Abstract
Under normal conditions, iron metabolism is carefully regulated to sustain normal cellular functions and the production of hemoglobin in erythroid cells. Perturbation to the erythropoiesis-iron metabolism axis can result in iron imbalances and cause anemia or organ toxicity. Various congenital and acquired diseases associated with abnormal red cell production are characterized by aberrant iron absorption. Several recent studies have shown that improvements in red blood cell production also ameliorate iron metabolism and vice versa. Many therapeutics are now under development with the potential to improve a variety of hematologic diseases, from β-thalassemia and iron-refractory iron deficiency anemia to anemia of inflammation and polycythemia vera. This review summarizes selected mechanisms related to red cell production and iron metabolism and describes potential therapeutics and their current uses. We also consider the potential application of the discussed therapeutics on various diseases, alone or in combination. The vast repertoire of drugs under development offers new opportunities to improve the clinical care of patients suffering from congenital or acquired red blood cell disorders with limited or no treatment options.
Erythropoiesis and iron metabolism
Erythropoiesis, the production of mature enucleated erythrocytes from early progenitor cells, is regulated by molecules that control proliferation and differentiation.1 For brevity, the descriptions of the molecules that regulate erythropoiesis and iron metabolism will be limited to those relevant to the diseases and potential therapies discussed in this review.
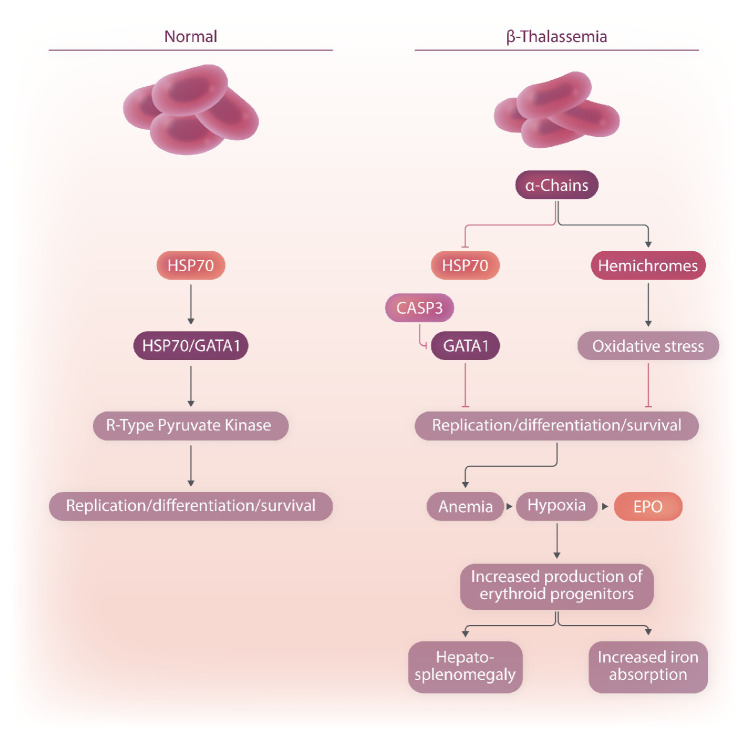
GATA1 is a key transcription factor essential for erythropoiesis (Figure 1).2 GATA1 promotes maturation and survival of erythroblasts, which develop into enucleated red cells.3 GATA1 stimulates several pathways, including those responsible for hemoglobin synthesis and stress erythropoiesis.4,5 The erythrocyte-specific isoform of pyruvate kinase, R-type pyruvate kinase (Figure 1), is regulated by GATA1 and is involved in the final steps of glycolysis, which leads to the formation of two adenosine triphosphate and two pyruvate molecules per glucose molecule (Figure 1).6,7 R-type pyruvate kinase is required for the survival of mature red cells and to sustain the tricarboxylic acid cyclein erythroid precursors.7- 9
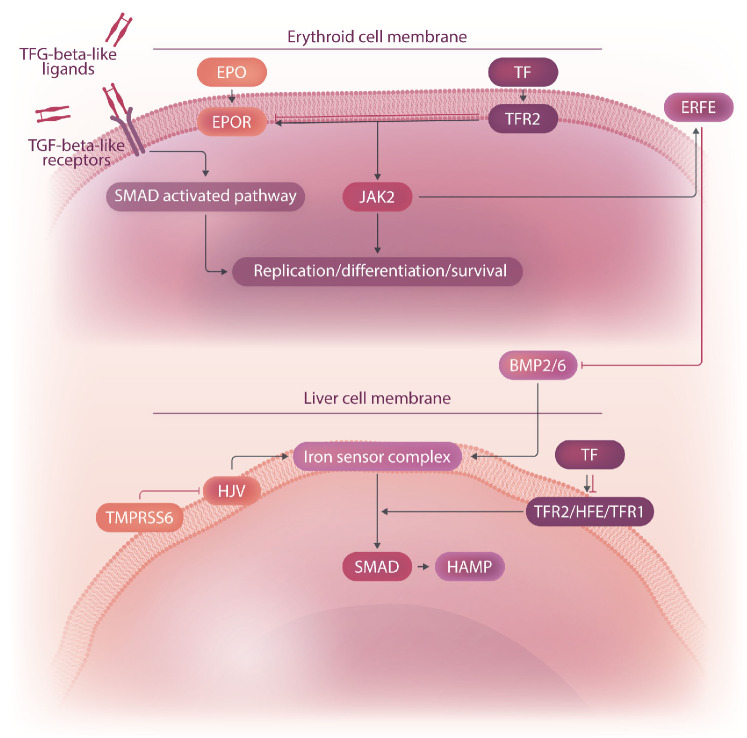
Erythropoietin (EPO) binds to the EPO receptor (EPOR) to regulate early-stage erythropoiesis, primarily by phosphorylation of the JAK2 kinase (Figure 2).10 Phosphorylated JAK2 kinase is responsible for the activation of downstream pathways that regulate expression of genes that control proliferation, survival, and iron metabolism, such as the hormone erythroferrone (ERFE) (Figure 2).11 A subset of transforming growth factor-β superfamily receptors, ligands and SMAD2/3 transcription factors may play a major role in late-stage erythropoiesis by modulation of differentiation and red cell production (Figure 2).12,13
Hepcidin (encoded by HAMP) is a liver-secreted hormone that plays a major role in iron homeostasis (Figure 1).14 It binds and inhibits the iron exporter ferroportin, preventing release of iron into the circulation from duodenal enterocytes, macrophages, and hepatocytes.15 Increased hepcidin expression causes anemia by limiting iron availability, whereas low hepcidin expression leads to iron overload.14,16
Figure 1.
The pathways of a subset of the molecules involved in red cell production and iron metabolism. Only those molecules discussed in this review are included in this schematic. HSP70: heat shock protein 70; CASP3: caspase 3; GATA1: GATA-binding factor 1; EPO: erythropoietin.
Activation of hepcidin transcription is mediated by the ironsensor complex on liver parenchymal cells, which includes bone morphogenetic protein (BMP) receptors from the TGF-β receptor superfamily as well as coreceptors, ligands, and modulators (Figure 2).16 Liver endothelial cells secrete the BMP ligands BMP2 and BMP6, which bind class I and II BMP receptors in hepatocytes and activate the SMAD1/5/8 complex and HAMP expression (Figure 2).16 Hemojuvelin (HJV) acts as a BMP coreceptor, whereas TMPRSS6 (which encodes for matriptase-2, a transmembrane serine protease) downregulates hepcidin by cleaving HJV (Figure 2).17
Additional proteins in the liver modulate the activity of the iron-sensor complex by sensing the level of iron bound to transferrin (TF), the main molecule responsible for the transportation of iron from the duodenum (Figure 2).16 Increasing amounts of diferric TF displace HFE from TF receptor 1.16 This not only enables iron uptake in the liver but also stabilizes TF receptor 2 (TFR2), potentiating signaling of the iron-sensor complex (Figure 2).16 Although TFR2 was thought to only modulate hepcidin synthesis in the liver, it was recently shown that TFR2 colocalizes with EPOR on erythroid precursor cells to modulate EPO sensitivity (Figure 2).16,18 The role of TFR2 in this setting is thought to be that of finetuning erythropoiesis based on the amount of iron bound to TF.19
Diseases associated with abnormal red cell synthesis and their pathophysiology
Erythropoiesis can be altered by genetic mutations that impair red cell production, such as in congenital anemias or polycythemia vera (PV), or by conditions that perturb normal iron metabolism, such as iron-refractory iron deficiency anemia (IRIDA).20 Conditions associated with chronic inflammation, such as chronic kidney disease, are also characterized by reduced red cell production and survival.21 In this review, we focus on diseases that illustrate key pathophysiological mechanisms responsible for altered red cell production, the role of iron metabolism, and strategies targeting these mechanisms that may serve as potential novel treatment approaches. Anemia is a consequence of inadequate functional red cells in circulation, which can result from reduced red cell production, increased red cell destruction, or a combination of these two processes. Diseases associated with genetic mutations that directly alter red cell production are generally characterized by defects at the erythroid cell-differentiation stage at which the mutated genes exert their functions. These mutations may also affect the quality and lifespan of surviving mature red cells.
β-thalassemia
β-thalassemia (BT) and a-thalassemia (AT) are two of the most widespread forms of congenital anemias.22 These diseases are triggered by mutations that reduce the synthesis of β-globin or a-globin, respectively.22 Heterozygosity for BT null mutations (BT trait) is usually clinically silent but becomes symptomatic with coinheritance of extra a-globin genes, as a consequence of a relatively increased amount of free a-globin protein.23,24 Hence, the globin-chain imbalance with an accumulation of free a-globin is a major determinant of BT pathophysiology and causes a variety of effects that impair red cell maturation and survival (Figure 2).22
Figure 2.
Schematic representation of normal and β-thalassemic erythropoiesis. A few consequences associated with ineffective erythropoiesis in β-thalassemia, such as iron overload and hepatosplenomegaly, are presented. EPO: erythropoietin; TF: transferrin; ERFE: erythroferrone; EPOR: erythropoietin receptor; TFR: transferrin receptor; JAK2: Janus kinase 2; BMP: bone morphogenetic protein; HJV: hemojuvelin; TMPRSS6: transmembrane protease, serine 6; HFE: homeostatic iron regulator; SMAD: small mothers against decapentaplegic; HAMP: hepcidin antimicrobial protein.
Excess α-globin chains precipitate as hemichromes, which are insoluble aggregates of α-chains and heme molecules that form large inclusion bodies and cause iron accumulation and free radical production (Figure 1).25,26 These events lead to premature destruction by apoptosis of a portion of red cell precursors in the bone marrow as well as the production of abnormal enucleated erythrocytes with reduced lifespan.27 In addition, excess α-globin chains also interfere with erythroid differentiation. In normal erythropoiesis, chaperone heat shock protein 70 (HSP70) protects GATA1 from caspase-3 cleavage (Figure 1).28 However, in BT, HSP70 interacts directly with free α-globin chains, and therefore HSP70 is sequestered and unable to protect GATA1, resulting in end-stage maturation arrest (Figure 1).28 Altogether, this mechanism limits the production of enucleated red cells and consequent oxygen delivery (Figure 1). The resulting hypoxia increases the production of EPO in the kidneys. EPO further stimulates the production and expansion of early erythroid progenitors without generating sufficient mature red cells (Figure 1).27 This leads to a net imbalance between nucleated and enucleated erythroid cells - the number of terminally differentiated red cells is disproportionally low compared with the number of progenitor red cells (Figure 1).27 This abnormal production of erythroid progenitors, combined with a hypoxic environment, profoundly affects iron metabolism. The production of ERFE, by the early progenitor red cells, increases as the number of erythroid precursor cells increases, leading to suppression of hepcidin and results in enhanced iron absorption (Figure 2).27 In addition, the hypoxic environment also triggers the synthesis of genes responsible for iron absorption in the duodenum, further exacerbating the process of iron overload in organs.27,29
Iron accumulation results in free radical damage to cells and organs and contributes to the morbidity and mortality of iron overload (Figure 1).30 Although the main source of extra iron in patients with severe BT comes from blood transfusions, the main cause of iron overload in patients who only sporadically receive transfused blood is from increased iron absorption.31 When total body iron is significantly elevated the TF iron-binding ability is exceeded and circulating non-TF-bound iron appears in the plasma. Non-TF-bound iron rises markedly when the TF saturation reaches high levels, and a reactive Fe2+ subspecies of non-TF-bound iron called labile plasma iron increases concomitantly.32,33 It is important to underline that clinical observations suggested that non-TF-bound iron was absent in non-transfusion-dependent thalassemia patients without previous transfusions.32,33 Further, labile plasma iron was not detected in most non-transfusion-dependent thalassemia patients, whereas other iron storage and turnover markers were elevated.32,34 Nevertheless, in both transfusion- and non-transfusion-dependent thalassemia patients, iron chelation is the only treatment to eliminate excess iron and prevent organ damage.31
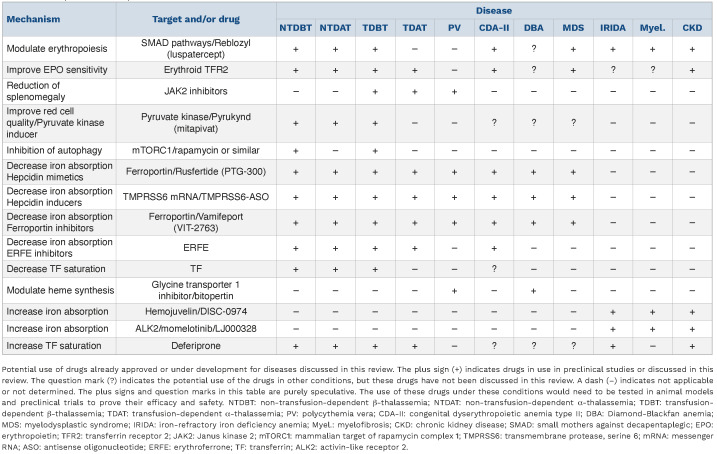
Following extensive studies in mouse models and humans, many novel drugs and genetic approaches are now in development to improve the quality of life of or provide a cure for patients with BT. These drugs can be loosely classified based on their mechanism of action, such as improvement of red cell quality and production (e.g., luspatercept, mitapivat, and drugs that target excess α-globin chains), improvement of anemia, suppression of erythropoiesis and improvement of splenomegaly (e.g., JAK2 inhibitors), limitation of iron absorption and/or erythroid iron intake (e.g., hepcidin agonists, hepcidin inducers, ferroportin or ERFE inhibitors, and TF therapy), all which lead to improvements of iron overload, and, potentially, improvement of red cell production over time (Table 1).35,36 In addition, novel gene therapy approaches and strategies aimed to pharmacologically reactivate fetal hemoglobin have the potential to significantly improve symptoms of BT or to cure BT, but these topics are beyond the scope of this review.
Drugs that improve red cell quality and production
Luspatercept (Reblozyl®) is a ligand trap made using the extracellular domain of the activin receptor type IIB (Table 1).37,38 This drug binds to a subset of TGF-β ligands to inhibit aberrant SMAD2/3 signaling and enhances late-stage erythropoiesis.37-39 Luspatercept has recently been approved in the USA and Europe for the treatment of adult patients with transfusion-dependent BT (TDBT).37 In the pivotal clinical trial, a significantly greater proportion of patients in the luspatercept group had a reduction in transfusion burden compared with the placebo group.37 Additional observations also suggest that patients with non-transfusion-dependent BT (NTDBT) may also benefit from this drug.38 Although luspatercept treatment reduced transfusion burden and iron loading in patients affected by TDBT, NTDBT, or myelodysplastic syndrome, likely caused by improved erythropoiesis and iron consumption, luspatercept treatment also increased ERFE levels and, consequently, reduced hepcidin levels.40 It is, therefore, possible that luspatercept may negatively modulate hepcidin transcription by binding TGF-β family ligands implicated in hepcidin synthesis.41 These observations suggest it could be beneficial to use luspatercept in combination with iron chelators or other therapeutics, as elaborated in the section “Anemias associated with impaired iron absorption.”
Mitapivat (AG-348; Pyrukynd®) is a small-molecule activator of R-type pyruvate kinase which enhances the affinity of R-type pyruvate kinase for its substrate, phosphoenolpyruvate (Table 1).42,43 Mitapivat was initially developed to treat patients with pyruvate kinase deficiency, as it can improve pyruvate kinase activity, increase red cell deformability, and reduce transfusion burden in some patients who receive regular blood transfusions.44 Additional observations found that mitapivat induces a rapid and sustained increase in hemoglobin levels in approximately 50% of patients with pyruvate kinase deficiency who did not receive regular blood transfusions.43 In mouse models of BT, oral mitapivat administration ameliorated ineffective erythropoiesis and anemia by improving red cell metabolism.9 Likely caused by improved erythropoiesis, mitapivat administration resulted in reduced levels of soluble ERFE, increased liver hepcidin expression, and diminished liver iron overload.9 Mitapivat also reduced duodenal iron absorption, suggesting that this drug could further reduce iron overload independently of its indirect effect on hepcidin expression.9,45 Ongoing observations in patients with non-transfusion-dependent AT (NTDAT) and NTDBT treated with mitapivat suggest that this drug could ameliorate anemia in these patients.45
Table 1.
Novel potential therapeutics.
Other studies are investigating the possibility of targeting pathways that enhance the removal of excess α-globin in BT erythroid precursor cells (Table 1).24 These types of detoxification strategies would allow BT to be treated similarly to other protein aggregation disorders.46 Pharmacological induction of protein-targeted quality control pathways and autophagy to eliminate relevant unstable proteins could be potentially beneficial in patients with BT.47, 4 8 As mTORC1 inhibits autophagy, systemic treatment with the mTORC1 inhibitor rapamycin reduces α-globin precipitates and lessens pathologies in BT mice.49 These findings may allow investigation of additional pathways that can be regulated by drugs for amelioration of BT symptoms. However, we do not know if rapamycin or similar drugs also improve iron metabolism in patients with BT, although rapamycin has been shown to increase hepcidin expression in wild-type mice.50
Selective deletion of Tfr2 exclusively in cells of the bone marrow in both NTDBT and TDBT mice significantly ameliorated anemia and highlight a major role for TFR2 in modulating the relationship between erythroid delivery of iron by TF, EPO sensitivity, and ineffective erythropoiesis (Table 1).51,52
Drugs that limit erythropoiesis to reduce splenomegaly
In BT, in response to anemia and hypoxia, high levels of EPO are associated with increased activation of the JAK2 pathway and increased numbers of erythroid progenitors in both the bone marrow and the spleen.53 This extramedullary hematopoiesis leads to hepatosplenomegaly, which enhances the entrapment of red cells in the spleen and exacerbates the anemia, further worsening the hepatosplenomegaly.27,53 Additionally, erythropoiesis occurs in extramedullary sites, most commonly resulting in a paraspinal mass.54 Unfortunately, splenectomy considerably increases the rate of thromboembolic complications.31 Although administration of JAK2 inhibitors does not reduce transfusion burden, the combination of blood transfusions with JAK2 inhibitors could be effective in reversing splenomegaly and could, therefore, be used as an alternative to splenectomy (Table 1).55,56
Drugs that limit iron absorption and heme synthesis
Identification of hepcidin and elucidation of the pathways that control its synthesis have been crucial to the development of pharmacological compounds that mimic the activity of hepcidin, increase hepcidin expression, and decrease ferroportin or ERFE activity, which all lead to decreased iron absorption and TF saturation.36 In a similar fashion, administration of exogenous apo-TF can also decrease levels of TF saturation.57 These potential therapeutics have reduced iron overload in animal models of BT.36 Intriguingly, in BT mice, the same drugs also lead to reduction in anemia.35,58 These drugs decreased not only iron absorption but also erythroid iron intake.35,59 In thalassemic erythroid progenitor cells, reduced iron uptake reduces the detrimental effects of oxidative stress triggered by the excess iron and heme that are not included in functional hemoglobin molecules.35,58 Overall, the consequences of iron restriction to erythroid cells improve the quality and lifespan of mature red cells and increase circulating hemoglobin levels.35,58 An ERFE antibody is also a potential therapeutic that targets a component of the iron-regulation pathway, as it prevented hepcidin suppression and corrected the iron-loading phenotype in a mouse model of BT.60 These observations suggested that iron-restricted erythropoiesis could benefit BT patients in a similar fashion, and a series of clinical trials were designed using drugs that restrict iron absorption. The hepcidin mimetic rusfertide® (PTG-300) can reduce iron absorption by targeting ferroportin.61 Rusfertide treatment improved iron metabolism and erythropoiesis in BT mice and decreased serum iron levels in patients with TDBT (https://www.protagonist-inc.com/investors-media/press-releases/default.aspx) but failed to improve symptoms in patients who received regular blood transfusions.62 The ferroportin inhibitor vamifeport® (VIT-2763) showed similar endpoints in BT mice. However, also in this case it failed to improve symptoms in NTDBT patients (h ttps://thalassaemia.o rg .c y/fr/clinical-trial-updates/ferroportin-inhibitors/#:~:text=A%20randomized%2C%20controlled%2C%20multi-centred%20phase%202%20proof-of-concept%20trial,serum%20iron%20for%20up%20to%2024%20 hours%20post-dose).63 Since, administration of these reagents in these clinical trials was associated with decreased levels of TF saturation, it is possible that the biology of human red cells may be less sensitive to the deleterious effect of hemichromes or that the effects of erythroid iron restriction on the viability of BT red cells may surpass the beneficial effects of reducing hemichrome formation. Alternatively, the use of these drugs may require some administration of EPO, as it has been shown to be very efficacious in BT mice.64 However, the ability of these reagents to modulate hemichrome formation in the red cells of BT patients has never been investigated. Although these drugs failed to improve anemia in BT patients, it is important to underline that they were efficacious in reducing dietary iron intake and TF saturation, which is a prerequisite to improving iron overload. This suggests that these drugs could be used to prevent iron overload or could be combined with other medications that improve erythropoiesis or reduce iron overload (e.g., luspatercept, mitapivat or iron chelators) in order to obtain a rapid reduction of iron overload, as shown in animal studies.35,64,65
Bitopertin is an oral inhibitor of glycine transporter type 1, a key membrane transporter required to supply red cells with sufficient glycine to support erythropoiesis.66 By limiting glycine uptake, bitopertin regulates downstream heme synthesis.66 In a mouse model of erythropoietic protoporphyria, bitopertin reduced the excessive production of protoporphyrin IX (a precursor of heme) and improved liver fibrosis.67 Based on these preclinical studies, bitopertin is currently being tested in patients with erythropoietic protoporphyria.67 Bitopertin also limited abnormal levels of heme and reduced anemia in BT mice.66 However, a clinical trial in patients with NTDBT failed to reverse the anemia, with decreased levels of mean corpuscular hemoglobin and hemoglobin.68
Additional conditions that may benefit from modulation of erythropoiesis or suppression of iron absorption
α-thalassemia
Patients with severe forms of AT who require chronic blood transfusion therapy for survival (i.e., who have TDAT) almost exclusively make hemoglobin H, which is a tetramer made of four β-globin chains that has extremely high oxygen affinity but provides poor oxygen delivery to tissues.69,70 These patients share several pathophysiological manifestations with patients with TDBT, such as iron overload and the requirement for iron chelation therapy.71 However, patients with TDAT require a more aggressive transfusion regimen than patients with TDBT.72 If not, patients with TDAT continue to have a high proportion of circulating non-functional hemoglobin H and exhibit features of hypoxia and EPO-driven increased erythropoietic activity.71,73 These observations suggest that ineffective erythropoiesis in patients with TDAT may be qualitatively and quantitively different from that observed in patients with TDBT, with potentially more enucleated but non-functional cells in circulation and increased iron absorption. If these predictions are confirmed by future studies, this will indicate that drugs that alter red cell quality may not be efficacious in patients with TDAT, although therapeutics that limit iron absorption or splenomegaly (such as JAK2 inhibitors) may be beneficial (Table 1). In contrast, the less severe forms of AT (i.e., NTDAT), in which some level of functional hemoglobin is present, may benefit from the same therapeutics that are being tested for patients with NTDBT, such as luspatercept and mitapivat. In a preliminary study, patients with NTDAT seemed to benefit from administration of mitapivat.45 As is the case with severe forms of AT, other drugs that limit iron absorption or erythroid iron intake could also be effective in the less severe forms of AT associated with iron overload (Table 1).
Polycythemia vera
There are conditions in which modulation of iron metabolism could be beneficial to reduce excessive production of red cells. Mutations in JAK2 can lead to PV, which is characterized by erythrocytosis, bone marrow erythroid and megakaryocytic hyperplasia, fatigue, and splenomegaly.74 Patients with PV are frequently iron deficient at the time of diagnosis, which is further exacerbated by therapeutic phlebotomies to maintain the hematocrit below 45% in order to decrease the risk of thrombosis.75,76
Studies in PV mice demonstrated that administration of exogenous hepcidin or induction of endogenous hepcidin using TMPRSS6-ASO, an antisense oligonucleotide to TMPRSS6 messenger RNA, was able to reverse erythrocytosis, decrease splenomegaly, and sequester iron in splenic macrophages, suggesting that approaches that limit erythroid iron absorption and/or heme synthesis could be beneficial in patients with PV (Table 1).35,77,78 Additionally, preliminary results from clinical trials that aimed to evaluate the safety and efficacy of the hepcidin mimetic rusfertide in phlebotomy-requiring patients with PV suggest that approaches that limit erythroid iron absorption and/or heme synthesis could eliminate phlebotomy requirements, increase systemic iron stores, and decrease systemic symptoms.79
Other conditions
In brief, some conditions, such as congenital dyserythropoietic anemia type II, myelodysplastic syndrome, and Diamond-Blackfan anemia, may benefit from therapeutics that modulate erythropoiesis, erythroid iron intake, heme synthesis, and/or decreased dietary iron absorption. For instance, a subset of patients affected by myelodysplastic syndrome benefit from luspatercept treatment and may also benefit from iron restriction.80 Similar approaches may also benefit a subset of patients with congenital dyserythropoietic anemia type II (mutations in SEC23B) who have anemia associated with increased ERFE expression, decreased hepcidin levels, and iron overload.81,82 Finally, erythroid cells in patients with Diamond-Blackfan anemia synthesize excess heme, which could be reduced by limiting erythroid iron intake and/or heme synthesis by treatment with a drug such as bitopertin.83
Anemias associated with impaired iron absorption
Other forms of anemia can help us to further understand the relationship between ineffective erythropoiesis and iron metabolism. In this part of the review, we will discuss informative forms of anemia and speculate on novel potential drugs to treat them.
Anemia of inflammation (AI) and IRIDA are two of the clinical manifestations associated with inappropriately high hepcidin levels.21,84,85 AI is common in inflammatory diseases with complex pathophysiological features, such as chronic kidney disease, autoimmune diseases, and some forms of cancer and myelofibrosis.21,85,86 Here, we review conditions in which inflammatory cytokines together with hepcidin contribute to iron-restricted erythropoiesis.
The primary clinical goal for patients with AI is to treat the cause of inflammation; however, treatment for anemia is often managed by administration of intravenous iron and erythropoiesis-stimulating agents,85 which is ineffective for some patients and is associated with adverse effects.85
Myelofibrosis is a myeloproliferative neoplasm characterized by splenomegaly, debilitating constitutional symptoms, and bone marrow failure.86 Disease-related anemia is common and associated with increased expression of hepcidin.86,87
IRIDA develops when mutations in TMPRSS6 lead to high hepcidin levels.20 Patients with IRIDA have microcytic anemia and low plasma-iron levels and are refractory to oral iron therapy but are partially responsive to parenteral iron administration.20,84
In all these conditions, high levels of hepcidin not only limit iron absorption but also sequester the iron provided to the patients by oral or intravenous administration to parenchymal cells and macrophages.16 Therefore, drugs that target proteins that interfere with the iron-sensor complex and decrease hepcidin levels or that can redistribute iron to TF may make iron more available for erythropoiesis. In addition, drugs that increase EPO sensitivity could also improve red cell production.
Activin-like receptor 2 (ALK2) belongs to the TGF-β receptor superfamily and is part of the iron-sensor complex.88,89 ALK2 is the target for the drugs momelotinib and LJ000328.90,91 These drugs can downregulate hepcidin expression and increase availability of iron for erythropoiesis.90,91 In clinical testing, momelotinib improved hemoglobin levels and reduced transfusion burden in patients with myelofibrosis and baseline anemia, while also reducing spleen size and symptom burden.91 In mice, LJ000328 repressed hepcidin activity and significantly reduced the symptoms of IRIDA.90
Mutations in HJV are associated with a severe form of hemochromatosis.20,92 Therefore, pharmacological targeting of HJV could lead to hepcidin suppression. Anti-HJV antibodies, such as DISC-0974, have been effective in suppressing hepcidin expression in normal individuals and in preclinical models of AI and IRIDA, in which the antibodies were shown to increase hemoglobin levels in both inflammatory and non-inflammatory states.93-96
It has recently been observed that luspatercept treatment in patients with BT or myelodysplastic syndrome resulted in the release of excess iron from stored organs into peripheral blood.40 This was associated with decreased hepcidin levels, suggesting that luspatercept (which targets a subset of TGF-β ligands) may also inhibit the activity of the iron-sensor complex, possibly by targeting BMP or BMP-like molecules involved in hepcidin synthesis.41 These observations provide a mechanistic rationale for evaluating luspatercept also in diseases of elevated hepcidin synthesis, such as AI and IRIDA.
Deferiprone (Ferriprox®) does not directly affect iron absorption, but it chelates iron and is used to treat iron overload in BT.97 Deferiprone readily reaches the major intracellular sites of iron accumulation, facilitating the extraction of iron deposited in organs.98 The iron affinity of deferiprone is lower than that of TF, so deferiprone could redistribute iron sequestered in organs to erythroid cells.99 Therefore, in patients with high hepcidin levels, deferiprone could shuttle the sequestered iron to unbound TF, paradoxically improving erythroid iron consumption and anemia in AI and IRIDA. This strategy may also require concurrent iron administration to provide the optimal amount of iron needed for efficient erythropoiesis.
Finally, it could be beneficial to patients with many of the above-mentioned conditions to target TFR2 on erythroid cells, as deletion of TFR2 in hematopoietic cells can improve EPO sensitivity and increase red cell production.100 Because deletion of TFR2 in the liver decreases hepcidin expression and leads to increased iron absorption, it is currently challenging to target TFR2 selectively to erythroid cells and improve anemic conditions associated with iron overload (e.g., AT and BT). However, it may be beneficial to target TFR2 on both erythroid cells and liver cells in conditions in which increases in both EPO sensitivity and iron absorption may be beneficial (i.e., chronic kidney disease).1
Additional emerging technologies
Current nucleoside-modified mRNA-lipid nanoparticle technology has successfully paved the way for next-generation vaccinations against severe acute respiratory syndrome coronavirus-2 during the COVID-19 pandemic.102 This emerging technology is also being used for additional applications, such as the ability to deliver small interfering RNA, messenger RNA, or plasmid DNA to treat most genetic diseases by silencing pathological genes, expressing therapeutic proteins, or gene editing. Using unmodified or targeted lipid nanoparticles encapsulating mRNA, several studies are underway to treat a variety of non-liver and liver diseases.103,104 As the pathways that control iron metabolism are primarily localized to the liver, it is only a question of time before this technology will be applied to modify the expression of iron-related genes and to improve iron overload or to correct genetic defects directly, as in IRIDA or HFE-related hemochromatosis.
Combinatorial therapies and conclusion
Future clinical trials should clarify which drugs benefit all or a subset of patients with diseases associated with defective erythropoiesis. Combination therapies may emerge as the best approach, not only to improve anemia and iron overload but also to enhance treatment efficacy in a larger number of patients. Drug combinations that prevent excess iron absorption and improve red cell production have already demonstrated clear additive benefits in animal models.64
In conclusion, a variety of promising novel drugs may expand the armory of therapeutics available to patients with diseases associated with defective erythropoiesis. If proven to be safe, selective, and effective, these drugs will increase the chance for treatment success, and competition between drug companies will likely diminish treatment costs.
Funding Statement
Funding: SR acknowledges the support of the Commonwealth Universal Research Enhancement (C.U.R.E.) Program Pennsylvania Department of Health and National Institute of Diabetes and Digestive and Kidney Diseases Institute of the National Institutes of Health (R01 DK090554, R01 DK095112), Institute for Translational Medicine and Therapeutics (ITMAT), Irish Health Research Board-Health Research Charities Ireland (HRCI-HRB) and The Sickle Cell and Red Cell Disorders Curative Therapy Center (CuRED)-Frontier Program. AG acknowledges the support of the Faculty CHOP Program.
References
- 1.An X, Schulz VP, Mohandas N, Gallagher PG. Human and murine erythropoiesis. Curr Opin Hematol. 2015;22(3):206-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fujiwara Y, Browne CP, Cunniff K, Goff SC, Orkin SH. Arrested development of embryonic red cell precursors in mouse embryos lacking transcription factor GATA-1. Proc Natl Acad Sci U S A. 1996;93(22):12355-12358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weiss MJ, Orkin SH. Transcription factor GATA-1 permits survival and maturation of erythroid precursors by preventing apoptosis. Proc Natl Acad Sci U S A. 1995;92(21):9623-9627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gutierrez L, Tsukamoto S, Suzuki M, et al. Ablation of Gata1 in adult mice results in aplastic crisis, revealing its essential role in steady-state and stress erythropoiesis. Blood. 2008;111(8):4375-4385. [DOI] [PubMed] [Google Scholar]
- 5.Liao R, Bresnick EH. Heme as a differentiation-regulatory transcriptional cofactor. Int J Hematol. 2022;116(2):174-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Max-Audit I, Eleouet JF, Romeo PH. Transcriptional regulation of the pyruvate kinase erythroid-specific promoter. J Biol Chem. 1993;268(8):5431-5437. [PubMed] [Google Scholar]
- 7.Zanella A, Fermo E, Bianchi P, Chiarelli LR, Valentini G. Pyruvate kinase deficiency: the genotype-phenotype association. Blood Rev. 2007;21(4):217-231. [DOI] [PubMed] [Google Scholar]
- 8.Max-Audit I, Kechemir D, Mitjavila MT, Vainchenker W, Rotten D, Rosa R. Pyruvate kinase synthesis and degradation by normal and pathologic cells during erythroid maturation. Blood. 1988;72(3):1039-1044. [PubMed] [Google Scholar]
- 9.Matte A, Federti E, Kung C, et al. The pyruvate kinase activator mitapivat reduces hemolysis and improves anemia in a betathalassemia mouse model. J Clin Invest. 2021;131(10):e144206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Witthuhn BA, Quelle FW, Silvennoinen O, et al. JAK2 associates with the erythropoietin receptor and is tyrosine phosphorylated and activated following stimulation with erythropoietin. Cell. 1993;74(2):227-236. [DOI] [PubMed] [Google Scholar]
- 11.Kautz L, Jung G, Valore EV, Rivella S, Nemeth E, Ganz T. Identification of erythroferrone as an erythroid regulator of iron metabolism. Nat Genet. 2014;46(7):678-684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dussiot M, Maciel TT, Fricot A, et al. An activin receptor IIA ligand trap corrects ineffective erythropoiesis in betathalassemia. Nat Med. 2014;20(4):398-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guerra A, Oikonomidou PR, Sinha S, et al. Lack of Gdf11 does not improve anemia or prevent the activity of RAP-536 in a mouse model of beta-thalassemia. Blood. 2019;134(6):568-572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nicolas G, Bennoun M, Devaux I, et al. Lack of hepcidin gene expression and severe tissue iron overload in upstream stimulatory factor 2 (USF2) knockout mice. Proc Natl Acad Sci U S A. 2001;98(15):8780-8785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nemeth E, Tuttle MS, Powelson J, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306(5704):2090-2093. [DOI] [PubMed] [Google Scholar]
- 16.Muckenthaler MU, Rivella S, Hentze MW, Galy B. A red carpet for iron metabolism. Cell. 2017;168(3):344-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silvestri L, Pagani A, Nai A, De Domenico I, Kaplan J, Camaschella C. The serine protease matriptase-2 (TMPRSS6) inhibits hepcidin activation by cleaving membrane hemojuvelin. Cell Metab. 2008;8(6):502-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nai A, Lidonnici MR, Rausa M, et al. The second transferrin receptor regulates red blood cell production in mice. Blood. 2015;125(7):1170-1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parrow NL, Li Y, Feola M, et al. Lobe specificity of iron binding to transferrin modulates murine erythropoiesis and iron homeostasis. Blood. 2019;134(17):1373-1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Finberg KE, Heeney MM, Campagna DR, et al. Mutations in TMPRSS6 cause iron-refractory iron deficiency anemia (IRIDA). Nat Genet. 2008;40(5):569-571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nemeth E, Ganz T. Hepcidin and iron in health and disease. Annu Rev Med. 2023;74:261-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steinberg MH. Thalassemia: molecular pathology and management. Am J Med Sci. 1988;296(5):308-321. [DOI] [PubMed] [Google Scholar]
- 23.Higgs DR. The thalassaemia syndromes. Q J Med. 1993;86(9):559-564. [PubMed] [Google Scholar]
- 24.Khandros E, Weiss MJ. Protein quality control during erythropoiesis and hemoglobin synthesis. Hematol Oncol Clin North Am. 2010;24(6):1071-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mannu F, Arese P, Cappellini MD, et al. Role of hemichrome binding to erythrocyte membrane in the generation of band-3 alterations in beta-thalassemia intermedia erythrocytes. Blood. 1995;86(5):2014-2020. [PubMed] [Google Scholar]
- 26.Rachmilewitz EA, Thorell B. Hemichromes in single inclusion bodies in red cells of beta thalassemia. Blood. 1972;39(6):794-800. [PubMed] [Google Scholar]
- 27.Rivella S. Iron metabolism under conditions of ineffective erythropoiesis in beta-thalassemia. Blood. 2019;133(1):51-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arlet JB, Ribeil JA, Guillem F, et al. HSP70 sequestration by free alpha-globin promotes ineffective erythropoiesis in betathalassaemia. Nature. 2014;514(7521):242-246. [DOI] [PubMed] [Google Scholar]
- 29.Anderson ER, Taylor M, Xue X, et al. Intestinal HIF2alpha promotes tissue-iron accumulation in disorders of iron overload with anemia. Proc Natl Acad Sci U S A. 2013;110(50):E4922-4930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cabantchik ZI, Breuer W, Zanninelli G, Cianciulli P. LPI-labile plasma iron in iron overload. Best Pract Res Clin Haematol. 2005;18(2):277-287. [DOI] [PubMed] [Google Scholar]
- 31.Musallam KM, Cappellini MD, Viprakasit V, Kattamis A, Rivella S, Taher AT. Revisiting the non-transfusion-dependent (NTDT) vs. transfusion-dependent (TDT) thalassemia classification 10 years later. Am J Hematol. 2021;96(2):E54-E56. [DOI] [PubMed] [Google Scholar]
- 32.Porter JB, Cappellini MD, Kattamis A, et al. Iron overload across the spectrum of non-transfusion-dependent thalassaemias: role of erythropoiesis, splenectomy and transfusions. Br J Haematol. 2017;176(2):288-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Porter JB, Walter PB, Neumayr LD, et al. Mechanisms of plasma non-transferrin bound iron generation: insights from comparing transfused Diamond Blackfan anaemia with sickle cell and thalassaemia patients. Br J Haematol. 2014;167(5):692-696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Origa R, Galanello R, Ganz T, et al. Liver iron concentrations and urinary hepcidin in beta-thalassemia. Haematologica. 2007;92(5):583-588. [DOI] [PubMed] [Google Scholar]
- 35.Casu C, Oikonomidou PR, Chen H, et al. Minihepcidin peptides as disease modifiers in mice affected by beta-thalassemia and polycythemia vera. Blood. 2016;128(2):265-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Casu C, Nemeth E, Rivella S. Hepcidin agonists as therapeutic tools. Blood. 2018;131(16):1790-1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cappellini MD, Viprakasit V, Taher AT, et al. A phase 3 trial of luspatercept in patients with transfusion-dependent betathalassemia. N Engl J Med. 2020;382(13):1219-1231. [DOI] [PubMed] [Google Scholar]
- 38.Piga A, Longo F, Gamberini MR, et al. Long-term safety and erythroid response with luspatercept treatment in patients with β-thalassemia. Ther Adv Hematol. 2022;13:20406207221134404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Attie KM, Allison MJ, McClure T, et al. A phase 1 study of ACE-536, a regulator of erythroid differentiation, in healthy volunteers. Am J Hematol. 2014;89(7):766-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ahsan A, Fang W, Ugidos M, et al. Investigations into the mechanisms and clinical implications of modulation of hepcidin levels by luspatercept in TD MDS and TD β-thalassemia. Blood. 2022;140:(Suppl 1): 8188-8189. [Google Scholar]
- 41.Arezes J, Foy N, McHugh K, et al. Erythroferrone inhibits the induction of hepcidin by BMP6. Blood. 2018;132(14):1473-1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kung C, Hixon J, Kosinski PA, et al. AG-348 enhances pyruvate kinase activity in red blood cells from patients with pyruvate kinase deficiency. Blood. 2017;130(11):1347-1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grace RF, Rose C, Layton DM, et al. Safety and efficacy of mitapivat in pyruvate kinase deficiency. N Engl J Med. 2019;381(10):933-944. [DOI] [PubMed] [Google Scholar]
- 44.Rab MAE, Van Oirschot BA, Kosinski PA, et al. AG-348 (mitapivat), an allosteric activator of red blood cell pyruvate kinase, increases enzymatic activity, protein stability, and ATP levels over a broad range of PKLR genotypes. Haematologica. 2021;106(1):238-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kuo KHM, Layton DM, Lal A, et al. Safety and efficacy of mitapivat, an oral pyruvate kinase activator, in adults with non-transfusion dependent alpha-thalassaemia or beta-thalassaemia: an open-label, multicentre, phase 2 study. Lancet. 2022;400(10351):493-501. [DOI] [PubMed] [Google Scholar]
- 46.Khandros E, Thom CS, D'Souza J, Weiss MJ. Integrated protein quality-control pathways regulate free alpha-globin in murine beta-thalassemia. Blood. 2012;119(22):5265-5275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lithanatudom P, Wannatung T, Leecharoenkiat A, Svasti S, Fucharoen S, Smith DR. Enhanced activation of autophagy in beta-thalassemia/Hb E erythroblasts during erythropoiesis. Ann Hematol. 2011;90(7):747-758. [DOI] [PubMed] [Google Scholar]
- 48.Ciechanover A, Kwon YT. Degradation of misfolded proteins in neurodegenerative diseases: therapeutic targets and strategies. Exp Mol Med. 2015;47(3):e147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lechauve C, Keith J, Khandros E, et al. The autophagy-activating kinase ULK1 mediates clearance of free alpha-globin in beta-thalassemia. Sci Transl Med. 2019;11(506):eaav4881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Colucci S, Pagani A, Pettinato M, et al. The immunophilin FKBP12 inhibits hepcidin expression by binding the BMP type I receptor ALK2 in hepatocytes. Blood. 2017;130(19):2111-2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Artuso I, Lidonnici MR, Altamura S, et al. Transferrin receptor 2 is a potential novel therapeutic target for beta-thalassemia: evidence from a murine model. Blood. 2018;132(21):2286-2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Di Modica SM, Tanzi E, Olivari V, et al. Transferrin receptor 2 (Tfr2) genetic deletion makes transfusion-independent a murine model of transfusion-dependent beta-thalassemia. Am J Hematol. 2022;97(10):1324-1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Libani IV, Guy EC, Melchiori L, et al. Decreased differentiation of erythroid cells exacerbates ineffective erythropoiesis in betathalassemia. Blood. 2008;112(3):875-885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tyler PA, Madani G, Chaudhuri R, Wilson LF, Dick EA. The radiological appearances of thalassaemia. Clin Radiol. 2006;61(1):40-52. [DOI] [PubMed] [Google Scholar]
- 55.Taher AT, Karakas Z, Cassinerio E, et al. Efficacy and safety of ruxolitinib in regularly transfused patients with thalassemia: results from a phase 2a study. Blood. 2018;131(2):263-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Casu C, Presti VL, Oikonomidou PR, et al. Short-term administration of JAK2 inhibitors reduces splenomegaly in mouse models of β-thalassemia intermedia and major. Haematologica. 2018;103(2):e46-e49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li H, Rybicki AC, Suzuka SM, et al. Transferrin therapy ameliorates disease in beta-thalassemic mice. Nat Med. 2010;16(2):177-182. [DOI] [PubMed] [Google Scholar]
- 58.Gardenghi S, Marongiu MF, Ramos P, et al. Ineffective erythropoiesis in beta-thalassemia is characterized by increased iron absorption mediated by down-regulation of hepcidin and up-regulation of ferroportin. Blood. 2007;109(11):5027-5035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Guo S, Casu C, Gardenghi S, et al. Reducing TMPRSS6 ameliorates hemochromatosis and beta-thalassemia in mice. J Clin Invest. 2013;123(4):1531-1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Arezes J, Foy N, McHugh K, et al. Antibodies against the erythroferrone N-terminal domain prevent hepcidin suppression and ameliorate murine thalassemia. Blood. 2020;135(8):547-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Langer AL, Esrick EB. β-Thalassemia: evolving treatment options beyond transfusion and iron chelation. Hematology Am Soc Hematol Educ Program. 2021;2021(1):600-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Taranath R, Bourne G, Zhao L, Frederick B, King C, Liu D. Regulation of iron homeostasis by PTG-300 improves disease parameters in mouse models for beta-thalassemia and hereditary hemochromatosis. Blood. 2019;134:(Suppl_1):3540. [Google Scholar]
- 63.Manolova V, Nyffenegger N, Flace A, et al. Oral ferroportin inhibitor ameliorates ineffective erythropoiesis in a model of beta-thalassemia. J Clin Invest. 2019;130(1):491-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Casu C, Pettinato M, Liu A, et al. Correcting beta-thalassemia by combined therapies that restrict iron and modulate erythropoietin activity. Blood. 2020;136(17):1968-1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Guerra A, Demsko P, McVeigh P, et al. Combination of a luspatercept-like drug (RAP-GRL) and Tmprss6-ASO is superior to either drug alone for correcting β-thalassemia. Blood. 2021;138(Suppl 1):2013. [Google Scholar]
- 66.Matte A, Federti E, Winter M, et al. Bitopertin, a selective oral GLYT1 inhibitor, improves anemia in a mouse model of betathalassemia. JCI Insight. 2019;4(22):e130111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wu M, Ducamp S, Xiang Y, et al. Bitopertin, a selective glycine transporter 1 inhibitor, reduced protoporphyrin IX (PPIX) level and improved liver fibrosis in a mouse model of erythropoietic protoporphyria (EPP). Blood. 2022;140:(Suppl 1):8192-8193. [Google Scholar]
- 68.Taher AT, Viprakasit V, Cappellini MD, et al. Haematological effects of oral administration of bitopertin, a glycine transport inhibitor, in patients with non-transfusion-dependent betathalassaemia. Br J Haematol. 2021;194(2):474-477. [DOI] [PubMed] [Google Scholar]
- 69.Papassotiriou I, Kanavakis E, Stamoulakatou A, Kattamis C. Tissue oxygenation in patients with hemoglobinopathy H. Pediatr Hematol Oncol. 1997;14(4):323-334. [DOI] [PubMed] [Google Scholar]
- 70.Mettananda S, Higgs DR. Molecular basis and genetic modifiers of thalassemia. Hematol Oncol Clin North Am. 2018;32(2):177-191. [DOI] [PubMed] [Google Scholar]
- 71.Amid A, Chen S, Athale U, et al. Iron overload in transfusiondependent survivors of hemoglobin Bart's hydrops fetalis. Haematologica. 2018;103(5):e184-e187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Amid A, Barrowman N, Odame I, Kirby-Allen M. Optimizing transfusion therapy for survivors of haemoglobin Bart's hydrops fetalis syndrome: defining the targets for haemoglobin-H fraction and "functional" haemoglobin level. Br J Haematol. 2022;197(3):373-376. [DOI] [PubMed] [Google Scholar]
- 73.Amid A, Chen S, Brien W, Kirby-Allen M, Odame I. Optimizing chronic transfusion therapy for survivors of hemoglobin Barts hydrops fetalis. Blood. 2016;127(9):1208-1211. [DOI] [PubMed] [Google Scholar]
- 74.Passamonti F, Rumi E, Pungolino E, et al. Life expectancy and prognostic factors for survival in patients with polycythemia vera and essential thrombocythemia. Am J Med. 2004;117(10):755-761. [DOI] [PubMed] [Google Scholar]
- 75.Ginzburg YZ, Feola M, Zimran E, Varkonyi J, Ganz T, Hoffman R. Dysregulated iron metabolism in polycythemia vera: etiology and consequences. Leukemia. 2018;32(10):2105-2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Barbui T, Passamonti F, Accorsi P, et al. Evidence- and consensus-based recommendations for phlebotomy in polycythemia vera. Leukemia. 2018;32(9):2077-2081. [DOI] [PubMed] [Google Scholar]
- 77.Grisouard J, Li S, Kubovcakova L, et al. JAK2 exon 12 mutant mice display isolated erythrocytosis and changes in iron metabolism favoring increased erythropoiesis. Blood. 2016;128(6):839-851. [DOI] [PubMed] [Google Scholar]
- 78.Casu C, Liu A, De Rosa G, et al. Tmprss6-ASO as a tool for the treatment of polycythemia vera mice. PLoS One. 2021;16(12):e0251995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pemmaraju N, Kuykendall A, Kremyanskaya M, et al. MPN-469 rusfertide (PTG-300) treatment interruption reverses hematological gains and upon reinitiation, restoration of clinical benefit observed in patients with polycythemia vera. Clin Lymphoma Myeloma Leuk. 2022;22(Suppl 2):S338-S339. [Google Scholar]
- 80.Zeidan AM, Platzbecker U, Garcia-Manero G, et al. Longer-term benefit of luspatercept in transfusion-dependent lower-risk myelodysplastic syndromes with ring sideroblasts. Blood. 2022;140(20):2170-2174. [DOI] [PubMed] [Google Scholar]
- 81.Russo R, Andolfo I, Manna F, et al. Increased levels of ERFE-encoding FAM132B in patients with congenital dyserythropoietic anemia type II. Blood. 2016;128(14):1899-1902. [DOI] [PubMed] [Google Scholar]
- 82.Iolascon A, Andolfo I, Russo R. Congenital dyserythropoietic anemias. Blood. 2020;136(11):1274-1283. [DOI] [PubMed] [Google Scholar]
- 83.Yang Z, Keel SB, Shimamura A, et al. Delayed globin synthesis leads to excess heme and the macrocytic anemia of Diamond Blackfan anemia and del(5q) myelodysplastic syndrome. Sci Transl Med. 2016;8(338):338ra367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Heeney MM, Finberg KE. Iron-refractory iron deficiency anemia (IRIDA). Hematol Oncol Clin North Am. 2014;28(4):637-652. [DOI] [PubMed] [Google Scholar]
- 85.Batchelor EK, Kapitsinou P, Pergola PE, Kovesdy CP, Jalal DI. Iron deficiency in chronic kidney disease: updates on pathophysiology, diagnosis, and treatment. J Am Soc Nephrol. 2020;31(3):456-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Birgegard G, Samuelsson J, Ahlstrand E, et al. Inflammatory functional iron deficiency common in myelofibrosis, contributes to anaemia and impairs quality of life. From the Nordic MPN Study Group. Eur J Haematol. 2019;102(3):235-240. [DOI] [PubMed] [Google Scholar]
- 87.Zhou A, Kong T, Fowles JS, et al. Hepcidin is elevated in primary and secondary myelofibrosis and remains elevated in patients treated with ruxolitinib. Br J Haematol. 2022;197(4):e49-e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Steinbicker AU, Bartnikas TB, Lohmeyer LK, et al. Perturbation of hepcidin expression by BMP type I receptor deletion induces iron overload in mice. Blood. 2011;118(15):4224-4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Traeger L, Gallitz I, Sekhri R, et al. ALK3 undergoes ligand-independent homodimerization and BMP-induced heterodimerization with ALK2. Free Radic Biol Med. 2018;129:127-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Belot A, Gourbeyre O, Fay A, et al. LJ000328, a novel ALK2/3 kinase inhibitor, represses hepcidin and significantly improves the phenotype of IRIDA. Haematologica. 2020;105(8):e385-e388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tremblay D, Mesa R. Momelotinib for the treatment of myelofibrosis with anemia. Future Oncol. 2022;18(20):2559-2571. [DOI] [PubMed] [Google Scholar]
- 92.Nili M, Shinde U, Rotwein P. Soluble repulsive guidance molecule c/hemojuvelin is a broad spectrum bone morphogenetic protein (BMP) antagonist and inhibits both BMP2- and BMP6-mediated signaling and gene expression. J Biol Chem. 2010;285(32):24783-24792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Boser P, Seemann D, Liguori MJ, et al. Anti-repulsive guidance molecule c (RGMc) antibodies increases serum iron in rats and Cynomolgus monkeys by hepcidin downregulation. AAPS J. 2015;17(4):930-938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kovac S, Boser P, Cui Y, et al. Anti-hemojuvelin antibody corrects anemia caused by inappropriately high hepcidin levels. Haematologica. 2016;101(5):e173-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Novikov N, Buch A, Yang H, et al. DISC-0974, an anti-hemojuvelin antibody, reduces hepcidin and mobilizes iron in healthy volunteers. Blood. 2022;140(Suppl 1):5339-5340. [Google Scholar]
- 96.Wu M, Wang K, MacDonald B. DISC-0974, an anti-hemojuvelin (HJV) monoclonal antibody, reduced hepcidin and improved anemia in a rat model of chronic kidney disease. Blood. 2022;140:(Suppl 1):8153-8154. [Google Scholar]
- 97.Kwiatkowski JL. Current recommendations for chelation for transfusion-dependent thalassemia. Ann N Y Acad Sci. 2016;1368(1):107-114. [DOI] [PubMed] [Google Scholar]
- 98.Glickstein H, El RB, Shvartsman M, Cabantchik ZI. Intracellular labile iron pools as direct targets of iron chelators: a fluorescence study of chelator action in living cells. Blood. 2005;106(9):3242-3250. [DOI] [PubMed] [Google Scholar]
- 99.Tricta F, Fradette C, Temin N, Rozova A, Lee D, Cabantchik I. Efficacy and safety of early-start deferiprone in infants and young children with transfusion-dependent beta thalassemia: evidence for iron shuttling to transferrin in a randomized, double-blind, placebo-controlled clinical trial (START). Hemasphere. 2022;6(Suppl):1439-1440. [DOI] [PubMed] [Google Scholar]
- 100.Silvestri L, Nai A, Pagani A, Camaschella C. The extrahepatic role of TFR2 in iron homeostasis. Front Pharmacol. 2014;5:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Olivari V, Di Modica SM, Lidonnici MR, et al. A single approach to targeting transferrin receptor 2 corrects iron and erythropoietic defects in murine models of anemia of inflammation and chronic kidney disease. Kidney Int. 2023;104(1):61-73. [DOI] [PubMed] [Google Scholar]
- 102.Oliver SE, Gargano JW, Marin M, et al. The Advisory Committee on Immunization Practices' interim recommendation for use of Pfizer-BioNTech COVID-19 vaccine - United States, December 2020. MMWR Morb Mortal Wkly Rep. 2020;69(50):1922-1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rizvi F, Everton E, Smith AR, et al. Murine liver repair via transient activation of regenerative pathways in hepatocytes using lipid nanoparticle-complexed nucleoside-modified mRNA. Nat Commun. 2021;12(1):613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rurik JG, Tombacz I, Yadegari A, et al. CAR T cells produced in vivo to treat cardiac injury. Science. 2022;375(6576):91-96. [DOI] [PMC free article] [PubMed] [Google Scholar]