Abstract
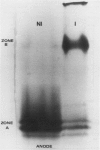
Increased activities of peroxidase and indole 3-acetic acid (IAA) oxidase were detected on root surfaces of bean (Phaseolus vulgaris) seedlings colonized with a soil saprophytic bacterium, Pseudomonas putida. IAA oxidase activity increased over 250-fold and peroxidase 8-fold. Enhancement was greater for 6-day-old than for 4- or 8-day-old inoculated plants No IAA oxidase or peroxidase activities were associated with the bacterial cells. Native polyacrylamide gel electrophoresis demonstrated that washes of P. putida-inoculated roots contained two zones of peroxidase activity. Only the more anodic bands were detected in washes from noninoculated roots. Ion exchange and molecular sizing gel chromatography of washes from P. putida-colonized roots separated two fractions of peroxidase activity. One fraction corresponded to the anodic bands detected in washes of P. putida inoculated and in noninoculated roots. A second fraction corresponded to the less anodic zone of peroxidase, which was characteristic of P. putida-inoculated plants. This peroxidase had a higher IAA oxidase to peroxidase ratio than the more anodic, common enzyme. The changes in root surface peroxidases caused by colonization by a saprophytic bacterium are discussed with reference to plant-pathogen interactions.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berger R. G., Drawert F., Kinzkofer A., Kunz C., Radola B. J. Proteins and peroxidase in callus and suspension cultures of apple : a study using ultrathin-layer isoelectric focusing, sensitive silver staining of proteins, and peroxidase isozyme visualization. Plant Physiol. 1985 Jan;77(1):211–214. doi: 10.1104/pp.77.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birecka H., Briber K. A., Catalfamo J. L. Comparative studies on tobacco pith and sweet potato root isoperoxidases in relation to injury, indoleacetic Acid, and ethylene effects. Plant Physiol. 1973 Jul;52(1):43–49. doi: 10.1104/pp.52.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. B., Varner J. E. Cross-linking of soluble extensin in isolated cell walls. Plant Physiol. 1984 Oct;76(2):414–417. doi: 10.1104/pp.76.2.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry S. C. Isodityrosine, a new cross-linking amino acid from plant cell-wall glycoprotein. Biochem J. 1982 May 15;204(2):449–455. doi: 10.1042/bj2040449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyle M. C. High resolution of peroxidase-indoleacetic Acid oxidase isoenzymes from horseradish by isoelectric focusing. Plant Physiol. 1977 Nov;60(5):787–793. doi: 10.1104/pp.60.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lee T. T. Changes in indoleacetic Acid oxidase isoenzymes in tobacco tissues after treatment with 2,4-dichlorophenoxyacetic Acid. Plant Physiol. 1972 Jun;49(6):957–960. doi: 10.1104/pp.49.6.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marugg J. D., van Spanje M., Hoekstra W. P., Schippers B., Weisbeek P. J. Isolation and analysis of genes involved in siderophore biosynthesis in plant-growth-stimulating Pseudomonas putida WCS358. J Bacteriol. 1985 Nov;164(2):563–570. doi: 10.1128/jb.164.2.563-570.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roby D., Toppan A., Esquerré-Tugayé M. T. Cell surfaces in plant-microorganism interactions : v. Elicitors of fungal and of plant origin trigger the synthesis of ethylene and of cell wall hydroxyproline-rich glycoprotein in plants. Plant Physiol. 1985 Mar;77(3):700–704. doi: 10.1104/pp.77.3.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Showalter A. M., Bell J. N., Cramer C. L., Bailey J. A., Varner J. E., Lamb C. J. Accumulation of hydroxyproline-rich glycoprotein mRNAs in response to fungal elicitor and infection. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6551–6555. doi: 10.1073/pnas.82.19.6551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sijmons P. C., Kolattukudy P. E., Bienfait H. F. Iron Deficiency Decreases Suberization in Bean Roots through a Decrease in Suberin-Specific Peroxidase Activity. Plant Physiol. 1985 May;78(1):115–120. doi: 10.1104/pp.78.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]