Abstract
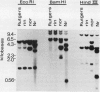
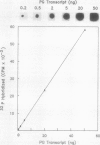
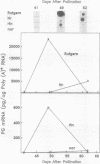
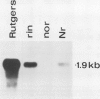
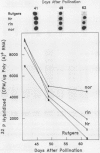
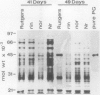
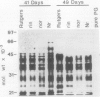
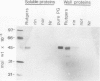
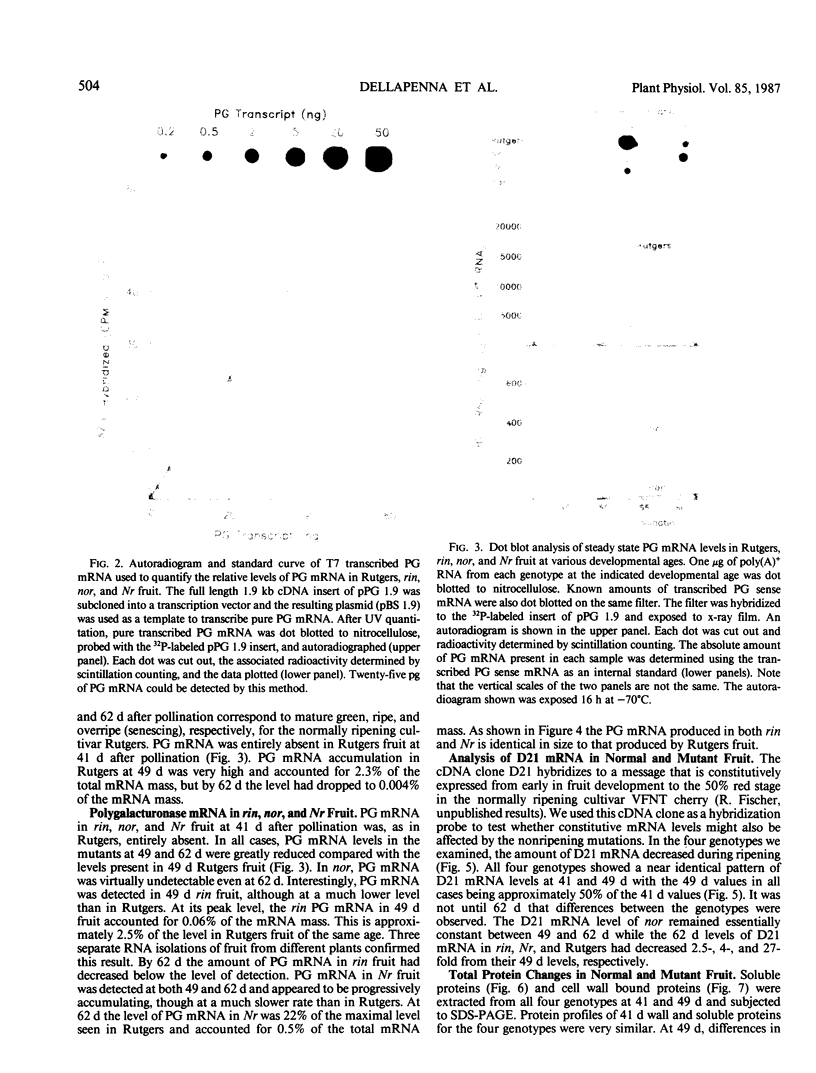
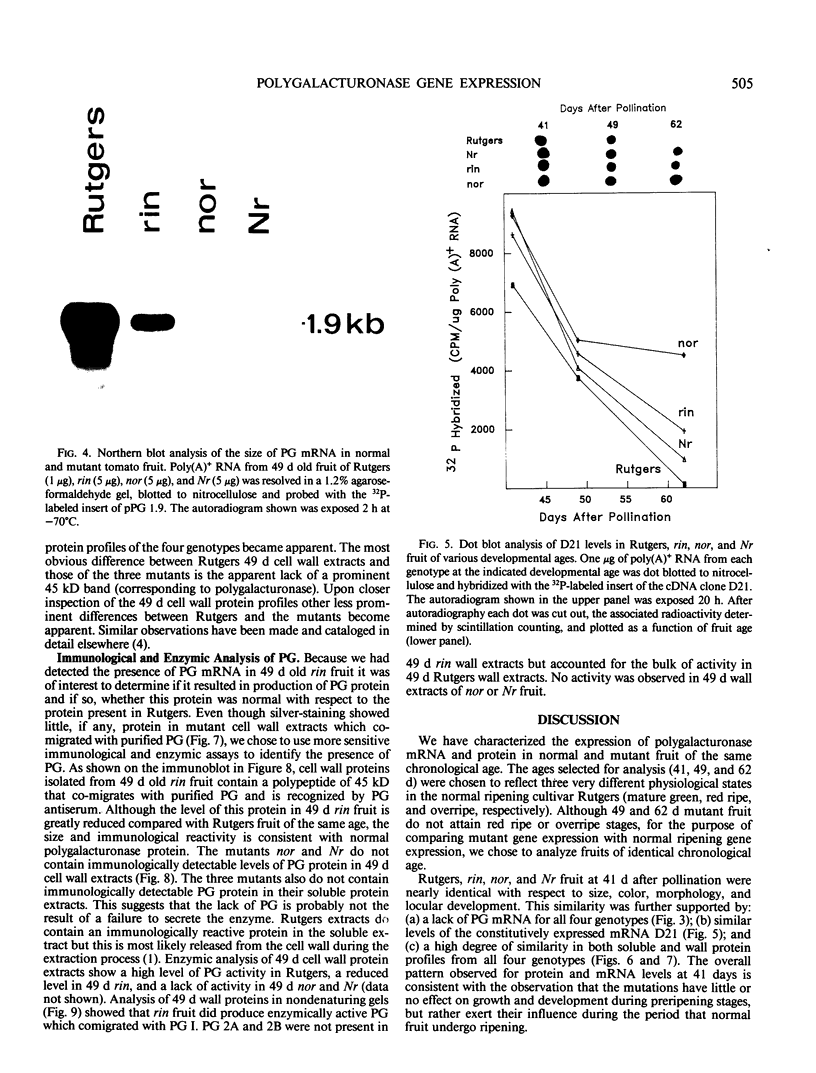
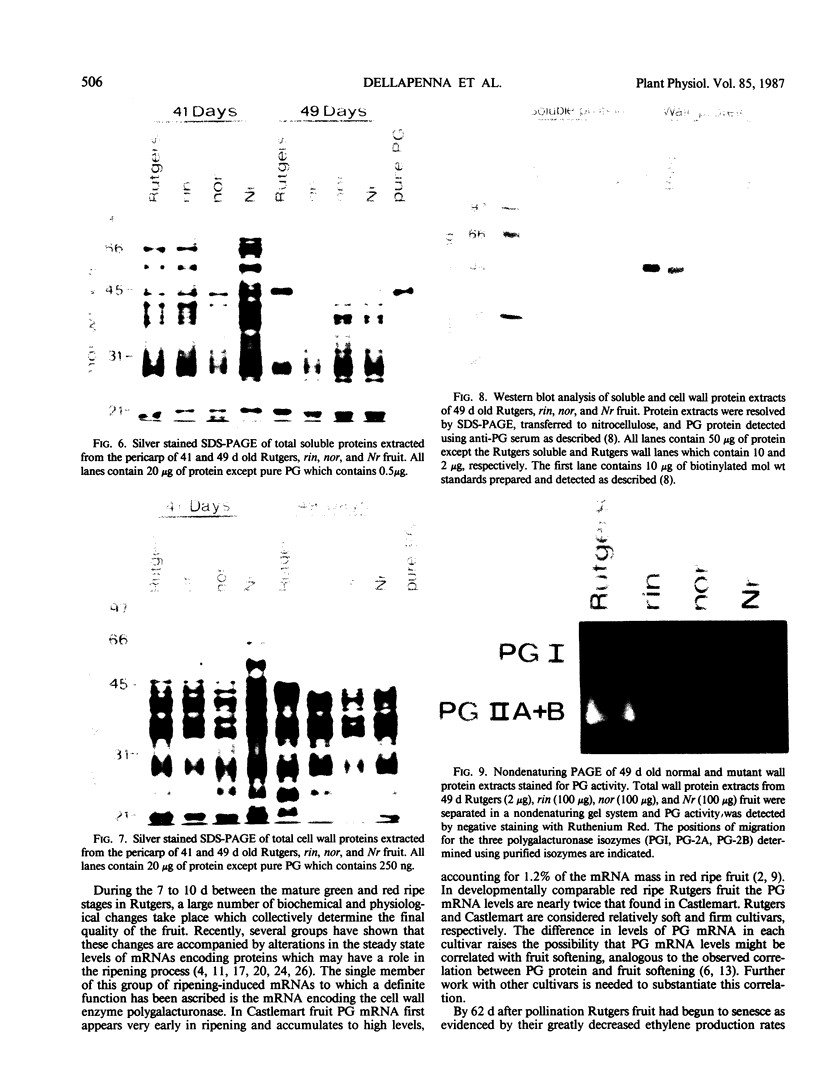
Polygalacturonase (PG) gene expression was studied in normally ripening tomato fruit (Lycopersicon esculentum Mill, cv Rutgers) and in three ripening-impaired mutants, rin, nor, and Nr. Normal and mutant fruit of identical chronological age were analyzed at 41, 49, and 62 days after pollination. These stages corresponded to mature-green, ripe, and overripe, respectively, for Rutgers. The amount of PG mRNA in Rutgers was highest at 49 days and accounted for 2.3% of the total mRNA mass but at 62 days had decreased to 0.004% of the total mRNA mass. In Nr, the amount of PG mRNA steadily increased between 41 and 62 days after pollination, reaching a maximum level of 0.5% of the total mRNA mass. The mutant nor exhibited barely detectable levels of PG mRNA at all stages tested. Surprisingly, PG mRNA, comprising approximately 0.06% of the mRNA mass, was detected in 49 day rin fruit. This mRNA accumulation occurred in the absence of elevated ethylene production by the fruit and resulted in the synthesis of enzymically active PG I. The different patterns of PG mRNA accumulation in the three mutants suggests that distinct molecular mechanisms contribute to reduced PG expression in each ripening-impaired mutant.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biggs M. S., Harriman R. W., Handa A. K. Changes in Gene Expression during Tomato Fruit Ripening. Plant Physiol. 1986 Jun;81(2):395–403. doi: 10.1104/pp.81.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della-Penna D., Christoffersen R. E., Bennett A. B. Biotinylated proteins as molecular weight standards on Western blots. Anal Biochem. 1986 Feb 1;152(2):329–332. doi: 10.1016/0003-2697(86)90417-3. [DOI] [PubMed] [Google Scholar]
- Dellapenna D., Alexander D. C., Bennett A. B. Molecular cloning of tomato fruit polygalacturonase: Analysis of polygalacturonase mRNA levels during ripening. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6420–6424. doi: 10.1073/pnas.83.17.6420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer R. L., Goldberg R. B. Structure and flanking regions of soybean seed protein genes. Cell. 1982 Jun;29(2):651–660. doi: 10.1016/0092-8674(82)90181-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lincoln J. E., Cordes S., Read E., Fischer R. L. Regulation of gene expression by ethylene during Lycopersicon esculentum (tomato) fruit development. Proc Natl Acad Sci U S A. 1987 May;84(9):2793–2797. doi: 10.1073/pnas.84.9.2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisker N., Retig N. Detection of polygalacturonase and pectin lyase isoenzymes in polyacrylamide gels. J Chromatogr. 1974 Sep 11;96(2):245–249. doi: 10.1016/s0021-9673(00)98570-4. [DOI] [PubMed] [Google Scholar]
- REISFELD R. A., LEWIS U. J., WILLIAMS D. E. Disk electrophoresis of basic proteins and peptides on polyacrylamide gels. Nature. 1962 Jul 21;195:281–283. doi: 10.1038/195281a0. [DOI] [PubMed] [Google Scholar]
- Wallner S. J., Bloom H. L. Characteristics of tomato cell wall degradation in vitro: implications for the study of fruit-softening enzymes. Plant Physiol. 1977 Aug;60(2):207–210. doi: 10.1104/pp.60.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallner S. J., Walker J. E. Glycosidases in Cell Wall-degrading Extracts of Ripening Tomato Fruits. Plant Physiol. 1975 Jan;55(1):94–98. doi: 10.1104/pp.55.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]