Antithrombin (AT) is a key anticoagulant serpin that efficiently inhibits multiple procoagulant proteases, mainly thrombin and FXa. Congenital AT deficiency is the strongest thrombophilia and a complete absence of AT causes embryonic lethality.1
The prevalence of AT deficiency, which is evaluated mainly by chromogenic methods using anti-FXa functional assays,2 is estimated at 0.02% to 0.2% of the general population,3 and the annual risk of venous thromboembolism (VTE) in this patient group is 2.3%.4
Most cases with AT deficiency are caused by SERPINC1 defects (chromosome 1q25.1). Nearly all changes affecting this sensitive serpin have functional or structural consequences, making them deleterious, and only few missense polymorphisms with low frequency have been described until now.1 Of note, the majority of variants causing AT deficiency are private or found only in a single family and recurrent variants are very rare among patients. The identification and characterization of SERPINC1 may help to understand the pathogenic mechanisms, and their prognostic relevance.1 Here we report the genetic identification and clinical characterization of a recurrent SERPINC1 variant with a founder effect in Polish patients with AT deficiency.
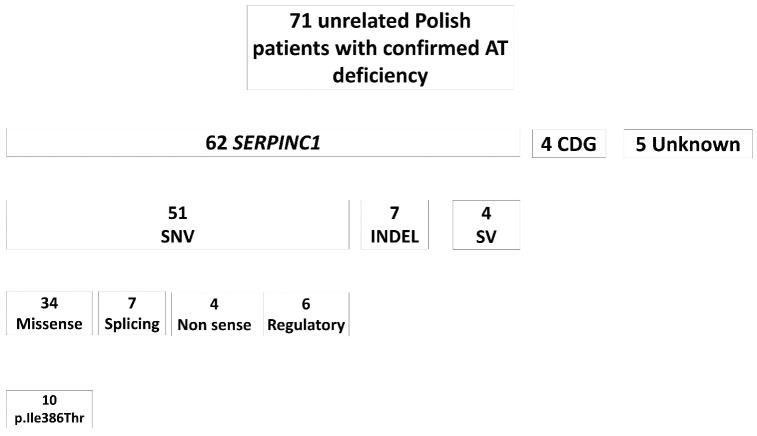
Figure 1 shows the results of the molecular characterization of the Polish cohort of 71 unrelated cases with AT deficiency, recruited within 9 years between 2013 and 2022. Relatives of selected probands were also assessed. Most cases are explained by single nucleotide variants (SNV); 34 causing missense, four nonsense, seven splicing, and six regulatory defects. We observed a considerable molecular heterogeneity as most variants were private or present in a single family and only four variants were identified in two or more unrelated probands. However, SERPINC1 (NM_000488.4): c.1157T>C (p.Ile386Thr) variant was found recurrently in ten Polish patients with AT deficiency (14.1% of the total cohort), always in a heterozygous state (Figure 2).
This variant was reported in the Human Gene Mutation database (HGMD): CM113684 (https://www.hgmd.cf.ac.uk/ ac/all.php). The variant was first identified in two patients from a German cohort of patients with AT deficiency,5 but it has also been sporadically reported in single Hungarian,6 Czech7 and French8 individuals, but not found it in 275 unrelated Spanish patients with AT deficiency. This distribution may be explained by the migration flow of the Polish population. All reported carriers belong to the Central-Eastern European population mainly consisting of Slavs. Archaeological, historical, and genetic evidence shows that Slavs arrived in the land of contemporary Poland in the sixth century.9 The Malopolska region where all our patients live did not undergo significant population movements during the last 300 years. The latest infiltration of about 18,000 inhabitants (3,000 families) from Germany occurred at the end of the eighth century,10 but it accounts for only 1% of inhabitants of this region and certainly had no influence on this population gene pool.
Figure 1.
Molecular defects identified in the cohort of 71 unrelated Polish patients with confirmed antithrombin deficiency. CDG: congenital disorders of glycosylation; Indels: insertions and deletions; SNV: single nucleotide variants; SV: structural variants.
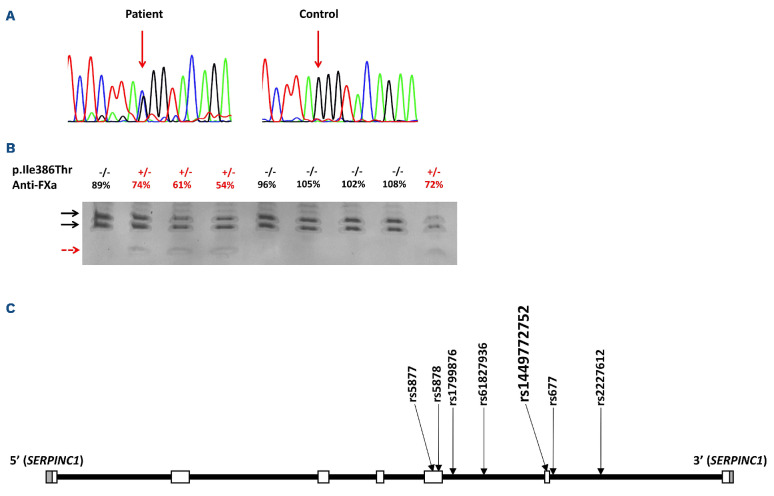
Interestingly, this variant was very rare in the general population, (rs1449772752: 1/251466 alleles; MAF: 3.98e-6) is reported as pathogenic/likely pathogenic.11 According to the American College of Medical Genetics (ACMG), the in silico predictions for the SERPINC1 c.1157T>C (p.Ile386Thr) variant supported a likely pathogenic effect (PP5 strong; PP3 moderate and PM2 supporting) (Online Supplementary Table S1). These data and the high prevalence of this variant in the Polish cohort of patients with AT deficiency strongly suggest a founder effect i.e., a genetic variant observed with a relatively high frequency in a specific population, in which one or more of the ancestors carried the altered version of the gene. A founder mutation is always embedded in a longer stretch of DNA identical to that of the founder, i.e., a haplotype and is often responsible for more or less infrequent monogenic disorders.12 In order to verify this hypothesis, we evaluated the SERPINC1 gene in carriers of this variant by nanopore sequencing using two approaches: whole genome sequencing and sequencing of the whole gene using long-range polymerase chain reaction (PCR). Sequencing of long fragments using this method allows reading all variants of the same allele, so phasing can be done to determine the haplotype linked to the variant. This study demonstrated that all carriers of the c.1157T>C (p.Ile386Thr) variant shared a common haplotype linked to the variant defined by six additional SNV, rs2227612, rs677, rs61827936, rs1799876, rs5878 and rs5877 (Figure 2C), which confirms the founder effect.
The c.1157T>C (p.Ile386Thr) variant causes a moderate AT deficiency according to the anticoagulant activity of carriers, with anti-FXa of 62.5±10.2% and anti-FIIa of 69.0±6.5%. Antigen levels were concordant with activity values 67.7±8.3% supporting a mild type I deficiency. However, an aberrant AT with slower electrophoretic mobility was observed in native gels (Online Supplementary Figure S1). Crossed immunoelectrophoresis confirmed the mild type I deficiency without increased levels of forms with low heparin affinity (Online Supplementary Figure S1). The variant affected one relatively conserved residue among the serpin superfamily located in the loop connecting helix I with strand s5A (Online Supplementary Figure S2).
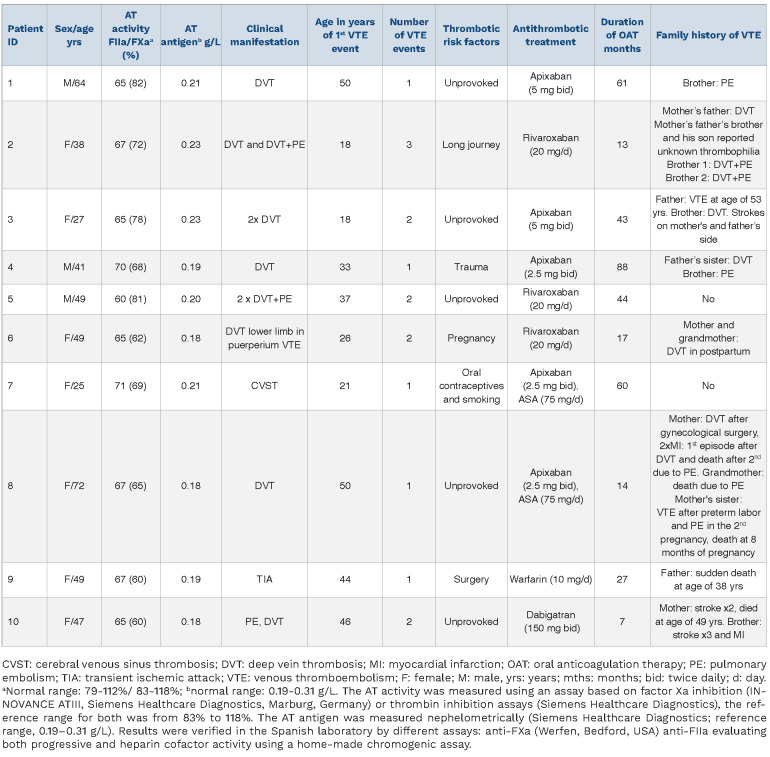
Clinical data of ten unrelated probands (representing 14.1% of the whole cohort of Polish patients with this disorder) is shown in Table 1.
The mean age of the first thromboembolic event was 34.3±12.3 years. Nine patients were without any other thrombophilia and one patient (patient 3) was heterozygous for the factor V Leiden polymorphism. Positive family history was found in eight (80.0%) patients. At the time of diagnosis, five (50.0%) probands had a history of one thromboembolic event, while the remainder experienced two (n=4, 40.0%) or three (n=1, 10%) recurrent VTE episodes. Five (50%) patients had provoked thromboembolic events. Nine (90.0%) probands received non-vitamin K antagonist oral anticoagulants (NOAC), most commonly apixaban (n=5, 50.0%), followed by rivaroxaban (n=3, 30.0%) and dabigatran (n=1, 10.0%). One (10%) patient received warfarin. During the median follow-up of 43 (interquartile range [IQR], 15.5-74.5) months, no thrombotic events were recorded. Three patients (patients 1, 3, 8) had COVID-19 with uninterrupted anticoagulation in March 2021, October 2020, and January 2022, respectively, without recurrent thromboembolism.
Figure 2.
Identification of the p.Ile386Thr variant. (A) Electropherogram of exon 6 of SERPINC1 in a patient carrying the variant and in a healthy control. In the patient, an arrow points the c.1157T>C variant. (B) Genotyping of the c.1157T>C variant in a representative family by polymerase chain reaction and and allele-specific restriction analysis (PCR-ASRA) using Rsa I. The restriction pattern of the wild-type allele is indicated by black arrows, while the presence of the variant generates a smaller fragment (red arrow). The proband is indicated by a green arrow. (C) Six polymorphisms defining the common haplotype shared by carriers of the variant of interest and the variant of interest itself plotted on the schematic representation of SERPINC1 gene; grey boxes - untranslated regions, white squares boxes - coding regions.
Analysis of 21 relatives carriers of the c.1157T>C (p.Ile386Thr) variant, showed that three (14%) experienced thromboembolic events (Table 1). This study is the first to show that the c.1157T>C (p.Ile386Thr) variant in SERPINC1, causing AT deficiency, seems to be restricted, or at least mainly found, in a Polish population. Indeed, gnomAD data revealed that this variant has been identified with a very low frequency (0.000008792) only in non-Finish Europeans and is completely absent in all other tested populations.
Table 1.
Characteristics of antithrombin deficient unrelated probands with p.Ile386Thr variant.
Our study supplies further data supporting the origin of this variant in the Polish population, specifically the presence of the common haplotype, which was obtained by a new method of long-range based sequencing with nanopore.13 Only three founder variants have been described as causative of AT deficiency, including two in Caucasian populations such as AT Budapest 3 (p.Leu131Phe) found in the Roma population14 and AT Basel (p.Pro73Leu) very frequent in the Finish population,15 and one African, p.Thr147Ala.16 These three previously described founder variants all causes type II deficiencies, which are characterized by a lower thrombotic risk than type I deficiencies.1,2 The Polish c.1157T>C (p.Ile386Thr) founder variant is the first one causing type I deficiency. However, we must point out that the deficiency caused by this variant is unusual, since the reduction of antigen (and anticoagulant activity) is not very severe (> 60%). Probably the variant caused an impaired secretion of the variant molecule having nearly normal anticoagulant activity. Indeed, the identification of traces of aberrant AT of lower electrophoretic mobility in plasma of the carriers and the relative conservation of the Ile386 residue in the serpin superfamily along with its structural localization (in the long loop connecting helix I with strand s5A) strongly suggest that this variant might have mild conformational consequences potentially favoring formation of oligomers that might be retained intracellularly. This hypothesis, which should be confirmed by further studies, could also explain the severe risk of thrombosis observed in carriers. Carriers of other conformational variants identified in SERPINC1 usually have a severe clinical phenotype with early and recurrent thrombotic events that usually require exogenous triggering factors to exacerbate the pathogenic consequences of the variant.16 We speculate that under normal conditions, the deficiency caused by the c.1157T>C (p.Ile386Thr) variant is mild and only increases the prothrombotic potential, which might be beneficial under certain conditions, thus representing a selective advantage for carriers that could also explain the expansion of the variant. This hypothesis has also been reported for the most common prothrombotic variant Factor V Leiden.16 However, under particular circumstances exacerbating the pathogenic consequences of the variant, the c.1157T>C (p.Ile386Thr)-related AT deficiency could probably lead to much higher risk of thrombosis, similar to that seen in the case of other SERPINC1 variants.18
The clinical characteristics of Polish patients with the recurrent c.1157T>C (p.Ile386Thr) variant differ from those of subjects carrying the previously described variants with a founder effect in the Hungarian, Finnish and African origin populations.13,15,19 First, in our cohort, in addition to dominant VTE episodes in typical locations, we observed one uncommon thrombosis, i.e., cerebral venous sinus thrombosis (CVST) in a very young female patient who is a smoker and is on oral contraceptives. Secondly, in our carriers, arterial thrombosis was less common. We recorded one (10%) transient ischemic attack in a 49-year-old patient, observed following major surgery despite thromboprophylaxis. In the study by Gindele et al.,19 in which the median age at the first thrombotic event in Hungarian heterozygous carriers of the p.Leu131Phe variant was similar to our cohort (33.0 vs. 34.4 years, respectively), 19.5% of all events were related to arterial thrombosis. Puurunen et al.15 in the study of large population of Finnish patients recorded a similar frequency of arterial thromboses, i.e., ten events (22% of all thrombotic episodes), including five strokes at a young age. Moreover, in our patients, obstetric complications were observed less frequently as well.
In conclusion, we report here that the c.1157T>C (p.Ile386Thr) variant is a pathogenic founder variant for AT-deficient Polish patients. The mutant allele shared a common haplotype involving six SERPINC1 SNV. It seems that under normal conditions, the deficiency caused by the p.Ile386Thr variant is mild and only increases the prothrombotic potential; however, when additional triggering factors are present, carriers of the p.Ile386Thr variant usually have a severe clinical phenotype with early and recurrent thrombotic events.
Supplementary Material
Funding Statement
Funding: This study was supported by grants (N41/DBS/000682 to AU) from Jagiellonian University School of Medicine and from Instituto de Salud Carlos III, FEDER and Next Generation UE (PI21/00174 & PMP21/00052).
References
- 1.Corral J, de la Morena-Barrio ME, Vicente V. The genetics of antithrombin. Thromb Res. 2018;169:23-29. [DOI] [PubMed] [Google Scholar]
- 2.Van Cott EM, Orlando C, Moore GW, Cooper PC, Meijer P, Marlar R. Subcommittee on Plasma Coagulation Inhibitors. Recommendations for clinical laboratory testing for antithrombin deficiency; communication from the SSC of the ISTH. J Thromb Haemost. 2020;18(1):17-22. [DOI] [PubMed] [Google Scholar]
- 3.Refaei M, Xing L, Lim W, Crowther M, Boonyawat K. Management of venous thromboembolism in patients with hereditary antithrombin deficiency and pregnancy: case report and review of the literature. Case Rep Hematol. 2017;2017:9261351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Croles FN, Borjas-Howard J, Nasserinejad K, Leebeek FWG, Meijer K. Risk of venous thrombosis in antithrombin deficiency: a systematic review and Bayesian meta-analysis. Semin Thromb Hemost. 2018;44(4):315-326. [DOI] [PubMed] [Google Scholar]
- 5.Luxembourg B, Delev D, Geisen C, et al. Molecular basis of antithrombin deficiency. Thromb Haemost. 2011;105(4):635-646. [DOI] [PubMed] [Google Scholar]
- 6.Gindele R, Selmeczi A, Oláh Z, et al. Clinical and laboratory characteristics of antithrombin deficiencies: a large cohort study from a single diagnostic center. Thromb Res. 2017;160:119-128. [DOI] [PubMed] [Google Scholar]
- 7.Provazníková D, Matýšková M, Cápová I, et al. Seventeen novel SERPINC1 variants causing hereditary antithrombin deficiency in a Czech population. Thromb Res. 2020;189:39-41. [DOI] [PubMed] [Google Scholar]
- 8.Alhenc-Gelas M, Plu-Bureau G, Hugon-Rodin J, Picard V, Horellou MH. GFHT study group on genetic thrombophilia. Thrombotic risk according to SERPINC1 genotype in a large cohort of subjects with antithrombin inherited deficiency. Thromb Haemost. 2017;2;117(6):1040-1051. [DOI] [PubMed] [Google Scholar]
- 9.Rębata K, Mikulich AI, Tsybovsky IS, et al. Y-STR variation among Slavs: evidence for the Slavic homeland in the middle Dnieper basin. J Hum Genet. 2007;52(5):406-414. [DOI] [PubMed] [Google Scholar]
- 10.Wieloucha A. Kolonizacja józefihska w galicyjskich Karpatach PŁAJ nr 19, wyd. Warszawa: Towarzystwo Karpackie; 1999. [Google Scholar]
- 11.National Center for Biotechnology Information. ClinVar; [VCV000863657.4], https://www.ncbi.nlm.nih.gov/clinvar/variation/VCV000863657.4. Accessed Aug. 25, 2022. [Google Scholar]
- 12.Evans JA. Old meets new: identifying founder mutations in genetic disease. CMAJ. 2015;187(2):93-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Orlando C, de la Morena-Barrio B, Pareyn I, et al. Antithrombin p.Thr147Ala: the first founder mutation in people of African origin responsible for inherited antithrombin deficiency. Thromb Haemost. 2021;121(2):182-191. [DOI] [PubMed] [Google Scholar]
- 14.Bereczky Z, Gindele R, Fiatal S, et al. Age and origin of the founder antithrombin Budapest 3 (p.Leu131Phe) mutation; its high prevalence in the Roma population and its association with cardiovascular diseases. Front Cardiovasc Med. 2021;5;7:617711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Puurunen M, Salo P, Engelbarth S, Javela K, Perola M. Type II antithrombin deficiency caused by a founder mutation Pro73Leu in the Finnish population: clinical picture. J Thromb Haemost. 2013;11(10):1844-1849. [DOI] [PubMed] [Google Scholar]
- 16.Corral J, Vicente V, Carrell RW. Thrombosis as a conformational disease. Haematologica. 2005;90:238-246. [PubMed] [Google Scholar]
- 17.Van Mens TE, Levi M, Middeldorp S. Evolution of Factor V Leiden. Thromb Haemost. 2013;110(1):23-30. [DOI] [PubMed] [Google Scholar]
- 18.De la Morena-Barrio ME, Suchon P, Jacobsen EM, et al. Two SERPINC1 variants affecting N-glycosylation of Asn224 cause severe thrombophilia not detected by functional assays. Blood. 2022;14;140(2):140-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gindele R, Oláh Z, Ilonczai P, et al. Founder effect is responsible for the p.Leu131Phe heparin-binding-site antithrombin mutation common in Hungary: phenotype analysis in a large cohort. J Thromb Haemost. 2016;14(4):704-715. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.