Abstract
Background
Ghrelin plays a critical role in regulating energy metabolism and homeostasis. The association between circulating ghrelin levels and gastric cancer has not been systematically analyzed.
Objective
This work explored the association between circulating ghrelin levels and gastric cancer.
Methods
The literature search for relevant articles published until November 2022 was performed using PubMed, Cochrane Library, EMBASE, and Web of Science with the keywords “ghrelin” and “gastric cancer”. Standardized mean differences (SMD) with 95% confidence intervals were used to measure the effectiveness. We assessed pooled data by use of a random-effects model.
Results
Of 5,302 identified studies, nine were included (N=3,196 participants). Circulating ghrelin levels were lower in gastric cancer patients (SMD=-0.255, 95%CI: -0.528 to 0.017, P < 0.00001), but with high heterogeneity (I2 = 88.8%).
Conclusion
The circulating ghrelin levels in patients with gastric cancer were lower than in controls. However, there was heterogeneity among results; therefore, studies with larger sample sizes are recommended.
Keywords: meta-analysis, ghrelin, gastric cancer, oncology, hunger hormone
Introduction
GLOBOCAN 2020 estimated 9,958,133 cancer deaths and 19,292,789 cancer cases globally (1). According to statistics, gastric cancer was the fifth most common cancer, with 1.09 million new cases (5.6%), and the fourth most common cause of cancer death, with 0.76 million deaths (7.7%) globally (1).
Gastric cancer is characterized by complex genetic and environmental interactions contributing to its initiation and progression (2). Risk factors included Helicobacter pylori infection, advanced age, male, smoking, high salt intake, and diets low in fruit and vegetables (3–5). The most common cause of sporadic distal gastric cancer is H. pylori infection (3, 4). In addition, many gastrointestinal hormones are associated with gastric cancer (6–8).
Ghrelin is an endogenous orexigenic peptide hormone of 28 amino acids that binds to the growth hormone secretagogue receptor 1α. Kojima et al. first reported this hormone (9–11). Ghrelin is secreted by the oxyntic glands of the stomach and GHSR is expressed in pituitary gland, hypothalamus, lung, kidney, liver, adipose tissue and endocrine pancreas (9, 12). The acylation of ghrelin is essential for binding and activating its receptor but most (80–90%) circulating ghrelin is non-acylated (12, 13). Ghrelin performs several physiological functions, including orexigenic effect, growth hormone secretion stimulation, insulin secretion inhibition, and anti-inflammatory activity (9, 12, 14–16). Reviews of the role of ghrelin in cancers established associations between ghrelin and tumor progression in many different tumor types (17–20). Other studies found that circulating ghrelin levels in patients with gastric cancer decreased after gastrectomy (21, 22). These findings suggest a strong link between ghrelin and gastric cancer.
We noticed that some clinical studies found patients with gastric cancer have low circulating ghrelin levels (21–23); however, other studies reached the opposite conclusions (24, 25). Therefore, the aim of our study was to explore the association between circulating ghrelin levels and gastric cancer.
Materials and methods
Date source and search strategy
We selected relevant studies published to November 1, 2022 by searching PubMed, Cochrane Library, EMBASE and Web of Science. Medical subject headings included “ghrelin” and “tumor”. We searched the following free terms in pubMed: Neoplasias, Neoplasm, Tumors, Cancer, Malignancy, Neoplasia, Malignancy, Malignant Neoplasms, Cancers, Malignant Neoplasm, Benign Neoplasm, GHRL Protein, Benign Neoplasms, Ghrelin-Obestatin Preprohormone, Ppghrelin, Ghrelin Obestatin Preprohormone, Motilin Related Peptide Precursor, Peptide Precursor, Motilin-Related, Precursor, Motilin-Related Peptide Precursor, Motilin-Related Peptide, Ghrelin Precursor, PpMTLRP, Precursor, Obestatin, Appetite-Regulating Hormone, Ghrelin, Motilin-Related Peptide, Motilin Related Peptide Appetite Regulating Hormone, and Gastric MLTRP.
Study selection and criteria
Eligible studies met the following criteria: (1) subjects were all adults; (2) studied gastric cancer; (3) subjects included both gastric cancer patients and controls; (4) original articles with≥20 subjects; (5) published in English.
The excluded research met the following criteria: (1) studies on animals; (2) studies without controls; (3) studies with substantial statistical errors or unreliable designs; (4) meta-analysis, reviews, comments and letters.
Data extraction and quality assessment
All studies were reviewed by 2 independent reviewers (Wang YX and Zhang CS) and data were extracted in a standardized format. The extracted data were as follows: study information (author, country, published year, number of men, and women study population); and subject characteristics (ghrelin levels, BMI, age, ghrelin type). The ghrelin levels were converted to unified units (pg/mL) as needed.
In the case-control study, we assessed three items using the Newcastle-Ottawa Scale (NOS): A: whether the definitions of gastric cancer were adequate; B: whether the cases were representative; C: whether the control groups were from the same community; D: whether the control subjects had a history of disease; E: Whether the designs or analyses were comparable between cases and controls; F: whether ascertainment of exposure included secure records or structured interviews that were blind to case/control status; G: whether cases and controls were ascertained identically; and H: whether the cases and controls showed identical non-response rates. An asterisk is assigned to each parameter, 0 (lowest) to 8 (highest). Studies with a score ≥7 were considered high quality, and other studies were classified as moderate quality.
Our meta-analysis includes data presented as an abstract in a meeting (26).
Data synthesis and analysis
Comparisons of ghrelin levels between patients with gastric cancer and controls were analyzed using a random effects model, which used mean values and standard deviations. We used standard mean difference and 95% confidence intervals to analyze continuous variables. Cochran’s (chi-square) test to measure heterogeneity and the I2 statistic to determine the extent of consistency: an I2 of over 75% indicates a high level of inconsistency, I2 of above 50% is moderate, and I2 of below 25% is low (27). Differences with p-values less than 0.05 were considered statistically significant. Subgroup analysis was performed according to the ghrelin type and race. Publication bias was assessed using Egger’s regression asymmetry test. Stata MP software (Version 17.0) was used for statistical analysis.
Results
The study inclusion procedure and study characteristics
The detailed steps of study screening are shown in Figure 1 . We extracted 5,302 potential literatures from PubMed, Embase, Cochrane Library, and the Web of Science. After a duplication check, 1,706 studies were removed. After review of titles and abstracts, 3,524 ineligible studies were removed. After review of the full text, 63 studies were removed. A total of 9 studies were included (21–24, 26, 28–31).
Figure 1.
Flow chart of literature search.
Tables 1 , 2 summarize the characteristics of the included studies. Pritchett et al. studied two groups in different regions in China (23). A total of 9 studies involving 10 groups and 3,196 patients were included. The studies were published from 2005 to 2022 and the sample sizes ranged from 27 to 1546. All subjects were adults. Each group was divided into those with gastric cancer and healthy controls. Three studies included subjects from Europe (21, 26, 28), and there were six from Asia (22–24, 29–31). Blood samples were taken under fasting conditions in six of these studies (21, 22, 28–31), and not stated in the remaining three studies (23, 24, 26). Blood samples were obtained from serum in one study (24) while the remaining eight were obtained from plasma. All the studies measured non-acylated ghrelin.
Table 1.
Characteristics of included studies.
| Author. Year | Race | Participants | Number | Blood sample | Gender (Male/Female) |
Age | BMI | Ghrelin |
|---|---|---|---|---|---|---|---|---|
| Changzhen Zhu et al., 2020 | Asian | Gastric cancer | 38 | Serum | 29/9 | Unspecified | Unspecified | 22.4 ± 6.2 |
| Qi Huang-Yue et al., 2007 | Asian | Gastric cancer | 58 | Plasma | 43/15 | 58.9 ± 13.3 | 21.8 ± 3.4 | 110.3 ± 92.1 |
| Natalie R. Pritchett et al., 2020 | Asian | Gastric cancer | 776 | Plasma | 467/309 | 55.3 ± 8 | 21.7 ± 2.2 | 917 ± 355.6 |
| Natalie R. Pritchett et al., 2020 | Asian | Gastric cancer | 249 | Plasma | Unspecified | 58 ± 9 | 24.6 ± 3.3 | 706 ± 259 |
| Hai-Tao Wang et al., 2008 | Asian | Gastric cancer | 42 | Plasma | 19/23 | 49.5 ± 6 | 24.6 ± 3.3 | 464.6 ± 126.5 |
| Anna Zub-Pokrowiecka et al., 2011 | European | Gastric cancer | 25 | Plasma | 14/11 | 49.5 ± 6 | 23.4 ± 2.8 | 191.1 ± 17.8 |
| Krike P. et al., 2018 | European | Gastric cancer | 196 | Plasma | Unspecified | Unspecified | Unspecified | 464.2 ± 268 |
| O Kemik et al., 2011 | European | Gastric cancer | 31 | Plasma | 14/17 | 46.8 ± 11.9 | 16.2 ± 1.5 | 723.7 ± 270.8 |
| Hajime Isomoto et al., 2005 | Asian | Gastric cancer | 23 | Plasma | 11/12 | 60 | 22.1 | 183.2 ± 121.2 |
| Hye-Kyung Jung et al., 2022 | Asian | Gastric cancer | 13 | Plasma | 8/5 | 59.2 ± 10.4 | 24.6 ± 2.8 | 631 ± 97 |
Table 2.
Characteristics of included studies.
| Author. Year | Race | Participants | Number | Blood sample | Gender (Male/Female) |
Age | BMI | Ghrelin |
|---|---|---|---|---|---|---|---|---|
| Changzhen Zhu et al., 2020 | Asian | Controls | 69 | Serum | Unspecified | Unspecified | Unspecified | 19 ± 5.8 |
| Qi Huang-Yue et al., 2007 | Asian | Controls | 24 | Plasma | 18/6 | 56.7 ± 12 | 24.1 ± 3.8 | 91.4 ± 73.6 |
| Natalie R. Pritchett et al., 2020 | Asian | Controls | 770 | Plasma | 255/515 | 51 ± 9 | 22.1 ± 2.6 | 1022 ± 386 |
| Natalie R. Pritchett et al., 2020 | Asian | Controls | 498 | Plasma | Unspecified | 58 ± 9 | 24.8 ± 3.6 | 743 ± 245 |
| Hai-Tao Wang et al., 2008 | Asian | Controls | 20 | Plasma | 8/12 | 40.4 ± 10.2 | 22.2 ± 2.2 | 472 ± 115.9 |
| Anna Zub-Pokrowiecka et al., 2011 | European | Controls | 25 | Plasma | 12/13 | 49.3 ± 11.5 | 23.8 ± 2.7 | 302.7 ± 76.7 |
| Krike P. et al., 2018 | European | Controls | 246 | Plasma | Unspecified | Unspecified | Unspecified | 545.9 ± 275.1 |
| O Kemik et al., 2011 | European | Controls | 40 | Plasma | 22/18 | 40.4 ± 11.3 | 21.5 ± 2.0 | 1104.3 ± 201.3 |
| Hajime Isomoto et al., 2005 | Asian | Controls | 39 | Plasma | 16/23 | 51 | 22.6 | 174.8 ± 125.1 |
| Hye-Kyung Jung et al., 2022 | Asian | Controls | 14 | Plasma | 7/7 | 49.6 ± 5.8 | 22.5 ± 2.3 | 555.8 ± 98.4 |
Overall analysis
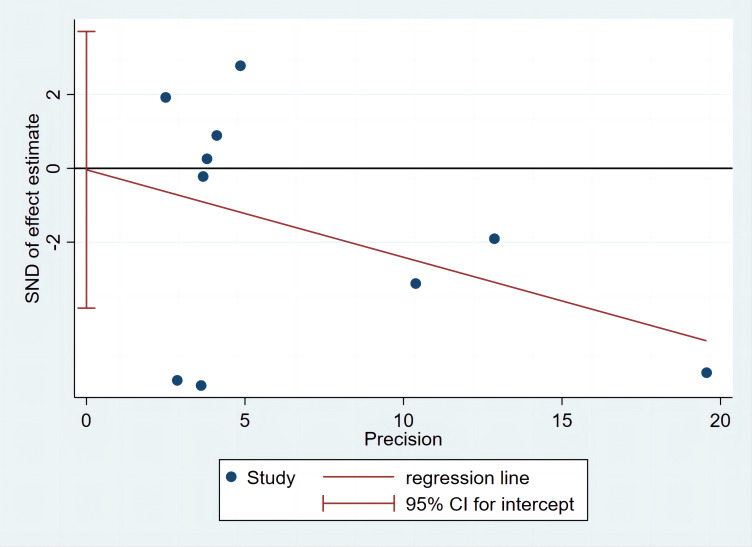
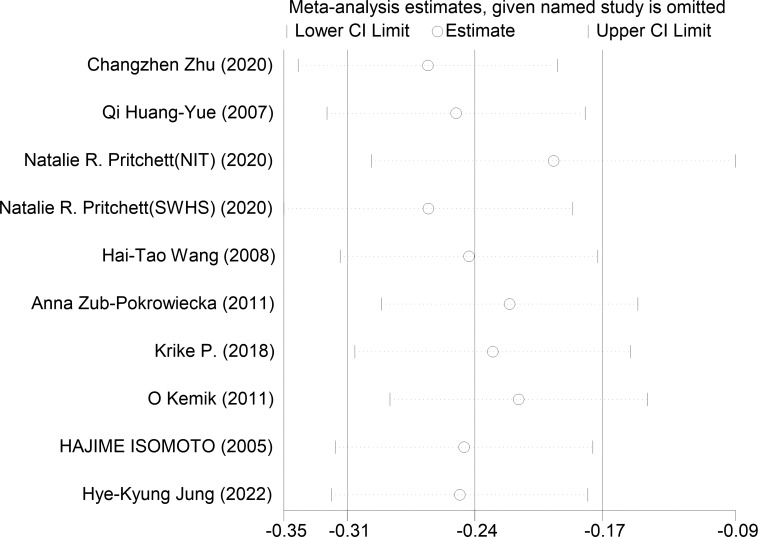
Ghrelin levels in patients were lower than in the control groups ( Figure 2 ). (SMD=-0.255, 95%CI: -0.528 to 0.017). However, standard mean differences showed significant heterogeneity when analyzed using the random-effects model (I2 = 88.8%, P < 0.00001). Publication bias was insignificant ( Figure 3 ; Egger’s test: P= 0.981). Sensitivity analysis demonstrated the stability of our meta-analysis ( Figure 4 ).
Figure 2.
Forest plot showing the effect size of association between circulating ghrelin and gastric cancer. CI, Confidence interval. Summary estimates were analyzed using a random-effects model.
Figure 3.
Egger’s publication bias plot.
Figure 4.
Impact analysis of a single study.
Subgroup analysis
Subgroup analysis was performed to determine the factors affecting heterogeneity. Subgroup analyses classified by blood sample (serum or plasma) or race (Asian or European) showed no significant reductions or differences in heterogeneity ( Table 3 ). This finding suggests that more information from different regions is needed for analysis.
Table 3.
Subgroup analysis.
| Groups | Participants | Random effects (95% Cl) | I2 (%) | |
|---|---|---|---|---|
| Overall | 10 | 3,196 | -0.255 (-0.528, 0.017) | 88.8 |
| Blood sample | ||||
| Plasma | 9 | 3089 | -0.349 (-0.618, -0.079) | 87.6 |
| Serum | 1 | 107 | 0.572 (0.169, 0.975) | – |
| Race | ||||
| Asian | 7 | 2633 | 0.062 (-0.175, 0.300) | 77.7 |
| European | 3 | 563 | -1.279 (-2.448, -0.110) | 94.9 |
CI, Confidence interval.
Discussion
Gastric cancer is a significant cause of cancer death worldwide, with a high mortality rate. Most gastric cancer is diagnosed at an advanced stage, with poor prognosis and limited treatment options (32). Gastric cancer outcomes are significantly related to the American Joint Committee on Cancer stage. The estimated adjusted 5-year survival rate after radical surgery was 41.3%, 82.9% for stage IA and stage IB, 62.8% for stage II, 17.8% for stage IIIA and stage IIIB, and 3.3% for stage IV (33). Therefore, early diagnosis is essential.
To our knowledge, the present study is the first meta-analysis to explore the association between circulating ghrelin levels and gastric cancer. Ghrelin levels were lower in gastric cancer patients than in controls. Our findings are consistent with those of Pritchett et al. (23). Ghrelin may be used as an early marker for gastric cancer screening after stratification of metabolic status including BMI and blood glucose of residents in high incidence areas of gastric cancer.
We performed subgroup analyses according to blood sample and race to examine sources of heterogeneity. There were no significant differences or reductions in heterogeneity. It should be noted that included studies were all from Europe or Asia. We attempted to obtain studies from other continents because of the limited number of studies but failed. Many other factors may influence circulating ghrelin levels.
(1) Ghrelin levels are inversely associated with BMI. Ghrelin levels are reduced in obese patients, suggesting a physiological adaptation to the positive energy balance associated with obesity (34, 35). Few studies in our meta-analysis adjusted for the association between circulating ghrelin levels and BMI, which varied among participants and influenced ghrelin levels. (2) Cancer-related cachexia causes weight loss (mainly from loss of skeletal muscle and body fat) and inflammation (36, 37). However, some studies in our meta-analysis did not separate gastric cancer patients due to cachexia (38, 39). (3) Gastric cancer patients follow different disease progressions, and few studies analyzed patients according to American Joint Committee on Cancer stage. (4) H. pylori is the main pathogen causing chronic active gastritis and plays a crucial role in gastric and duodenal ulcers and gastric cancer (40). H. pylori infection inhibits the expression of ghrelin active cells in the gastric mucosa, thereby reducing the level of ghrelin in the circulation. After eradication of H. pylori infection, ghrelin levels return to pre-infection levels (41–43). Similarly, few studies separated gastric cancer patients according to the presence or absence of H. pylori infection. (5) There are few related such studies, and ghrelin measurement methods are not standardized; furthermore, the normal range of ghrelin has not been determined. In many studies published in recent years, researchers have used methods to inhibit proteases followed by acidification of samples and obtained relatively accurate results (44, 45). Unfortunately, not all studies have used such preservation techniques. As in the table, experimental data measured by different experimenters differed significantly.
The surgical options for non-early operable gastric cancer are subtotal or total gastrectomy (46). Patients undergoing gastrectomy often suffer from weight loss, which reduces their quality of life, increasing the risk of contracting other diseases and affecting long-term survival. Several studies showed that ghrelin levels decreased significantly after gastrectomy but recovered over time. The concentrations of ghrelin decreased to 12–29% of the preoperative levels in total gastrectomy patients and 39–71% of the preoperative levels within three days of the gastrectomy (14).
Ghrelin is the only hormone known to promote appetite; researchers noted a possible role for ghrelin in cancer-associated cachexia (47). Two studies, one in patients after gastrectomy and one in patients with advanced cancer, demonstrated the beneficial effect of a large dose of exogenous ghrelin injection on increasing energy intake (43, 47). However, GHSR is known to express in many cancer cell types and may be upregulated in some cancers, including breast and colon cancer (48–50). Therefore, more studies are needed to demonstrate the safety of exogenous ghrelin injection.
Gastric cancer patients have different degrees of gastric atrophy, which is often accompanied by decreased ghrelin secretion (51). Therefore, the reduction of ghrelin may be a defense mechanism to limit the progression of gastric cancer. One limitation of this analysis is the small sample sizes of some studies, large-scale studies are needed to improve the accuracy of this meta-analysis. Many studies did not classify patients according to whether they had H. pylori infection or cachexia. Therefore, we could not make more accurate subgroup analyses based on H. pylori infection and cachexia. In addition, the literature search was performed using PubMed, Cochrane Library, EMBASE, and the Web of Science; Thus, language limitations may have increased the risk of publication bias.
In summary, we found that the circulating ghrelin levels were lower in patients with gastric cancer than in controls. Although the pooled results had high heterogeneity, the findings in trials with larger sample sizes agreed with our conclusion (23, 26). When ghrelin is used as an early marker for screening a large population in regions and countries with high incidence of gastric cancer, attention should be paid to factors affecting ghrelin levels, including the relationship between ghrelin and BMI and whether patients have H. pylori infection.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
YuxW: Data curation, Formal analysis, Investigation, Methodology, Software, Writing- original draft, Writing- review & editing. CZ: Data curation, Investigation, Methodology, Software, Supervision, Writing- review & editing, Writing- original draft. QZ: Data curation, Investigation, Methodology, Software, Writing- review & editing. JY: Data curation, Methodology, Software, Writing- review & editing. YukW: Data curation, Software, Writing- review & editing. YX: Data curation, Supervision, Writing- review & editing. JD: Formal analysis, Funding acquisition, Methodology, Resources, Supervision, Writing- review & editing.
Funding Statement
This work was supported by grants (to JD) from the Science Foundation of Shandong Province of China (No.ZR201807070189) and the National Natural Science Foundation of China (No.31872791).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2. McLean MH, El-Omar EM. Genetics of gastric cancer. Nat Rev Gastroenterol hepatology. (2014) 11(11):664–74. doi: 10.1038/nrgastro.2014.143 [DOI] [PubMed] [Google Scholar]
- 3. Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet (London England) (2020) 396(10251):635–48. doi: 10.1016/S0140-6736(20)31288-5 [DOI] [PubMed] [Google Scholar]
- 4. Van Cutsem E, Sagaert X, Topal B, Haustermans K, Prenen H. Gastric cancer. Lancet (London England) (2016) 388(10060):2654–64. doi: 10.1016/S0140-6736(16)30354-3 [DOI] [PubMed] [Google Scholar]
- 5. Machlowska J, Baj J, Sitarz M, Maciejewski R, Sitarz R. Gastric cancer: epidemiology, risk factors, classification, genomic characteristics and treatment strategies. Int J Mol Sci (2020) 21(11):4012. doi: 10.3390/ijms21114012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lamers CB, Jansen JB, Woutersen RA. Cholecystokinin and gastrointestinal cancer. J Steroid Biochem Mol Biol (1990) 37(6):1069–72. doi: 10.1016/0960-0760(90)90467-y [DOI] [PubMed] [Google Scholar]
- 7. Morris DL, Watson SA, Durrant LG, Harrison JD. Hormonal control of gastric and colorectal cancer in man. Gut (1989) 30(4):425–9. doi: 10.1136/gut.30.4.425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chopin L, Walpole C, Seim I, Cunningham P, Murray R, Whiteside E, et al. Ghrelin and cancer. Mol Cell Endocrinol. (2011) 340(1):65–9. doi: 10.1016/j.mce.2011.04.013 [DOI] [PubMed] [Google Scholar]
- 9. Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature (1999) 402(6762):656–60. doi: 10.1038/45230 [DOI] [PubMed] [Google Scholar]
- 10. Kojima M, Kangawa K. Ghrelin: structure and function. Physiol Rev (2005) 85(2):495–522. doi: 10.1152/physrev.00012.2004 [DOI] [PubMed] [Google Scholar]
- 11. Date Y, Kojima M, Hosoda H, Sawaguchi A, Mondal MS, Suganuma T, et al. Ghrelin, a novel growth hormone-releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology (2000) 141(11):4255–61. doi: 10.1210/endo.141.11.7757 [DOI] [PubMed] [Google Scholar]
- 12. van der Lely AJ, Tschöp M, Heiman ML, Ghigo E. Biological, physiological, pathophysiological, and pharmacological aspects of ghrelin. Endocrine Rev (2004) 25(3):426–57. doi: 10.1210/er.2002-0029 [DOI] [PubMed] [Google Scholar]
- 13. Pacifico L, Poggiogalle E, Costantino F, Anania C, Ferraro F, Chiarelli F, et al. Acylated and nonacylated ghrelin levels and their associations with insulin resistance in obese and normal weight children with metabolic syndrome. Eur J Endocrinol. (2009) 161(6):861–70. doi: 10.1530/EJE-09-0375 [DOI] [PubMed] [Google Scholar]
- 14. Takiguchi S, Takata A, Murakami K, Miyazaki Y, Yanagimoto Y, Kurokawa Y, et al. Clinical application of ghrelin administration for gastric cancer patients undergoing gastrectomy. Gastric Cancer Off J Int Gastric Cancer Assoc Japanese Gastric Cancer Assoc (2014) 17(2):200–5. doi: 10.1007/s10120-013-0300-8 [DOI] [PubMed] [Google Scholar]
- 15. Shintani M, Ogawa Y, Ebihara K, Aizawa-Abe M, Miyanaga F, Takaya K, et al. Ghrelin, an endogenous growth hormone secretagogue, is a novel orexigenic peptide that antagonizes leptin action through the activation of hypothalamic neuropeptide Y/Y1 receptor pathway. Diabetes (2001) 50(2):227–32. doi: 10.2337/diabetes.50.2.227 [DOI] [PubMed] [Google Scholar]
- 16. Li WG, Gavrila D, Liu X, Wang L, Gunnlaugsson S, Stoll LL, et al. Ghrelin inhibits proinflammatory responses and nuclear factor-kappaB activation in human endothelial cells. Circulation (2004) 109(18):2221–6. doi: 10.1161/01.CIR.0000127956.43874.F2 [DOI] [PubMed] [Google Scholar]
- 17. Kotta AS, Kelling AS, Corleto KA, Sun Y, Giles ED. Ghrelin and cancer: examining the roles of the ghrelin axis in tumor growth and progression. Biomolecules (2022) 12(4):483. doi: 10.3390/biom12040483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Soleyman-Jahi S, Sadeghi F, Pastaki Khoshbin A, Khani L, Roosta V, Zendehdel K. Attribution of ghrelin to cancer; attempts to unravel an apparent controversy. Front Oncol (2019) 9:1014. doi: 10.3389/fonc.2019.01014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Au CC, Furness JB, Brown KA. Ghrelin and breast cancer: emerging roles in obesity, estrogen regulation, and cancer. Front Oncol (2016) 6:265. doi: 10.3389/fonc.2016.00265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ginter G, Ceranowicz P, Warzecha Z. Protective and healing effects of ghrelin and risk of cancer in the digestive system. Int J Mol Sci (2021) 22(19):10571. doi: 10.3390/ijms221910571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zub-Pokrowiecka A, Rembiasz K, Konturek PC, Budzyński A, Konturek SJ, Winiarski M, et al. Ghrelin and gastrin in advanced gastric cancer before and after gastrectomy. World J Gastroenterol. (2011) 17(4):449–58. doi: 10.3748/wjg.v17.i4.449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang HT, Lu QC, Wang Q, Wang RC, Zhang Y, Chen HL, et al. Role of the duodenum in regulation of plasma ghrelin levels and body mass index after subtotal gastrectomy. World J Gastroenterol. (2008) 14(15):2425–9. doi: 10.3748/wjg.14.2425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pritchett NR, Maziarz M, Shu XO, Kamangar F, Dawsey SM, Fan JH, et al. Serum ghrelin and esophageal and gastric cancer in two cohorts in China. Int J Cancer (2020) 146(10):2728–35. doi: 10.1002/ijc.32597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhu C, Liu Y, Kang W, Zhang Z, Zeng Z, Liu D. Exploration of the role of serum ghrelin in the diagnosis and treatment of digestive tract Malignancies. J Int Med Res (2020) 48(5):300060520920441. doi: 10.1177/0300060520920441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Isomoto H, Ueno H, Saenko VA, Mondal MS, Nishi Y, Kawano N, et al. Impact of Helicobacter pylori infection on gastric and plasma ghrelin dynamics in humans. Am J Gastroenterol. (2005) 100(8):1711–20. doi: 10.1111/j.1572-0241.2005.41492.x [DOI] [PubMed] [Google Scholar]
- 26. Krike P, Rudzite D, Polaka I, Kojalo I, Isajevs S, Leja M. Decrease of trefoil factor 3 and ghrelin in gastric cancer patients. Helicobacter (2018) 23:e12525. doi: 10.1111/hel.12525 [DOI] [Google Scholar]
- 27. Bowden J, Tierney JF, Copas AJ, Burdett S. Quantifying, displaying and accounting for heterogeneity in the meta-analysis of RCTs using standard and generalised Q statistics. BMC Med Res Methodol. (2011) 11:41. doi: 10.1186/1471-2288-11-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kemik O, Kemik AS, Begenik H, Erdur FM, Emre H, Sumer A, et al. The relationship among acute-phase responce proteins, cytokines, and hormones in various gastrointestinal cancer types patients with cachectic. Hum Exp Toxicol. (2012) 31(2):117–25. doi: 10.1177/0960327111417271 [DOI] [PubMed] [Google Scholar]
- 29. Isomoto H, Ueno H, Nishi Y, Yasutake T, Tanaka K, Kawano N, et al. Circulating ghrelin levels in patients with various upper gastrointestinal diseases. Digestive Dis Sci (2005) 50(5):833–8. doi: 10.1007/s10620-005-2648-z [DOI] [PubMed] [Google Scholar]
- 30. Huang Q, Fan YZ, Ge BJ, Zhu Q, Tu ZY. Circulating ghrelin in patients with gastric or colorectal cancer. Digestive Dis Sci (2007) 52(3):803–9. doi: 10.1007/s10620-006-9508-3 [DOI] [PubMed] [Google Scholar]
- 31. Jung HK, Tae CH, Lee HA, Lee KE, Moon CM, Kim SE, et al. Association between gut regulatory hormones and post-operative weight loss following gastrectomy in patients with gastric cancer. J Neurogastroenterol Motil. (2022) 28(3):409–17. doi: 10.5056/jnm21145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Necula L, Matei L, Dragu D, Neagu AI, Mambet C, Nedeianu S, et al. Recent advances in gastric cancer early diagnosis. World J Gastroenterol. (2019) 25(17):2029–44. doi: 10.3748/wjg.v25.i17.2029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Spataro V, Genoni M, Maurer C, Müller W. [Stomach cancer: 10 years experiences with surgical treatment and possibilities for improving the prognosis]. Helv Chirurgica Acta (1993) 59(4):589–95. [PubMed] [Google Scholar]
- 34. Park HS, Lee KU, Kim YS, Park CY. Relationships between fasting plasma ghrelin levels and metabolic parameters in children and adolescents. Metabolism: Clin experimental. (2005) 54(7):925–9. doi: 10.1016/j.metabol.2005.02.007 [DOI] [PubMed] [Google Scholar]
- 35. Tschöp M, Weyer C, Tataranni PA, Devanarayan V, Ravussin E, Heiman ML. Circulating ghrelin levels are decreased in human obesity. Diabetes (2001) 50(4):707–9. doi: 10.2337/diabetes.50.4.707 [DOI] [PubMed] [Google Scholar]
- 36. Baracos VE, Martin L, Korc M, Guttridge DC, Fearon KCH. Cancer-associated cachexia. Nat Rev Dis Primers (2018) 4:17105. doi: 10.1038/nrdp.2017.105 [DOI] [PubMed] [Google Scholar]
- 37. Argilés JM, Busquets S, Stemmler B, López-Soriano FJ. Cancer cachexia: understanding the molecular basis. Nat Rev Cancer (2014) 14(11):754–62. doi: 10.1038/nrc3829 [DOI] [PubMed] [Google Scholar]
- 38. Akamizu T, Kangawa K. Ghrelin for cachexia. J Cachexia Sarcopenia Muscle (2010) 1(2):169–76. doi: 10.1007/s13539-010-0011-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Garcia JM, Garcia-Touza M, Hijazi RA, Taffet G, Epner D, Mann D, et al. Active ghrelin levels and active to total ghrelin ratio in cancer-induced cachexia. J Clin Endocrinol Metab (2005) 90(5):2920–6. doi: 10.1210/jc.2004-1788 [DOI] [PubMed] [Google Scholar]
- 40. Marshall BJ, McGechie DB, Francis GJ, Utley PJ. Pyloric campylobacter serology. Lancet (London England) (1984) 2(8397):281. doi: 10.1016/S0140-6736(84)90318-0 [DOI] [PubMed] [Google Scholar]
- 41. Tatsuguchi A, Miyake K, Gudis K, Futagami S, Tsukui T, Wada K, et al. Effect of Helicobacter pylori infection on ghrelin expression in human gastric mucosa. Am J Gastroenterol (2004) 99(11):2121–7. doi: 10.1111/j.1572-0241.2004.30291.x [DOI] [PubMed] [Google Scholar]
- 42. Isomoto H, Ueno H, Nishi Y, Wen CY, Nakazato M, Kohno S. Impact of Helicobacter pylori infection on ghrelin and various neuroendocrine hormones in plasma. World J Gastroenterol (2005) 11(11):1644–8. doi: 10.3748/wjg.v11.i11.1644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Adachi S, Takiguchi S, Okada K, Yamamoto K, Yamasaki M, Miyata H, et al. Effects of ghrelin administration after total gastrectomy: a prospective, randomized, placebo-controlled phase II study. Gastroenterology (2010) 138(4):1312–20. doi: 10.1053/j.gastro.2009.12.058 [DOI] [PubMed] [Google Scholar]
- 44. Lund LH, Hage C, Pironti G, Thorvaldsen T, Ljung-Faxén U, Zabarovskaja S, et al. Acyl ghrelin improves cardiac function in heart failure and increases fractional shortening in cardiomyocytes without calcium mobilization. Eur Heart J (2023) 44(22):2009–25. doi: 10.1093/eurheartj/ehad100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kleftaki SA, Simati S, Amerikanou C, Gioxari A, Tzavara C, Zervakis GI, et al. Pleurotus eryngii improves postprandial glycaemia, hunger and fullness perception, and enhances ghrelin suppression in people with metabolically unhealthy obesity. Pharmacol Res (2022) 175:105979. doi: 10.1016/j.phrs.2021.105979 [DOI] [PubMed] [Google Scholar]
- 46. Joshi SS, Badgwell BD. Current treatment and recent progress in gastric cancer. CA: Cancer J Clin (2021) 71(3):264–79. doi: 10.3322/caac.21657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Blum D, de Wolf-Linder S, Oberholzer R, Brändle M, Hundsberger T, Strasser F. Natural ghrelin in advanced cancer patients with cachexia, a case series. J Cachexia Sarcopenia Muscle (2021) 12(2):506–16. doi: 10.1002/jcsm.12659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chopin LK, Seim I, Walpole CM, Herington AC. The ghrelin axis–does it have an appetite for cancer progression? Endocrine Rev (2012) 33(6):849–91. doi: 10.1210/er.2011-1007 [DOI] [PubMed] [Google Scholar]
- 49. Waseem T, Javaid Ur R, Ahmad F, Azam M, Qureshi MA. Role of ghrelin axis in colorectal cancer: a novel association. Peptides. (2008) 29(8):1369–76. doi: 10.1016/j.peptides.2008.03.020 [DOI] [PubMed] [Google Scholar]
- 50. Grönberg M, Nilsson C, Markholm I, Hedenfalk I, Blomqvist C, Holmberg L, et al. Ghrelin expression is associated with a favorable outcome in male breast cancer. Sci Rep (2018) 8(1):13586. doi: 10.1038/s41598-018-31783-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, et al. Helicobacter pylori infection and the development of gastric cancer. New Engl J Med (2001) 345(11):784–9. doi: 10.1056/NEJMoa001999 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.