Treatment of patients with acute myeloid leukemia (AML) fit to receive intensive treatment and under 65 years of age consists of one or two cycles of high-dose induction (“3+7”) chemotherapy (C1 or C2) followed by different options of post-remission treatment.1 These options include allogeneic stem cell transplantation (allo-SCT), continuation with chemotherapy or high-dose chemotherapy with autologousSCT (auto-SCT).2 Choosing the right consolidation treatment is a trade-off between anti-leukemic effect and treatment safety. Relapse chances are lowest after allo-SCT, but this treatment modality is also associated with considerable therapy-related morbidity (e.g., graft-versus-host disease), reduced quality of life, and higher procedure-related mortality.3,4 Therefore, for patients with a relatively favorable outcome (i.e., core-binding factor AML), allo-SCT is often avoided as first-line consolidation treatment. In contrast, for patients with an adverse-risk disease (if deemed feasible) this additional anti-leukemic effect is needed, making alloSCT the preferred post-remission treatment option. For patients with intermediate-risk AML, the optimal post-remission therapy is still a subject of debate.5 Measurable residual disease (MRD) assessment, by multiparameter flow cytometry (MFC) and/or by NPM1 gene mutation reverse transcriptase polymerase chain reaction (RT-PCR), has been proposed to guide this decision due to the strong prognostic value and the ability to predict relapse when applied in complete remission (CR) (or CR with incomplete hematologic recovery [CRi], according to the 2017 European LeukemiaNet [ELN] classification) after C2.1,6 Therefore, the presence of MRD at this time point may warrant an allo-SCT as additional intensive therapy.2 In contrast, patients in CRi without MRD before transplant have a relatively low risk of relapse and therefore allo-SCT may be omitted.7 According to the protocol of the HOVON-SAKK132 trial (HO132: registered as NTR4376; available from the Netherlands Trial Register at https://trialsearch.who.int/), the choice of post-remission therapy in intermediate-risk patients was guided by MRD status defined by MFC (>0.1%) and mutant NPM1 (>10-4). Patients with MRD were recommended to receive allo-SCT while patients without MRD were recommended to proceed with less intensive non-allo treatment (auto-SCT or a third cycle of chemotherapy).1 Notably, the previously reported analysis on the HO132 trial showed no difference in relapsefree survival for the ELN-2017 intermediate-risk category, which may suggest the positive effect of MRD guidance.8
In order to better understand the influence of MRD guidance, we present a more detailed analysis of the results of MRD-guided post-remission therapy for intermediate-risk AML or high-risk myelodysplastic syndrome (MDS) patients in relation to treatment outcome in the HO132 trial, including a per protocol analysis. In addition, since the HO132 was guided by MRD status, we compared this MRD-guided cohort to an unguided cohort using a propensity score match (PSM) analysis. This unguided matched control group was derived from previous HOVON-SAKK trials that had no MRD-guided post-remission therapy.
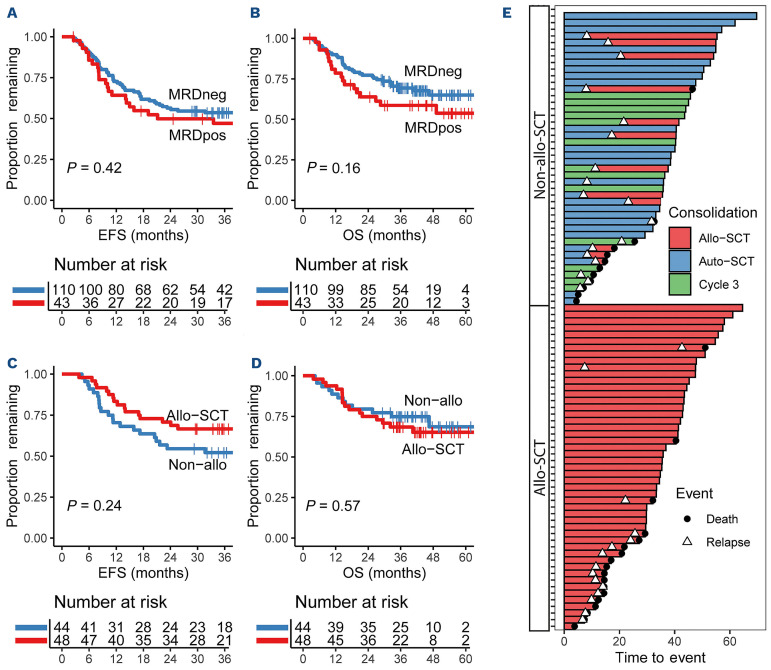
A total of 153 ELN intermediate-risk patients in the HO132 trial (also including patients enrolled in the run-in phase) were in CRi and had a MRD result after C2 as assessed by either leukemia-associated immunophenotype detection with MFC and/or by RT-PCR for mutated NPM1, according to earlier published guidelines.2,9 Of these 153 patients, 110 (72%) were MRD-negative (by both techniques), of which 44/110 (40%) received, as advised per protocol, non-allo-SCT consolidation therapy and 48/110 (44%) patients received an allo-SCT. The other 18 patients (16%) received no consolidation therapy, mainly due to an early relapse. Reasons for deviating from the advised protocol treatment were not systematically collected, but MRD-negative patients who received allo-SCT had significantly more complex karyotype (45.8% vs. 4.5%) and were more often in first CR after C2 instead of C1 (33.3% vs. 4.5%), compared to MRD-negative non-allo consolidated patients. Protocol adherence was better for MRD-positive patients, with 36/43 (84%) receiving the per protocol recommended allo-SCT. Survival differences were analyzed using Kaplan-Meier curves for event-free survival (EFS) and overall survival (OS), with Cox regression accounting for clustering. There was no significant difference in EFS, defined as the time between MRD-assessment after C2 in CRi and relapse or death, between the patients with and without MRD (hazards ratio [HR]: 1.24; 95% CI: 0.75-2.00; P=0.42) (Figure 1A), with an EFS after 36 months of 47% compared to 54%, respectively. OS (defined as the time between MRD-assessment in CRi and death or censoring) (HR: 1.50; 95% CI: 0.85-2.64; P=0.16) (Figure 1B) seems to be slightly worse for MRD-positive patients (5-year OS 54% compared to 65% for MRD-negative), although not statistically significant. Both EFS and OS were in line with recently published HOVON-SAKK trials.8,11 For MRD-negative patients, we also compared the patients who, contrary to trial protocol, received allo-SCT with patients who received non-allo-SCT treatment according to protocol. Between these two groups, there were no apparent significant differences in EFS (HR: 0.69; 95% CI: 0.37-1.29; P=0.24) (Figure 1C) nor in OS (HR: 1.24; 95% CI: 0.59-2.63; P=0.57) (Figure 1D). However, the sequence of events did differ between MRD-negative patients treated with allo-SCT versus non-allo-SCT. A total of 15/48 (31%) of allo-SCT treated patients relapsed within three years after CR, of whom most (93%, 14/15) died within ten months after relapse. Although in the non-all-SCT group 18/44 (41%) relapsed, 12 patients could be successfully salvaged with a (delayed) allo-SCT, which was followed by a long leukemia-free follow-up for 10/18 (55%) patients (Figure 1E). Importantly, for MRD-negative patients, 32 allo-SCT could be averted and 12 postponed without negatively effecting EFS and OS compared to the patients treated with allo-SCT. Therefore, based on these results from the HO132 trial, non-allo-SCT treatment options seem to be justified for intermediate-risk MRD-negative patients.
Figure 1.
Survival of European LeukemiaNet (ELN) intermediate-risk patients in the measurable residual disease (MRD)-guided HO132 trial and subgroup analyses for MRD-negative patients afer two cycles of induction chemotherapy. (A) In the HO132 trial, a total of 153 patients with an MRD result were ELN intermediate-risk, of which 110 (72%) were (MRDneg) after two cycles of chemotherapy. There was no significant difference in event-free survival (EFS) assessed using Cox regression between MRDneg and MRDpos patients (HR: 1.24; 95% CI: 0.75-2.00; P=0.42). (B) Neither was there any significant difference in overall survival (OS) between MRDneg and MRDpos patients (HR: 1.50; 95% CI: 0.85-2.64; P=0.16). (C) A subgroup analysis of the intermediate-risk MRDneg patients in the HO132 trial showed no difference in EFS between 44 patients who received the recommended non-allo (cycle 3 or auto-SCT) consolidation treatment compared to 48 patients who received an allo-SCT (HR: 0.69; 95% CI: 0.37-1.29; P=0.24). (D) The same subgroup also showed no difference in OS between patients treated with non-allo and allo-SCT (HR: 1.24; 95% CI: 0.59-2.63; P=0.57). (E) Swimmer plot of MRDneg patients in the HO132 (MRD-guided) study ordered by first post-remission therapy (y-axis) and OS (x-axis). The non-allo-stem cell transplant (SCT) group consists of 30 patients who received an auto-SCT and 14 patients who received a third cycle of chemotherapy. The majority (67%, 12/18 patients) of the relapsed patients (symbolized by a triangle) who initially received a non-allo consolidation therapy were able to undergo a delayed allo-SCT after successful salvage therapy (red beam). (The HO132 trial is registered as NTR4376; available from the Netherlands Trial Register at https://trialsearch.who.int/)
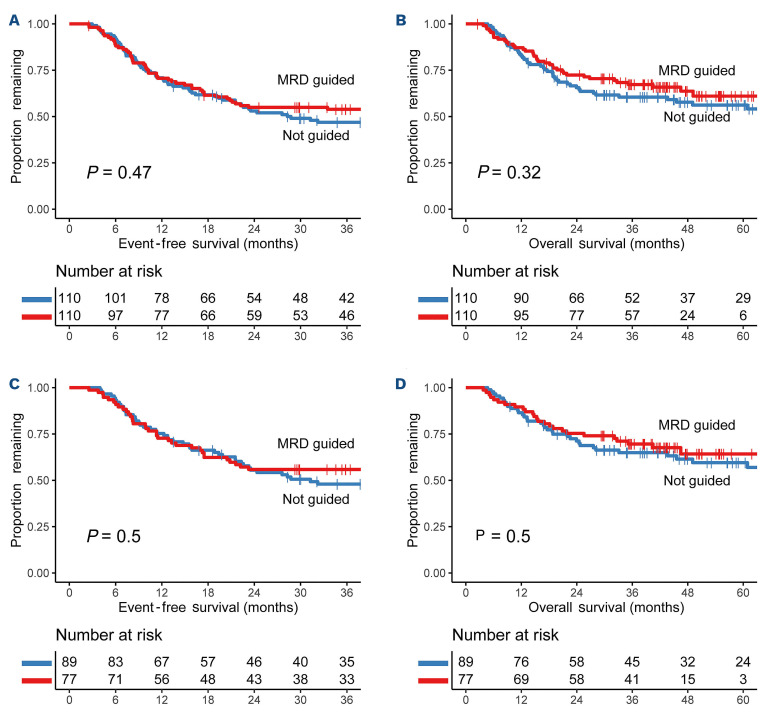
Although benefits of MRD-guided therapy would preferably be evaluated in a randomized controlled trial (RCT), we do not consider this realistic due to the extensive use of MRD in daily practice and the current evidence for the prognostic value of MRD. In addition, no such AML trials are currently reported to be ongoing or planned. Hence, we simulated the analysis by comparing survival of the MRD-guided intermediate-risk patients from the HO132 trial (conducted from 2014 to 2017) to an MRD-unguided cohort from HOVON-SAKK phase II/III trials (HO42A: NTR230; HO81: NTR904; HO92: NTR1446; HO102: NTR2187; all available from the Netherlands Trial Register at https://trialsearch.who.int/), conducted from 2006 to 2013.7,10-12 The principle of measuring MRD and gating strategy remained the same across the studies and followed a strict protocol.9 Via PSM,13 the intermediate-risk patients derived from the unguided studies in CR with MRD measurement after C2 (n=150) were matched to the HO132 MRD-guided patients using six baseline variables that are associated with survival: age, white blood cell count at diagnosis, WHO classification, karyotype, NPM1 status, and FLT3-ITD status (Online Supplementary Figure S1). The MRD-guided and unguided studies included in our analyses were randomized for an investigational treatment, but no significant differences in EFS or OS were observed between the standard and the investigational arms for included patients (Online Supplementary Figure S2); hence, investigational treatment was omitted in the matching. We used the ‘nearest neighbor’ matching technique with a caliper (maximum distance between cases) of 0.25, because this rendered the lowest standardized mean difference of 0.09.14 This resulted in 110 matches with similar patient characteristics. All clinical features between the MRD-guided and matched control group were comparable, except for more karyotype abnormalities in the MRD-guided cohort (Online Supplementary Table S1). EFS after 36 months was comparable between the MRD-guided group (54%) and the historical control cohort (47%) (HR: 0.87; 95% CI: 0.60-1.26; P=0.47) (Figure 2A). In addition, the same comparable results were found for OS with a 61% survival rate for MRD-guided and 56% for the unguided cohort after 60 months (HR: 0.80; 95% CI: 0.52-1.24; P=0.32) (Figure 2B). Between the two cohorts, preferred consolidation treatment had only changed for MRD-negative patients. In former HOVON-SAKK trials, all intermediate-risk patients had been advised to receive an allo-SCT, which changed to only for the MRD-positive patients in the HO132 trial. Therefore, a separate subgroup analysis was carried out for only MRD-negative patients, which showed that there was no significant difference in EFS after three years (HR: 0.86; 95% CI: 0.56-1.33; P=0.50) or OS after five years (HR: 0.84; 95% CI: 0.50-1.40; P=0.50) between unguided and guided MRD-negative patients (Figure 2C, D). These results again suggest that MRD-guidance for consolidation selection in intermediate-risk patients allows allo-SCT treatment to be safely curcumvented without having a negative impact on EFS or OS, which is in accordance with previous data provided by the GIMEMA AML1310 trial.15
Figure 2.
Survival by propensity score match analysis between measurable residual disease (MRD)-unguided and MRD-guided groups, and subgroup analysis of only MRD-negative patients. (A) Event-free survival (EFS) after 36 months was 47% for the MRD-unguided group and 54% for the MRD-guided group (Hazard Ratio [HR]: 0.87; 95% confidence interval [CI]: 0.60-1.26; P=0.47). (B) Overall survival (OS) after 60 months was 56% in the MRD-unguided group and 61% in the MRD-guided group (HR: 0.80; 95% CI: 0.52-1.24; P=0.32). (C) EFS for MRD-negative patients after 36 months was 48% in the unguided group compared to 56% in the MRD-guided group (HR: 0.86; 95% CI: 0.56-1.33; P=0.50). (D) OS after 60 months was 60% in the unguided group, compared to 64% in the MRD-guided group (HR: 0.84; 95% CI: 0.50-1.40; P=0.50).
We wish to add that the PSM method is a valuable alternative for RCT to compare two groups, although it is less preferred because of possible unequal distributions of unknown confounding factors. In addition, due to matching with historical data, the time frame in which patients were treated differed, which may have influenced survival due to changes in patient care, such as supportive care. Here, these effects seem relatively limited since OS did not deviate between patients included from different studies (Online Supplementary Figure S2). Unfortunately, for both cohorts, the exact reasons for choosing a specific consolidation treatment are not known. The conclusions of the retrospective PSM-based comparison with historical non-guided data are based on non-statistical significant data with a broad confidence interval. Nevertheless, these conclusions are substantiated by the results from the in-depth subgroup analysis of the MRD-guided patients in the HO132 trial, which also support the value of MRD-negativity for selecting a less intensive consolidation treatment than allo-SCT for intermediate-risk patients. Future improvements to MRD assays can potentially further increase appropriate MRD-guided post remission therapy.
Supplementary Material
Acknowledgments
We would like to thank the MRD-team of the Amsterdam UMC and Erasmus MC for their help in measuring all samples.
Funding Statement
Funding: This study was supported by the Dutch Cancer Society (n. ALPE-2013-6371).
References
- 1.Dohner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129(4):424-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heuser M, Freeman SD, Ossenkoppele GJ, et al. 2021 update measurable residual disease in acute myeloid leukemia: European LeukemiaNet Working Party Consensus Document. Blood. 2021;138(26):2753-2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Versluis J, Kalin B, Zeijlemaker W, et al. Graft-versus-leukemia effect of allogeneic stem-cell transplantation and minimal residual disease in patients with acute myeloid leukemia in first complete remission. JCO Precis Oncol. 2017;1:1-13. [DOI] [PubMed] [Google Scholar]
- 4.Andersson I, Ahlberg K, Stockelberg D, Persson LO. Patients' perception of health-related quality of life during the first year after autologous and allogeneic stem cell transplantation. Eur J Cancer Care (Engl). 2011;20(3):368-379. [DOI] [PubMed] [Google Scholar]
- 5.Ganzel C, Rowe JM. Revisiting autologous transplantation in acute myeloid leukemia. Curr Opin Hematol. 2018;25(2):95-102. [DOI] [PubMed] [Google Scholar]
- 6.Short NJ, Zhou S, Fu C, et al. Association of measurable residual disease with survival outcomes in patients with acute myeloid leukemia: a systematic review and meta-analysis. JAMA Oncol. 2020;6(12):1890-1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Terwijn M, van Putten WL, Kelder A, et al. High prognostic impact of flow cytometric minimal residual disease detection in acute myeloid leukemia: data from the HOVON/SAKK AML 42A study. J Clin Oncol. 2013;31(31):3889-3897. [DOI] [PubMed] [Google Scholar]
- 8.Löwenberg B, Pabst T, Maertens J, et al. Addition of lenalidomide to intensive treatment in younger and middle-aged adults with newly diagnosed AML: the HOVON-SAKK-132 trial. Blood Adv. 2021;5(4):1110-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cloos J, Harris JR, Janssen JJWM, et al. Comprehensive protocol to sample and process bone marrow for measuring measurable residual disease and leukemic stem cells in acute myeloid leukemia. J Vis Exp. 2018;(133):56386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ossenkoppele GJ, Stussi G, Maertens J, et al. Addition of bevacizumab to chemotherapy in acute myeloid leukemia at older age: a randomized phase 2 trial of the Dutch-Belgian Cooperative Trial Group for Hemato-Oncology (HOVON) and the Swiss Group for Clinical Cancer Research (SAKK). Blood. 2012;120(24):4706-4711. [DOI] [PubMed] [Google Scholar]
- 11.Löwenberg B, Pabst T, Maertens J, et al. Therapeutic value of clofarabine in younger and middle-aged (18-65 years) adults with newly diagnosed AML. Blood. 2017;129(12):1636-1645. [DOI] [PubMed] [Google Scholar]
- 12.Randomized study to assess the added value of laromustine in combination with standard remission-induction chemotherapy in patients aged 18-65 years with previously untreated acute myeloid leukemia (AML) or myelodysplasia (MDS) (RAEB with IPSS >= 1.5). 2013. https://trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2008-000404-92-NL. Accessed January 14 2022. [Google Scholar]
- 13.Cenzer I, Boscardin WJ, Berger K. Performance of matching methods in studies of rare diseases: a simulation study. Intractable Rare Dis Res. 2020;9(2):79-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lunt M. Selecting an appropriate caliper can be essential for achieving good balance with propensity score matching. Am J Epidemiol. 2014;179(2):226-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Venditti A, Piciocchi A, Candoni A, et al. GIMEMA AML1310 trial of risk-adapted, MRD-directed therapy for young adults with newly diagnosed acute myeloid leukemia. Blood. 2019;134(12):935-945. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.