Acute myeloid leukemia (AML) is an aggressive hematological malignancy more common in the elderly. While selected older patients can receive intensive chemotherapy resulting in a 50-60% complete remission (CR) rate, they are generally not eligible for hematopoietic stem cell transplantation (HSCT) and median overall survival (OS) is less than 1 year due to frequent relapse. Therefore, renewed interest in maintenance strategies recently emerged to improve survival among this population. Several maintenance approaches including treatment with histamine dihydrochloride-IL2, gemtuzumab ozogamycin, cytarabine or norethandronolone showed limited impact.1– 3 Hypomethylating agents (azacitidine [AZA] and decitabine) are DNA methyltransferase inhibitors with a favorable efficacy/safety profile in the treatment of myelodysplastic syndromes and AML.4 Recently, AZA maintenance demonstrated survival gain in AML, either using a conventional subcutaneous administration route or a newly available oral formulation (CC-486) in clinical trials.5–7 In this study, we report our single-center experience on the use of subcutaneous AZA maintenance in a real-world cohort of 39 patients with AML in first CR after intensive induction chemotherapy, with a focus on the impact of leukemic cell genotype on survival.
We retrospectively investigated a cohort of AML patients treated with intensive chemotherapy frontline, who received subcutaneous AZA maintenance after CR achievement from 2012 to 2022.
Characteristics of the 39 patients treated from 2012 to 2022 are reported in Table 1. Median age was 72 years (range, 23-78) and 44% had secondary AML. Conventional cytogenetic analysis was available at diagnosis for 36 patients, who were classified according to the national comprehensive cancer network (NCCN) cytogenetic risk categories8 as favorable, intermediate or adverse in 5%, 72% and 15%, respectively. Moreover, most patients were classified as intermediate (41%) or adverse (44%) risk according to the 2017 European Leukemia Network (ELN) guidelines.9 The genomic landscape of leukemic samples at diagnosis is provided (Online Supplementary Figure S1). All patients were in CR after one (87%) or two (13%) cycles of intensive induction chemotherapy with idarubicin (64%) or daunorubicin (26%) combined with cytarabine, and 33% received at least one consolidation cycle before AZA maintenance initiation (Table 1). During AZA maintenance, 14 patients received other treatments including venetoclax (n=5), tyrosine kinase inhibitor (n=4) and other (n=5), and two patients had HSCT after one and two AZA cycles. Patients received a median number of six (range, 1-38) AZA maintenance cycles. Initial AZA dosing was 75 mg/m2/day during 7 days in all patients, and dose-reduction occurred in 36% of patients during follow-up (Online Supplementary Table S1). The most common adverse events during AZA maintenance were thrombocytopenia and/or neutropenia, leading to AZA dose reduction and discontinuation in 23% and 8% of patients, respectively (Online Supplementary Table S1). After a median follow-up of 20.2 (range, 4-102) months, only 10% patients remained on-therapy and the main causes of AZA discontinuation were relapse (36%) and persistence of CR (18%) (Figure 1; Online Supplementary Table S1).
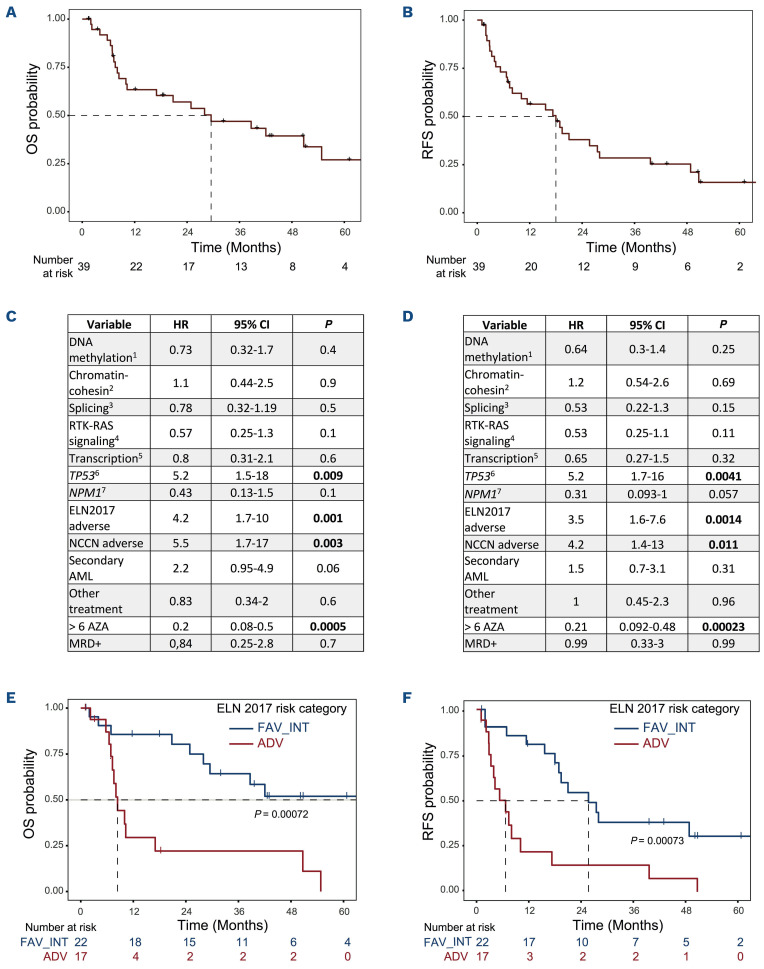
From AZA maintenance onset, median RFS and OS were 18 and 30 months, respectively, for the whole cohort (Figure 2A, B). We performed subgroup analyses and observed using a univariate Cox model that presence of a TP53 alteration (mutation and/or deletion), adverse ELN 2017 or cytogenetic NCCN risk categories, and administration of less than six cycles of AZA maintenance were significantly associated with decreased RFS and OS probabilities (Figure 2C, D). Particularly, ELN 2017 risk classifier, which aggregates several of these variables was highly predictive of survival (Figure 2E, F). Finally, comparison of variant allele frequency at diagnosis and post-AZA relapse suggested patterns of clonal evolution with reappearance of the initial clone in most cases and occasional subclone loss or gain (Online Supplementary Figure S2).
In our single-center retrospective real-world study, we found that subcutaneous AZA maintenance therapy was feasible in selected elderly patients with AML in CR after intensive induction chemotherapy. Moreover, the 2-year survival probabilities were similar to those observed in prospective clinical trials including the HOVON97 (subcutaneous AZA, 2-year RFS 44%) and QUAZAR-AML-001 (CC-486, median OS 24 months) trials.5,7 In contrast to a recent large real-world study on AZA treatment in MDS/AML showing inferior survival in comparison with results from clinical trials,10 our retrospective study confirmed the results of two large prospective phase III trials of AZA maintenance in AML, suggesting that the benefits of this strategy is also found in the routine practice. This could also have reflected an important selection of patients eligible for this strategy both in clinical trials and in routine practice due to the use of intensive chemotherapy induction. Representing a potential bias to this study, 36% patients received concomitant treatment during AZA maintenance including 13% with venetoclax. However, these time-limited associations had no impact on survival in this small cohort (Figure 2C, D).
Table 1.
Patient characteristics.
HOVON97 and QUAZAR-AML-001 trials focused on the comparison of AZA maintenance with observation or placebo, respectively, and did not specifically investigate variables predicting AZA efficacy. Here, we showed that adverse ELN 2017 risk group predicted shorter survival upon AZA maintenance therapy even in a small-sized cohort of patients with AML. While ELN 2017 risk categories are strongly predictive of treatment response in patients with AML undergoing intensive chemotherapy,11 the validation of our finding in larger cohorts could represent an important predictive tool in the context of AZA mainten ance as well. We also identified that patients receiving more than six cycles of AZA maintenance had improved survival, suggesting either that early treatment failure led to AZA discontinuation, or that the anti-leukemic activity of AZA required sufficient exposure to reach significant clinical benefit in patients. In addition, we observed that mutational status correlated with survival in our cohort, especially the presence of TP53 alterations. We also observed that NPM1 mutations were associated with a trend to improved RFS, in agreement with the results of the QUAZAR-AML-001 trial.12 Together these results suggest that cytogenetic and molecular biomarkers, such as aggregated in the ELN 2017 risk stratification represent important predictive tools in the context of AZA maintenance therapy in AML. Recently, the combination of AZA with venetoclax (VEN), a BCL-2 inhibitor demonstrated a high activity in elderly patients with AML.13 This strategy opens new perspectives currently evaluated in clinical trials, including the comparison of single-agent oral AZA maintenance versus oral AZA+VEN maintenance in older patients and the comparison of subcutaneous AZA+VEN in patients under 65 years old versus observation after CR (clinicaltrials gov. Identifiers, respectively: NCT04102020, NCT05404906), and will represent new opportunities to investigate predictive biomarkers as initiated in our current study.
Figure 1.
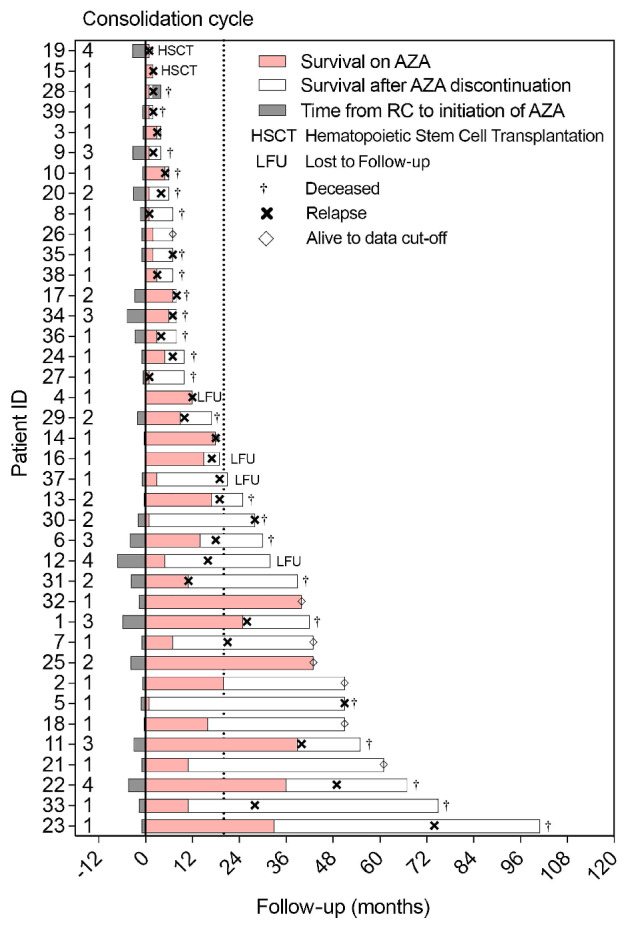
Swimmer plot representing time from CR (complete remission) to initiation of azacitidine (AZA), duration of AZA maintenance (beginning on day 1 of cycle one and ending at day 1 of the last cycle), survival afer AZA discontinuation and time of confirmed relapse. Some patients who were lost to follow-up may have relapsed, and some may have relapsed after the end of follow-up (relapse date unknown).
Figure 2.
Survival analysis of patients with acute myeloid leukemia receiving azacitidine maintenance. (A, B) Survival analyses: from the onset of azacitidine (AZA) maintenance until the next event, defined as death for overall survival (OS) (A), and as death or disease progression for relapse-free survival (RFS) (B), and estimated using the Kaplan-Meier method. Patients were censored at the time of allogenic stem cell transplantation. (C, D) Univariate subgroup analysis of OS (C), and RFS (D). HR: hazard ratio; CI: confidence interval, 1: mutation in IDH1, IDH2, TET2 or DNMT3A; 2: mutation in ASXL1, EZH2, BCORL1, BCOR or STAG2; 3: mutation in SRSF2, SETBP1, SF3B1, ZRZR2 or U2AF1; 4: mutation in FLT3, KRAS, NRAS, PTPN11, CBL, JAK2, CSF3R, MPL or RIT1; 5: mutation in CEBPA, RUNX1, GATA2, ETV6 or WT1; 6: mutation/deletion in TP53; 7: NPM1 mutation; >6 AZA: patients who received more than 6 AZA maintenance cycles; MRD+: positivity of minimal residual disease. (E, F) Survival stratified on ELN 2017 risk category. Kaplan Meyer representations for OS (E) and RFS (F).
To summarize, we showed that subcutaneous AZA maintenance is an effective option in patients with AML achieving CR in a real-world setting. We also observed that the ELN 2017 classifier efficiently predicted survival during AZA maintenance. While the duration of maintenance remains controversial, our data suggest that at least six cycles of AZA should be given to patients with AML achieving post-induction CR. The recent availability of oral AZA will probably result in a switch to a large use of this more convenient formulation, and future studies should investigate the impact of cytogenetic and molecular markers in large cohorts of patients with AML treated with oral AZA.
Supplementary Material
Acknowledgments
To the memory of our dear colleague Eugenie Duroyon, who extensively contributed to this work and many others.
References
- 1.Brune M, Castaigne S, Catalano J, et al. Improved leukemia-free survival after postconsolidation immunotherapy with histamine dihydrochloride and interleukin-2 in acute myeloid leukemia: results of a randomized phase 3 trial. Blood. 2006;108(1):88-96. [DOI] [PubMed] [Google Scholar]
- 2.Löwenberg B, Beck J, Graux C, et al. Gemtuzumab ozogamicin as postremission treatment in AML at 60 years of age or more: results of a multicenter phase 3 study. Blood. 2010;115(13):2586-2591. [DOI] [PubMed] [Google Scholar]
- 3.Pigneux A, Béné MC, Guardiola P, et al. Addition of androgens improves survival in elderly patients with acute myeloid leukemia: A GOELAMS Study. J Clin Oncol. 2017;35(4):387-393. [DOI] [PubMed] [Google Scholar]
- 4.Dombret H, Seymour JF, Butrym A, et al. International phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with >30% blasts. Blood. 2015;126(3):291-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huls G, Chitu DA, Havelange V, et al. Azacitidine maintenance after intensive chemotherapy improves DFS in older AML patients. Blood. 2019;133(13):1457-1464. [DOI] [PubMed] [Google Scholar]
- 6.Burnett A, Russell N, Freeman S, et al. A comparison of limited consolidation chemotherapy therapy or not, and demethylation maintenance or not in older patients with AML and high risk MDS: long term results of the UK Ncri Aml16 Trial. EHA Library. Burnett A. 06/13/15;103225;S513. [Google Scholar]
- 7.Wei AH, Döhner H, Pocock C, et al. Oral azacitidine maintenance therapy for acute myeloid leukemia in first remission. N Engl J Med. 2020;383(26):2526-2537. [DOI] [PubMed] [Google Scholar]
- 8.Pollyea DA, Bixby D, Perl A, et al. NCCN guidelines insights: acute myeloid leukemia, version 2.2021. J Natl Compr Cancer Netw. 2021;19(1):16-27. [DOI] [PubMed] [Google Scholar]
- 9.Döhner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129(4):424-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mozessohn L, Cheung MC, Fallahpour S, et al. Azacitidine in the ‘real-world’: an evaluation of 1101 higher-risk myelodysplastic syndrome/low blast count acute myeloid leukaemia patients in Ontario, Canada. Br J Haematol. 2018;181(6):803-815. [DOI] [PubMed] [Google Scholar]
- 11.Herold T, Rothenberg-Thurley M, Grunwald VV, et al. Validation and refinement of the revised 2017 European LeukemiaNet genetic risk stratification of acute myeloid leukemia. Leukemia. 2020;34(12):3161-3172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Döhner H, Wei AH, Roboz GJ, et al. Prognostic impact of NPM1 and FLT3 mutations at diagnosis and presence of measurable residual disease (MRD) after intensive chemotherapy (IC) for patients with acute myeloid leukemia (AML) in remission: outcomes from the QUAZAR AML-001 trial of oral azacitidine (Oral-AZA) maintenance. Blood. 2021;138(Suppl 1):S804. [Google Scholar]
- 13.DiNardo CD, Jonas BA, Pullarkat V, et al. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med. 2020;383(7):617–629. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.