death or neurodevelopmental impairment than placebo and was associated with a higher rate of serious adverse events. (Funded by the National Institute of Neurological Disorders and Stroke; ClinicalTrials.gov number, NCT02811263.)
NEONATAL HYPOXIC–ISCHEMIC ENCEPH alopathy refers to neurologic dysfunction resulting from a reduction of oxygen and blood flow to a fetus’s brain near the time of birth and is an important cause of brain injury in term and near-term infants. Hypoxic–ischemic encephalopathy affects more than 10,000 infants each year in the United States (1 to 3 per 1000 births1) and accounts for 22% of neonatal deaths worldwide.2 Therapeutic hypothermia is the only neuroprotective therapy that improves neurodevelopmental outcomes in large trials involving humans; however, up to 40% of infants who received this therapy during a clinical trial either died or had long-term disabilities, including cerebral palsy and intellectual impairment.3
Recombinant human erythropoietin, a cytokine with neuroprotective and neuroregenerative effects in preclinical models of neonatal hypoxia–ischemia,4–9 has been proposed as a potential adjuvant therapy for neonatal hypoxic–ischemic encephalopathy. In a study involving 24 infants with hypoxic–ischemic encephalopathy undergoing therapeutic hypothermia, erythropoietin administered intravenously at a dose of 1000 U per kilogram of body weight resulted in plasma levels that are neuroprotective in animals.10 A phase 2 trial involving 50 infants undergoing therapeutic hypothermia who were randomly assigned to receive five doses of erythropoietin (1000 U per kilogram) or placebo suggested less injury on neonatal magnetic resonance imaging (MRI) of the brain and better motor function at 1 year of age in the erythropoietin group.11 However, evidence supporting the use of erythropoietin for neonatal neuroprotection is limited by the small size and short follow-up period of previous studies.11–13 Thus, we conducted the High-Dose Erythropoietin for Asphyxia and Encephalopathy (HEAL) trial, a phase 3 double-blind, randomized, placebo-controlled trial, to determine the safety and efficacy of high doses of erythropoietin in conjunction with therapeutic hypothermia for neuroprotection in newborn infants with hypoxic–ischemic encephalopathy.
METHODS
PATIENTS, TRIAL DESIGN, AND OVERSIGHT
Details of the trial protocol have been published previously.14 Infants were eligible if they met all four of the following criteria: birth at 36 weeks or more of gestation; one or more signs of perinatal depression (including an Apgar score of <5 at 10 minutes, cardiorespiratory resuscitation received beyond 10 minutes of age, and a pH of <7.00 or a base deficit of ≥15 mmol per liter in an umbilical-cord or infant arterial or venous gas obtained within 60 minutes after birth); moderate or severe encephalopathy, according to Sarnat criteria, present at 1 to 6 hours of age14 (Table S1 in the Supplementary Appendix, available with the full text of this article at NEJM.org); and receipt of passive or active therapeutic hypothermia that was started within 6 hours after birth and was continued for 72 hours. Exclusion criteria were a birthweight of less than 1800 g, a head circumference of less than 30 cm, a genetic or congenital condition affecting neurodevelopment, a hematocrit of more than 65.0%, parents considering redirection to palliative care, encephalopathy attributed to a postnatal event, a guardian of diminished cognitive capacity, a surviving twin undergoing therapeutic hypothermia, and anticipation that the child would be unavailable for evaluation at 2 years of age.
A central data coordinating center produced randomization sequences that were provided to the research pharmacy, where a trial binder linked all infant identification numbers to their randomization assignments. Randomization was stratified according to trial site and severity of encephalopathy. After parents provided written informed consent, infants were randomly assigned to receive erythropoietin (Epogen, Amgen; 4000 U per milliliter) at a dose of 1000 U per kilogram or an equal volume of saline placebo intravenously before 26 hours of age and at 2, 3, 4, and 7 days of age. All trial staff were unaware of the trial-group assignments except the pharmacists at the trial sites and the statistician at the data coordinating center. The trial was approved by the institutional review boards at all participating sites and was registered with the Food and Drug Administration (FDA) (Investigational New Drug application 102,138). The protocol and statistical analysis plan are available at NEJM.org. The first, second, fifth, and last authors vouch for the accuracy and completeness of the data and for the fidelity of the trial to the protocol.
PRIMARY OUTCOME
The primary outcome was death or neurodevelopmental impairment of any severity at 22 to 36 months of age. Neurodevelopmental impairment was defined as any of the following: cerebral palsy, a Gross Motor Function Classification System15 (GMFCS) level of at least 1 (on a scale of 0 [normal] to 5 [most impaired]) (Fig. S1), or a cognitive score of less than 90 (which corresponds to 0.67 SD below the mean, with higher scores indicating better performance) on the Bayley Scales of Infant and Toddler Development, third edition (BSID-III). Cerebral palsy was determined by means of a validated standardized neurologic examination.16 The GMFCS is a simple tool that was used to classify the degree of motor impairment in all children regardless of the presence of cerebral palsy. All neurologic and BSID-III examiners were centrally trained and certified on an annual basis. To capture all severities of neurodevelopmental impairment, our primary outcome definition included milder degrees of impairment than were included in the hypothermia trials.3 Because of the coronavirus disease 2019 (Covid-19) pandemic, we extended the window for assessment of the primary outcome from 22 to 26 months to 22 to 36 months of age. This modification was instituted on May 8, 2020, and approved by the FDA and the institutional review boards at all sites.
SECONDARY OUTCOMES
Prespecified secondary outcomes were death; cerebral palsy; the GMFCS level; BSID-III cognitive and language scores; a four-level ordinal outcome consisting of death, moderate or severe neurodevelopmental impairment (i.e., a GMFCS level of 1 and cerebral palsy, a GMFCS level of ≥2, quadriplegic cerebral palsy, or a BSID-III cognitive score of <85 corresponding to 1 SD below the mean), mild impairment (i.e., impairment not meeting criteria for moderate or severe impairment), or no impairment; parental report of seizures, vision impairment, and hearing loss; and behavioral abnormalities (e.g., aggressive behavior) as indicated by an externalizing score on the Child Behavior Checklist of 65 or more.17 We performed MRI of the brain using a harmonized neuroimaging protocol18 at 4 to 6 days after birth when possible. The severity of brain injury was determined by three independent readers with the use of a validated MRI hypoxic–ischemic encephalopathy scoring system in which values ranged from 0 to 144 (higher scores indicate greater injury).11,19
SERIOUS ADVERSE EVENTS
We recorded the incidence of prespecified serious adverse events: death, hypertension warranting medical treatment, a hematocrit of more than 65.0%, thrombosis unrelated to central catheters that warranted anticoagulation or involved a major vessel, disseminated intravascular coagulation with clinical bleeding and warranting transfusion of blood products, pulmonary hypertension warranting inhaled nitric oxide or extracorporeal membrane oxygenation, intracranial hemorrhage (defined as intraparenchymal or intraventricular blood seen on ultrasonography or MRI of the head), cardiopulmonary arrest, and other unexpected life-threatening events. We also recorded the incidence of common complications of hypoxic–ischemic encephalopathy: respiratory, cardiovascular, hematologic, renal, and hepatic dysfunction.
STATISTICAL ANALYSIS
Using phase 2 trial data,11 we estimated the cumulative incidence of death or neurodevelopmental impairment (primary outcome) to be 49% among children who received placebo. On the basis of animal,8 phase 1,10 and phase 2 data,11 we estimated the cumulative incidence to be 31 to 35% among those who received erythropoietin (relative risk with erythropoietin, 0.65 to 0.71). Assuming a 90% follow-up rate at 22 to 26 months, we estimated that 500 infants should be enrolled for the trial to have greater than 90% power to detect a relative risk difference of 33%.
Analyses were based on a modified intention-to-treat approach, in which all the infants who underwent randomization and received at least one dose of erythropoietin or placebo were included. The primary analysis evaluated equality of the incidence of death or neurodevelopmental impairment across the two groups with the use of a likelihood-ratio test based on logistic regression, with adjustment for trial site and severity of encephalopathy (moderate or severe). Subgroup-specific treatment effects were computed according to sex and severity of encephalopathy. In sensitivity analyses, we included children with outcome assessments performed outside the follow-up window and those whose outcomes required adjudication owing to incomplete data. For the remaining children with missing data on neurodevelopmental outcomes, we used the multivariate imputation by chained equations (MICE) algorithm in R software20 (10 imputations) to impute missing data on neurodevelopmental impairment with the use of baseline and telephone follow-up variables associated with missing primary outcome data (see the Supplementary Appendix).
Proportional-odds regression models were used to compare the ordered categorical secondary long-term outcome (death, moderate or severe neurodevelopmental impairment, mild impairment, or no impairment) and the ordered MRI-based injury severity between the trial groups. We used a Poisson regression model with robust standard errors to compare total serious adverse events between the groups and logistic regression for individual serious adverse events, adjusting for the severity of hypoxic–ischemic encephalopathy and trial site in each model. All other secondary outcomes were evaluated with the use of adjusted linear (continuous) or logistic (binary) regression models. No adjustment was made for the multiplicity of secondary outcomes; thus, the 95% confidence intervals around these odds ratios and relative risks should not be used to infer definitive treatment effects.
A two-sided significance level of 0.05 was used for the prespecified efficacy hypothesis test and comparisons of serious adverse events between the two groups. A National Institutes of Health–assigned data and safety monitoring board monitored trial conduct and safety. As prespecified in the protocol, after 125, 250, and 375 infants had been enrolled, the data and safety monitoring board compared mortality and rates of serious adverse events according to trial group using appropriate small-sample methods such as Fisher’s exact test. Mortality was monitored as a primary safety outcome, with the overall significance level controlled with the use of O’Brien–Fleming boundaries and a significance threshold of 0.031 at the final analysis.21 An interim analysis of efficacy or futility was not conducted, because most patients had already undergone randomization at the time the primary measure of treatment efficacy could be assessed. Statistical analyses were performed with the use of R software, version 4.0.2.
RESULTS
PATIENTS
A total of 501 infants underwent randomization from January 25, 2017, through October 9, 2019, at 17 sites involving 23 hospitals. Because 1 infant who was not receiving therapeutic hypothermia was erroneously enrolled and did not receive erythropoietin or placebo, 500 infants were included in the modified intention-to-treat analysis: 257 in the erythropoietin group and 243 in the placebo group (Fig. 1). The severity of encephalopathy was moderate in 387 infants (77.4%) and severe in 113 (22.6%). The evaluation of efficacy at 22 to 36 months of age included 240 children (93.4%) in the erythropoietin group and 222 (91.4%) in the placebo group. Primary outcome assessments conducted after 26 months of age were evenly balanced between the erythropoietin group (26.1%) and the placebo group (25.3%). A total of 74 children were assessed at 27 to 30 months of age, and 31 were assessed at 31 to 36 months of age.
Figure 1. Trial Enrollment and Follow-up.
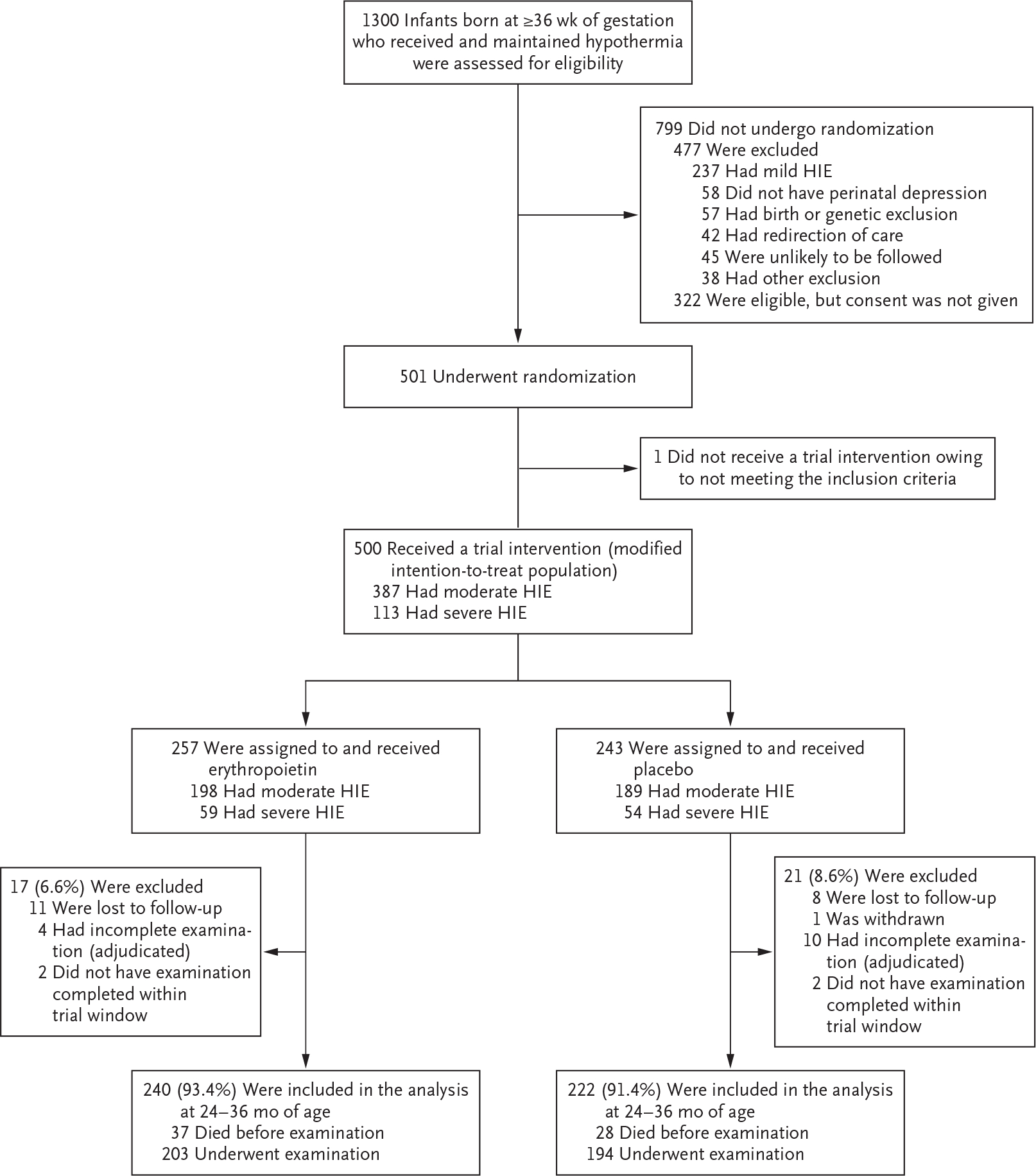
HIE denotes hypoxic–ischemic encephalopathy.
The baseline characteristics of mothers and infants and any complications related to pregnancy and delivery are shown in Tables 1 and S2. The percentage of infants who received all five doses of erythropoietin or placebo did not differ substantially according to trial group (87.9% in the erythropoietin group and 92.2% in the placebo group). The median age at which infants reached the target temperature was also similar in the erythropoietin group (4.4 hours) and the placebo group (4.3 hours).
Table 1.
Maternal, Pregnancy, and Infant Characteristics at Baseline.*
| Characteristic | Erythropoietin (N = 257) | Placebo (N = 243) |
|---|---|---|
|
| ||
| Maternal characteristics | ||
| Race — no. (%)† | ||
| White | 183 (71.2) | 173 (71.2) |
| Black | 28 (10.9) | 38 (15.6) |
| Asian | 18 (7.0) | 15 (6.2) |
| Other | 28 (10.9) | 17 (7.0) |
| Hispanic ethnic group — no. (%)† | 63 (24.5) | 59 (24.3) |
| Age — yr | 29.6±6.3 | 30.0±6.6 |
| Education, high school or less — no. (%) | 102 (39.7) | 83 (34.2) |
| Parity of 1, including trial infant — no. (%) | 145 (56.4) | 141 (58.0) |
| Pregnancy and delivery complications — no. (%) | ||
| Maternal chorioamnionitis or fever | 45 (17.5) | 32 (13.2) |
| Maternal preeclampsia or eclampsia | 22 (8.6) | 23 (9.5) |
| Gestational diabetes | 33 (12.8) | 25 (10.3) |
| Maternal obesity: body-mass index >30‡ | 47 (18.3) | 42 (17.3) |
| Sentinel event§ | 71 (27.6) | 72 (29.6) |
| Shoulder dystocia | 14 (5.4) | 18 (7.4) |
| Placental abruption | 40 (15.6) | 31 (12.8) |
| Prolapsed cord | 10 (3.9) | 13 (5.3) |
| Uterine rupture | 13 (5.1) | 11 (4.5) |
| Cesarean section delivery | 170 (66.1) | 159 (65.4) |
| Outborn delivery | 214 (83.3) | 201 (82.7) |
| Infant characteristics | ||
| Female sex — no. (%) | 122 (47.5) | 103 (42.4) |
| Birth weight — g | 3332±572 | 3414±614 |
| Gestational age — wk | 39.1±1.4 | 39.2±1.5 |
| Median 5-min Apgar score (IQR) | 3 (2–5) | 3 (2–5) |
| Median 10-min Apgar score (IQR) | 5 (3–7) | 5 (3.8–6) |
| Continued resuscitation at 10 min — no. (%)¶ | 243 (94.6) | 217 (89.3) |
| Lowest pH‖ | 6.95±0.17 | 6.91±0.17 |
| Worst base deficit‖ | 18.0±6.0 | 18.5±6.8 |
| Severe encephalopathy — no. (%)** | 59 (23.0) | 54 (22.2) |
| Median age at randomization — hr (IQR) | 14 (10–19) | 14 (10–20) |
| Age at first treatment — hr | 17.6±5.5 | 17.6±5.5 |
Plus–minus values are means ±SD. IQR denotes interquartile range.
Race and ethnic group were reported by the mother or father.
The body-mass index is the weight in kilograms divided by the square of the height in meters.
A sentinel event was defined as shoulder dystocia, placental abruption, prolapsed cord, or uterine rupture.
Ongoing resuscitation with chest compressions, mechanical ventilation, or both was warranted at 10 minutes of age.
Shown is the lowest pH or worst base deficit among cord arterial, cord venous, and arterial blood gas samples obtained tained before 60 minutes of age.
Severe encephalopathy was defined according to Sarnat criteria (Table S1 in the Supplementary Appendix).
PRIMARY OUTCOME
Death or neurodevelopmental impairment at 22 to 36 months of age occurred in 126 of 240 children (52.5%) in the erythropoietin group and in 110 of 222 children (49.5%) in the placebo group (relative risk, 1.03; 95% confidence interval [CI], 0.86 to 1.24; P = 0.74) (Fig. 2). Death occurred in 37 of 257 children (14.4%) and in 28 of 243 children (11.5%), respectively (relative risk, 1.24; 95% CI, 0.78 to 1.99), and neurodevelopmental impairment occurred in 89 of 203 children (43.8%) and in 82 of 194 children (42.3%), respectively (relative risk, 0.97; 95% CI, 0.77 to 1.23). There was no material modification of treatment effect according to sex, severity of encephalopathy, or age at first dose of erythropoietin or placebo. There was no meaningful treatment effect observed in sensitivity analyses of the primary outcome when we excluded children who were assessed after 26 months of age (105 children) or who later received a diagnosis of a genetic condition or congenital anomaly that could affect neurodevelopment (20 children). The effect of erythropoietin was essentially unchanged (relative risk, 1.06; 95% CI, 0.89 to 1.27) when outcome results were included that were collected outside the trial window (4 children), adjudicated (14 children), or multiply imputed (20 children) owing to the child being lost to follow-up.
Figure 2. Forest Plot of Death or Any Neurodevelopmental Impairment, with Stratification According to Sex and the Severity of Encephalopathy.
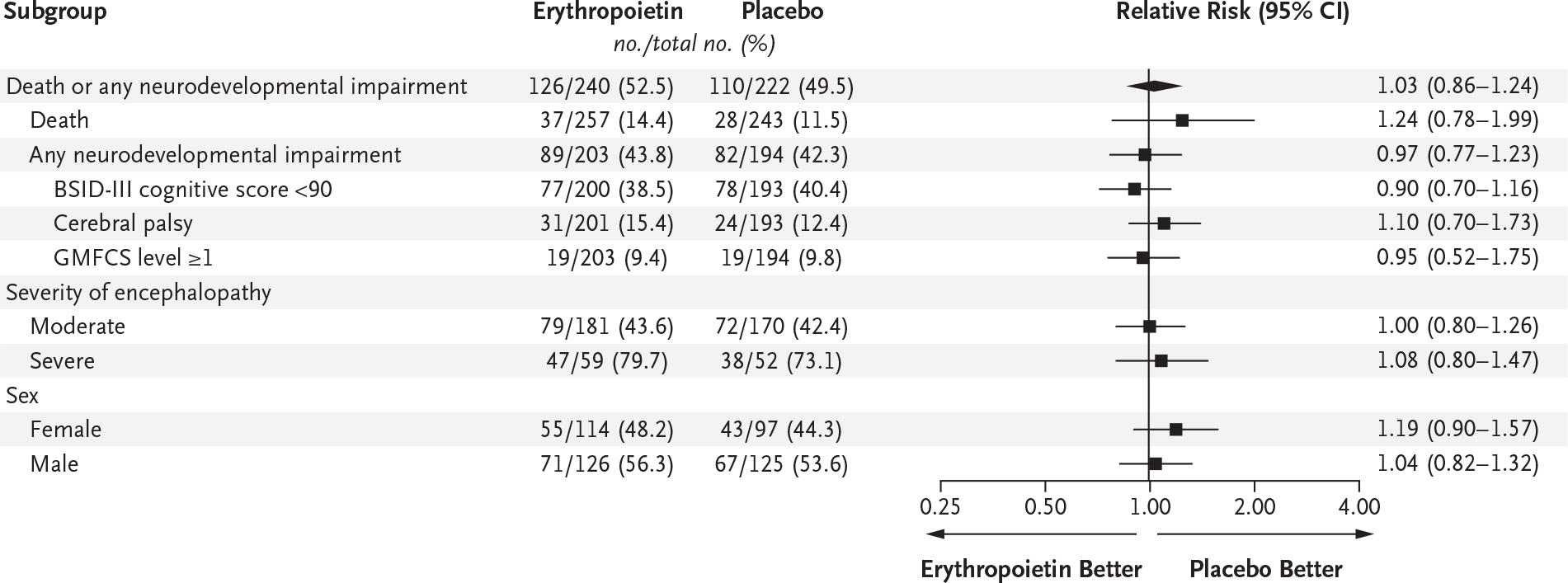
A cognitive score of less than 90 on the Bayley Scales of Infant and Toddler Development, third edition (BSID-III), corresponds to 0.67 SD below the mean, with higher scores indicating better performance. Gross Motor Function Classification System (GMFCS) levels range from 0 (normal) to 5 (most impaired).15
SECONDARY OUTCOMES
The results for the four-level ordinal outcome measure (i.e., death, moderate or severe neurodevelopmental impairment, mild impairment, or no impairment) did not differ materially according to trial group (odds ratio, 1.05; 95% CI, 0.73 to 1.51). The percentage of children with a high externalizing behavior score (≥65) was 7.2% in the erythropoietin group and 1.5% in the placebo group (relative risk, 4.86; 95% CI, 1.46 to 16.17). Other secondary outcomes were similar across the two trial groups (Table 2). A total of 473 infants (94.6%) received a brain MRI at a median age of 4.9 days (interquartile range, 4.5 to 5.8). There were no appreciable between-group differences in brain-injury score on MRI, the severity of brain injury, or the pattern of brain injury (Table 2).
Table 2.
Secondary Outcomes and Neonatal Brain MRI Findings.
| Outcome | Erythropoietin | Placebo | Group Comparison (95% CI) |
|---|---|---|---|
|
| |||
| Secondary outcomes | |||
| Ordinal secondary outcome — no./total no. (%) | 1.05 (0.73 to 1.51)* | ||
| No neurodevelopmental impairment | 114/240 (47.5) | 112/222 (50.5) | |
| Mild neurodevelopmental impairment | 26/240 (10.8) | 23/222 (10.4) | |
| Moderate or severe neurodevelopmental impairment | 63/240 (26.2) | 59/222 (26.6) | |
| BSID-III cognitive score of <85† | 55/200 (27.5) | 56/193 (29.0) | |
| Moderate or severe cerebral palsy | 20/201 (10.0) | 19/193 (9.8) | |
| GMFCS level of ≥2‡ | 15/203 (7.4) | 13/194 (6.7) | |
| Death | 37/240 (15.4) | 28/222 (12.6) | |
| Death or moderate or severe neurodevelopmental impairment — no./total no. (%) | 100/239 (41.8) | 86/220 (39.1) | 1.04 (0.84 to 1.31)§ |
| BSID-III cognitive scored | 89.1±15.7 | 89.5±17.5 | 0.2 (−2.9 to 3.2)‖ |
| BSID-III language scored | 87.9±20.4 | 86.7±20.8 | 1.8 (−2.0 to 5.7)‖ |
| Score for externalizing problems on Childhood Behavior Checklist of ≥65 — no./total no. (%)** | 15/207 (7.2) | 3/201 (1.5) | 4.86 (1.46 to 16.17)†† |
| Taking antiseizure medication according to parental report — no./total no. (%) | 12/211 (5.7) | 12/204 (5.9) | 0.96 (0.44 to 2.10)†† |
| Vision problems according to parental report — no./total no. (%) | 19/211 (9.0) | 20/203 (9.9) | 0.91 (0.50 to 1.66)‖ |
| Hearing problems according to parental report — no./total no. (%) | 10/211 (4.7) | 13/203 (6.4) | 0.74 (0.33 to 1.64)‖ |
| Neonatal brain MRI findings | |||
| Median brain-injury score (IQR)‡‡ | 8 (2 to 26) | 7 (2 to 20) | 1 (−2 to 4) †† |
| Severity of brain injury — no. (%)§§ | 1.10 (0.78 to 1.53)* | ||
| None | 49/242 (20.2) | 51/231 (22.1) | |
| Mild | 91/242 (37.6) | 86/231 (37.2) | |
| Moderate | 46/242 (19.0) | 51/231 (22.1) | |
| Severe | 56/242 (23.1) | 43/231 (18.6) | |
| Pattern of brain injur¶¶ | |||
| Central gray or posterior limb of internal capsule | 96/242 (39.7) | 78/231 (33.8) | 1.17 (0.92 to 1.48)†† |
| Peripheral watershed | 57/242 (23.6) | 61/231 (26.4) | 0.88 (0.64 to 1.22)†† |
| Other | 23/242 (9.5) | 32/231 (13.9) | 0.70 (0.42 to 1.16)†† |
Shown is the odds ratio estimated with a proportional-odds regression model, with adjustment for trial group, severity of encephalopathy, and recruitment site.
A score of less than 85 on the Bayley Scales of Infant and Toddler Development, third edition (BSID-III), corresponds to 1 SD below the mean, with higher scores indicating better performance.
Gross Motor Function Classification System (GMFCS) levels range from 0 (normal) to 5 (most impaired).
Shown is the bootstrapped difference in medians.
BSID-III cognitive scores (available for 200 children in the erythropoietin group and 192 in the placebo group) and language scores (available for 198 and 188 children, respectively) are continuous scores with a norm-based mean of 100; lower scores indicate greater impairment.
Shown is the adjusted difference in means estimated with linear regression.
Scores for externalizing problems on the Child Behavior Checklist above 65 (i.e., >95th percentile) are indicative of borderline clinically significant behavior abnormalities.
Shown is the relative risk estimated with a generalized linear model, with adjustment for trial group, severity of encephalopathy, and recruitment site.
Scores range from 0 to 138, with higher scores indicating greater injury. Data were available for 242 children in the erythropoietin group and 231 in the placebo group.
The severity of brain injury on MRI was categorized by means of the global injury score as follows: none, score of 0; mild, score of 1 to 11; moderate, score of 12 to 32; and severe, score of 33 to 138.19
Patterns of brain injury on MRI were not mutually exclusive.
ADVERSE EVENTS
The percentage of children who had any single type of serious adverse event did not differ substantially according to trial group (Fig. 3). However, the mean number of serious adverse events per child was higher in the erythropoietin group than in the placebo group (0.86 vs. 0.67; relative risk, 1.26; 95% CI, 1.01 to 1.57), as was the percentage of children with at least one serious adverse event (53.3% vs. 43.6%; relative risk, 1.21; 95% CI, 1.00 to 1.45). The incidence of other common complications of hypoxic–ischemic encephalopathy was not substantially higher among children who received erythropoietin. Among infants surviving to discharge, the percentage with abnormal neurologic examination at discharge was similar in the two groups. The median length of stay was 14 days (interquartile range, 10 to 23) in the erythropoietin group and 13 days (interquartile range, 9 to 22.5) in the placebo group.
Figure 3. Adverse Events and Serious Adverse Events.
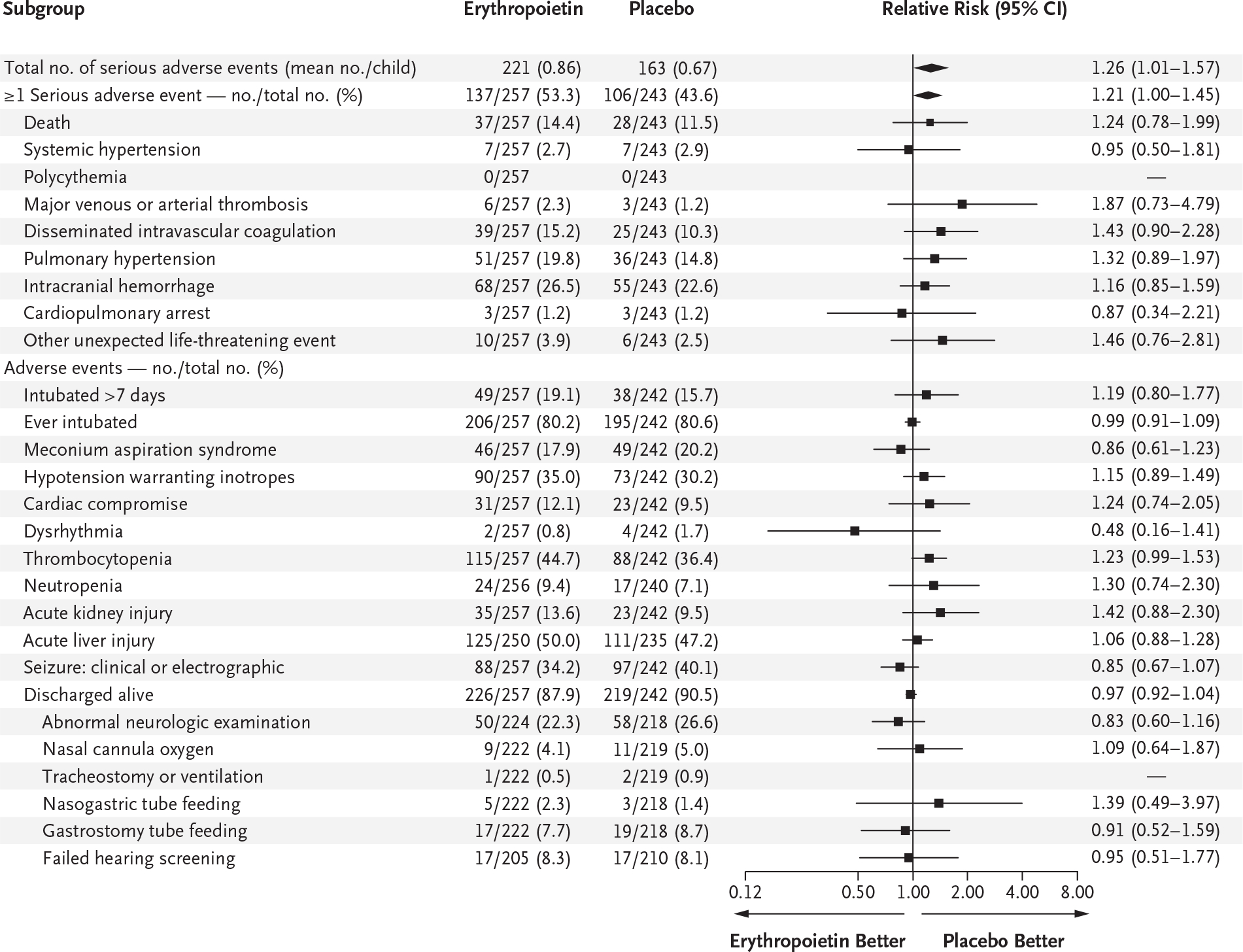
Intracranial hemorrhage was defined as intraparenchymal or intraventricular blood seen on ultrasonography or MRI of the head. Cardiac compromise was defined as an elevated troponin level or decreased cardiac function on echocardiography. Thrombocytopenia was defined as a platelet count of less than 100,000 per cubic millimeter. Neutropenia was defined as an absolute neutrophil count of less than 1500 per cubic millimeter. Acute kidney injury was defined as a plasma creatinine level of more than 1.5 times the baseline level. Acute liver injury was defined as a serum aspartate aminotransferase level of more than 200 U per liter or a serum alanine aminotransferase level of more than 100 U per liter.
DISCUSSION
We found that multiple high doses of erythropoietin given with therapeutic hypothermia to term and near-term newborn infants with moderate or severe hypoxic–ischemic encephalopathy did not significantly affect the incidence of death or neurodevelopmental impairment at 2 to 3 years of age. Furthermore, infants who received erythropoietin were more likely to have at least one serious adverse event and had a greater number of serious adverse events than those who received placebo. These findings contrast with those of small trials in which erythropoietin appeared both safe and effective.10,11 In some countries, 13 to 27% of recently surveyed hospitals are using erythropoietin to treat hypoxic–ischemic encephalopathy in infants, with or without hypothermia.22,23 The lack of benefit observed in our large randomized, placebo-controlled trial calls into question the practice of giving multiple high doses of erythropoietin to infants undergoing therapeutic hypothermia for hypoxic–ischemic encephalopathy.
In the absence of hypothermia, erythropoietin has been shown to improve histologic and functional outcomes in a wide range of animal models of neonatal hypoxic–ischemic brain injury.24 However, preclinical studies evaluating the effect of erythropoietin in the context of hypothermia have had mixed results, with some benefit shown in nonhuman primates but little to no benefit in rodents, piglets, or sheep.8,25–30 Because both hypothermia and erythropoietin may initiate similar neuroprotective pathways during the acute phase of hypoxic–ischemic injury,29 including reduction of apoptotic, inflammatory, and excitotoxic injury, the use of hypothermia in our trial may have reduced any potential for additional benefit from erythropoietin. Other possible explanations for our negative findings include toxic effects of erythropoietin administration early in the injury cascade when combined with therapeutic hypothermia; suboptimal dosage or timing of administration, because later doses may be most effective31,32; and differences in injury mechanisms between preclinical models of hypoxic–ischemic encephalopathy and human hypoxic–ischemic encephalopathy.
The present results contrast with those of previous studies involving neonates receiving multiple high doses of erythropoietin that suggested that this treatment is safe.10,11,33 The causes underlying the present results are unclear; no single type of serious adverse event was substantially more common in the erythropoietin group than in the placebo group, including recognized adverse effects of long-term use of erythropoietin in adults, such as hypertension, thrombosis, and polycythemia. A trial involving adults with ischemic stroke showed more deaths among those who received high doses of erythropoietin but was also unable to attribute this finding to a single mechanism.34 Additional safety data from an ongoing clinical trial testing high-dose erythropoietin and therapeutic hypothermia (PAEAN; ClinicalTrials.gov number, NCT03079167) may further inform safety concerns raised in our trial. We also unexpectedly observed a higher incidence of behavioral abnormalities at 2 years of age among children in the erythropoietin group than among those in the placebo group, but our findings were not adjusted for multiplicity of testing. We found no appreciable between-group differences in findings on MRI of the brain or functional outcomes.
In preclinical models, erythropoietin improves regeneration of brain tissue after the acute phase of hypoxic–ischemic injury by enhancing neurogenesis, oligodendrogenesis, and angiogenesis7,35 and by stimulating growth factors including brain-derived neurotrophic factor.36 These delayed cytoprotective effects may explain why erythropoietin appears to be neuroprotective in rodents even when treatment is not started until 3 to 7 days after focal ischemic stroke.31,32 Studies are under way to examine whether delayed erythropoietin treatment confers benefit to term infants with arterial ischemic stroke37 and to premature infants with intraventricular brain hemorrhage.38
Our trial has limitations. We are unable to address the usefulness of erythropoietin in the absence of therapeutic hypothermia, such as in low- and middle-income countries where hypothermia is unavailable or ineffective,39 or in infants with milder forms of hypoxic–ischemic encephalopathy.40 Some children were evaluated in a delayed fashion owing to the Covid-19 pandemic; however, this is unlikely to have altered our findings because the BSID-III is normed through an age of 42 months and the neurologic examination and GMFCS are valid at both 2 and 3 years of age. Finally, our patient sample is representative of infants with hypoxic–ischemic encephalopathy in the United States (Table S3), but our findings are generalizable only to the population of infants with hypoxic–ischemic encephalopathy in this country.
In our trial, multiple high doses of erythropoietin administered during the first week of age to newborns undergoing therapeutic hypothermia for moderate or severe hypoxic–ischemic encephalopathy did not result in a lower risk of death or neurodevelopmental impairment than placebo.
Supplementary Material
Acknowledgments
We thank Donna Ferriero and Roberta Ballard for their roles as study advisors; John Feltner, Kelleen Nelson, and Stephanie Hauge for their contributions as central study coordinators; Ashok Panigrahy for his assistance in harmonizing the brain MRI studies; Ronnie Guillet, Jody Ciolino, Michael Cotten, Robin Ohls, Renee Shellhaas, and Janet Soul for their contributions as members of the data and safety monitoring board; and the parents of the infants enrolled in this trial for being willing to take part in this research effort, which would not have been possible otherwise.
Supported by the National Institute of Neurological Disorders and Stroke of the National Institutes of Health under award numbers U01NS092764 and U01NS092553.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
A data sharing statement provided by the authors is available with the full text of this article at NEJM.org.
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The members of the HEAL Consortium are listed in the Supplementary Appendix, available at NEJM.org.
Contributor Information
Y.W. Wu, Departments of Neurology, University of California, San Francisco, San Francisco, California Departments of Pediatrics, University of California, San Francisco, San Francisco, California.
B.A. Comstock, Department of Biostatistics, University of Washington, Seattle
F.F. Gonzalez, Departments of Pediatrics, University of California, San Francisco, San Francisco, California
D.E. Mayock, Department of Pediatrics, University of Washington School of Medicine, Seattle
A.M. Goodman, Departments of Neurology, University of California, San Francisco, San Francisco, California
N.L. Maitre, Department of Pediatrics, Children’s Healthcare of Atlanta, Atlanta Department of Pediatrics, Emory University, Atlanta.
T. Chang, Division of Neurology, Children’s National Hospital, Washington, D.C. Department of Neurology, George Washington School of Medicine and Health Sciences, Washington, D.C.
K.P. Van Meurs, Department of Pediatrics, Stanford University School of Medicine, Stanford, California
A.L. Lampland, Department of Neonatology, Children’s Minnesota, St. Paul, Minnesota
E. Bendel-Stenzel, Department of Pediatric and Adolescent Medicine, Mayo Clinic, Rochester, Minnesota
A.M. Mathur, Department of Pediatrics, Saint Louis University School of Medicne, St. Louis
T.-W. Wu, Departments of Pediatrics, Children’s Hospital Los Angeles, California Departments of Pediatrics, University of Southern California Keck School of Medicine, Los Angeles, California.
D. Riley, Department of Pediatrics, Cook Children’s Medical Center, the Department of Pediatrics, Texas Christian University, Texas Department of Pediatrics, University of North Texas Health Science Center, Ft. Worth, Texas.
U. Mietzsch, Department of Pediatrics, University of Washington School of Medicine, Seattle Department of Pediatrics, Indiana University School of Medicine, Indianapolis, Pennsylvania.
L. Chalak, Department of Pediatrics, University of Texas Southwestern Medical Center, Dallas, Texas
J. Flibotte, Department of Pediatrics, Children’s Hospital of Philadelphia, Pennsylvania Department of Pediatrics, University of Pennsylvania Perelman School of Medicine, Philadelphia, Pennsylvania.
J.-H. Weitkamp, Department of Pediatrics, Vanderbilt University Medical Center, Nashville, Ohio
K.A. Ahmad, Department of Pediatrix Medical Group of San Antonio, Children’s Hospital of San Antonio, and Methodist Children’s Hospital, San Antonio, Texas
T.D. Yanowitz, Department of Pediatrics, University of Pittsburgh School of Medicine, Pennsylvania Department of Pediatrics, UPMC Children’s Hospital of Pittsburgh, Pennsylvania; Department of Pediatrics, UPMC Magee-Womens Hospital, Pittsburgh, Pennsylvania.
M. Baserga, Department of Pediatrics, University of Utah, Salt Lake City, Ohio
B.B. Poindexter, Department of Pediatrics, Emory University, Atlanta
E.E. Rogers, Departments of Pediatrics, University of California, San Francisco, San Francisco, California
J.R. Lowe, Department of Pediatrics, University of New Mexico School of Medicine, Albuquerque, Ohio
K.C.K. Kuban, Department of Pediatrics, Boston University Medical Center, Boston, Ohio
T.M. O’Shea, Department of Pediatrics, University of North Carolina at Chapel Hill School of Medicine, Chapel Hill, Ohio
J.L. Wisnowski, Departments of Pediatrics, Children’s Hospital Los Angeles, California Departments of Radiology, Children’s Hospital Los Angeles, California; Departments of Pediatrics, University of Southern California Keck School of Medicine, Los Angeles, California; Departments of Radiology, University of Southern California Keck School of Medicine, Los Angeles, California.
R.C. McKinstry, Departments of Radiology, Washington University in St. Louis School of Medicine, St. Louis.
S. Bluml, Departments of Radiology, Children’s Hospital Los Angeles, California Departments of Radiology, University of Southern California Keck School of Medicine, Los Angeles, California.
S. Bonifacio, Department of Pediatrics, Stanford University School of Medicine, Stanford, California
K.L. Benninger, Department of Pediatrics, Nationwide Children’s Hospital, Columbus, Ohio
R. Rao, Departments of Pediatrics, Washington University in St. Louis School of Medicine, St. Louis
C.D. Smyser, Departments of Pediatrics, Washington University in St. Louis School of Medicine, St. Louis Departments of Radiology, Washington University in St. Louis School of Medicine, St. Louis; Departments of Neurology, Washington University in St. Louis School of Medicine, St. Louis.
G.M. Sokol, Department of Pediatrics, Indiana University School of Medicine, Indianapolis, Pennsylvania
S. Merhar, Department of Pediatrics, Cincinnati Children’s Hospital Medical Center, Ohio Department of Pediatrics, University of Cincinnati College of Medicine, Cincinnati, Ohio.
M.D. Schreiber, Department of Pediatrics, University of Chicago, Chicago
H.C. Glass, Departments of Neurology, University of California, San Francisco, San Francisco, California Departments of Pediatric, University of California, San Francisco, San Francisco, California; Departments of Epidemiology, University of California, San Francisco, San Francisco, California.
P.J. Heagerty, Department of Biostatistics, University of Washington, Seattle
S.E. Juul, Department of Pediatrics, University of Washington School of Medicine, Seattle.
REFERENCES
- 1.Kurinczuk JJ, White-Koning M, Badawi N. Epidemiology of neonatal encephalopathy and hypoxic-ischaemic encephalopathy. Early Hum Dev 2010;86:329–38. [DOI] [PubMed] [Google Scholar]
- 2.Black RE, Cousens S, Johnson HL, et al. Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet 2010;375:1969–87. [DOI] [PubMed] [Google Scholar]
- 3.Azzopardi DV, Strohm B, Edwards AD, et al. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N Engl J Med 2009;361:1349–58. [DOI] [PubMed] [Google Scholar]
- 4.Juul SE, McPherson RJ, Bammler TK, Wilkerson J, Beyer RP, Farin FM. Recombinant erythropoietin is neuroprotective in a novel mouse oxidative injury model. Dev Neurosci 2008;30:231–42. [DOI] [PubMed] [Google Scholar]
- 5.van der Kooij MA, Groenendaal F, Kavelaars A, Heijnen CJ, van Bel F. Neuroprotective properties and mechanisms of erythropoietin in in vitro and in vivo experimental models for hypoxia/ischemia. Brain Res Rev 2008;59:22–33. [DOI] [PubMed] [Google Scholar]
- 6.Gonzalez FF, McQuillen P, Mu D, et al. Erythropoietin enhances long-term neuroprotection and neurogenesis in neonatal stroke. Dev Neurosci 2007;29:321–30. [DOI] [PubMed] [Google Scholar]
- 7.Gonzalez FF, Larpthaveesarp A, McQuillen P, et al. Erythropoietin increases neurogenesis and oligodendrogliosis of subventricular zone precursor cells after neonatal stroke. Stroke 2013;44:753–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Traudt CM, Juul SE. Erythropoietin as a neuroprotectant for neonatal brain injury: animal models. Methods Mol Biol 2013;982:113–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gonzalez FF, Abel R, Almli CR, Mu D, Wendland M, Ferriero DM. Erythropoietin sustains cognitive function and brain volume after neonatal stroke. Dev Neurosci 2009;31:403–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu YW, Bauer LA, Ballard RA, et al. Erythropoietin for neuroprotection in neonatal encephalopathy: safety and pharmacokinetics. Pediatrics 2012;130:683–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu YW, Mathur AM, Chang T, et al. High-dose erythropoietin and hypothermia for hypoxic-ischemic encephalopathy: a phase II trial. Pediatrics 2016;137(6):e20160191. [DOI] [PubMed] [Google Scholar]
- 12.Rogers EE, Bonifacio SL, Glass HC, et al. Erythropoietin and hypothermia for hypoxic-ischemic encephalopathy. Pediatr Neurol 2014;51:657–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garg B, Sharma D, Bansal A. Systematic review seeking erythropoietin role for neuroprotection in neonates with hypoxic ischemic encephalopathy: presently where do we stand. J Matern Fetal Neonatal Med 2018;31:3214–24. [DOI] [PubMed] [Google Scholar]
- 14.Juul SE, Comstock BA, Heagerty PJ, et al. High-Dose Erythropoietin for Asphyxia and Encephalopathy (HEAL): a randomized controlled trial — background, aims, and study protocol. Neonatology 2018;113:331–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palisano R, Rosenbaum P, Walter S, Russell D, Wood E, Galuppi B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol 1997;39:214–23. [DOI] [PubMed] [Google Scholar]
- 16.Kuban KCK, Allred EN, O’Shea M, et al. An algorithm for identifying and classifying cerebral palsy in young children. J Pediatr 2008;153:466–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Achenbach TM, Rescorla LA. Manual for the ASEBA preschool forms & profiles: child behavior checklist for ages 1.5–5. Burlington: University of Vermont Research Center for Children, Youth, & Families, 2001. [Google Scholar]
- 18.Wisnowski JL, Bluml S, Panigrahy A, et al. Integrating neuroimaging biomarkers into the multicentre, high-dose erythropoietin for asphyxia and encephalopathy (HEAL) trial: rationale, protocol and harmonisation. BMJ Open 2021;11(4):e043852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trivedi SB, Vesoulis ZA, Rao R, et al. A validated clinical MRI injury scoring system in neonatal hypoxic-ischemic encephalopathy. Pediatr Radiol 2017;47:1491–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Buuren S, Groothuis-Oudshoorn K. mice: multivariate imputation by chained equations in R. J Stat Softw 2011;45:1–67. [Google Scholar]
- 21.O’Brien PC, Fleming TR. A multiple testing procedure for clinical trials. Biometrics 1979;35:549–56. [PubMed] [Google Scholar]
- 22.Bolte K, Maier RF. Survey on clinical use and non-use of recombinant human erythropoietin in European neonatal units. J Perinat Med 2020;48: 744–50. [DOI] [PubMed] [Google Scholar]
- 23.Wang Z, Zhang P, Zhou W, et al. Neonatal hypoxic-ischemic encephalopathy diagnosis and treatment: a national survey in China. BMC Pediatr 2021;21:261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Juul S Recombinant erythropoietin as a neuroprotective treatment: in vitro and in vivo models. Clin Perinatol 2004;31:129–42. [DOI] [PubMed] [Google Scholar]
- 25.Pang R, Avdic-Belltheus A, Meehan C, et al. Melatonin and/or erythropoietin combined with hypothermia in a piglet model of perinatal asphyxia. Brain Commun 2020;3(1): fcaa211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spasojevic SD, Stojanovic VD, Barisic NA, Doronjski AR, Zikic DR, Babovic SM. Neuroprotective effects of hypothermia and erythropoietin after perinatal asphyxia in newborn rats. J Matern Fetal Neonatal Med 2013;26:1506–9. [DOI] [PubMed] [Google Scholar]
- 27.Fan X, van Bel F, van der Kooij MA, Heijnen CJ, Groenendaal F. Hypothermia and erythropoietin for neuroprotection after neonatal brain damage. Pediatr Res 2013;73:18–23. [DOI] [PubMed] [Google Scholar]
- 28.Fang AY, Gonzalez FF, Sheldon RA, Ferriero DM. Effects of combination therapy using hypothermia and erythropoietin in a rat model of neonatal hypoxia-ischemia. Pediatr Res 2013;73:12–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wassink G, Davidson JO, Crisostomo A, et al. Recombinant erythropoietin does not augment hypothermic white matter protection after global cerebral ischaemia in near-term fetal sheep. Brain Commun 2021;3(3):fcab172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dhillon SK, Wassink G, Lear CA, Davidson JO, Gunn AJ, Bennet L. Adverse neural effects of delayed, intermittent treatment with rEPO after asphyxia in preterm fetal sheep. J Physiol 2021;599:3593–609. [DOI] [PubMed] [Google Scholar]
- 31.Larpthaveesarp A, Georgevits M, Ferriero DM, Gonzalez FF. Delayed erythropoietin therapy improves histological and behavioral outcomes after transient neonatal stroke. Neurobiol Dis 2016;93:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reitmeir R, Kilic E, Kilic U, et al. Post-acute delivery of erythropoietin induces stroke recovery by promoting perilesional tissue remodelling and contralesional pyramidal tract plasticity. Brain 2011;134:84–99. [DOI] [PubMed] [Google Scholar]
- 33.Juul SE, Comstock BA, Wadhawan R, et al. A randomized trial of erythropoietin for neuroprotection in preterm infants. N Engl J Med 2020;382:233–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ehrenreich H, Weissenborn K, Prange H, et al. Recombinant human erythropoietin in the treatment of acute ischemic stroke. Stroke 2009;40(12):e647–e656. [DOI] [PubMed] [Google Scholar]
- 35.Osredkar D, Sall JW, Bickler PE, Ferriero DM. Erythropoietin promotes hippocampal neurogenesis in in vitro models of neonatal stroke. Neurobiol Dis 2010;38:259–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang L, Zhang Z, Wang Y, Zhang R, Chopp M. Treatment of stroke with erythropoietin enhances neurogenesis and angiogenesis and improves neurological function in rats. Stroke 2004;35:1732–7. [DOI] [PubMed] [Google Scholar]
- 37.Benders MJ, van der Aa NE, Roks M, et al. Feasibility and safety of erythropoietin for neuroprotection after perinatal arterial ischemic stroke. J Pediatr 2014;164(3):481.e1–486.e1. [DOI] [PubMed] [Google Scholar]
- 38.Rüegger CM, Hagmann CF, Bührer C, Held L, Bucher HU, Wellmann S. Erythropoietin for the repair of cerebral injury in very preterm infants (EpoRepair). Neonatology 2015;108:198–204. [DOI] [PubMed] [Google Scholar]
- 39.Malla RR, Asimi R, Teli MA, Shaheen F, Bhat MA. Erythropoietin monotherapy in perinatal asphyxia with moderate to severe encephalopathy: a randomized placebo-controlled trial. J Perinatol 2017;37:596–601. [DOI] [PubMed] [Google Scholar]
- 40.DuPont TL, Baserga M, Lowe J, Zamora T, Beauman S, Ohls RK. Darbepoetin as a neuroprotective agent in mild neonatal encephalopathy: a randomized, placebo-controlled, feasibility trial. J Perinatol 2021;41:1339–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


