Abstract
In this communication we document the reproducible protocols for the purification of milligram quantities of cytochrome b5 and NADH-cytochrome b5 reductase from the microsomal fraction of Pisum sativum. The cytochrome b5 component of this NADH linked electron transport chain was found to have a molecular mass of 16,400 daltons and the reductase a molecular mass of 34,500 daltons. These components could be reconstituted into a functional NADH oxidase activity active in the reduction of exogenous cytochrome c or ferricyanide. In the latter assay the purified reductase exhibited a turnover number of 22,000 per minute. The amino-terminal amino acid sequence of the cytochrome b5 component was determined by sequential Edmund degredation, thus providing crucial information for the efficient cloning of this central protein of plant microsomal electron transfer.
Full text
PDF
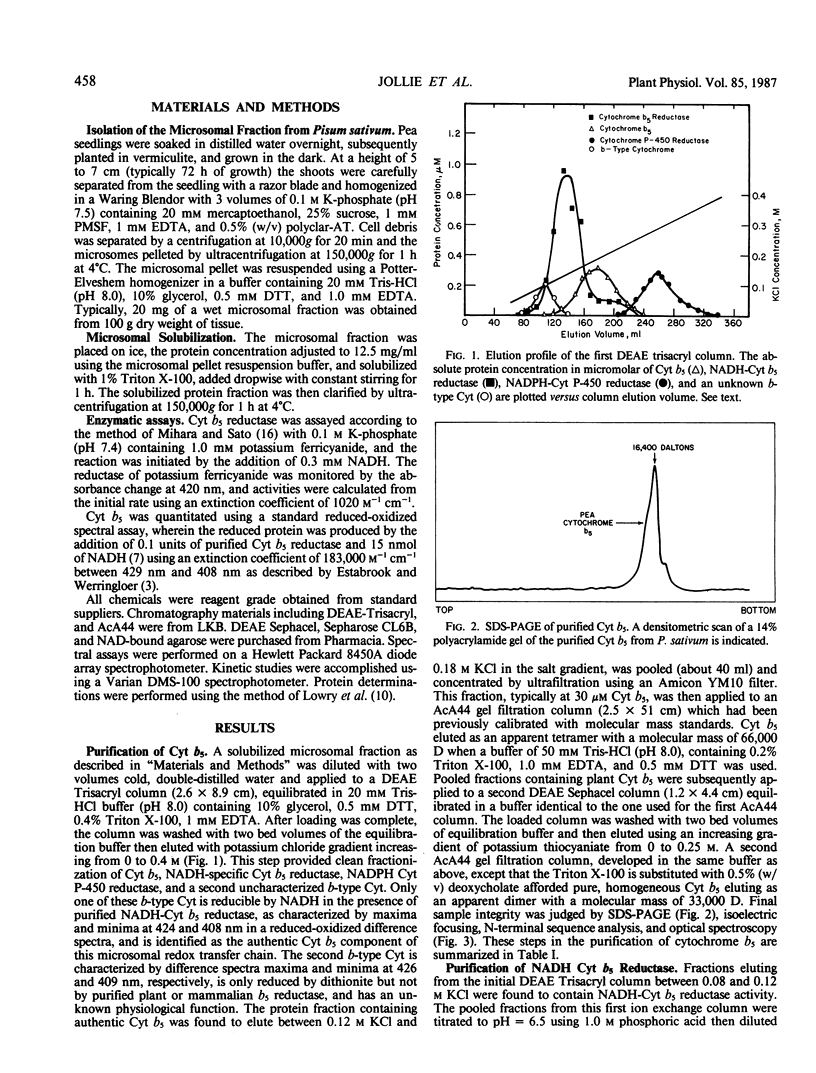
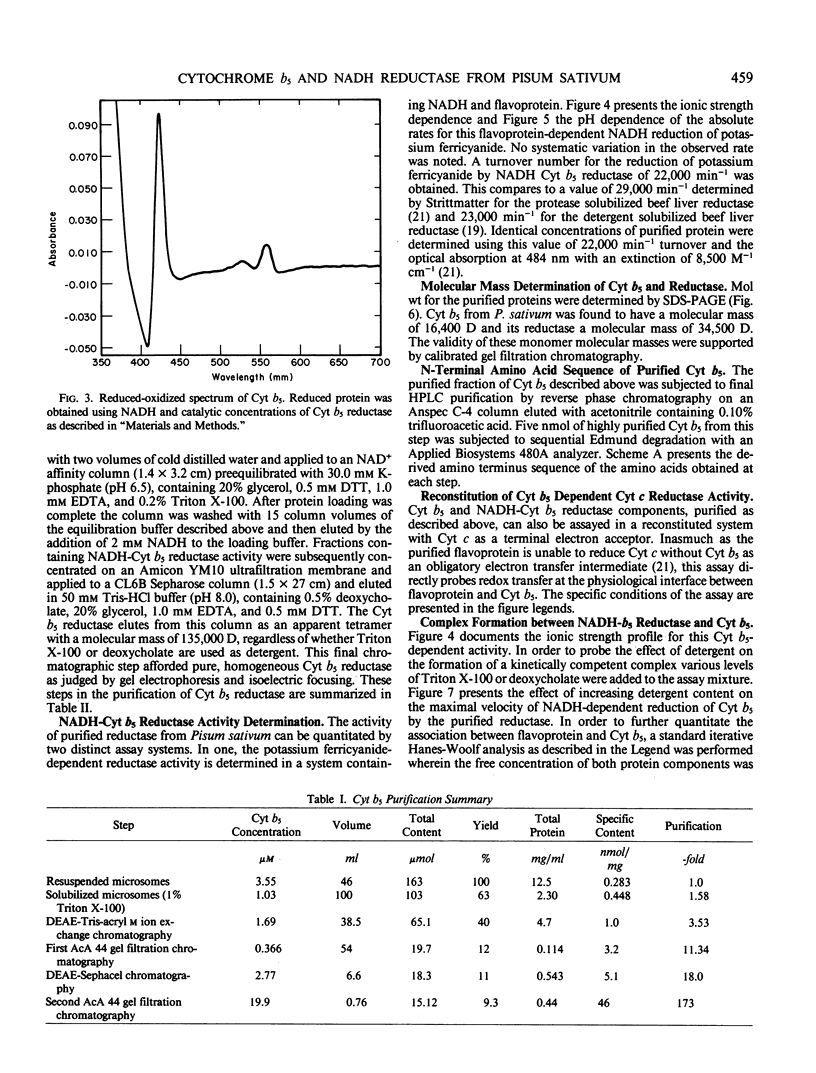
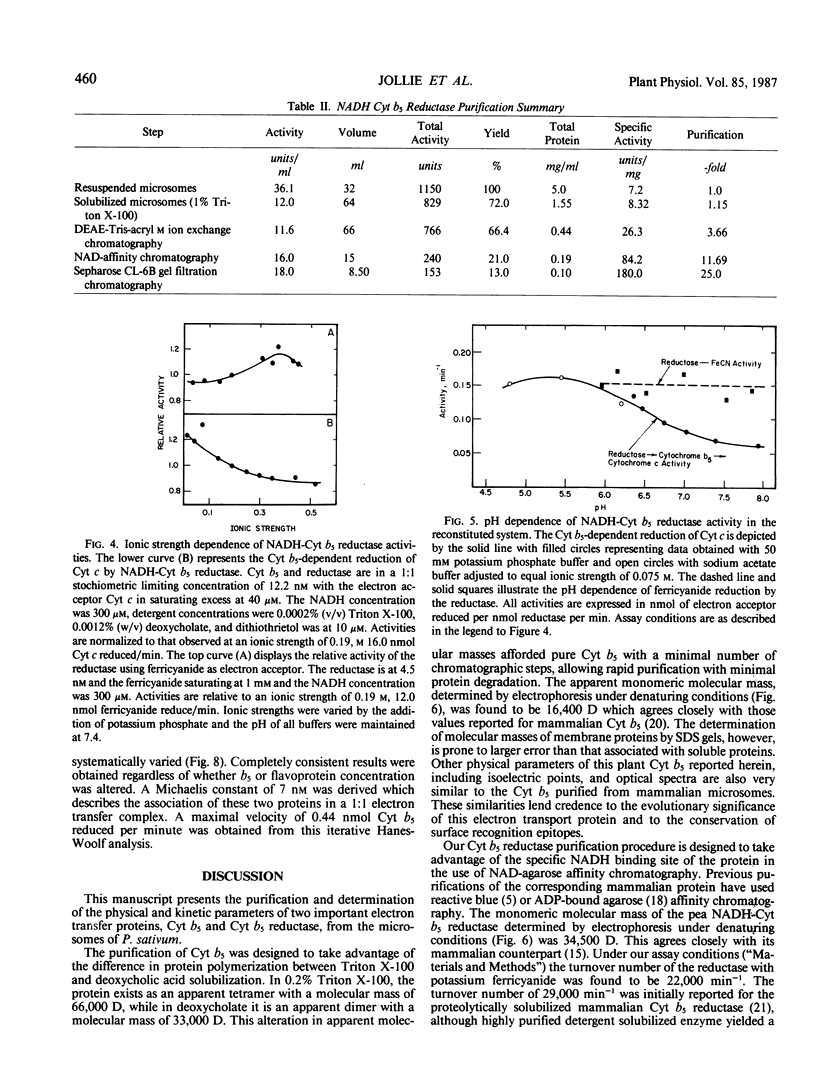

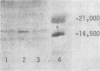
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beck von Bodman S., Schuler M. A., Jollie D. R., Sligar S. G. Synthesis, bacterial expression, and mutagenesis of the gene coding for mammalian cytochrome b5. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9443–9447. doi: 10.1073/pnas.83.24.9443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste I., Gabriac B., Durst F. Purification and characterization of the NADPH-cytochrome P-450 (cytochrome c) reductase from higher-plant microsomal fraction. Biochem J. 1986 Apr 15;235(2):365–373. doi: 10.1042/bj2350365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnerot C., Galle A. M., Jolliot A., Kader J. C. Purification and properties of plant cytochrome b5. Biochem J. 1985 Feb 15;226(1):331–334. doi: 10.1042/bj2260331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estabrook R. W., Werringloer J. The measurement of difference spectra: application to the cytochromes of microsomes. Methods Enzymol. 1978;52:212–220. doi: 10.1016/s0076-6879(78)52024-7. [DOI] [PubMed] [Google Scholar]
- Galle A. M., Bonnerot C., Jolliot A., Kader J. C. Purification of a NADH-ferricyanide reductase from plant microsomal membranes with a zwitterionic detergent. Biochem Biophys Res Commun. 1984 Aug 16;122(3):1201–1205. doi: 10.1016/0006-291x(84)91219-1. [DOI] [PubMed] [Google Scholar]
- Ito A., Sato R. Purification by means of detergents and properties of cytochrome b5 from liver microsomes. J Biol Chem. 1968 Sep 25;243(18):4922–4923. [PubMed] [Google Scholar]
- Klein R. R., Burke J. J. Separation Procedure and Partial Characterization of Two NAD(P)H Dehydrogenases from Cauliflower Mitochondria. Plant Physiol. 1984 Oct;76(2):436–441. doi: 10.1104/pp.76.2.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Loverde A., Strittmatter P. The role of lysyl residues in the structure and reactivity of cytochrome B5 reductase. J Biol Chem. 1968 Nov 10;243(21):5779–5787. [PubMed] [Google Scholar]
- Madyastha K. M., Coscia C. J. Detergent-solubilized NADPH-cytochrome c(P-450) reductase from the higher plant, Catharanthus roseus. Purification and characterization. J Biol Chem. 1979 Apr 10;254(7):2419–2427. [PubMed] [Google Scholar]
- Madyastha K. M., Krishnamachary N. Purification and partial characterization of microsomal cytochrome b555 from the higher plant Catharanthus roseus. Biochem Biophys Res Commun. 1986 Apr 29;136(2):570–576. doi: 10.1016/0006-291x(86)90478-x. [DOI] [PubMed] [Google Scholar]
- Mathews F. S., Argos P., Levine M. The structure of cytochrome b 5 at 2.0 Angstrom resolution. Cold Spring Harb Symp Quant Biol. 1972;36:387–395. doi: 10.1101/sqb.1972.036.01.050. [DOI] [PubMed] [Google Scholar]
- Mihara K., Sato R. Detergent-solubilized NADH-cytochrome b5 reductase. Methods Enzymol. 1978;52:102–108. doi: 10.1016/s0076-6879(78)52011-9. [DOI] [PubMed] [Google Scholar]
- Mihara K., Sato R. Purification and properties of the intact form of NADH-cytochrome b5 reductase from rabbit liver microsomes. J Biochem. 1975 Nov;78(5):1057–1073. doi: 10.1093/oxfordjournals.jbchem.a130983. [DOI] [PubMed] [Google Scholar]
- Neville D. M., Jr Molecular weight determination of protein-dodecyl sulfate complexes by gel electrophoresis in a discontinuous buffer system. J Biol Chem. 1971 Oct 25;246(20):6328–6334. [PubMed] [Google Scholar]
- STRITTMATTER P., VELICK S. F. The purification and properties of microsomal cytochrome reductase. J Biol Chem. 1957 Oct;228(2):785–799. [PubMed] [Google Scholar]
- Schafer D. A., Hultquist D. E. Purification of bovine liver microsomal NADH-cytochrome b5 reductase using affinity chromatography. Biochem Biophys Res Commun. 1980 Jul 16;95(1):381–387. doi: 10.1016/0006-291x(80)90749-4. [DOI] [PubMed] [Google Scholar]
- Spatz L., Strittmatter P. A form of reduced nicotinamide adenine dinucleotide-cytochrome b 5 reductase containing both the catalytic site and an additional hydrophobic membrane-binding segment. J Biol Chem. 1973 Feb 10;248(3):793–799. [PubMed] [Google Scholar]
- Strittmatter P., Fleming P., Connors M., Corcoran D. Purification of cytochrome b5. Methods Enzymol. 1978;52:97–101. doi: 10.1016/s0076-6879(78)52010-7. [DOI] [PubMed] [Google Scholar]
- Yasukochi Y., Masters B. S. Some properties of a detergent-solubilized NADPH-cytochrome c(cytochrome P-450) reductase purified by biospecific affinity chromatography. J Biol Chem. 1976 Sep 10;251(17):5337–5344. [PubMed] [Google Scholar]