Graphical abstract
Keywords: Scleroderma, Systemic sclerosis, Cardiomyopathy, Heart failure
Highlights
-
•
Scleroderma-related heart disease is usually secondary to lung disease or PH.
-
•
Scleroderma rarely causes systolic HF in young patients or those without CAD.
-
•
A multimodality strategy should be used to characterize scleroderma cardiomyopathy.
Introduction
Systemic sclerosis, also known as scleroderma, is a rare cause of systolic heart failure (HF) and predominantly occurs in patients with pulmonary hypertension (PH), long-standing autoimmune disease, and concomitant coronary artery disease (CAD). We present a unique case of rapidly progressing systemic sclerosis in a 22-year-old man that manifested with primary scleroderma cardiomyopathy. A multimodality strategy, including echocardiography, computed tomography, cardiac magnetic resonance (CMR) imaging, and histopathologic analysis, is demonstrated to fully characterize the extent of the patient’s disease.
Case Report
A 22-year-old formerly obese man presented to their surgeon’s office with a year of oral intolerance. They had undergone a successful laparoscopic sleeve gastrectomy 2 years prior, which resulted in weight loss of nearly 200 pounds, and they had no other medical history. As a result of the coronavirus pandemic, they avoided seeking treatment for over a year. Given a concern for severe malnutrition and overall poor health, they were directed to the hospital’s emergency department.
Over the past year, the patient had dramatically reduced their food intake due to a feeling of discomfort while swallowing, including a sensation of food becoming caught in their chest. During the same timeframe, the patient started becoming short of breath with exertion. They also developed hypopigmented skin changes over their chest, followed by their face, mouth, and upper extremities. The skin around the joints in their fingers became thick, and the patient was unable to fully open or close their hands into a fist. Their fingertips changed colors when exposed to the cold. Their symptoms worsened, and they started having chest tightness as well as feeling dizzy with very mild exertion. During the 2 weeks prior to presentation, the patient developed signs and symptoms of volume overload.
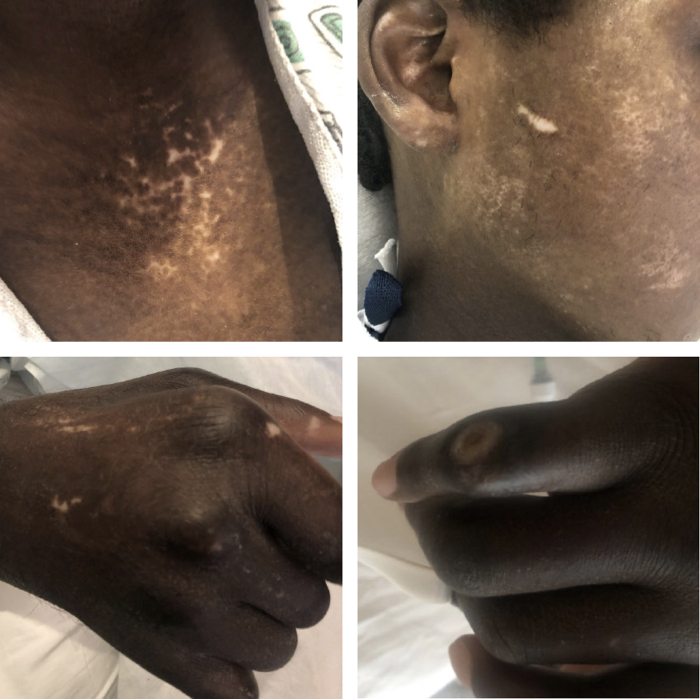
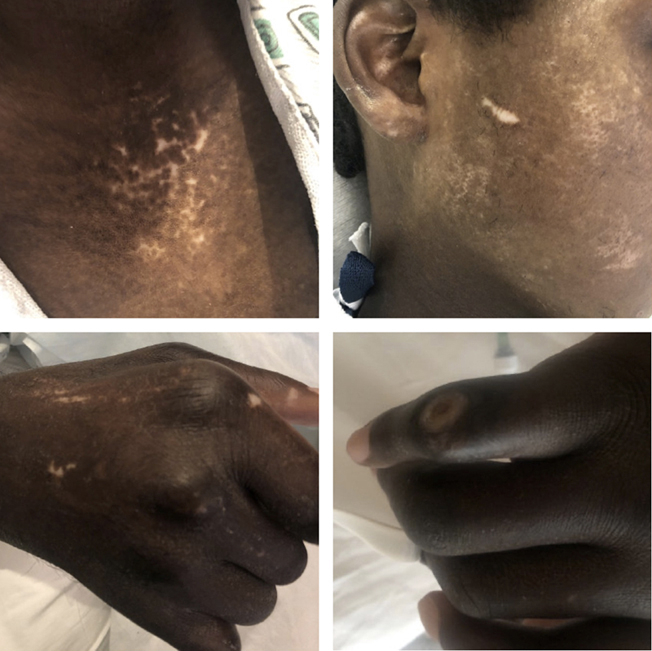
At the time of initial presentation, the patient’s vitals were blood pressure 96/75 mm Hg, pulse 65 bpm, respiratory rate 20 breaths per minute, oxygen saturation of 98% on room air, and temperature of 36.7 °C. Their exam was remarkable for jugular venous distension and anasarca. Their skin was notable for peristomal hypopigmentation, multiple hypopigmented lesions on the thorax and abdomen, metacarpal phalangeal joint deformities, thickened skin of all fingers, finger ulcerations, and skin tightening with loss of print markings on the fingertips (Figure 1). The patient also had decreased range of motion in all joints distal to their shoulders, including an inability to straighten their arms or make a fist.
Figure 1.
Skin findings present at the time of initial presentation, including hypopigmented lesions on the chest (top left) and face (top right). Bottom left demonstrates similar lesions with thickened skin and inability to make a fist. Bottom right demonstrates one of the patient’s finger ulcerations.
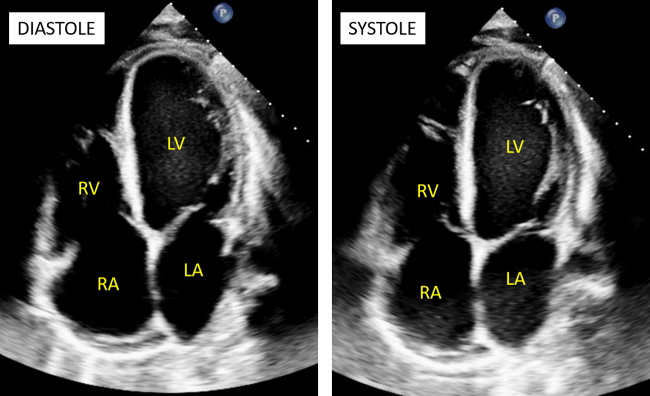
Initial laboratory findings were notable for mild normocytic anemia (12.5 g/dL), a normal comprehensive metabolic panel, a troponin-I of 0.11, and a brain-type natriuretic peptide level of 925 pg/mL. Their electrocardiogram showed sinus rhythm with an incomplete right bundle branch block. A transthoracic echocardiogram (TTE; Video 1, Video 2, Video 3, Figure 2) performed at the time of initial presentation showed a left ventricular (LV) ejection fraction of 15% by visual estimation, LV dilation (LV end-diastolic volume index of 80 mL/m2 and LV end-diastolic diameter of 5.6 cm), LV wall hypokinesis, and paradoxical septal motion. There was grade III LV diastolic dysfunction with mitral E/A of 4.8 and E/E’ of 20.0. The aortic valve was bicuspid without aortic stenosis and only mild aortic regurgitation. Pulmonary hypertension was detected, with a pulmonary artery systolic pressure of 50 mm Hg and a pulmonary artery diastolic pressure of 27 mm Hg. The right ventricle (RV) was hypokinetic with a low tricuspid annular plane systolic excursion (0.5 cm). Peak tricuspid valve annular tissue Doppler S-wave velocity was low (8 cm/sec). The right atrial (RA) pressure was elevated to 15 mm Hg and dilated with an RA volume index of 35 mL/m2.
Figure 2.
Two-dimensional TTE apical 4- chamber views in diastole (left) and systole (right) demonstrate LV dilation (LV end-diastolic volume index 80 mL/m2; LV end-systolic volume index 61 mL/m2) with severely reduced biventricular systolic function.
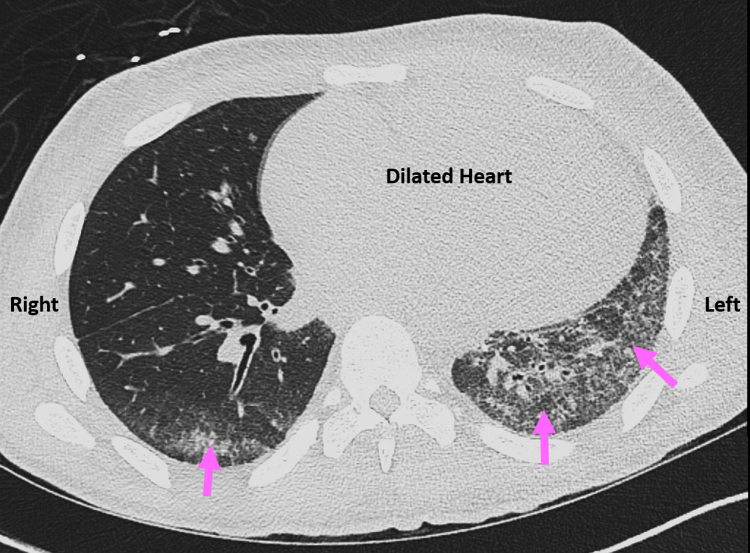
Further laboratory studies found a mildly elevated ferritin level (377 ng/mL [ref 22-248]) and normal levels of thyroid-stimulating hormone, thiamine, selenium, vitamin B6, vitamin B12, zinc, copper, and vitamin D. Titers returned very elevated levels of antinuclear antibody (>1:640 [ref <1:40]) and SCL-70 antibody (DNA topoisomerase 1; >8 [ref <1:1]). Computed tomography of the chest without contrast revealed confluent interstitial markings involving the left lower lobe and lingula and mild right lower lobe with septal thickening consistent with asymmetric pulmonary edema. There were no findings suggesting interstitial pulmonary fibrosis (Figure 3).
Figure 3.
Computed tomography chest without contrast, axial image, demonstrates confluent interstitial markings (pink arrows) involving the left lower lobe and lingula as well as the right lower lobe with septal thickening consistent with asymmetric pulmonary edema. The patient’s dilated heart is also seen.
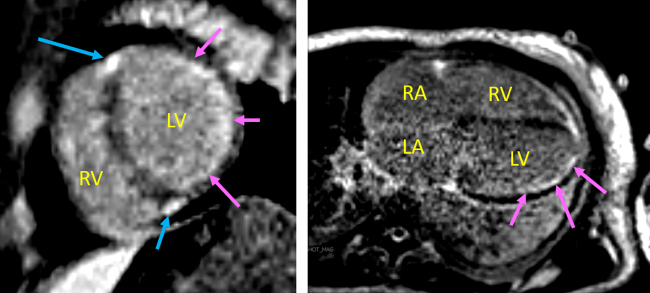
The patient underwent pharmacologic diuresis. Cardiovascular magnetic resonance imaging using single-shot phase-sensitive inversion recovery technique found a moderately dilated left ventricle (LV; LV end-diastolic volume index = 111 mL/m2) with severely diminished systolic function as well as a qualitatively mildly dilated RV with severely diminished systolic function and a mildly dilated right atrium (RA). T2-weighted images were limited in quality but did not show any regions with increased signal to suggest acute inflammation. Delayed contrast enhanced images (Figure 4) noted extensive subendocardial late gadolinium enhancement (LGE) in the basal and midinferior and inferolateral walls (up to approximately 25% thickness of the myocardium), extending to the apical lateral wall (approximately 25%-50% thickness of the myocardium). In addition, there was midwall LGE of the LV at the right ventricular insertion points (basal/midanteroseptum and basal/midinferoseptum). Three days later, a right heart catheterization, coronary angiography, and endomyocardial biopy demonstrated angiographically normal coronary arteries, mild PH, normal cardiac filling pressures (RA pressure = 4 mm Hg; pulmonary capillary wedge pressure = 13 mm Hg), and normal cardiac output. Histopathologic results from the endomyocardial biopy showed myocardial hypertrophy with patchy interstitial fibrosis (Figure 5). The patient’s American College of Rheumatology/European League against Rheumatism Score—a criterion including symptoms, imaging findings, and serologic results—was 23 (diagnostic when ≥9), consistent with scleroderma/systemic sclerosis (SSc). The patient was started on rituximab and cyclophosphamide1 with plans to eventually initiate mycophenolate mofetil as an outpatient. They were successfully discharged home.
Figure 4.
Cardiac magnetic resonance delayed contrast enhanced images, single-shot phase-sensitive inversion recovery technique, basal short-axis (left) and oblique axial 4-chamber (right) views, demonstrate extensive subendocardial LGE extending to the anterior, lateral, and inferior LV segments (pink arrows). Late gadolinium enhancement is also present at the RV insertion points (blue arrows). LA, Left atrium.
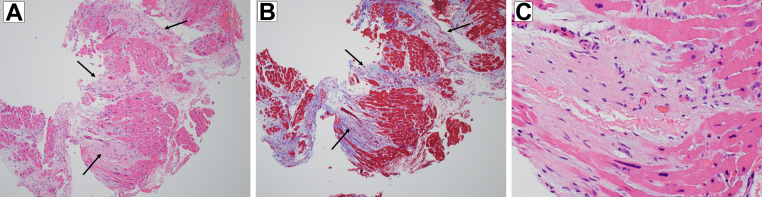
Figure 5.
Histopathology. (A) Hypertrophied myocardium with patchy interstitial fibrosis (black arrows; hematoxylin and eosin; ×10 magnification). (B) The same section of hypertrophied myocardium using a trichrome stain to highlight areas of fibrosis in blue (black arrows; ×10 magnification). (C) A close-up of interstitial fibrosis within the myocardium (hematoxylin and eosin; ×40 magnification).
The patient started following in our institution’s HF clinic and was initiated on guideline-directed medical therapy. They reported frequent gaps in medication adherence during their visits. Three months later, the patient presented to an outside institution with cardiogenic shock and died shortly thereafter.
Discussion
Among patients with scleroderma, cardiac manifestations are associated with significantly more aggressive disease as well as higher rates of mortality.2, 3, 4 While SSc patients most commonly have heart disease that is secondary to interstitial lung disease or PH, nearly 70% of SSc patients develop echocardiographic evidence of primary cardiac involvement as their disease progresses, most frequently manifesting as diastolic dysfunction.2 This case is unique given the patient’s young age, rapidly progressive scleroderma, and HF with a severely reduced ejection fraction.
The prevalence of cardiac involvement in SSc is difficult to estimate as most patients do not manifest cardiac symptoms.2,5, 6, 7 While the exact mechanism underlying cardiac scleroderma is not fully understood, most theories implicate immune dysregulation resulting in microvascular injury or ischemia and subsequent negative remodeling with fibrosis of cardiac tissues.6, 7, 8 However, a portion of patients do eventually develop progressive symptoms of HF with preserved ejection fraction.2 Risk factors for scleroderma HF with preserved ejection fraction include those for traditional cardiovascular disease (older age, men, uncontrolled hypertension) as well as long-standing scleroderma and scleroderma with diffuse cutaneous involvement.6
Left ventricular systolic dysfunction resulting in acute decompensated HF with a reduced ejection fraction (HFrEF) due to scleroderma is very uncommon.2,3 Despite some studies noting a reduced ejection fraction in 5% to 11% of SSc patients, most of these patients remain in a clinically compensated state without congestive symptoms.2, 3, 4 Systolic dysfunction in scleroderma is thought to be linked to accelerated progression of concomitant CAD of the epicardial circulation or cardiomyopathy following myocarditis.9 Compared with scleroderma patients with normal LV ejection fraction, registry analyses of scleroderma patients suggest HFrEF tends to occur in older male patients with prior cardiovascular disease, long-standing diffuse cutaneous disease characterized by digital ulcerations, and previous renal crises.4,10
The use of multimodality imaging, including CMR, is crucial for comprehensively evaluating patients with scleroderma cardiomyopathy. Although not obtained in our patient, emerging CMR imaging techniques known as T1 and T2 mapping may add significant value in assessing the myocardium in patients with scleroderma.11, 12, 13 Abnormal T2 mapping suggests chronic inflammation associated with some SSc patients.13 T1 mapping along with extracellular volume measurements can detect early myocardial involvement and may be useful for risk stratification or even as a guide for treatment, although more investigation in this area is warranted.11 With delayed contrast enhanced imaging, the pattern of LGE in scleroderma can be quite variable and may affect the subendocardium, midwall, or subepicardial regions of the LV.14,15 Evidence suggests that when the inferolateral and anterolateral segments are affected, they most often exhibit a subendocardial pattern similar to that in our patient.14
The patient in our case does not match previously reported characteristics of patients with HFrEF from primary scleroderma cardiomyopathy. They were young, and their scleroderma originally presented less than a year before. They had no clear risk factors or history of cardiovascular disease. Their coronary angiogram revealed no evidence of epicardial CAD, and they did not have long-standing uncontrolled hypertension or prior episodes of renal crises. Cardiac magnetic resonance imaging did not suggest past myocarditis; however, it did have an LGE pattern that suggested scleroderma.
Conclusion
This case highlights the devastating consequences of rapidly progressing scleroderma cardiomyopathy. Primary cardiac scleroderma is a rare cause of HfrEF and presents heterogeneously. Clinicians should be aware of this disease entity and rely on multimodality imaging, including CMR, when evaluating patients with nonischemic cardiomyopathy. Prospective data on scleroderma cardiomyopathy are lacking and should be the subject of future study.
Consent Statement
The authors declare that since this was a non-interventional, retrospective, observational study utilizing de-identified data, informed consent was not required from the patient under an IRB exemption status.
Ethics Statement
The authors declare that the work described has been carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans.
Funding Statement
The authors declare that this report did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure Statement
The authors report no conflict of interest.
Acknowledgments
We thank the following individuals for their tireless efforts in caring for the patient presented in this case report: George A. Fielding, MD, Michael DiVita, MD, Nikhil Sikand, MD, Sean T. Chen, MD, Andre L. Moreira, MD, PhD, and Adriana Quinones-Camacho, MD. We are also grateful for the entire noninvasive cardiology and echocardiography laboratory at NYU Langone Health.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.case.2023.03.006.
Supplementary Data
Two-dimensional TTE parasternal long-axis view demonstrates biventricular dilation and the severely reduced LV systolic (LV ejection fraction < 20% by visual estimation).
Two-dimensional parasternal short-axis view at the level of the mitral valve demonstrates severe global LV hypokinesis and paradoxical septal motion.
Two-dimensional TTE apical 4-chamber view demonstrates LV dilation (LV end-diastolic volume index 80 mL/m2; LV end-systolic volume index 61 mL/m2) and severely reduced biventricular systolic function.
References
- 1.Sircar G., Goswami R.P., Sircar D., Ghosh A., Ghosh P. Intravenous cyclophosphamide vs rituximab for the treatment of early diffuse scleroderma lung disease: open label, randomized, controlled trial. Rheumatology (Oxford) 2018;57:2106–2113. doi: 10.1093/rheumatology/key213. [DOI] [PubMed] [Google Scholar]
- 2.Champion H.C. The heart in scleroderma. Rheum Dis Clin North Am. 2008;34:181–190. doi: 10.1016/j.rdc.2007.12.002. viii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parks J.L., Taylor M.H., Parks L.P., Silver R.M. Systemic sclerosis and the heart. Rheum Dis Clin North Am. 2014;40:87–102. doi: 10.1016/j.rdc.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 4.Bissell L.A., Md Yusof MY, Buch M.H. Primary myocardial disease in scleroderma-a comprehensive review of the literature to inform the UK Systemic Sclerosis Study Group cardiac working group. Rheumatology (Oxford) 2017;56:882–895. doi: 10.1093/rheumatology/kew364. [DOI] [PubMed] [Google Scholar]
- 5.de Groote P., Gressin V., Hachulla E., Carpentier P., Guillevin L., Kahan A., et al. Evaluation of cardiac abnormalities by Doppler echocardiography in a large nationwide multicentric cohort of patients with systemic sclerosis. Ann Rheum Dis. 2008;67:31–36. doi: 10.1136/ard.2006.057760. [DOI] [PubMed] [Google Scholar]
- 6.Hinchcliff M., Desai C.S., Varga J., Shah S.J. Prevalence, prognosis, and factors associated with left ventricular diastolic dysfunction in systemic sclerosis. Clin Exp Rheumatol. 2012;30(2 Suppl 71):S30–S37. [PMC free article] [PubMed] [Google Scholar]
- 7.Fernandes F., Ramires F.J., Arteaga E., Ianni B.M., Bonfa E.S., Mady C. Cardiac remodeling in patients with systemic sclerosis with no signs or symptoms of heart failure: an endomyocardial biopsy study. J Card Fail. 2003;9:311–317. doi: 10.1054/jcaf.2003.51. [DOI] [PubMed] [Google Scholar]
- 8.Allanore Y., Meune C. Primary myocardial involvement in systemic sclerosis: evidence for a microvascular origin. Clin Exp Rheumatol. 2010;28(5 Suppl 62):S48–S53. [PubMed] [Google Scholar]
- 9.Mok M.Y., Lau C.S., Chiu S.S., Tso A.W., Lo Y., Law L.S., et al. Systemic sclerosis is an independent risk factor for increased coronary artery calcium deposition. Arthritis Rheum. 2011;63:1387–1395. doi: 10.1002/art.30283. [DOI] [PubMed] [Google Scholar]
- 10.Allanore Y., Meune C., Vonk M.C., Airo P., Hachulla E., Caramaschi P., et al. Prevalence and factors associated with left ventricular dysfunction in the EULAR Scleroderma Trial and Research group (EUSTAR) database of patients with systemic sclerosis. Ann Rheum Dis. 2010;69:218–221. doi: 10.1136/ard.2008.103382. [DOI] [PubMed] [Google Scholar]
- 11.Poindron V., Chatelus E., Canuet M., Gottenberg J.E., Arnaud L., Gangi A., et al. T1 mapping cardiac magnetic resonance imaging frequently detects subclinical diffuse myocardial fibrosis in systemic sclerosis patients. Semin Arthritis Rheum. 2020;50:128–134. doi: 10.1016/j.semarthrit.2019.06.013. [DOI] [PubMed] [Google Scholar]
- 12.Mavrogeni S., Pepe A., Gargani L., Bruni C., Quaia E., Kitas G.D., et al. Cardiac inflammation and fibrosis patterns in systemic sclerosis, evaluated by magnetic resonance imaging: an update. Semin Arthritis Rheum. 2023;58:152126. doi: 10.1016/j.semarthrit.2022.152126. [DOI] [PubMed] [Google Scholar]
- 13.Ntusi N.A., Piechnik S.K., Francis J.M., Ferreira V.M., Rai A.B., Matthews P.M., et al. Subclinical myocardial inflammation and diffuse fibrosis are common in systemic sclerosis--a clinical study using myocardial T1-mapping and extracellular volume quantification. J Cardiovasc Magn Reson. 2014;16:21. doi: 10.1186/1532-429X-16-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meduri A., Di Molfetta D.V., Natale L., Manfredi R. Cardiac magnetic resonance in systemic sclerosis patients with cardiac symptoms. Eur Rev Med Pharmacol Sci. 2017;21:4797–4803. [PubMed] [Google Scholar]
- 15.Hachulla A.L., Launay D., Gaxotte V., de Groote P., Lamblin N., Devos P., et al. Cardiac magnetic resonance imaging in systemic sclerosis: a cross-sectional observational study of 52 patients. Ann Rheum Dis. 2009;68:1878–1884. doi: 10.1136/ard.2008.095836. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Two-dimensional TTE parasternal long-axis view demonstrates biventricular dilation and the severely reduced LV systolic (LV ejection fraction < 20% by visual estimation).
Two-dimensional parasternal short-axis view at the level of the mitral valve demonstrates severe global LV hypokinesis and paradoxical septal motion.
Two-dimensional TTE apical 4-chamber view demonstrates LV dilation (LV end-diastolic volume index 80 mL/m2; LV end-systolic volume index 61 mL/m2) and severely reduced biventricular systolic function.