Abstract
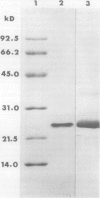
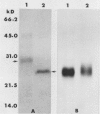
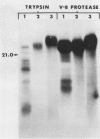
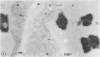
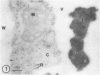
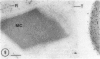
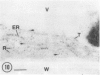
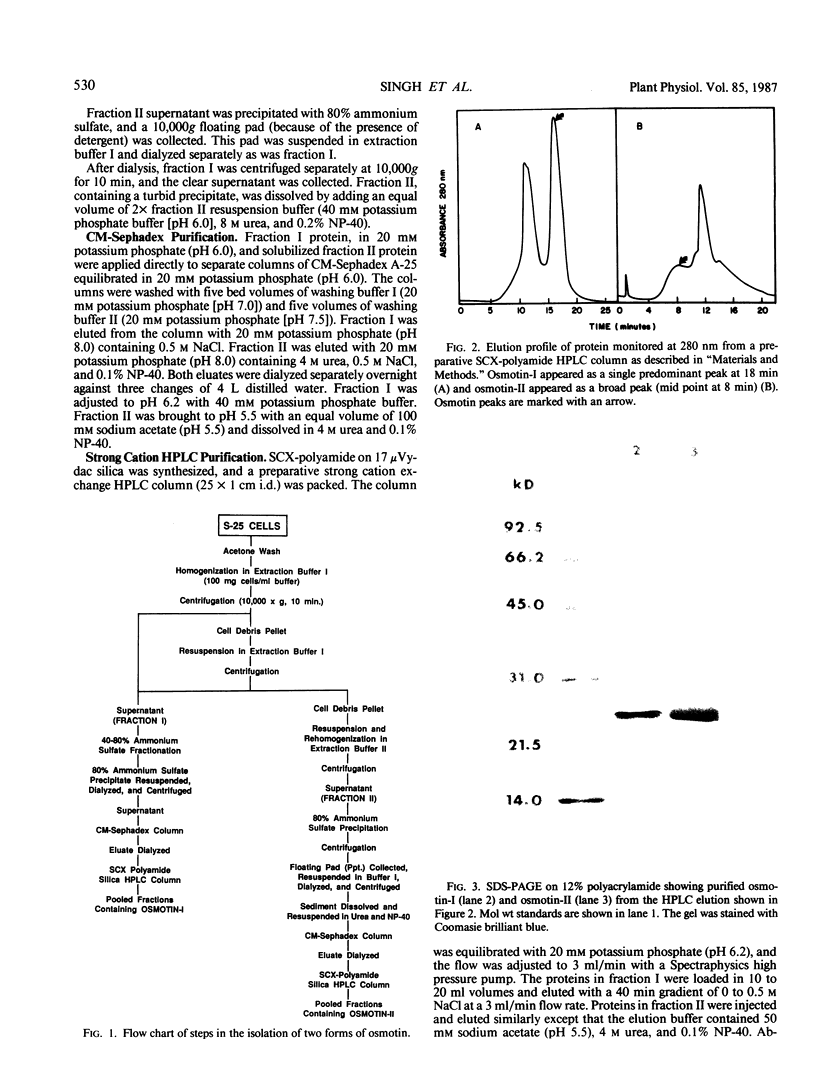
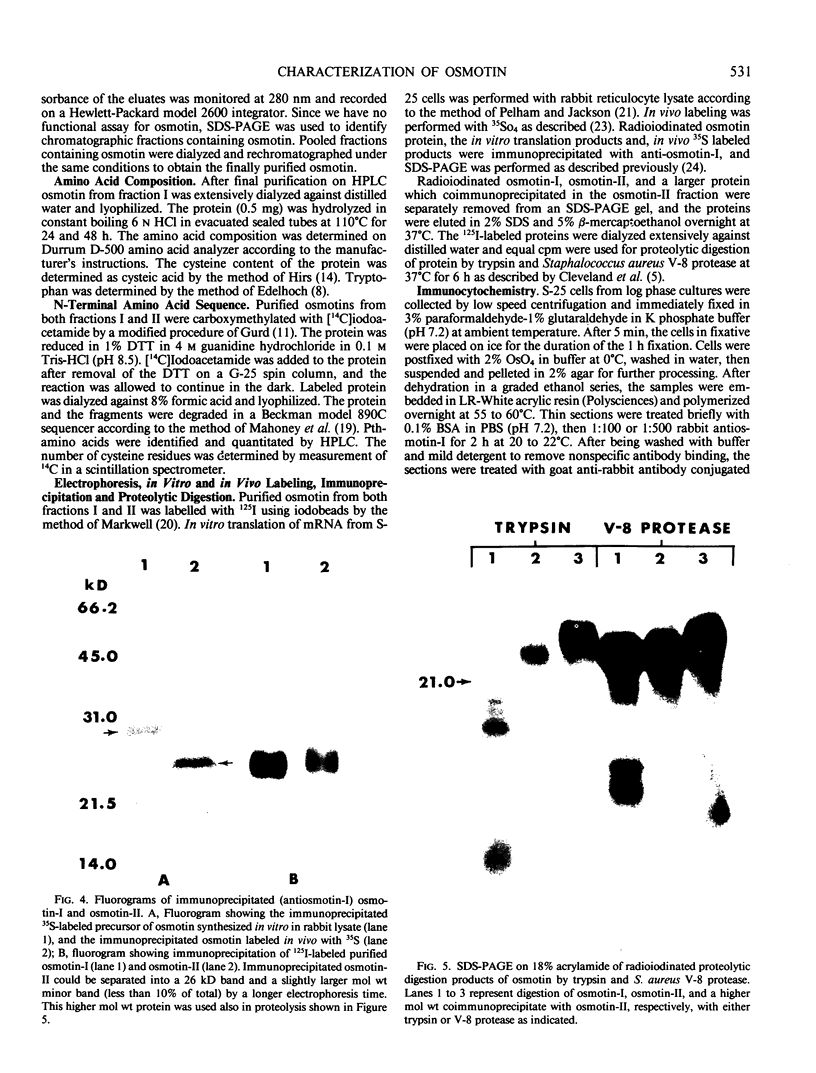
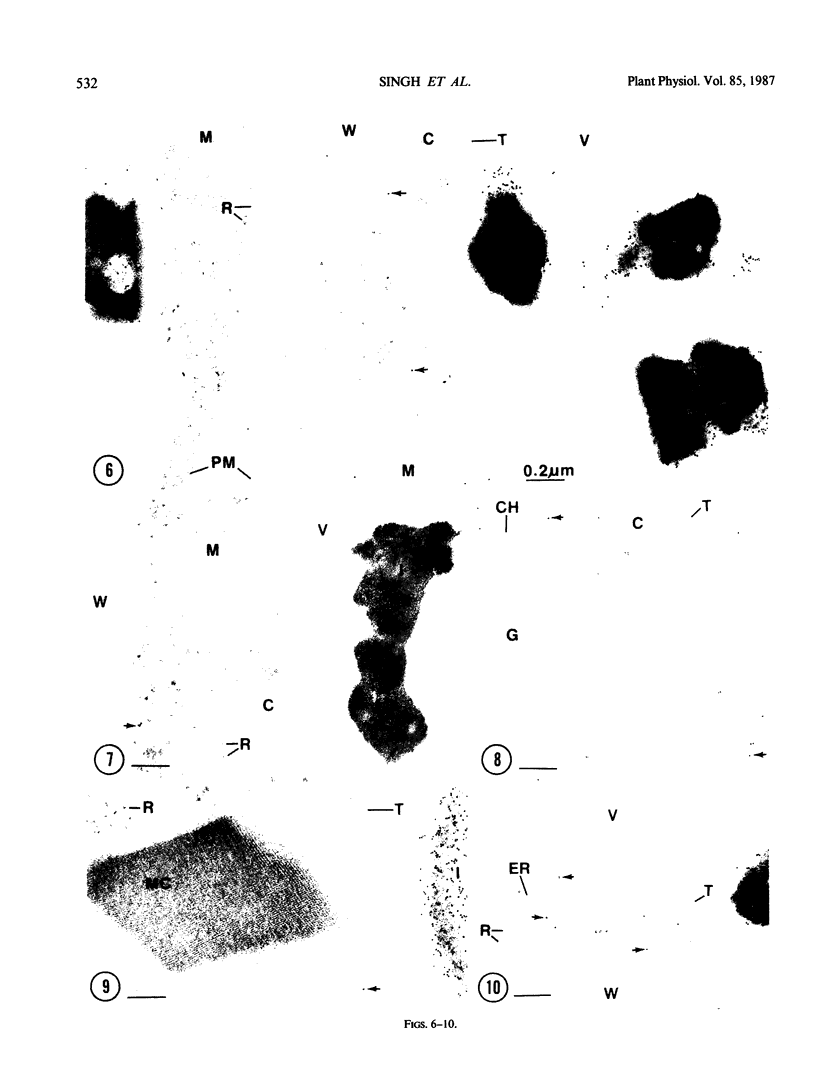
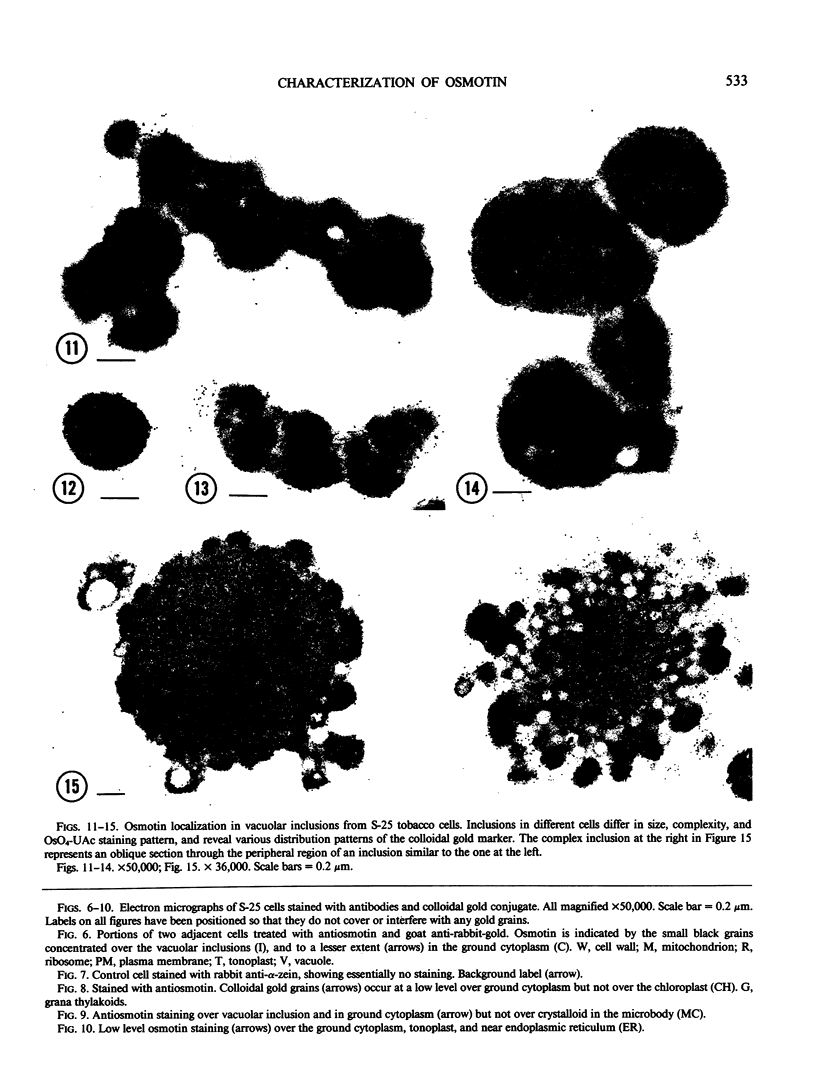
Cultured tobacco (Nicotiana tabacum var Wisconsin 38) cells adapted to grow under osmotic stress synthesize and accumulate a 26 kilodalton protein (osmotin) which can constitute as much as 12% of total cellular protein. In cells adapted to NaCl, osmotin occurs in two forms: an aqueous soluble form (osmotin-I) and a detergent soluble form (osmotin II) in the approximate ratio of 2:3. Osmotin-I has been purified to electrophoretic homogeneity, and osmotin-II has been purified to 90% electrophoretic homogeneity. The N-terminal amino acid sequences of osmotins I and II are identical through position 22. Osmotin-II appears to be much more resistant to proteolysis than osmotin-I. However, it cross-reacts with polyclonal antibodies raised in rabbits against osmotin-I. Osmotin strongly resembles the sweet protein thaumatin in its molecular weight, amino acid composition, N-terminal sequence, and the presence of a signal peptide on the precursor protein. Thaumatin does not cross-react with antiosmotin. An osmotin solution could not be detected as sweet at a concentration at least 100 times that of thaumatin which could be detected as sweet. Immunocytochemical detection of osmotin revealed that osmotin is concentrated in dense inclusion bodies within the vacuole. Although antiosmotin did not label organelles, cell walls, or membranes, osmotin appeared sparsely distributed in the cytoplasm.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Binzel M. L., Hasegawa P. M., Handa A. K., Bressan R. A. Adaptation of Tobacco Cells to NaCl. Plant Physiol. 1985 Sep;79(1):118–125. doi: 10.1104/pp.79.1.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Cornelissen B. J., Hooft van Huijsduijnen R. A., Bol J. F. A tobacco mosaic virus-induced tobacco protein is homologous to the sweet-tasting protein thaumatin. 1986 May 29-Jun 4Nature. 321(6069):531–532. doi: 10.1038/321531a0. [DOI] [PubMed] [Google Scholar]
- Edelhoch H. Spectroscopic determination of tryptophan and tyrosine in proteins. Biochemistry. 1967 Jul;6(7):1948–1954. doi: 10.1021/bi00859a010. [DOI] [PubMed] [Google Scholar]
- Edens L., Heslinga L., Klok R., Ledeboer A. M., Maat J., Toonen M. Y., Visser C., Verrips C. T. Cloning of cDNA encoding the sweet-tasting plant protein thaumatin and its expression in Escherichia coli. Gene. 1982 Apr;18(1):1–12. doi: 10.1016/0378-1119(82)90050-6. [DOI] [PubMed] [Google Scholar]
- Ericson M. C., Alfinito S. H. Proteins Produced during Salt Stress in Tobacco Cell Culture. Plant Physiol. 1984 Mar;74(3):506–509. doi: 10.1104/pp.74.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyengar R. B., Smits P., van der Ouderaa F., van der Wel H., van Brouwershaven J., Ravestein P., Richters G., van Wassenaar P. D. The complete amino-acid sequence of the sweet protein thaumatin I. Eur J Biochem. 1979 May 2;96(1):193–204. doi: 10.1111/j.1432-1033.1979.tb13029.x. [DOI] [PubMed] [Google Scholar]
- Larosa P. C., Handa A. K., Hasegawa P. M., Bressan R. A. Abscisic Acid accelerates adaptation of cultured tobacco cells to salt. Plant Physiol. 1985 Sep;79(1):138–142. doi: 10.1104/pp.79.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Mahoney W. C., Hogg R. W., Hermodson M. A. The amino acid sequence of the D-galactose-binding protein from Escherichia coli B/r. J Biol Chem. 1981 May 10;256(9):4350–4356. [PubMed] [Google Scholar]
- Markwell M. A. A new solid-state reagent to iodinate proteins. I. Conditions for the efficient labeling of antiserum. Anal Biochem. 1982 Sep 15;125(2):427–432. doi: 10.1016/0003-2697(82)90025-2. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Singh N. K., Handa A. K., Hasegawa P. M., Bressan R. A. Proteins Associated with Adaptation of Cultured Tobacco Cells to NaCl. Plant Physiol. 1985 Sep;79(1):126–137. doi: 10.1104/pp.79.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N. K., Larosa P. C., Handa A. K., Hasegawa P. M., Bressan R. A. Hormonal regulation of protein synthesis associated with salt tolerance in plant cells. Proc Natl Acad Sci U S A. 1987 Feb;84(3):739–743. doi: 10.1073/pnas.84.3.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Wel H., Loeve K. Isolation and characterization of thaumatin I and II, the sweet-tasting proteins from Thaumatococcus daniellii Benth. Eur J Biochem. 1972 Dec 4;31(2):221–225. doi: 10.1111/j.1432-1033.1972.tb02522.x. [DOI] [PubMed] [Google Scholar]