Abstract
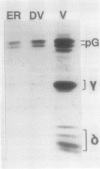
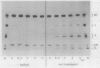
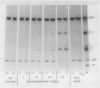
The enzymic conversion of proglobulin to globulin catalyzed by the extracts of vacuoles isolated from developing pumpkin (Cucurbita sp. cv Kurokawa Amakuri Nankin) cotyledons was investigated. The endoplasmic reticulum fraction isolated from the developing cotyledons pulselabeled with [35S]methionine was shown to contain mainly the radiolabeled proglobulin, which was used as a substrate for assaying the proteolytic processing in vitro. The vacuolar extracts catalyzed the proteolytic processing of the proglobulin molecule to produce globulin containing two kinds of polypeptide chains, γ and δ. The pH optimum for the vacuole-mediated conversion was at pH 5.0. The proteolytic processing of proglobulin by the vacuolar extracts was inhibited in the presence of various thiol reagents, e.g. p-chloromercuribenzoate, N-ethylmaleimide, iodoacetic acid, Hg2+, and Cu2+, but not phenylmethylsulfonyl fluoride, EDTA, o-phenanthroline, leupeptin, antipain, pepstatin, chymostatin, or pumpkin trypsin inhibitor, and was activated in the presence of dithiothreitol and cysteine, indicating that the processing enzyme is a thiol protease. The suborganellar fractionation of the vacuoles showed that the processing activity was localized in the matrix fraction, but not in the membrane or crystalloid fractions. During the seed development, the enzyme was shown to increase, exhibiting the maximal activity at the late developmental stage. The matrix fraction of the protein bodies isolated from the dry castor bean (Ricinus communis) exhibited the processing activity toward the pumpkin proglobulin molecules in the same manner as that by the matrix fraction of pumpkin vacuoles.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowles D. J., Marcus S. E., Pappin D. J., Findlay J. B., Eliopoulos E., Maycox P. R., Burgess J. Posttranslational processing of concanavalin A precursors in jackbean cotyledons. J Cell Biol. 1986 Apr;102(4):1284–1297. doi: 10.1083/jcb.102.4.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docherty K., Carroll R. J., Steiner D. F. Conversion of proinsulin to insulin: involvement of a 31,500 molecular weight thiol protease. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4613–4617. doi: 10.1073/pnas.79.15.4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher D. J., Quigley J. P., Bauer G. E., Noe B. D. Characterization of proinsulin- and proglucagon-converting activities in isolated islet secretory granules. J Cell Biol. 1981 Aug;90(2):312–322. doi: 10.1083/jcb.90.2.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara-Nishimura I., Nishimura M., Akazawa T. Biosynthesis and Intracellular Transport of 11S Globulin in Developing Pumpkin Cotyledons. Plant Physiol. 1985 Mar;77(3):747–752. doi: 10.1104/pp.77.3.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara-Nishimura I., Nishimura M., Matsubara H., Akazawa T. Suborganellar localization of proteinase catalyzing the limited hydrolysis of pumpkin globulin. Plant Physiol. 1982 Sep;70(3):699–703. doi: 10.1104/pp.70.3.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay R., Böhni P., Gasser S. How mitochondria import proteins. Biochim Biophys Acta. 1984 Jan 27;779(1):65–87. doi: 10.1016/0304-4157(84)90004-2. [DOI] [PubMed] [Google Scholar]
- Lamb F. I., Roberts L. M., Lord J. M. Nucleotide sequence of cloned cDNA coding for preproricin. Eur J Biochem. 1985 Apr 15;148(2):265–270. doi: 10.1111/j.1432-1033.1985.tb08834.x. [DOI] [PubMed] [Google Scholar]
- Momma T., Negoro T., Hirano H., Matsumoto A., Udaka K., Fukazawa C. Glycinin A5A4B3 mRNA: cDNA cloning and nucleotide sequencing of a splitting storage protein subunit of soybean. Eur J Biochem. 1985 Jun 18;149(3):491–496. doi: 10.1111/j.1432-1033.1985.tb08951.x. [DOI] [PubMed] [Google Scholar]
- Robinson C., Ellis R. J. Transport of proteins into chloroplasts. The precursor of small subunit of ribulose bisphosphate carboxylase is processed to the mature size in two steps. Eur J Biochem. 1984 Jul 16;142(2):343–346. doi: 10.1111/j.1432-1033.1984.tb08292.x. [DOI] [PubMed] [Google Scholar]
- Stinissen H. M., Peumans W. J., Chrispeels M. J. Posttranslational processing of proteins in vacuoles and protein bodies is inhibited by monensin. Plant Physiol. 1985 Feb;77(2):495–498. doi: 10.1104/pp.77.2.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tully R. E., Beevers H. Protein bodies of castor bean endosperm: isolation, fractionation, and the characterization of protein components. Plant Physiol. 1976 Dec;58(6):710–716. doi: 10.1104/pp.58.6.710. [DOI] [PMC free article] [PubMed] [Google Scholar]